Summary
Background
Childhood tuberculosis (TB) remains underdiagnosed largely because of limited awareness and poor access to all or any of specimen collection, molecular testing, clinical evaluation, and chest radiography at low levels of care. Decentralising childhood TB diagnostics to district hospitals (DH) and primary health centres (PHC) could improve case detection.
Methods
We conducted an operational research study using a pre-post intervention cross-sectional study design in 12 DHs and 47 PHCs of 12 districts across Cambodia, Cameroon, Côte d’Ivoire, Mozambique, Sierra Leone and Uganda. The intervention included 1) a comprehensive diagnosis package at patient-level with tuberculosis screening for all sick children and young adolescents <15 years, and clinical evaluation, Xpert Ultra-testing on respiratory and stool samples, and chest radiography for children with presumptive TB, and 2) two decentralisation approaches (PHC-focused or DH-focused) to which districts were randomly allocated at country level. We collected aggregated and individual data. We compared the proportion of tuberculosis detection in children and young adolescents <15 years pre-intervention (01 August 2018–30 November 2019) versus during intervention (07 March 2020–30 September 2021), overall and by decentralisation approach. This study is registered with ClinicalTrials.gov, NCT04038632.
Findings
TB was diagnosed in 217/255,512 (0.08%) children and young adolescent <15 years attending care pre-intervention versus 411/179,581 (0.23%) during intervention, (OR: 3.59 [95% CI 1.99–6.46], p-value<0.0001; p-value = 0.055 after correcting for over-dispersion). In DH-focused districts, TB diagnosis was 80/122,570 (0.07%) versus 302/86,186 (0.35%) (OR: 4.07 [1.86–8.90]; p-value = 0.0005; p-value = 0.12 after correcting for over-dispersion); and 137/132,942 (0.10%) versus 109/93,395 (0.11%) in PHC-focused districts, respectively (OR: 2.92 [1.25–6.81; p-value = 0.013; p-value = 0.26 after correcting for over-dispersion).
Interpretation
Decentralising and strengthening childhood TB diagnosis at lower levels of care increases tuberculosis case detection but the difference was not statistically significant.
Funding source
Unitaid, Grant number 2017-15-UBx-TB-SPEED
Keywords: Decentralisation, Child, Tuberculosis, Diagnosis
Research in context.
Evidence before this study
We searched PubMed for decentralization of TB services from Jan 1 2000 to Dec 31 2022, with the terms ((decentral∗[Title]) AND (TB [Title] OR tb [Title]) AND (servic∗[Title] OR child∗[Title]) AND (“2000/01/01” [Date–Entry]: “2022/12/31” [Date–Entry])) restricted to English. This search yielded 15 articles; studies that were not focused on decentralizing childhood TB services were excluded leaving 2 studies. One study showed a positive impact on TB detection while one study showed increased screening but not diagnosis of multidrug resistant TB. These studies demonstrating the positive impact of decentralizing TB services were limited to one country and geographical region. A study by Zawedde et al. in Uganda using a Before-and-after analysis showed that the proportion of child TB among all TB notified increased from 8.8% to 15%. Another study by Seddon et al. showed that decentralized care led to an increased number of child contacts being evaluated for MDR TB. None of the published studies addressed the important question of what, where and how much to decentralize while considering the health system structures and task shifting. In 2022, WHO recommended decentralised models of care to deliver TB services to children and adolescents. The recommendation referred to enhancing child and adolescent TB services at the peripheral health level nearer to the communities alongside the specialised paediatric TB services at higher levels. The WHO guideline development group rated the overall certainty of evidence as “very low” and emphasized key implementation considerations to decentralise TB services.
Added value of this study
This multi-country implementation study using a pre-post design assessed the effect on TB detection among sick children by decentralizing a comprehensive childhood TB diagnosis package at primary health centres (PHC) and/or district hospital (DH) levels in districts from six countries with high or very TB incidence using two decentralised approaches. Implementing systematic screening for TB among sick children <15 years attending care, and a full clinical evaluation, Ultra testing of NPA and stool or expectorated sputum, and chest X-Ray using a standardised approach in those identified with presumptive TB led to nearly tripled the detection of children with TB as compared to pre-intervention data. After correcting for overdispersion the difference between the pre- and post-intervention TB detection was not statistically significant due to the variability in the contexts of the different countries. The DH-focused approach had a larger effect on the detection of children with TB than the PHC-focused approach. This study provides evidence across several countries with varying health systems strength, on the feasibility and effectiveness of decentralising childhood TB diagnosis.
Implications of all the available evidence
Together with evidence from previous studies assessing the feasibility of decentralising a single component, the effectiveness and feasibility of decentralising a comprehensive childhood TB diagnosis package in different settings and health systems further strengthens evidence supporting the WHO recommendation on decentralising childhood TB services. Decentralising childhood TB diagnosis is likely to reduce the gap between the number of estimated TB in children and the number notified to WHO, but the implementation of the decentralisation needs to be adapted to the relative low frequency of childhood TB and limited resources available in peripheral setting in order to maximize the access of children to molecular testing and Chest X-ray.
Introduction
The burden of childhood tuberculosis (TB) in children and young adolescents (aged below 15 years) is high worldwide, with an estimated 1.1 million new tuberculosis cases among children and 209,000 deaths in 2021.1 Between 2018 and 2021, only 54% of the estimated childhood tuberculosis cases were notified to the World Health Organisation (WHO).1
Low detection of tuberculosis in children is classically attributed to the difficulty to collect sputum in young children and to the low yield of current tuberculosis microbiological tests, due to the paucibacillary nature of tuberculosis in children.2, 3, 4 Alternative sputum specimen collection methods such as induced sputum or gastric aspirate are either poorly tolerated by children or difficult to implement in resource-limited settings. Another important factor for low detection of TB in children is structural. Childhood tuberculosis services are mostly centralized at high levels of care, thus poorly accessible to most children living in high incidence and resource-limited countries. Child-adapted respiratory specimen collection methods and rapid molecular testing are often lacking at lower levels of care. Chest X-ray, a useful tool for diagnosis of non-microbiologically confirmed tuberculosis, is often only available at referral hospitals, not affordable to patients, of poor quality and inadequately interpreted. In a survey of 15 secondary hospitals in Kenya, only 2% of children presenting with presumptive tuberculosis benefited from microbiological evaluation and a chest X-Ray.5 Few healthcare workers at district level are trained on childhood TB diagnosis resulting in low awareness of childhood TB and confidence in making a clinical diagnosis.
Recent advances in diagnostic approaches could contribute to improved childhood TB diagnosis at low levels of care and Clinical diagnosis of TB in children remains central. Simple symptom-based screening could identify children requiring further TB evaluation and the use of stool samples and nasopharyngeal aspirate (NPA) could improve microbiological sample collection among outpatients.6 Testing of NPA and stool samples with the newly developed rapid molecular assay Xpert MTB/RIF Ultra (Ultra; Cepheid, Sunnyvale, CA, USA), was recently recommended by WHO for the diagnosis of pulmonary TB in children.7 Combining NPA and stool testing using Xpert MTB/RIF achieves similar detection yield as gastric aspirates or induced sputum samples.8,9 Furthermore, there is evidence that the GeneXpert machines can be decentralized in peripheral centres using the battery-operated GeneXpert G1 Edge device.10,11 Lastly, simple digital system added to existing radiography could contribute to solve the problem of poor-quality chest X-ray, largely due to poor quality of reagents, and facilitate the interpretation and the transfer of images for double reading and quality assessment.
WHO recently recommended that decentralised models of care may be used to deliver TB services to children and adolescents in high TB incidence settings.7 This was a conditional recommendation with a low level of certainty due to limited high quality evidence. Providing evidence on the effect of decentralising childhood TB diagnosis to district hospitals (DH)- and primary health centres (PHC)-levels and sharing of experience on diagnosis components to be decentralised is expected to contribute to informed decision-making by national TB programs (NTPs) in resource-limited settings.
