Abstract
Introduction
Endodontic treatment failures often stem from the presence of microbial pathogens, particularly Enterococcus faecalis and Candida albicans. This study systematically assesses the prevalence of E. faecalis and C. albicans in endodontic retreatment cases, aiming to explore their impact on treatment outcomes.
Methods
Employing a systematic sampling approach, 30 patients with a history of previous endodontic treatment were selected. Rigorous clinical and radiographic assessments were conducted, following standardized protocols for root canal sample collection. Microbiological analysis, utilizing selective culture media, was employed to identify and quantify E. faecalis and C. albicans. Statistical analyses, including chi-square and logistic regression tests, were performed.
Results
The study involved 30 patients undergoing endodontic retreatment, with comprehensive clinical and radiographic evaluations for cases with and without periradicular lesions. Microbiological analysis unveiled a significant prevalence of E. faecalis and C. albicans, establishing a robust association between these pathogens and retreatment failure. These findings underscore the critical need for targeted antimicrobial interventions to enhance the overall success rates of endodontic retreatment procedures.
Conclusion
This study highlights the substantial prevalence of E. faecalis and C. albicans in endodontic retreatment cases, emphasizing the importance of identifying and effectively managing these pathogens for successful treatment outcomes. The notable association between these microbial agents and retreatment failure underscores the imperative for tailored antimicrobial strategies to enhance the efficacy of endodontic retreatment procedures.
Keywords: Enterococcus faecalis, Candida albicans, Endodontic retreatment, Prevalence, Treatment failure
1. Introduction
Endodontic retreatment, a procedure undertaken for patients grappling with persistent or recurring infections within the root canal system (Siqueira et al., 2001). Endodontic retreatment faces challenges associated with microbial pathogens, prominently E.faecalis and C.albicans, known culprits in endodontic treatment failures (Figdor et al (2007)). This study delves into assessing the prevalence of E. faecalis and C. albicans in endodontic retreatment cases, aiming to unravel their impact on treatment outcomes (Ørstavik,2020).Fig. 1.Fig. 2.Fig. 3.Fig. 4.Table 1.Table 2..
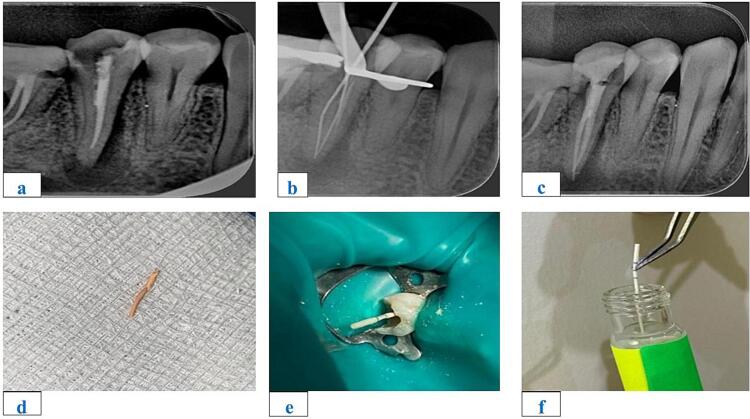
Fig. 1.
Images of Sample Extraction Procedure - a) Preoperative b) Working Length c) Obturation d) Old Gutta-percha e) Paper point f) Transfer to Sterile Test Tube.
Fig. 2.
Batches - a) Batch One Before Incubation b) Batch Two Before Incubation C) Batch Three Before Incubation d) Batch One After Incubation e) Batch Two After Incubation f) Batch Three After Incubation.
Fig. 3.
Microbial identification of the E. feacalis - a) GAMA hemolytic colonies on Blood Agar Plate b) Gram positive cocci in chains c) Bile esculin – Negative d) Bile esculin – Positive e) Pyruvate fermentation – Negative f) Pyruvate fermentation – Positive.
Fig. 4.
Microbial identification of the C.albicans – a) Pale white ovoid colonies on Sabauraud’s Dextrose Agar b) Gram positive oval yeast budding cells c) Germ Tube Test.
Table 1.
