Abstract
While degrading 2,4-dichlorophenol, two strains of Gloeophyllum striatum, a basidiomycetous fungus causing brown rot decay of wood, simultaneously produced 4-chlorocatechol and 3,5-dichlorocatechol. These metabolites were identified by comparing high-performance liquid chromatography retention times and mass spectral data with those of chemically synthesized standards. Under similar conditions, 3-hydroxyphthalic hydrazide was generated from phthalic hydrazide, a reaction assumed to indicate hydroxyl radical formation. Accordingly, during chemical degradation of 2,4-dichlorophenol by Fenton's reagent, identical metabolites were formed. Both activities, the conversion of 2,4-[U-14C]dichlorophenol into 14CO2 and the generation of 3-hydroxyphthalic hydrazide, were strongly inhibited by the hydroxyl radical scavenger mannitol and in the absence of iron. These results provide new evidence in favor of a Fenton-type degradation mechanism operative in Gloeophyllum.
Basidiomycetous fungi causing brown rot decay of wood play an important role in the carbon cycle. They depolymerize the cellulose component of wood while lignin remains as an amorphous brown residue. In this process, an extracellular Fenton-type mechanism, providing hydroxyl radicals via Fe2+ and hydrogen peroxide, has long been implicated (15). However, the potential of species like Gloeophyllum striatum and Gloeophyllum trabeum in the degradation of xenobiotics was explored only recently: two fluoroquinolone antibacterial drugs, polyethylene glycol, and two chlorophenols have been shown to be decomposed (9, 14, 23, 24). G. striatum produced three remarkable hydroxylated congeners of enrofloxacin, which could also be generated via Fenton's reaction (23). An extracellular 2,5-dimethoxyhydroquinone-driven Fenton reaction in G. trabeum, employed to depolymerize polyethylene glycol, represents the most recent mechanistic evidence (14).
Wheat straw cultures of G. striatum catalyzed CO2 production from the environmental pollutant 2,4-dichlorophenol (2,4-DCP). Moreover, in a defined mineral medium lacking carbon, nitrogen, and phosphate, G. striatum expressed a degradation capacity similar to that shown on wheat straw (9, 23). Here, our aim was to provide further evidence for a Fenton-type reaction in G. striatum by (i) identifying primary metabolites of 2,4-DCP formed by the fungus and in Fenton's reaction as well; (ii) testing for hydroxylation of phthalic hydrazide (PH) giving 3-hydroxyphthalic hydrazide (3-OHPH), which has been postulated to specifically indicate hydroxyl radical formation (1, 2, 19); and (iii) inhibiting the conversion of 2,4-[U-14C]DCP to 14CO2 as well as hydroxylation of PH under conditions antagonizing hydroxyl radical activity. Such data are intended to help to firmly establish the capability of some higher fungi to employ a Fenton-type reaction in the degradation of xenobiotics. This reaction may even take different forms in the few genera investigated so far (13, 14).
MATERIALS AND METHODS
Organisms.
The source and maintenance of G. striatum DSM 9592 and G. striatum DSM 10335 have been described in reference 23.
Degradation of 2,4-[U-14C]DCP by G. striatum or Fenton's reagent.
2,4-[U-14C]DCP (Sigma-Aldrich Chemie, Steinheim, Germany) had a specific activity of 9.3 mCi per mmol and a radiochemical purity of >95%, assessed as described previously (9). G. striatum, pregrown on malt medium, was transferred into a mineral medium (23) containing 20 μM FeSO4. After addition of 20 μM 2,4-DCP (labeled with 0.3 kBq), culture vessels were incubated at 100 rpm and 24°C in the dark. Uninoculated flasks served as controls. 14CO2 and 14C-labeled volatile organic compounds were quantified as described in reference 9. Radioactivity bound to mycelia was extracted three times with 5 ml of methanol. Aliquots of 1 ml of combined extracts and of culture supernatants were subjected to liquid scintillation counting (9). Dry weight was determined as described in reference 11. Mycelia were combusted in an OX 500 biological oxidizer (Zinsser Analytik, Frankfurt, Germany). Fenton's reagent (method A) consisted of 0.5 mM FeSO4 and 1% hydrogen peroxide in 10 ml of 5 mM sodium acetate buffer, pH 4.5, 2,4-DCP was applied as described above.