In this study we assessed the effect of deploying a comprehensive childhood TB diagnosis package on TB detection at DH and PHCs in six high TB incidence and resource-limited countries. We also compared the uptake of different components of the diagnostic package along the cascade of TB care within two specific decentralisation approaches.
Methods
Study design and setting
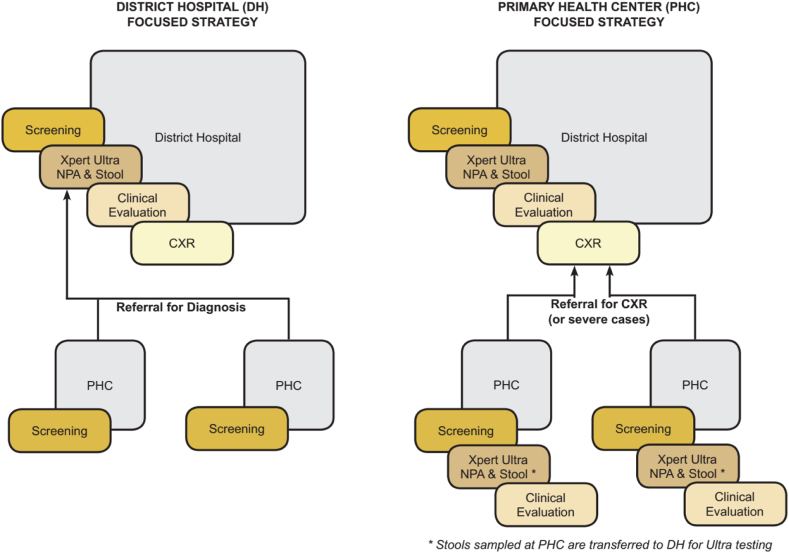
The methods adhere to the WHO operational reporting guidelines.12 We conducted an operational research study using a pre- and post-intervention cross-sectional design in two rural or semi-urban districts of six countries with high TB incidence (100–300/100,000 population, Cameroon, Côte d’Ivoire, Cambodia) or very high TB incidence (>300/100,000 population, Mozambique, Sierra Leone Uganda). The intervention was executed at two levels: at patient-level where a comprehensive childhood TB diagnosis package was implemented, and at health systems level where two distinct decentralisation approaches for deploying the patient-level intervention were randomly assigned to districts and compared. The two decentralisation approaches included (Fig. 1): i) the DH-focused approach in which the DH implemented the diagnosis package, while PHCs implemented TB screening only and referred children with presumptive TB to DH for further diagnostic assessment, and ii) the PHC-focused approach in which both the DH and PHCs implemented the childhood TB diagnosis package, except for CXR that was available at DH only. Two separate districts were selected to prevent risk of spillover effect between the two approaches. In each country, the two districts were randomly assigned to either the DH or PHC-focused approach based on a district randomization done by the central statistician stratified by country, at mid of the observation period.
Fig 1.
Study decentralisation approaches and health facility levels. DH focused strategy in which the PHCs only screen and refer presumptive TB to DH but the DH conducts screening, systematic CXRs, Xpert ultra on stool and NPA, and clinical evaluation for TB. PHC focused strategy in which at the PHC screening, xpert ultra testing on NPA and clinical evaluation are performed Participants are referred to the DH for CXR when indicated and the collected stool is referred to DH for testing. DH, District Hospital; PHC, Primary Health Centre; NPA, Nasopharyngeal aspirate; CXR, Chest Xray.
Participating districts were selected with each country NTP, based on a baseline assessment of district capacities and on pre-defined selection criteria.13 Overall the study was implemented in 12 DH—of which 10 had analogue radiography available prior to the study; and 47 PHCs—of which 40 (85%) were rural, and 16 (34%) were TB diagnostic and treatment units (see Supplementary Appendix).
The comprehensive childhood TB diagnosis package, consisted of; 1) systematic screening for TB among sick children and young adolescents <15 years attending care, and, for those identified with presumptive TB: 2) a full clinical evaluation, 3) microbiological testing of NPA and stool or expectorated sputum using Ultra, and 4) chest X-Ray using a simplified and standardized interpretation approach.
The study comprised: 1) pre-intervention (01 August 2018–30 November 2019); an observation period in which we documented 3-month prospective and 9-month retrospective facility attendance and TB detection, 2) a lead–in phase for district and health facility set-up and capacity building, and 3) an intervention period (07 March 2020–30 September 2021) during which we decentralized the comprehensive childhood TB diagnosis package and documented TB diagnosis at facility- and at individual-level. The start and end dates of periods varied across countries. The study included a nested prospective cohort to assess the diagnostic performance of the patient-level intervention and an implementation research component (data not presented).
During the observation period, field research assistants collected aggregated data on children attendance from Outpatient department registers and TB diagnosis from unit TB registers, without interfering with the routine childhood TB diagnosis processes. Extracted aggregated data were recorded in paper study forms. The data was checked and verified NTP District TB focal person and country project managers. During the lead–in phase, we set up clinical and coordination study teams, equipment, patient and sample flow systems, and trained health care workers on TB screening, clinical diagnosis, NPA and stool sample collection, Ultra and CXR interpretation. Simple digital system and quality assurance of CXR reading by national re-readers was also set-up.
During the intervention period, the comprehensive childhood TB diagnosis package was implemented within routine programmatic setting, without hiring of any additional staff. Sick children were screened for presumptive TB by either a healthcare worker or a community health volunteer at the health facility outpatient department entry point. At DH, children with presumptive TB were fully evaluated for TB and those at PHCs were either i) referred to the DH for full diagnostic assessment in DH-focused districts, or ii) assessed on site in PHC-focused districts. In DH-focused districts, in PHCs that were already diagnosing TB before the study intervention, only children not diagnosed as per routine were referred to the DH for further evaluation. The study strengthened existing referral systems and provided no funds for transport to the DH at the initial visit.
Once consent and assent for enrolment of children with presumptive tuberculosis, was obtained, children had NPA collected immediately and were provided a container for stool samples or expectorated sputum in older children. NPA and expectorated sputum were tested with Ultra at the PHC using GeneXpert G1 Edge battery-operated devices or at DH laboratory using regular GeneXpert 4-module devices. Stool samples collected at PHCs were referred to the DH for Ultra testing after specimen processing using a centrifuged-based sucrose floatation method. Children had a clinical evaluation and those enrolled at DH a systematic chest X-Ray. Children were initiated on TB treatment as per national guidelines with support of diagnosis algorithms adapted from the Uganda TB guidelines.14 Children had another clinical assessment at day 7. At PHC, those not diagnosed with TB whose symptoms persisted at day 7 were referred to DH for CXR with transportation costs covered by the study.
Support supervision visits were conducted jointly by NTP and country research units to oversee study activities, identify challenges and discuss solutions with health facility teams. Clinical mentoring visits were conducted alongside supervision visits to sustain clinical skills through patient and CXR discussions.
All sick children aged below 15 years were included in the analysis of the effect on TB detection. Children with presumptive TB and parental/guardian consent as well as individual assent, as per national ethics guidelines, obtained were enrolled for individual data collection. We excluded children who had received TB treatment in the previous 6 months. Presumptive TB was defined by a positive TB screening (either cough for ≥2 weeks, fever for ≥2 weeks, documented weight loss, or history of TB contact with any duration of cough) or by clinician's decision, irrespective of the mentioned screening criteria.
Outcomes
The primary outcome for the study was the proportion of children with TB detected among sick children attending outpatient services overall. Secondary outcomes for comparing decentralization approaches included the proportion of i) children screened for TB among sick children; ii) presumptive TB among children screened; iii) children with TB detected among children with presumptive TB; iv) enrolled children with presumptive TB receiving the different components of the diagnostic package, v) children with positive Ultra; vi) children initiating TB treatment among TB diagnosed; and vii) time from TB positive screening (enrolment) to treatment initiation.