Study on the radiographic changes and teeth with multiple root canals.
| Sample No. | Age/sex | Symptoms | Radiographic changes | Time since obturation (years) | Tooth No. | Number of root canals |
|---|---|---|---|---|---|---|
| 1 | 56/M | Asymptomatic | PR | 5 | 14 | 2 |
| 2 | 30/F | Symptomatic | PR | 2 | 22 | 1 |
| 3 | 22/F | Asymptomatic | PDL | 3 | 11 | 1 |
| 4 | 37/M | Symptomatic | PR | 2 | 21 | 1 |
| 5 | 28/F | Symptomatic | PDL | 4 | 35 | 2 |
| 6 | 44/F | Symptomatic | PR | 6 | 24 | 2 |
| 7 | 33/F | Symptomatic | PDL | 3 | 15 | 2 |
| 8 | 47/M | Asymptomatic | PR | 4 | 21 | 1 |
| 9 | 25/F | Asymptomatic | PR | 2 | 22 | 1 |
| 10 | 20/M | Asymptomatic | PR | 2 | 44 | 2 |
| 11 | 40/F | Asymptomatic | PR | 5 | 35 | 1 |
| 12 | 23/M | Symptomatic | PR | 4 | 14 | 2 |
| 13 | 42/F | Asymptomatic | PDL | 2 | 23 | 1 |
| 14 | 33/M | Symptomatic | PR | 2 | 16 | 4 |
| 15 | 19/F | Asymptomatic | PDL | 3 | 46 | 4 |
| 16 | 26/F | Asymptomatic | PDL | 3 | 37 | 3 |
| 17 | 45/M | Asymptomatic | PR | 4 | 24 | 2 |
| 18 | 50/M | Asymptomatic | PR | 2 | 21 | 1 |
| 19 | 27/F | Symptomatic | PR | 3 | 12 | 1 |
| 20 | 24/M | Asymptomatic | PDL | 3 | 21 | 1 |
| 21 | 36/F | Symptomatic | PR | 1 | 11 | 1 |
| 22 | 30/M | Symptomatic | PDL | 3 | 45 | 1 |
| 23 | 27/F | Symptomatic | PR | 1 | 22 | 1 |
| 24 | 24/M | Asymptomatic | PR | 2 | 44 | 1 |
| 25 | 38/F | Symptomatic | PR | 5 | 24 | 2 |
| 26 | 35/F | Asymptomatic | PR | 3 | 34 | 1 |
| 27 | 24/M | Symptomatic | PR | 4 | 14 | 2 |
| 28 | 28/F | Symptomatic | PDL | 3 | 15 | 1 |
| 29 | 30/M | Symptomatic | PR | 2 | 16 | 4 |
| 30 | 40/M | Asymptomatic | PDL | 2 | 23 | 1 |
PR – Periapical Radiolucency.
PDL – Widened PDL space.
Table 2.
Microbiological Test Results.
| TUBES | ENTEROCOCCUS | CANDIDA | NORMAL FLORA |
|---|---|---|---|
| 1 | Staphylococcus | ||
| 2 | + | Staphylococcus | |
| 3 | Coliforms | ||
| 4 | + | Coliforms | |
| 5 | Coliforms | ||
| 6 | + | Staphylococcus | |
| 7 | Staphylococcus | ||
| 8 | + | Staphylococcus | |
| 9 | Coliforms | ||
| 10 | + | Staphylococcus | |
| 11 | + | Staphylococcus | |
| 12 | Coliforms | ||
| 13 | + | Staphylococcus | |
| 14 | + | Coliforms | |
| 15 | + | Staphylococcus | |
| 16 | + | Staphylococcus | |
| 17 | + | Coliforms | |
| 18 | + | Staphylococcus | |
| 19 | + | Coliforms | |
| 20 | + | Staphylococcus | |
| 21 | + | Coliforms | |
| 22 | + | Staphylococcus | |
| 23 | + | Staphylococcus | |
| 24 | + | Coliforms | |
| 25 | Staphylococcus | ||
| 26 | + | Coliforms | |
| 27 | + | Coliforms | |
| 28 | Staphylococcus | ||
| 29 | + | Coliforms | |
| 30 | + | Staphylococcus |
The field of endodontics, constituting the foundation of dental expertise, underscores the paramount importance of early identification of endodontic microflora and a comprehensive understanding of its role in chronic root canal infections (Torabinejad et al., 2009). Microbiota within the root canal system plays a pivotal role in the pathogenesis of periapical lesions, navigating challenges posed by root canal irrigation and intracranial medication (Chávez et al, (2003)). The core principle of root canal treatment hinges on the complete elimination of microflora, including fungi, achieved through instrumentation and antimicrobial irrigation (Stuart et al., 2006). The primary goal is to prevent and address apical periodontitis (Haapasalo et al, (1989)). Persistent periapical lesions in previously treated teeth indicate the continuation or recurrence of apical periodontitis, paving the way for non-surgical endodontic retreatment, offering favorable long-term outcomes in cases of root canal failure (Samaranayake et al., 2018).