Hydroxylation of PH by G. striatum or in Fenton's reaction.
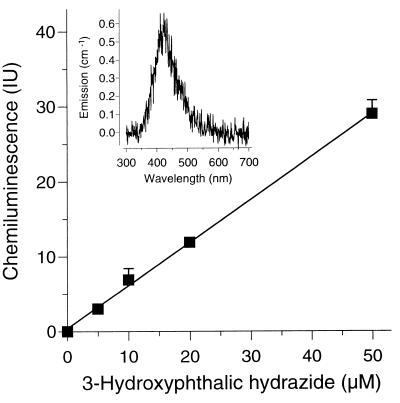
The formation of 3-OHPH was detected by chemiluminescence (1), recorded on an RF-5001 PC fluorophotometer (Shimadzu, Duisburg, Germany) at a λem of 300 to 700 nm (λmax = 415 nm [2]). Fenton's reagent was modified to contain 50 mM acetate buffer (method B). PH was applied at a final concentration of 0.1 g/liter (1). Fungal cultures or Fenton's reagent without PH served as a blank. Chemically prepared 3-OHPH (6) (purity, >98%, as checked by high-performance liquid chromatography [HPLC] at 310 nm) was employed to calibrate the chemiluminescence signal (Fig. 1) and to identify, by HPLC, the 3-OHPH produced, using a Merck-Hitachi HPLC system (method I) described before (11). PH and 3-OHPH were separated at an isocratic flow rate of 0.5 ml/min, using methanol and 0.1% phosphoric acid (45:55 [vol/vol]).
FIG. 1.
Dependence of chemiluminescence on the concentration of 3-OHPH. Its intensity is expressed as integration units (IU) over the emission range of 350 to 550 nm. Values given are the means ± standard deviations for triplicate measurements. The inset shows the emission spectrum of chemiluminescence in a typical sample.
Isolation of metabolites.
Cultures of G. striatum in mineral medium (23) as well as Fenton's reagent (method C; now consisting of 5 μM FeSO4 and 0.01% hydrogen peroxide in 100 ml of 0.5 mM acetate buffer, pH 4.5) were supplemented with 250 μM 2,4-DCP and incubated for 48 h. Thereafter, fungal mycelia were removed by filtration. Culture supernatants and Fenton assay mixtures were adjusted to pH 2.0 with H3PO4. After three extractions with ethyl acetate, combined extracts were dried over anhydrous sodium sulfate. Following evaporation of the solvent at 40°C, dry matter was redissolved in methanol. To detect metabolites of 2,4-DCP, analytical HPLC was performed on an HPLC system, HP 1050 (method II), as described in reference 23. Metabolites were eluted by employing a linear gradient over 30 min.
HPLC-electrospray ionization mass spectrometry (HPLC-MS).
HPLC-MS was performed on HPLC system 140B (Applied Biosystems Inc., Foster City, Calif.) equipped with a Kromasil 100 C18 column and linked to a MAT 900 MS as described in reference 24. The elution solvent consisted of 0.01% formic acid and acetonitrile. Starting at 100%, formic acid was linearly decreased to 0% over 30 min. The effluent of 200 μl/min was passed on to the MS interface without splitting. The range of m/z, 138 to 1,400, was scanned in the negative mode within 3 s.
Chemicals.
3,5-Dichlorocatechol, synthesized by Schwien et al. (20), was kindly provided by S. Kaschabeck (Stuttgart, Germany). 4-Chlorocatechol is available from Chemos GmbH (Regenstauf, Germany). All other chemicals were purchased from Sigma-Aldrich Chemie or Merck (Darmstadt, Germany).