Statistical analysis
We estimated the study power based on an estimated number of 260,000 sick children attending outpatient departments of the participating health facilities at study period (observation and intervention), assuming the following estimates: 10% presumptive TB among sick children, 20% TB among presumptive TB, 20% TB detection pre-intervention and an increase to 50% post-intervention, i.e., TB detection in sick children increasing from 0.4% to 1.0%, and an intra-class correlation (ICC) of 0.005. With a significance level of 0.05, and with the estimated attendance, we would have >0.99 power to show the expected difference of the primary endpoint.
We analysed the primary and secondary outcomes using unadjusted comparisons using Pearson's chi-squared test and adjusted comparison generalized linear mixed effects model (GLMM) with binomial function. For the primary outcome analysis, the model included study period (12-months observation versus 12-months intervention) as fixed effect, and facility, districts, and countries as random effects to account for repeated measures and multi-level (countries, districts, sites) study design. For the secondary endpoint analyses comparing the two decentralisation approaches, the model included decentralisation approaches as fixed effects, and districts and countries as random effects. Sites-level random effect was added if there was significant effect on the outcome. We systematically verified for over-dispersion to account for greater variability in data than what would be expected in a binomial distribution and used quasi-binomial model when over-dispersion occurred.15
We conducted post hoc analyses of our study outcomes by levels of facility, i.e. PHC-level versus DH-level instead of PHC-focused versus DH-focused approaches to further explain the variability of the primary outcome results between countries and between decentralisation approaches. We also estimated post-hoc our study power using the observed attendance, proportion of children with TB, and ICC calculated using a one-way ANOVA.
A significance level of 0.05 was used for all analyses. No imputation was done and all analyses were complete case analyses. There was no strategy to mitigate impact of missing data. Analyses were done using the R software 4.1.2.
The study protocol was approved by the WHO's and the sponsor's (Inserm) Ethics Review Committees, as well as the country ethics committees (See Supplementary material). Administrative clearance was obtained from the NTPs, districts and the institutions of the participating countries. This study is registered with clinicaltrials.gov (NCT04038632).
Role of the funding source
The study funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
During the pre-intervention period 255,512 children attended care in the 59 participating health facilities, including 122,570 and 132,942 in DH-focused and PHC-focused districts, respectively. During the intervention period from 7 March 2020 to 31 September 2021, with a 3-month study interruption due to COVID-19 from April 2020 to June 2020, 177,166 children attended care, including 86,367 and 90,799 in DH-focused and PHC-focused districts, respectively (Table 1).
Table 1.
Comparison of tuberculosis case detection between pre-intervention and intervention periods–overall, by country and by DH-focused or PHC-focused decentralisation approach.
| Pre-intervention |
Intervention period |
Variation of TB case proportion delta in point (minimum; maximum) | Unadjusted p-valuea | Impact of decentralisation (Reference = Pre-intervention) b |
|||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | Corrected p-value for overdispersion | |||||||
| Facility attendance | TB cases n (%) | Facility attendance | TB cases n (%) | ||||||
| Overall | 255,512 | 217 (0.08) | 177,166 | 419 (0.24) | 0.16 (−0.22; 3.22) | <0.0001 | 3.60 (2.0–6.48) | <0.0001 | 0.054 |
| Cambodia | 32,837 | 14 (0.04) | 18,441 | 18 (0.1) | 0.06 (−0.13; 3.22) | 0.100 | 3.97 (1.89–8.35) | 0.0003 | 0.142 |
| Cameroon | 8114 | 15 (0.2) | 6866 | 29 (0.4) | 0.2 (−0.22, 1.27) | 0.045 | 2.30 (1.23–4.31) | 0.009 | 0.170 |
| Côte d’Ivoire | 54,863 | 5 (0.01) | 36,816 | 89 (0.2) | 0.19 (0; 1.02) | <0.0001 | 24.80 (10.07–61.10) | <0.0001 | 0.0008 |
| Mozambique | 67,609 | 114 (0.2) | 37,889 | 89 (0.2) | 0 (−0.15; 0.5) | 0.111 | 1.50 (1.14–1.97) | 0.004 | 0.308 |
| Sierra Leone | 39,856 | 43 (0.1) | 31,883 | 152 (0.5) | 0.4 (0.05; 0.6) | <0.0001 | 4.75 (3.37–6.70) | <0.0001 | 0.0004 |
| Uganda | 52,233 | 26 (0.05) | 45,271 | 42 (0.09) | 0.04 (−0.08; 0.2) | 0.067 | 1.86 (1.14–3.04) | 0.013 | 0.301 |
| DH-focused districts | 122,570 | 80 (0.07) | 86,367 | 311 (0.4) | 0.33 (−0.13; 1.93) | <0.0001 | 4.06 (1.85–8.91) | 0.0005 | 0.122 |
| PHC-focused districts | 132,942 | 137 (0.1) | 90,799 | 108 (0.1) | 0 (−0.22; 3.22) | 0.293 | 2.92 (1.26–6.79) | 0.013 | 0.256 |
DH, district hospital; PHC, primary health centre.
Pearson's chi-squared test.
GLMM modelling impact of decentralisation approach.
Overall, 217/255,512 (0.08%) children were diagnosed with TB pre-intervention and 419/177,166 (0.24%) post-intervention (OR: 3.60; 95% CI: 2.00–6.48; p-value<0.0001; p-value = 0.054 after correcting for overdispersion). Facility attendance and children with TB detected in pre-intervention and intervention periods varied widely across countries (Supplement, Table S1).
In DH-focused districts, 80/122,570 (0.07%) and 311/86,367 (0.36%) children were diagnosed with TB pre-intervention and post-intervention, respectively (OR: 4.07; 95% CI: 1.85–8.91; p-value = 0.0005; p-value = 0.122 after correcting for overdispersion). In PHC-focused districts, 137/132,942 (0.10%) and 108/90,799 (0.12%) children were diagnosed with TB pre-intervention and post-intervention, respectively (OR: 2.92; 95% CI: 1.26–6.79; p-value = 0.013; p-value = 0.256 after correcting for overdispersion) (See country details in Supplement, Table S1). A posteriori power was estimated to be 41.50%, and the ICC to be 0.015.
During the intervention period, 149,599 (84.4%) of 177,166 children attending care were screened, and 3849 (2.6%) were identified as presumptive TB (Table 2). There was no difference in screening rates (83.4% versus 85.4%, p-value = 0.281) and presumptive TB rates (2.7% versus 2.5%, p-value = 0.359) between DH-focused and PHC-focused districts. TB screening rates were above 80% in most countries. The presumptive TB rate was below 3% in all countries except Cameroon where it reached 10% (Supplement, Table S2). Among children with presumptive TB, 311 (16.3%) and 108 (5.6%) were diagnosed with TB in the DH-focused and PHC-focused districts, respectively (OR: 0.43; 95% CI: 0.21–0.87; p-value = 0.019; p-value = 0.369 after correcting for overdispersion) (Supplement, Table S2).
Table 2.
Screening to diagnosis cascade during the intervention period by decentralisation approach (aggregated data).
| DH-focused approach n (% on upper level) | PHC-focused approach n (% on upper level) | Unadjusted p-valuea | Impact of decentralisation approach (Ref = DH-focused) b |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | Corrected p-value for overdispersion | ||||
| Facility attendance | 86,367 | 90,799 | ||||
| At DHs | 35,447 | 25,180 | ||||
| At PHCs | 50,920 | 65,619 | ||||
| Screened | 72,036 (83.4) | 77,563 (85.4) | <0.0001 | 4.32 (0.30, 61.61) | 0.281 | 0.929 |
| At DHs | 28,585 (80.6) | 19,686 (78.2) | ||||
| At PHCs | 43,451 (85.3) | 57,877 (88.2) | ||||
| Presumptive TB | 1910 (2.7) | 1939 (2.5) | 0.067 | 0.80 (0.51, 1.28) | 0.359 | 0.872 |
| At DHs | 1099 (3.8) | 666 (3.4) | ||||
| At PHCs | 811 (1.9) | 1273 (2.2) | ||||
| TB cases | 311 (16.3) | 108 (5.6) | <0.0001 | 0.43 (0.21, 0.87) | 0.019 | 0.369 |
| At DHs | 249 (22.5) | 66 (9.7) | ||||
| At PHCs | 62 (7.7) | 42 (3.4) | ||||
DH, district hospital; PHC, primary health centre.