E.faecalis, distinguished by its ability to form biofilms, emerges as a formidable microbial species, exhibiting a resistance to phagocytosis and antimicrobials compared to non-biofilm-producing microorganisms(Muazzam et al., 2020). The retreatment process involves regaining access to the apical region, removing previous obturation material, and executing biomechanical preparation, culminating in the three-dimensional obturation of the entire root canal system (Abraham et al., 2020).
Despite strides in root canal treatment, post-treatment endodontic infections persist as a challenge for dentists (Segura et al., 2012). Limited information on the prevalence of E. faecalis and C.albicans in endodontic retreatment cases within the Saudi Arabian population prompted this study. The rationale was to assess their prevalence in this specific population, employing microbiological culturing techniques.
The study aimed to evaluate the incidence of E. faecalis and C. albicans in endodontic retreatment cases through microbiological culturing techniques. The primary objectives were to scrutinize the correlation between the presence of E. faecalis, with or without periradicular lesions, and to assess a similar correlation for C. albicans. Endodontic retreatment, addressing failed or persistent infections in previously treated root canals, remains imperative despite advancements in techniques and materials (Zoletti et al., 2006).
Among microorganisms commonly associated with endodontic treatment failure, E. faecalis and C. albicans emerge as recurrent isolates (Poptani et al., 2013). E. faecalis, a facultative anaerobic bacterium, presents challenges due to its survival in hostile environments and resistance to disinfection methods (Sundqvist et al., 1998). The frequent recovery of E. faecalis from root canals with persistent or recurrent infections underscores its resilience. C. albicans, a dimorphic fungus, can transition between yeast and hyphal forms, turning pathogenic under specific conditions and contributing to endodontic treatment failure, particularly in cases linked to periradicular lesions and chronic symptoms (Molander et al., 1998).
The prevalence of E. faecalis and C. albicans in endodontic retreatment cases assumes significance for devising effective treatment strategies (Hancock et al., 2001). Hence, this study endeavors to analyze the prevalence of these pathogens in a cohort of endodontic retreatment cases within the Saudi Arabian population and assess their impact on treatment outcomes.
2. Materials and method
2.1. Study design and sample Selection
Conducted at a specialized dental clinic focusing on endodontics, this cross-sectional study engaged a sample of 30 patients requiring endodontic retreatment between October 1, 2022, and March 31, 2023. Informed consent was secured from all participants, and ethically approved by the institutional ethics committee.
2.2. Inclusion Criteria
The study included patients aged 20 to 70 years, having undergone endodontic retreatment at least two years prior, identified through patient history, clinical symptoms, percussion tenderness, and the presence or absence of periapical radiolucency. Teeth with insufficient root canal obturation within 2 mm of the apex or voids in the obturation were also considered.
2.3. Exclusion Criteria
Patients with systemic fungal infections, undergoing antifungal medication, immunodeficiency diseases, and specific dental conditions such as difficulty in applying a rubber dam, advanced mobility, posts in the root canal, separated instruments, extreme root canal curvature, and radicular fractures were excluded.
2.4. Clinical and radiographic Evaluation
Clinical records detailed previous endodontic treatments, while examinations assessed pain on percussion, facial or localized swelling, sinus tract, defective restoration, and recurrent caries. Periapical radiographs were examined for periapical radiolucency, missed canals, and the quality of previous obturation.
2.5. Sample collection and Microbiological Analysis
Microbial samples, obtained under aseptic conditions, involved isolating teeth with a rubber dam after oral prophylaxis. Disinfection with hydrogen peroxide and neutralization with sodium thiosulfate preceded the removal of coronal restorations. Gutta-percha removal utilized Gates Glidden burs and Protaper universal retreatment files without chemical solvents. Sterile saline irrigated the canals before collecting samples using standardized paper points, immediately transferred to sterile test tubes containing Stuart transport medium for transportation to the Microbiology laboratory within 2 h.