RESULTS
Metabolites of 2,4-DCP formed by G. striatum or in Fenton's reaction.
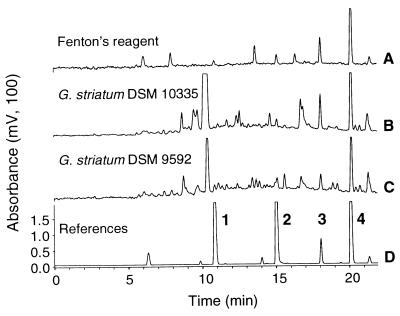
HPLC analysis of supernatants of 48-h-old cultures of G. striatum DSM 9592 and G. striatum DSM 10335 provided profiles of metabolites of 2,4-DCP (Fig. 2). A profile obtained from Fenton's reaction after 48 h was also included; structures of primary congeners have not yet been reported (21). Two metabolites (2 and 3) were present in all profiles and had retention times identical to those of the standards, 4-chlorocatechol and 3,5-dichlorocatechol. MS analysis of these metabolites revealed molecular weights of 144 and 178, deduced from characteristic patterns of pseudomolecular ions due to the isotopy of chlorine and in accordance with its natural abundance. 4-Chlorocatechol could clearly be distinguished from a potential structural alternative, 2-chlorohydroquinone, by its retention time (Fig. 2). The structures of other metabolites produced by G. striatum remain to be elucidated. Apparently, some of those were also generated in Fenton's reaction or were already present as impurities in the mixture of standards, most likely indicating its oxidative decomposition.
FIG. 2.
HPLC elution profiles indicating metabolites of 2,4-DCP produced either by chemical degradation with Fenton's reagent (A) or by G. striatum DSM 10335 (B) or G. striatum DSM 9592 (C). A mixture of standard compounds (D) consisted of 2-chlorohydroquinone (1), 4-chlorocatechol (2), 3,5-dichlorocatechol (3), and 2,4-DCP (4), detected at retention times of 10.8, 15.0, 18.0, and 20.1 min, respectively.
14CO2 formation from 2,4-[U-14C]DCP by G. striatum.
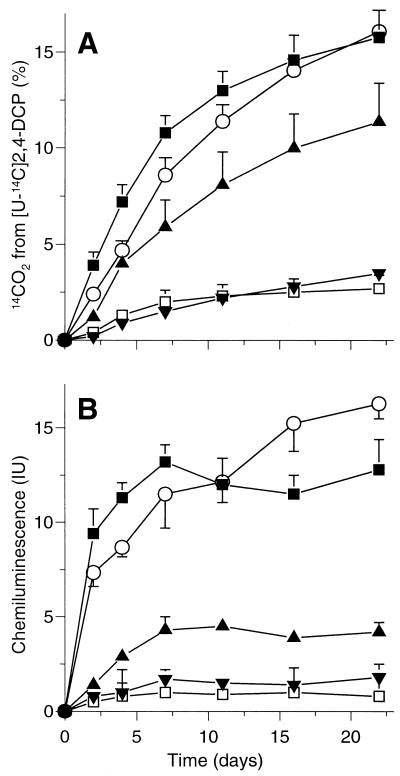
The time course of 14CO2 production from 2,4-[U-14C]DCP by liquid cultures of G. striatum DSM 9592 is depicted in Fig. 3A. In mineral medium containing 20 μM FeSO4, 15.8% of the applied radioactivity was liberated as 14CO2 during 22 days. When mannitol was present at 5.6 or 56 mM, 14CO2 production was inhibited by 28 and 78%, respectively. In contrast, 56 mM glucose only delayed 14CO2 formation. When FeSO4 was omitted from the medium, 14CO2 formation was inhibited by 83%; residual activity was likely due to contaminating iron.