Pearson's chi-squared test.
GLMM modelling impact of decentralisation approach.
Overall, 1189/1910 (62.3%) children with presumptive TB in DH-focused districts and 1915/1939 (98.8%) children in PHC-focused districts were enrolled in the study. There were more children presenting with severe acute malnutrition (22.0% versus 13.9%; p-value <0.0001) and with TB suggestive signs (81.8% versus 73.4%, p < 0.001) in DH-focused versus PHC-focused districts (Table 3). Sample collection and testing rates were similar, with 1175/1189 (98.8%) children in DH-focused districts and 1906/1915 (99.5%) children in PHC-focused districts with at least one sample collected (p-value = 0.357), and 1126 (94.7%) and 1890 (98.7%) with at least one Ultra test performed, respectively (p-value = 0.141). There were more children with Ultra positive samples in the DH-focused districts than in the PHC-focused districts [37/1126 (3.3%) versus 34/1891 (1.8%); p-value: 0.053] (Table 4). Overall, 273/1189 (23.0%) children enrolled in DH-focused districts were diagnosed with TB versus 106/1915 (5.5%) in the PHC-focused districts (p-value: <0.0001), with 37/273 (3.1%) microbiologically confirmed TB in DH-focused districts and 34/106 (1.8%) in PHC-focused districts. TB detection rates ranged between 5.9% in Cameroon (40/677) and Uganda (43/732), and 32.7% (133/407) in Sierra Leone (Supplement, Table S3).
Table 3.
Characteristics of enrolled children (intervention period).
| Overall |
DH-focused approach |
PHC-focused approach |
p-value | ||||
|---|---|---|---|---|---|---|---|
| Available in N (if #N′) | n (%) (N’ = 3104) | Available in N (if #N′) | n (%) (N’ = 1189) | Available in N (if #N′) | n (%) (N’ = 1915) | ||
| Age (median) | 3094 | 3.75 (1.33–7.42) | 1189 | 3.54 (1.33–7.17) | 1915 | 3.83 (1.33–7.65) | 0.336 |
| Age <5 years | 3094 | 1813 (58.6) | 1184 | 716 (60.5) | 1910 | 1097 (57.4) | 0.095 |
| Gender female | 1490 (48) | 557 (46.8) | 933 (48.7) | 0.310 | |||
| Gender male | 1614 (52.0) | 632 (53.2) | 982 (51.3) | 0.310 | |||
| First care seeking facility level | <0.0001 | ||||||
| District hospital | 1555 (50.1) | 808 (67.9) | 747 (39.0) | ||||
| Primary health centre | 1549 (49.9) | 381 (32.0) | 1168 (61.0) | ||||
| Severe acute malnutritiona | 3074 | 522 (16.8) | 1180 | 259 (22.0) | 1894 | 263 (13.9) | <0.0001 |
| WHZ <−3 SD (age <5 years) | 1732 | 303 (9.8) | 695 | 157 (22.6) | 1037 | 146 (14.1) | <0.0001 |
| MUAC <115 (age 6–59 months) | 1502 | 216 (7.0) | 588 | 121 (20.6) | 914 | 95 (10.4) | <0.0001 |
| BMI for age Z score <−3 SD (age ≥5 years) | 1280 | 141 (4.5) | 468 | 62 (13.3) | 812 | 79 (9.7) | 0.0528 |
| TB contact history | 990 (31.9) | 492 (41.4) | 498 (26.0) | <0.0001 | |||
| Contact with smear or Xpert positive case in the previous year | 644 (20.8) | 312 (26.2) | 332 (17.3) | <0.0001 | |||
| Cough >2 weeks | 1956 (63) | 850 (71.5) | 1106 (57.8) | <0.0001 | |||
| Unremittent cough | 2521 | 1303 (51.7) | 981 | 523 (53.3) | 1540 | 780 (50.6) | 0.192 |
| Fever >2 weeks | 1109 (35.7) | 578 (48.6) | 531 (27.7) | <0.0001 | |||
| Lethargy/reduced appetite | 831 (26.8) | 503 (42.3) | 328 (17.1) | <0.0001 | |||
| Weight loss documented | 3103 | 1085 (34.9) | 533 (44.8) | 1914 | 552 (28.8) | <0.0001 | |
| TB symptoms per child (med (IQR)) | 2 (1–4) | 3 (1–4) | 2 (1–3) | <0.0001 | |||
| At least 1 TB symptomb | 2801 (90.2) | 1072 (90.2) | 1729 (90.3) | 0.907 | |||
Defined as WHZ <−3 SD in those aged ≤5 or BMI for age <−3 SD in those aged >5 years, or MUAC <115 in those aged 6–59 months.
Cough, Fever, Weight loss, Lethargy or fatigue.
Table 4.
Uptake and results of the diagnostic evaluation process in enrolled children by decentralization approach.
| DH-focused approach |
PHC-focused approach |
Unadjusted p-valueg | Effect of decentralisation approach (Reference = DH-focused)h |
||||
|---|---|---|---|---|---|---|---|
| Available in N (if #N′) | n (%) (N’ = 1189) | Available in N (if #N′) | n (%) (N’ = 1915) | OR (95% CI) | p-value | ||
| Sample collection and testing | |||||||
| NPA collected | 1100 (92.5) | 1876 (98.0) | <0.0001 | 3.92 (1.00, 15.36) | 0.049 | ||
| Stool collected | 957 (80.5) | 1278 (66.7) | <0.0001 | 0.52 (0.10, 2.74) | 0.437 | ||
| Sputum collected | 76 (6.4) | 275 (14.4) | <0.0001 | 1.32 (0.19, 9.32) | 0.782 | ||
| Stool or sputum collected | 978 (82.3) | 1504 (78.5) | 0.014 | 0.87 (0.18, 4.34) | 0.869 | ||
| At least 1 sample collected | 1175 (98.8) | 1906 (99.5) | 0.043 | 1.57 (0.60, 4.14) | 0.357 | ||
| ≥2 samples collected | 925 (77.8) | 1488 (77.7) | 0.987 | 1.09 (0.22, 5.35) | 0.914 | ||
| ≥1 specimen tested with Ultra | 1126 (94.7) | 1890 (98.7) | <0.0001 | 1.54 (0.87, 2.72) | 0.141 | ||
| Ultra positive result on any sample | 1126 | 37 (3.3) | 1891 | 34 (1.8) | 0.013 | 0.60 (0.36, 1.01) | 0.053 |
| Time to 1st Xpert result | 1126 | 1 (0–3)a | 1890 | 0 (0–1)b | <0.0001 | 1.63 (0.97, 2.74) | 0.066 |
| Clinical evaluation | |||||||
| Clinical evaluation performede | 1189 (100) | 1915 (100) | NA | NA | NA | ||
| Presumptive TB clinical featuresf | 1018 (85.6) | 1529 (79.8) | <0.0001 | 0.46 (0.15, 1.38) | 0.166 | ||
| Presumptive TB clinical features except weight loss | 973 (81.8) | 1406 (73.4) | <0.0001 | 0.35 (0.06, 2.06) | 0.247 | ||
| D7 visit done | 665 (55.9) | 1550 (80.9) | <0.0001 | 5.70 (1.76, 18.46) | 0.004 | ||
| Still symptomatic at day 7 | 663 | 241 (36.4) | 1550 | 575 (37.1) | 0.775 | 0.53 (0.11, 2.44) | 0.414 |
| Chest X-ray (CXR) | |||||||
| CXR done | 1011 (85.0) | 625 (32.6) | <0.0001 | 0.01 (0.001, 0.08) | <0.0001 | ||
| CXR result interpreted | 1011 (85.0) | 625 (32.6) | <0.0001 | 0.01 (0.001, 0.08) | <0.0001 | ||
| CXR suggestive of TB | 1011 | 318 (31.5) | 625 | 92 (14.7) | <0.0001 | 0.47 (0.16, 1.41) | 0.180 |
| TB diagnosis and treatment | |||||||
| TB diagnosed | 273 (23.0) | 106 (5.5) | <0.0001 | 0.19 (0.09, 0.42) | <0.0001 | ||
| TB microbiologically diagnosed | 37 (3.1) | 34 (1.8) | 0.022 | 0.61 (0.36, 1.02) | 0.062 | ||
| TB clinically diagnosed | 236 (19.9) | 72 (3.8) | <0.0001 | 0.15 (0.06, 0.43) | 0.0003 | ||
| TB treatment initiated | 255 (21.5) | 87 (4.5) | <0.0001 | 0.15 (0.07, 0.36) | <0.0001 | ||
| Time to treatment initiation (days) | 255 | 1 [0–4]c | 87 | 2 [0–7]d | 0.125 | 0.83 (0.54, 1.29) | 0.400 |
NA, not applicable.