FIG. 3.
Total 14CO2 production from 2,4-[U-14C]DCP (A) and chemiluminescence indicating the formation of 3-OHPH from PH (B) by liquid cultures of G. striatum DSM 9592 in a mineral medium (■) and in mineral medium supplemented with either 56 mM glucose (○), 5.6 mM mannitol (▴), or 56 mM mannitol (▾). Activities resulting upon omission of FeSO4 from mineral medium (□) are shown. Values shown are the means ± standard deviations for triplicate cultures.
Total amounts of 14CO2 produced, mycelial dry weight determined after 22 days of incubation, and dry weight-based 14CO2 production are summarized in Table 1. Although similar absolute amounts of 14CO2 were obtained in mineral medium and in mineral medium containing 56 mM glucose, in the latter fungal dry weight had almost tripled, indicating extensive growth of G. striatum. Hence, dry weight-based 14CO2 production was markedly reduced. Mannitol, supporting growth to a lesser extent and, most likely, acting as hydroxyl radical scavenger (7), caused massive inhibition of dry weight-based 14CO2 production. A similarly low value was observed when iron was omitted from the medium.
TABLE 1.
14CO2 produced from 2,4-[U-14C]DCP by G. striatum DSM 9592 after 22 daysa
| Medium | Total 14CO2 (nmol) | Fungal dry wt (mg) | 14CO2/fungal dry wt (nmol/mg) |
|---|---|---|---|
| Mineral medium | 189.6 ± 16.8 | 23.4 ± 2.1 | 8.1 ± 3.7 |
| Glucose added at 56 mM | 193.2 ± 13.2 | 68.3 ± 2.3b | 2.8 ± 1.7 |
| Mannitol added at 56 mM | 42.0 ± 3.6b | 44.4 ± 1.5b | 0.9 ± 0.6 |
| FeSO4 omitted | 32.4 ± 8.4b | 30.8 ± 1.7 | 1.0 ± 1.5 |
Values given are the means ± standard deviations for triplicate cultures. Standard deviations for dry weight-based production of 14CO2 were calculated according to Gauss' law of errors.
Significantly different from values obtained in mineral medium (P < 0.02), as obtained by Tukey's honestly significant difference test.
Balance of radioactivity.
The distribution of 14C label in the compartments of cultures of G. striatum DSM 9592 is shown in Table 2. 14CO2 formation was highest in mineral medium, but it represented only a small fraction when mannitol was present or when iron was omitted. Compared to cultures in mineral medium, radioactivity collected in the traps for volatile compounds was approximately doubled in the presence of 56 mM mannitol or in the absence of FeSO4. This indicated conversion of 2,4-DCP into congeners having a higher volatility than the parent compound. About 40% of the applied radioactivity remained in the supernatants of cultures in unsupplemented and glucose-amended mineral medium. This amount was reduced to 30%, if the medium was devoid of FeSO4. Negligible radioactivity was recovered by methanolic extraction of mycelia, while between 5.0 and 8.6% of 14C was detected upon their combustion.
TABLE 2.
Distribution of radioactivity (percent) within cultures of G. striatum DSM 9592 after 22 days of incubation in mineral mediuma
| Fraction | Uninoculated control | No modification | Mannitol added at 56 mM | FeSO4 omitted |
|---|---|---|---|---|
| 14CO2 | 0.2 ± 0.1 | 15.8 ± 1.4b | 3.5 ± 0.3b | 2.7 ± 0.7b |
| Volatile organic compounds | 9.2 ± 1.5 | 18.6 ± 1.0b | 27.7 ± 1.8bc | 41.8 ± 2.3bc |
| Culture supernatant | 81.9 ± 0.5 | 40.3 ± 1.7b | 39.0 ± 3.4b | 30.1 ± 0.5bc |
| Methanol extract | 0.9 ± 0.2 | 2.0 ± 0.3 | 1.3 ± 0.2 | |
| Mycelium | 8.6 ± 0.4 | 7.1 ± 1.7 | 5.0 ± 0.1 | |
| Sum | 91.3 ± 3.6 | 84.2 ± 2.5 | 79.3 ± 4.2 | 80.9 ± 2.5 |
Values given are the means ± standard deviations for triplicate cultures. Standard deviations for the sum of radioactivity were calculated according to Gauss' law of errors.