Range (min, max) = (0, 374).
Range (min, max) = (−238, 59).
Range (min, max) = (0, 193).
Range (min, max) = (0, 385).
At least one TB sign assessed.
Presumptive TB clinical features defined as having at least one of the following symptoms: Cough more than 2 weeks, Fever more than 2 weeks, Night sweat, Lethargy, Weight loss.
Pearson's chi-squared test or Wilcoxon test.
GLMM modelling impact of decentralisation approach.
In facility level analysis, systematic screening rates were similar in DH and PHCs (79.6% versus 87.0%; p-value = 0.618) but there were higher rates of presumptive TB at DH compared to PHCs (3.7% versus 2.1%; p-value = 0.0001) (Table 5). Overall, the 1936 children enrolled at DH had more clinical TB features than the 1168 children enrolled at PHC, with higher proportions of severe acute malnutrition, cough >2 weeks, and history of TB exposure. Samples were collected in more than 98% of children both at DH and PHC, but more children had positive Ultra at DH than at PHC (58/1860, 3.1% versus 13/1157, 1.1%, p-value = 0.005). Additionally, there were more children clinically diagnosed with TB at DH than at PHC, 280/1936 (14.5%) versus 28/1168 (2.4%) (p-value = 0.0002).
Table 5.
Diagnosis cascade, patient characteristics and uptake by facility level.
| District hospital |
Primary health centre |
Unadjusted p-valuee |
Effect of type of facility (Ref = DH)f |
|||||
|---|---|---|---|---|---|---|---|---|
| N | n/N (%) | N | n/N (%) | OR (95% CI) | p-value | Corrected p-value for overdispersion | ||
| Diagnosis cascade (aggregated data) | ||||||||
| OPD attendance | 60,627 | 116,539 | ||||||
| Screened | 60,627 | 48,271 (79.6) | 116,539 | 101,328 (87.0) | <0.0001 | 1.21 (0.58, 2.51) | 0.618 | 0.888 |
| Presumptive TB | 48,271 | 1765 (3.7) | 101,328 | 2084 (2.1) | <0.0001 | 0.38 (0.23, 0.63) | 0.0001 | 0.254 |
| Treated for TB | 1765 | 315 (0.52) | 2084 | 104 (0.09) | <0.0001 | 0.30 (0.23, 0.39) | <0.0001 | <0.0001 |
|
Characteristics of children enrolled (individual data) |
(N’ = 1936) |
(N’ = 1168) |
||||||
| Age <5 years | 1929 | 1148 (59.5) | 1165 | 665 (57.0) | 0.169 | |||
| Gender female | 907 (46.8) | 583 (49.9) | 0.098 | |||||
| Gender male | 1029 (53.2) | 585 (50.1) | 0.098 | |||||
| SAMg | 1917 | 386 (20.1) | 1157 | 136 (11.8) | <0.0001 | |||
| TB contact history | 766 (39.6) | 224 (19.2) | <0.0001 | |||||
| Contact with smear positive adult in the previous year | 488 (25.2) | 156 (13.4) | <0.0001 | |||||
| Cough >2 weeks | 1310 (67.7) | 646 (55.3) | <0.0001 | |||||
| Fever >2 weeks | 828 (42.8) | 281 (24.1) | <0.0001 | |||||
| Lethargy/reduced appetite | 606 (31.3) | 225 (19.3) | <0.0001 | |||||
| Weight loss documented | 729 (37.6) | 1167 | 356 (30.5) | 0.0001 | ||||
| Sample collection and testing | ||||||||
| NPA collected | 1867 | 1829 (98.0) | 1150 | 1147 (99.7) | 0.0001 | 4.61 (0.62, 34.36) | 0.136 | NA |
| Stool collected | 1506 (77.8) | 729 (62.4) | <0.0001 | 0.83 (0.27, 2.54) | 0.739 | NA | ||
| At least 1 sample collected | 1917 (99.0) | 1164 (99.7) | 0.073 | 2.05 (0.67, 6.27) | 0.211 | NA | ||
| ≥2 samples collected | 1517 (78.4) | 896 (76.7) | 0.306 | 1.11 (0.43, 2.87) | 0.824 | NA | ||
| ≥1 specimen tested with Ultra | 1859 (96.0) | 1157 (99.1) | <0.0001 | 1.89 (0.95, 3.75) | 0.068 | NA | ||
| Ultra positive result on any sample | 1860 | 58 (3.1) | 1157 | 13 (1.1) | 0.0007 | 0.40 (0.21, 0.75) | 0.005 | NA |
| Clinical evaluation | ||||||||
| Clinical evaluation performedc | 1937 (100) | 1168 (100) | NA | NA | NA | NA | ||
| TB suggestive clinical featuresd | 1595 (82.4) | 952 (81.5) | 0.568 | 1.29 (0.43, 3.81) | 0.648 | NA | ||
| TB suggestive clinical features without weight loss | 1521 (78.6) | 858 (73.5) | 0.001 | 1.07 (0.35, 3.25) | 0.900 | NA | ||
| D7 visit done | 1237 (63.9) | 978 (83.7) | <0.0001 | 5.21 (1.99, 13.67) | 0.0008 | NA | ||
| Still symptomatic at day 7 | 1228 | 535 (43.6) | 970 | 281 (29.0) | <0.0001 | 0.32 (0.10, 1.07) | 0.064 | NA |
| CXR | ||||||||
| CXR done | 1563 (80.7) | 73 (6.3) | <0.0001 | 0.01 (0.01, 0.03) | <0.0001 | NA | ||
| TB suggestive CXR features | 1563 | 387 (24.8) | 73 | 23 (31.5) | 0.245 | 4.34 (2.39, 7.86) | <0.0001 | NA |
| TB diagnosis and treatment | ||||||||
| TB diagnosed | 338 (17.5) | 41 (3.5) | <0.0001 | 0.35 (0.23, 0.52) | <0.0001 | NA | ||
| TB microbiologically diagnosed | 58 (3.0) | 13 (1.1) | 0.001 | 0.41 (0.22, 0.76) | 0.005 | NA | ||
| TB clinically diagnosed | 280 (14.5) | 28 (2.4) | <0.0001 | 0.18 (0.07, 0.44) | 0.0002 | NA | ||
| TB treatment initiation | 313 (16.2) | 29 (2.5) | <0.0001 | 0.16 (0.16, 0.16) | <0.0001 | NA | ||
| Time to treatment initiation (days) | 313 | 1 [0–5]a | 29 | 7 [1–14]b | 0.003 | 0.60 (0.39, 0.94) | 0.025 | NA |
NA, not applicable.