Significantly different from the uninoculated control (P < 0.02), as obtained by Tukey's honestly significant difference test.
Significantly different from mineral medium without modification (P < 0.02).
Hydroxylation of PH by G. striatum.
When PH (instead of 2,4-DCP) was present in mineral medium, G. striatum DSM 9592 formed 3-OHPH; its oxidation under alkaline conditions gives rise to chemiluminescence (1). This was detected throughout the incubation period of 22 days (Fig. 3B). The identity of 3-OHPH was confirmed by HPLC analysis: its retention time of 9.8 min (compared to 6.7 min for PH) and its UV spectrum could be verified using chemically prepared 3-OHPH. In agreement with earlier reports, 3-OHPH showed one major and two minor absorbance maxima at 321, 334, and 277 nm, respectively (16), whereas PH exhibited a major maximum at 292 nm and a minor one at 262 nm (8). The addition of 56 mM (and even of 5.6 mM) mannitol strongly inhibited chemiluminescence (Fig. 3B). Glucose at 56 mM caused only a slight reduction in its intensity during the first days (transient quenching), and chemiluminescence tended to be highest from day 10 onward. Probably, this was due to consumption of glucose and an increased biomass. The omission of FeSO4 from the medium resulted in drastically reduced chemiluminescence.
Decomposition of 2,4-DCP and PH by Fenton's reagent.
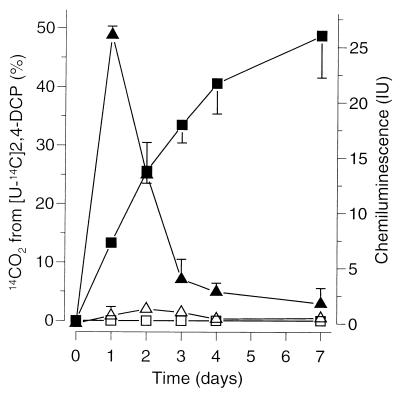
Cumulative 14CO2 production from 2,4-[U-14C]DCP caused by Fenton's reagent is depicted in Fig. 4. After 7 days, 48.8% of the applied radioactivity could be attributed to 14CO2 (9). No 14CO2 was liberated when FeSO4 was omitted. Chemiluminescence was most prominent until day 2. Again, the formation of 3-OHPH was confirmed by HPLC analysis. Upon exposure of 3-OHPH (instead of PH) to Fenton's reagent, essentially no chemiluminescence was detected. Instead, the reaction mixture turned brownish, indicating decomposition of 3-OHPH. No chemiluminescence was detected in the absence of FeSO4. Glucose had been shown before to strongly inhibit the formation of 3-OHPH (19).
FIG. 4.
Total 14CO2 production from 2,4-[U-14C]DCP (squares) and chemiluminescence indicating the formation of 3-OHPH (triangles) by Fenton's reagent in the presence (closed symbols) and in the absence (open symbols) of FeSO4. Values shown are the means ± standard deviations for triplicate experiments.
DISCUSSION
During the degradation of 2,4-DCP by either G. striatum or Fenton's reagent, two dihydroxylated metabolites, 4-chlorocatechol and 3,5-dichlorocatechol, were formed simultaneously (Fig. 5). In aerobic bacteria and imperfect fungi, decomposition of chlorophenols is initiated by hydroxylating enzymes which have been located intracellularly. In these organisms, 3,5-dichlorocatechol is a known metabolite of 2,4-DCP (12, 18, 20), while 4-chlorocatechol was detected in Penicillium frequentans as a congener of 4-chlorophenol (12). Intracellular hydroxylation was also observed in white rot fungi: Phanerochaete chrysosporium produced 4,5-dichlorocatechol from 3,4-dichlorophenol (5).