Range (min, max) = (0, 385).
Range (min, max) = (0, 88).
At least one TB sign assessed.
Presumptive TB clinical features defined as having at least one of the following symptoms: Cough more than 2 weeks, Fever more than 2 weeks, Night sweat, Lethargy, Weight loss.
Pearson's chi-squared test or Wilcoxon test.
GLMM modelling impact of type of facility.
Defined as WHZ <−3 SD in those aged ≤5 or BMI for age <−3 SD in those aged >5 years, or MUAC <115 in those aged 6–59 months.
Discussion
This multi-country implementation study showed that decentralising childhood TB diagnosis at PHC and DH levels increased detection of children with TB. Overall, the DH-focused approach had a larger effect on detection of children with TB than the PHC-focused approach.
Implementing a comprehensive childhood TB diagnosis package at DH and PHC level nearly tripled the detection of children with TB with variability between countries. The use of systematic screening at facility entry-point to identify children with presumptive TB together with the strengthening of clinical skills and better access to microbiological tests and CXR contributed to reducing missed opportunities for diagnosis. Overall, our findings are similar to those of the DETECT TB project that showed increase in proportions of children among all TB notification from 9% to 15% after strengthening and decentralisation of TB services within routine integrated child health services in Uganda.16 Unlike DETECT TB that was implemented in a few sites in one country, our study was deployed in six countries with different TB incidences and health systems organization. At country level, the effect of the intervention was larger in countries with no or limited prior decentralization of childhood TB services, such as in Cameroon, Côte d’Ivoire and Sierra Leone where there were almost no childhood TB screening activities at PHC level. On the other hand, in Mozambique and Uganda, children with presumptive TB could be diagnosed and treated for TB at PHC level and only complicated or severe cases were referred to DH. The variability in TB care practice, facility attendance and TB incidence may explain differences in TB detection rates between countries, as well as the data heterogeneity underlying the (need of correcting for) overdispersion.
The effect of the DH-focused approach on TB detection was higher than that of the PHC-focused approach. There are several possible explanations for our study findings. First, the screening and presumptive TB rates were similar between the two approaches and thus do not account for this finding. Second, in DH-focused districts, a large proportion of children referred from PHC did not reach DH and most likely the sickest reached. Indeed, there were more children with TB suggestive symptoms and severe acute malnutrition, hence more likely to be diagnosed with TB, at DH than at PHC. Third, skills of medical doctors at DH for clinical assessment may account for higher TB detection than that done by clinical officers or nurses at PHC. Despite training and clinical mentoring in the study, clinicians at PHC may have not been empowered enough to initiate treatment for TB without microbiological confirmation, as implied by the higher proportion of microbiologically confirmed TB in children at PHC level (32%) compared to DH (17%). This is also likely to explain the low proportion of children started on treatment at PHC in the PHC-focused approach where the diagnosis is made by a nurse as compared to the DH-focused approach in which children with presumptive TB from PHC were referred to the DH where diagnosis was made by a doctor. Finally, all children enrolled in DH-focused districts had a CXR performed while in PHC-focused districts only children with persisting symptoms after 7 days were referred for CXR at DH; this is likely to have contributed to higher TB detection in DH-focused districts.
Contextual factors probably played a role in low TB detection in PHC-focused districts. As the intervention was deployed in a small number of health facilities per country, site-specific events related to human resources and management, might have led to variations in the implementation of the intervention and/or negatively impacted the effect of the intervention globally. The impact of the COVID-19 pandemic, including movement restrictions (with limited access to transport means, and increase in transport costs), may explain the decrease in facility attendance and the decline in the number of children detected with TB, with heterogeneous effect between and within countries.17
This study brings evidence about the feasibility and yield of different diagnostic interventions at lower levels of healthcare. Systematic screening for TB among children attending care was feasible both at DH and PHC levels. Nonetheless up to a quarter of children presenting at DH were not screened, which may indicate challenges in patient flows or constraints in implementing the simple question-based screening, in often overburdened district hospitals.18 Except in Cameroon, the presumptive TB rate after systematic screening was lower for all countries than we had hypothesized (10%). The uptake of NPA and stools collection as well as Ultra testing on NPA was high at PHC level, showing the feasibility of decentralising child-adapted sample collection and molecular testing.
Our study has limitations. First, the pre-post design has inherent weaknesses as it does not allow to distinguish between the effect of the intervention, the effect of time, change of policy, and other confounding factors such as the COVID-19 pandemic to reliably establish the causal effect of the intervention on TB detection in children. Second, aiming to maximize the external validity of the study, we included many countries, thereby increasing heterogeneity, which contributed to increasing data over-dispersion and diluted the intervention effect when we corrected for it in analysis. Third, our study included a low number of districts per country, which may have hampered documentation of country specificities as well as negatively impacted study results. Lastly, we were unable to properly document details of the standard of care for diagnosis of TB in children during the observation period hence unable to document changes in clinical practices compared to the intervention period.
Despite its limitations, our study conducted in six high TB incidence countries across different regions in Africa and in South-East Asia using an integrated approach within routine care provides key evidence to further strengthen the WHO recommendation on decentralising childhood TB services. Beyond previous studies10,16,19 assessing the feasibility of decentralising a single diagnostic component, we documented the effectiveness and feasibility of decentralizing a comprehensive childhood TB diagnosis package. Furthermore, considering the potential low TB detection at PHC level, the diagnostic approach chosen in our study could be further adapted depending on local resources and health systems organization and alternative hybrid decentralisation models proposed. For example, despite its low detection yield in children, decentralising Ultra testing can be highly relevant and effective for TB diagnosis in adults and thus could be integrated with other services for diagnosis or quantification of other pathogens.20 Alternatively, child-adapted sample collection could be implemented at PHC with sample transportation and testing in centralized hubs at DH-level.21 Given the persisting poor microbiological detection yield from respiratory samples, clinical diagnosis remains the cornerstone of TB diagnosis in children. Access to CXR remains a major problem in many countries and alternative approaches using portable X-rays and computer aided detection programs need to be further evaluated for diagnosis of pulmonary TB in children. Task shifting from physicians to nurses at PHC, that was shown to be effective in the field of malaria and HIV, may be more challenging for TB in the absence of highly sensitive TB point of care test and poorly specific clinical presentation.22 Treatment decision algorithm (TDA), as recently recommended by WHO, are likely to help TB treatment decision at low level of care but warrants further research on their effectiveness and feasibility.7 Our cost effectiveness and budget impact analyses also provide important information for adapting decentralization approaches and models to country resources. Additional implementation research analyses are specifically assessing the feasibility and perceived sustainability of the different diagnosis package components and evaluating implementation strategies used to deploy diagnosis at decentralised levels; these findings will be useful to NTPs in their operational and financial planning to scale-up and sustain decentralised services.
Overall, our study supports the recent WHO recommendation on decentralising TB services by providing evidence on its effect on TB detection and feasibility and showing that decentralising and strengthening childhood TB diagnosis at lower levels of care increases tuberculosis detection, especially at DH-levels accompanied by screening activities at PHC levels. The difference disappeared after adjusting for over-dispersion with marginal significance due the high variability of the TB diagnosis between the countries. Though not statistically significant, implying there could be a real effect going on, the three-fold increase in the absolute TB cases is programmatically significant. Although not shown by the effectiveness results, decentralisation at PHC level could be interesting in setting with high TB prevalence and high facility testing volumes as suggested by the cost-effectiveness results.