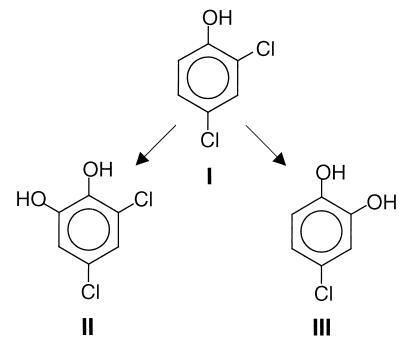
FIG. 5.
Primary metabolites of 2,4-DCP (I) produced by the brown rot basidiomycete G. striatum. 3,5-Dichlorocatechol (II) and 4-chlorocatechol (III) are proposed to be formed simultaneously in a hydroxyl radical-driven reaction resulting in hydroxylation of the positions ortho to the phenolic group of 2,4-DCP.
Aromatic hydroxylation involving chemically generated hydroxyl radicals has been studied with a variety of compounds (10, 21, 22). From salicylic acid, congeners hydroxylated in ortho and para positions were formed: the generation of 2,3-dihydroxybenzoate and 2,5-dihydroxybenzoate has been used to detect hydroxyl radicals (7, 13). If exposed to hydroxyl radicals generated by gamma radiolysis of water, para-substituted phenols were hydroxylated predominantly in the ortho position. In addition, the amount of catechols thus formed was increased by electron-withdrawing substituents such as cyano or nitro groups in the para position (22). Because of the chlorine at C-4, the formation of 3,5-dichlorocatechol from 2,4-DCP could be expected in Fenton's reaction. However, concurrent formation of 4-chlorocatechol, indicating hydroxyl radical-driven dechlorination, appears to fully reflect the ortho-specific action of hydroxyl radicals. In Gloeophyllum, this activity is most likely located extracellularly (14). The formation of 3,5-dichlorocatechol and 4-chlorocatechol from 2,4-DCP parallels the hydroxylation of enrofloxacin by G. striatum and in Fenton's reaction in the ortho position to the piperazine moiety, i.e., at C-6, eliminating fluorine, and at C-8 (23).
The production of 14CO2 from 2,4-[U-14C]DCP by G. striatum (and Fenton's reagent) indicates ring cleavage of the catechol intermediates. Alternatively, chlorocatechols can be degraded further by intracellular catechol-1,2-dioxygenases (12, 18, 20). However, no such enzymes were detected in the brown rot species Daedala quercina, Fomes pinicola, and Lenzites trabea, whereas all of these strains catalyzed an intradiol cleavage of 1,2,4-trihydroxybenzene (3), another potential metabolite of 2,4-DCP formed by G. striatum. The presence of a Fenton-type reaction mechanism in G. striatum is further supported by the effect of the hydroxyl radical scavenger mannitol (7), which strongly inhibited 14CO2 production from 2,4-[U-14C]DCP as well as hydroxylation of PH. Moreover, Fe2+ is an essential component of Fenton's reagent in serving as an electron donor for hydrogen peroxide, thus causing hydroxyl radical formation (7, 13). Its addition to cultures of G. striatum greatly enhanced mineralization of 2,4-DCP as well as hydroxylation of PH. Recently, studies have shown that the brown rot fungus G. trabeum produced 2,5-dimethoxyhydroquinone and 4,5-dimethoxycatechol, which have been proposed to mediate Fe3+ reduction and hydrogen peroxide production by redox cycling (14, 17). All of these findings are in support of an extracellular Fenton-type reaction mechanism in Gloeophyllum.