Contributors
OM, MB, EW conceived and designed the study. MN coordinated and contributed to the implementation of the study protocol. OM, MB, EW, AD, MS, GB, RM, J-VT, and JOG contributed to the development of the study protocol. OM, MB, and EW led the study at international level. LB and TEM led the study in Cambodia, J-VT and MB led the study in Cameroon, RM led the study in Côte d’Ivoire, CK led the study in Mozambique, EW and JM-A led the study in Uganda, AM led the study in Sierra Leone, ML and EN coordinated laboratory aspects at international level, ADL and BD coordinated study implementation in Cambodia, SKN coordinated study implementation in Cameroon, EAK coordinated study implementation in Côte d’Ivoire, SC coordinated study implementation in Mozambique, NN coordinated study implementation in Uganda. PN, LT developed the chest X-ray section and course. JP coordinated the TB-Speed project at international level. BJ conducted implementation research work. AD provided scientific guidance and expertise for the study. J-VT, JM-A, AM, CK, JM, NN, GB, ADL and SC implemented the study and enrolled participants. ST, SS, LF, KKA, IM supported study implementation in country. EB developed and maintained the study database. MH did the statistical analysis. OM and MH accessed and verified the data and prepared the report. AD, SV provided scientific expertise and guidance. OM, MN, MH, MB, AD, JOG and EW contributed to the interpretation of the results. EW wrote the first draft and all authors reviewed and approved the final version of the manuscript. OM, MB, EW were responsible for the decision to submit the manuscript.
Data sharing statement
Study data will not be publicly available. Data could be made available by the sponsor (Inserm) to interested researchers on request to the corresponding author under a data transfer agreement.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
We thank the Ministries of Health and NTPs of participating countries for their support. We thank members of the TB-Speed Scientific Advisory Board who gave technical advice on the design of the study and approved the protocol: Anneke Hesseling (Stellenbosch University, Cape Town, South Africa), Luis Cuevas (Liverpool School of Tropical Medicine, Liverpool, UK), Malgorzata Grzemska (WHO, Geneva, Switzerland), Philippa Musoke (Makerere University, Kampala, Uganda), and Mark Nicol (University of Western Australia, Perth, WA, Australia). We thank all the children and their families who participated to the study and the healthcare workers of the participating hospitals and laboratories.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102527.
Contributor Information
Eric Wobudeya, Email: ewobudeya@mujhu.org.
TB-Speed Decentralisation study group:
Borand Laurence, de Lauzanne Agathe, Dim Bunnet, Heang Seyla, Kaing Sanary, Keang Chanty, L.Y. Socheat, Meas Pichpiseth, Nhoueng Sovann, Pring Long, Sreng Vouchleang, Yin Song, Sovan Saren, Phan Chanvirak, Chreng Chanra, Khoun Ratha, Rin Monicando, Pal Sophea, Nang Boraneath, Pom Rathakrun, Mao Tan Eang, Chhim Simoy, Touch Huot, Suon Kosal, Chum Saronn, Tok Kimhong, Pring Kimchorn, Krouch Satya, Chok Chean, Seun Sunleng, Phon Savtey, Nang Mai, Hun Kimda, Hong Vanny, Sok Dara, Chea Kosal, Chheang Bunthoeun, Sem Rino, Lay Lam, Say Haysan, Kem Pholly, Meng Sreyphal, Phorn Sokheng, Him Sreyvann, Pheach Peakdey, Kive Dalai, Sar Moeur, Kong Sreydy, Kong Seyha, Yorn Sreytouch, Tes Soam, Kep Sophal, Leng Seroeung HENG Thy, Neak Savorn, Seng Sim, Pay Pheakna, Suon Sithan, Chan Sophanna, Um Dyna, Sin Savuth, Phan Sam, Kum Sarim, Khath Sokheng, Phem Pong, Sok Seyha, Ny Chanty, Leim Van, Pich Sereyvuth, Chheang Sengkry, Eang Nhin, Sao Vannareth, Sim Vannak, Som Sopheak, Pong Ney, Van Sokha, Seng Sreyleap, Yoeurng Vanna, Toem Kakada, Keo Thida, Sem Vuochny, Veng Sophal, Rin Chanthol, Seang Vanny, Lok Kiri, Mao Khemra, Ouk Keovanna, Min Maiya, Morm Suomun, Koy Rattana, Chhann Sreypov, Set Sreytouch, Amboua Schouame Audrey, Babey Clifford, Eden Ngu Masama, Guenou Etienne, Kwedi Nolna Sylvie, Mbang Massom Douglas, Melingui Bernard Fortune, Nga Elomo Nadia, Mvetumbo Moïse, Nkembe Angeline, Ebo Krystel, Kuate Kuate Albert, Choupa Michelline, Mbede Maggi, Donkeng Valerie, Kamgaing Nelly, Taguebue Jean-Voisin, Touha Yannick Achille, Abomo Zang Estelle, Bakoa Rosette, Mimbouombela Leger Esperance, Eleme Sabine, Eteme Marie Gabrielle, Elouna Nkoa Thierry, Ndzana Dieudonné, Bitti Christophe, Fotsing Olivier, Lani Boko Charlotte, Mbonga Mathieu, Njakou Sagang Ghislain Ulrich, Onomo Innocent, Essaga Hortense Charlotte, Mboudi Kouang Daniel Desiré, Eloundou Léon, Kengne Helene, Mbassi Felix, Bidjeme Juliette, Toua Eteme Hortense, Essama Nadege, Belinga Balla Roger, Tassi Norbert, Nguiko Elsa Roline, Mekongo Leonard, Eyebe Ayissi Fabien, Biloa Anaba Francine Christelle, Nsom Philomène, Yam Essola Celestin Géraud, Mame Moo Edwige Léa, Makon Noé, Nounkep Yanghu Arllette Rita, Ebanga Frank, Assiga Ntsama Antoinette, Kamguia Djuimsop Carlyle Sorelle, Mbabou Diane, Maguip Abanda Marie, Nguemafouo Doummene Rosine Berthe, Mekone Amos, Konfor Blessing Ngwankfu, Mimboe Jérome, Tiona Virginie, Beleck Roland, Zam Sairou, Adibone Nicole, Biaback Jean Claude, Bessong Denis, Aminou Gilbert, Bille Bonga Jeremie Pagnol, Fotso Monkap Aubin, Hitekelek Epse NGON Annie, Sebe Vitrice, Makon Leo, Sebe Vitrice, Ennah Marie, Paul Boyolo Mpie, Metchoum Diane Viviane, Nzambe Celin, Dado Arnaud, Mbengang Milobert, Eyebi Marceline, Ngah Vanessa, Mballa Batonga Alice, Ayouba Solange, Ebode Pierrette, Mamou Majino, Botomogne Bomba Marguerite, Ngnet Salametou, Essengue Ngono Augustine Florence, Odionoloba Charles Rolland, Bisso Bernice, Balemaken Ingrid Suzy, Mandoki a Bilong Marie Louise, Ndeng Ayouba Gertrude, Ngon Josue, Aka Bony Roger, Bah Kacou Michel, Bakayoko Dro, Baki Aimee Rolande, Banga Marie-France Larissa, Bouzié Olivier, Brou Kan, Coulibaly Pan, Danho Serge, Deli Flavien, Dion Alphonse, Do Bi, Dohoun Armand, Edjeme William, Falé Cathérine, Gogoua Saulé Melissa, Kesse Constant, Komena Auguste Eric, Kouadio Christian, Kouame Abel Arkason, Moh Raoul, Nguessan Marcelle Sandrine, Siloué Bertine, Soua Nina, Yao Yapi Cyrille Prisca, Ouassa Timothée, Kouakou Jacquemin, Balestre Eric, Beuscart Aurélie, Charpin Aurélie, D'elbee Marc, Font Hélène, Joshi Basant, Koskas Nicolas, Marcy Olivier, Occelli Estelle, Orne-Gliemann Joanna, Poublan Julien, Vernoux Elodie, Bonnet Maryline, Chauvet Savine, Lounnas Manon, Breton Guillaume, Norval Pierre-Yves, Cassy Sheyla, Chambal Verna, Chiúle Valter, Chimbanje Supinho, Cumbe Saniata, Matsinhe Mércia, Khosa Celso, Mabote Nairo, Machava Salvador, Machonisse Emelva, Macuácua Verónica, Milice Denise, Ribeiro Jorge, Tivane Elcídio, Uetela Dorlim, Voss de Lima Yara, Zandamela Américo, Zita Alcina, Manhiça Ivan, José