Finally, the formation of metabolites of higher volatility than 2,4-DCP was enhanced by the addition of the hydroxyl radical quencher mannitol or by omitting Fe2+. The structures of such metabolites remain to be elucidated. The production of 3,4-dichloroanisole from 3,4-dichlorophenol by Phanerochaete chrysosporium, e.g., was attributed to cell-associated methylation (3), a process frequently observed in basidiomycetes (4). In conclusion, the simultaneous occurrence of 4-chlorocatechol and 3,5-dichlorocatechol, the dependence on iron and the effect of mannitol on CO2 production from 2,4-DCP, and the generation of 3-OHPH from PH are in support of an extracellular Fenton-type degradation mechanism employed by G. striatum. However, straw cultures of G. striatum did not show 14CO2 formation from 2,4,6-[U-14C]trinitrotoluene or [U-14C]pyrene (K. Fahr and D. Schlosser, unpublished data), which may indicate mechanistic limitations. Hence, the general role of Fenton chemistry-based reactions in the environmental fate of xenobiotics remains to be defined.
ACKNOWLEDGMENTS
This work was supported by Thüringer Ministerium für Wissenschaft, Forschung und Kultur (grant B 303-95004).
We thank A. Orthaus and J. Schneider for excellent technical assistance.
Footnotes
Dedicated to G. Gottschalk, University of Göttingen, on the occasion of his 65th birthday.
REFERENCES
- 1.Backa S, Gierer J, Reitberger T, Nilsson T. Hydroxyl radical activity in brown-rot fungi studied by a new chemiluminescence method. Holzforschung. 1992;46:61–67. [Google Scholar]
- 2.Backa S, Jansbo K, Reitberger T. Detection of hydroxyl radicals by a chemiluminescence method—a critical review. Holzforschung. 1997;51:557–564. [Google Scholar]
- 3.Buswell J A, Eriksson K-E, Gupta J K, Hamp S G, Nordh I. Vanillic acid metabolism by selected soft-rot, brown-rot, and white-rot fungi. Arch Microbiol. 1982;131:366–374. [Google Scholar]
- 4.De Jong E, Field J A. Sulfur tuft and turkey tail: biosynthesis and biodegradation of organohalogens by basidiomycetes. Annu Rev Microbiol. 1997;51:375–414. doi: 10.1146/annurev.micro.51.1.375. [DOI] [PubMed] [Google Scholar]
- 5.Deschler C, Duran R, Junqua M, Landou C, Salvado J-C, Goulas P. Involvement of 3,4-dichlorophenol hydroxylase in degradation of 3,4-dichlorophenol by the white rot fungus Phanerochaete chrysosporium. J Mol Catal B Enzym. 1998;5:423–428. [Google Scholar]
- 6.Drew H D K, Pearmann F H. Chemiluminescent organic compounds. Part II. The effect of substituents on the closure of phthalhydrazides to 5- and 6-membered rings. J Chem Soc. 1937;1937:26–33. [Google Scholar]
- 7.Elstner E F. Der Sauerstoff. Biochemie, Biologie, Medizin. Mannheim, Germany: Wissenschaftsverlag; 1990. pp. 22–40. [Google Scholar]
- 8.Elvidge J A, Redman A P. 1,4-Dimethoxyphthalazine and the other O- and N-methyl derivatives of phthalhydrazide. J Chem Soc. 1960;1960:1710–1714. [Google Scholar]
- 9.Fahr K, Wetzstein H-G, Grey R, Schlosser D. Degradation of 2,4-dichlorophenol and pentachlorophenol by two brown rot fungi. FEMS Microbiol Lett. 1999;175:127–132. doi: 10.1111/j.1574-6968.1999.tb13611.x. [DOI] [PubMed] [Google Scholar]
- 10.Gierer J, Yang E, Reitberger T. The reactions of hydroxyl radicals with aromatic rings in lignins, studied with creosol and 4-methylveratrol. Holzforschung. 1992;46:495–504. [Google Scholar]
- 11.Grey R, Höfer C, Schlosser D. Degradation of 2-chlorophenol and formation of 2-chloro-1,4-benzoquinone by mycelia and cell-free crude culture liquids of Trametes versicolor in relation to extracellular laccase activity. J Basic Microbiol. 1998;38:371–382. [PubMed] [Google Scholar]
- 12.Hofrichter M, Bublitz F, Fritsche W. Unspecific degradation of halogenated phenols by the soil fungus Penicillium frequentans Bi 7/2. J Basic Microbiol. 1994;34:163–172. doi: 10.1002/jobm.3620340306. [DOI] [PubMed] [Google Scholar]
- 13.Hyde S M, Wood P M. A mechanism for the production of hydroxyl radicals by the brown-rot fungus Coniophora putanea: Fe(II) reduction by cellobiose dehydrogenase and Fe(II) oxidation at a distance from the hyphae. Microbiology. 1997;143:259–266. doi: 10.1099/00221287-143-1-259. [DOI] [PubMed] [Google Scholar]
- 14.Kerem Z, Jensen K A, Hammel K E. Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven Fenton reaction. FEBS Lett. 1999;446:49–54. doi: 10.1016/s0014-5793(99)00180-5. [DOI] [PubMed] [Google Scholar]
- 15.Koenigs J W. Hydrogen peroxide and iron: a proposed system for decomposition of wood by brown-rot basidiomycetes. Wood Fiber. 1974;6:66–80. [Google Scholar]
- 16.Ojima H. Chemiluminescence and metal chelate formation properties of 5-hydroxyphthalohydrazide. Bull Aichi Univ Educ. 1987;36:39–48. [Google Scholar]
- 17.Paszczynski A, Crawford R, Funk D, Goodell B. De novo synthesis of 4,5-dimethoxycatechol and 2,5-dimethoxyhydroquinone by the brown rot fungus Gloeophyllum trabeum. Appl Environ Microbiol. 1999;65:674–679. doi: 10.1128/aem.65.2.674-679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins E J, Gordon M P, Caceres O, Lurquin P F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990;172:2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiller J, Arnold J, Schwinn J, Sprinz H, Brede O, Arnold K. Reactivity of cartilage and selected carbohydrates with hydroxyl radicals. An NMR study to detect degradation products. Free Radic Res. 1998;28:215–228. doi: 10.3109/10715769809065806. [DOI] [PubMed] [Google Scholar]
- 20.Schwien U, Schmidt E, Knackmuss H-J, Reineke W. Degradation of chlorosubstituted aromatic compounds by Pseudomonas sp. strain b13: fate of 3,5-dichlorocatechol. Arch Microbiol. 1988;150:78–84. [Google Scholar]
- 21.Tang W Z, Huang C P. 2,4-Dichlorophenol oxidation kinetics by Fenton's reagent. Environ Technol. 1996;17:1371–1376. [Google Scholar]
- 22.Urano Y, Higuchi T, Hirobe M. Unusual substituent effects in the hydroxylation of phenols by a Cu2+-ascorbic acid-O2 system, γ-radiolysis, and microsomes. Biochem Biophys Res Commun. 1993;192:568–574. doi: 10.1006/bbrc.1993.1453. [DOI] [PubMed] [Google Scholar]
- 23.Wetzstein H-G, Schmeer N, Karl W. Degradation of the fluoroquinolone enrofloxacin by the brown rot fungus Gloeophyllum striatum: identification of metabolites. Appl Environ Microbiol. 1997;63:4272–4281. doi: 10.1128/aem.63.11.4272-4281.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetzstein H-G, Stadler M, Tichy H-V, Dalhoff A, Karl W. Degradation of ciprofloxacin by basidiomycetes and identification of metabolites generated by the brown rot fungus Gloeophyllum striatum. Appl Environ Microbiol. 1999;65:1556–1563. doi: 10.1128/aem.65.4.1556-1563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]