Benedita, Rego Dalila, Buck W. Chris, Kasembe Kapoli, Massangaie Atália, Sitoe Assa, Argola Ambostique, Miambo Césio, Nhatsave Presequila, Sitoe Gilda, Vesta Charifito, Dimande Salvador, Mazembe Lázaro, Amade Nilza, Chavela Manuela, Macheque Nomsa, Comé Salomão, Machava Eulália, Mucavele Narciso, Nhabanga Jacinto, Nicolau Marlene, Simbine Natércia, Uendela Lina, Juaio Micaela, Saíde Abiba, Macie Naira, Mondlane Fernando, Simango Stélio, Beyan Prince, Flomo Benjamin M, Jalloh Joseph Abubakarr, Kamara Ishmael, Koroma Monica G, Lamin Mohamed, Matata Lena, Mugisha Jacob Ross, Senesie Christiana M, S.E.S.A.Y. Sheriff, Tamba Kamara Egerton, Mustapha Ayeshatu, Foray Lynda, Agondeze Sandra, Kobusingye Agnes, Nanfuka Mastula, Namulinda Faith, Wobudeya Eric, Arinaitwe Rinah, Kaitano Rodney, Kasujja Martin, Mwanga-Amumpaire Juliet, Mwesigwa Evans, Natukunda Naome, Nuwamanya Simpson, Nyangoma Miria, Orikiriza Patrick, Tumwijukye Johnbosco, Turyashemererwa Esther, Dan Nyehangane, Ivan Mugisha, Biryeri Winnie, Naika George, Ongwara O. Robert, Najjuko Allen, Kayiira Augustine, Yairo Samuel, Tumwebaze Immaculate, Nalwoga Goreth, Nsiyaleta Paul, Agaba Annet, Mpimbaza M. Martin, Akampurira Norbert, Tugumisirize Agatha, Ariyo Evans, Agaba Julius, Natukunda Yovita, Musazi Nelson, Musinguzi Edmund, Baluku Julius Brown, Sekadde Moorine, Turyahabwe Stavia, Chabala Chishala, Cuevas Luis, Delacourt Christophe, Graham Steve, Grzemska Malgorzata, Verkuijl Sabine, Hesseling Anneke, Maleche-Obimbo Elizabeth, Nicol Mark, and Mao Tan Eang
Appendix A. Supplementary data
References
- 1.Bagcchi S. WHO's global tuberculosis report 2022. Lancet Microbe. 2023;4(1) doi: 10.1016/S2666-5247(22)00359-7. [DOI] [PubMed] [Google Scholar]
- 2.Wobudeya E., Bonnet M., Walters E.G., et al. Diagnostic advances in childhood tuberculosis-improving specimen collection and yield of microbiological diagnosis for intrathoracic tuberculosis. Pathogens. 2022;11(4):389. doi: 10.3390/pathogens11040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gous N., Scott L.E., Khan S., Reubenson G., Coovadia A., Stevens W. Diagnosing childhood pulmonary tuberculosis using a single sputum specimen on Xpert MTB/RIF at point of care. S Afr Med J. 2015;105:1044–1048. doi: 10.7196/SAMJ.2015.v105i12.8585. [DOI] [PubMed] [Google Scholar]
- 4.Zar H.J., Workman L., Isaacs W., et al. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clin Infect Dis. 2012;55(8):1088–1095. doi: 10.1093/cid/cis598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliwa J.N., Gathara D., Ogero M., et al. Diagnostic practices and estimated burden of tuberculosis among children admitted to 13 government hospitals in Kenya: an analysis of two years' routine clinical data. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0221145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens S., Abdel-Rahman I.E., Balyejusa S., et al. Nasopharyngeal aspiration for diagnosis of pulmonary tuberculosis. Arch Dis Child. 2007;92(8):693–696. doi: 10.1136/adc.2006.108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . WHO operational handbook on tuberculosis: module 5: management of tuberculosis in children and adolescents. 2022. WHO operational handbook on tuberculosis: module 5: management of tuberculosis in children and adolescents. [PubMed] [Google Scholar]
- 8.Marcy O., Ung V., Goyet S., et al. Performance of xpert MTB/RIF and alternative specimen collection methods for the diagnosis of tuberculosis in HIV-infected children. Clin Infect Dis. 2016;62(9):1161–1168. doi: 10.1093/cid/ciw036. [DOI] [PubMed] [Google Scholar]
- 9.Song R., Click E.S., McCarthy K.D., et al. Sensitive and feasible specimen collection and testing strategies for diagnosing tuberculosis in young children. JAMA Pediatr. 2021;175(5) doi: 10.1001/jamapediatrics.2020.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehme C.C., Nicol M.P., Nabeta P., et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theron G., Zijenah L., Chanda D., et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(9915):424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 12.Hales S., Lesher-Trevino A., Ford N., Maher D., Ramsay A., Tran N. Reporting guidelines for implementation and operational research. Bull World Health Organ. 2016;94(1):58–64. doi: 10.2471/BLT.15.167585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wobudeya E., Niangoran S., Borand L., et al. 50th World conference on lung health of the international union against tuberculosis and lung disease (The Union) 2019. Childhood TB diagnostic capacities in primary healthcare facilities in high TB-burden countries: results from the TB-Speed cross-sectional descriptive survey. Hyderabad. [Google Scholar]
- 14.MoH . 2015. Uganda management of tuberculosis in children; a health worker guide. [Google Scholar]
- 15.Hinde J., Demétrio C.G. Overdispersion: models and estimation. Comput Stat Data Anal. 1998;27(2):151–170. [Google Scholar]
- 16.Zawedde-Muyanja S., Nakanwagi A., Dongo J.P., et al. Decentralisation of child tuberculosis services increases case finding and uptake of preventive therapy in Uganda. Int J Tuberc Lung Dis. 2018;22(11):1314–1321. doi: 10.5588/ijtld.18.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abayneh M., HaileMariam S., Asres A. Low tuberculosis (TB) case detection: a health facility-based study of possible obstacles in kaffa zone, southwest district of Ethiopia. Can J Infect Dis Med Microbiol. 2020;2020 doi: 10.1155/2020/7029458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deya R.W., Masese L.N., Jaoko W., et al. Yield and coverage of active case finding interventions for tuberculosis control: a systematic Review and meta-analysis. Tuberc Res Treat. 2022;2022 doi: 10.1155/2022/9947068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei X., Liang X., Liu F., Walley J.D., Dong B. Decentralising tuberculosis services from county tuberculosis dispensaries to township hospitals in China: an intervention study. Int J Tuberc Lung Dis. 2008;12(5):538–547. [PubMed] [Google Scholar]
- 20.Ndlovu Z., Fajardo E., Mbofana E., et al. Multidisease testing for HIV and TB using the GeneXpert platform: a feasibility study in rural Zimbabwe. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyaga C., Sendagire H., Joseph E., et al. Uganda's new national laboratory sample transport system: a successful model for improving access to diagnostic services for early infant HIV diagnosis and other programs. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0078609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penazzato M., Davies M.A., Apollo T., Negussie E., Ford N. Task shifting for the delivery of pediatric antiretroviral treatment: a systematic review. J Acquir Immune Defic Syndr. 2014;65(4):414–422. doi: 10.1097/QAI.0000000000000024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.