2.6. Microbial culturing for e. Faecalis:
Inoculation on sterile blood agar plates, incubation at 37 °C for 2 to 3 days, and Gram staining identified gram-positive cocci, with bile esculin agar revealing E. faecalis through the hydrolysis of esculin to escluetin, observed as blackening of the media (Maqbul et al., 2022).
2.7. Microbial culturing for C. albicans
Inoculation on sterile Sabouraud's Dextrose Agar plates, incubation at 37 °C for 2 to 3 days, and Gram staining identified yeast oval colonies. KOH and lactophenol tests detected yeast budding cells, with the Germ tube test confirming C. albicans through characteristic germ tube formation.
2.8. Statistical Analysis
Software-based statistical analysis calculated the prevalence of E. faecalis and C. albicans with 95 % confidence intervals. The chi-square test assessed the association between pathogen presence and retreatment failure. Logistic regression analysis determined odds ratios (OR) and 95 % CIs for retreatment failure in cases positive for E. faecalis and C. albicans, adjusting for relevant covariates.
3. Result
3.1. Clinical and radiographic Evaluation
The presented tabulated results in the Table-1 encapsulate the findings of an in-depth study focusing on radiographic changes and symptoms in teeth boasting multiple root canals. The investigation discerned that teeth featuring multiple root canals exhibit a heightened likelihood of developing periapical radiolucency (PR) and widened periodontal ligament space (PDL) in comparison to their single-rooted counterparts. PR, indicating inflammation or infection at the root tip, and widened PDL, signifying an enlargement between the tooth and surrounding bone, emerge as reliable indicators of underlying dental issues. The intricate nature of teeth with multiple root canals contributes to a greater likelihood of widened periodontal ligament space (PDL) development than teeth with a single root canal. The inherent complexity exposes a larger surface area to potential bacterial infiltration and infection. Moreover, the root canals in multi-rooted teeth pose challenges in terms of thorough cleaning and disinfection, escalating the risk of infection. To facilitate comprehension, a visual representation of the sample extraction procedure from the root canals is presented in Figure-1, organized in a collage manner for enhanced understanding. The study further established that teeth with multiple root canals are more prone to exhibiting symptoms compared to their single-rooted counterparts. The infections causing PR and PDL can manifest as pain, swelling, and tenderness, and in severe cases, may lead to conditions such as abscess formation. Result images in the Figure-2 of the three batches of tubes before and after incubation were provided.
3.2. Microbiological Identification
The growth of E. faecalis was observed, characterized by gamma hemolytic colonies on Blood Agar. Gram staining revealed Gram-positive cocci arranged in chains, with subsequent tests confirming its identity, notably a positive outcome for the Bile Esculin Test. Bile esculin agar was used to detect the presence of E. faecalis. After overnight incubation at 37 °C, a presumptive positive reaction was observed as blackening of the media due to the hydrolysis of esculin to escluetin by E. faecalis in the presence of ferric citrate [Muazzam et al., 2020]. Images in the Figure-3 of microbial identification highlighted its distinctive characteristics, including metabolic traits and environmental adaptability.
The growth of C.albicans appeared as pale white ovoid colonies on Sabouraud's Dextrose Agar (SDA) plates. Gram staining disclosed Gram-positive oval yeast cells with budding formations, confirmed by subsequent tests, including the germ tube confirmatory test. Images in the Figure-4 provided a visual insight into the microbial identification process.
3.3. Microbial test Results
E. faecalis was predominant, isolated in 13 tubes, signifying the highest pathogenic concentration in the sample. C.albicans was present in 9 tubes, indicating a significant concentration. Normal flora, represented by Staphylococcus and Coliform, were isolated in 17 and 13 tubes, respectively. The presence of normal flora might suggest sample contamination during collection or processing, although coexistence with pathogens remains plausible. The presence of normal microbiota was consistent in all samples and does not alter our conclusion that many of these individuals had infections with E. faecalis and C. albicans. The tabular column Table-2 shows the isolated samples from the patients.
3.4. Statistical Analysis
The study encompassed patients aged 20 to 70 from the Saudi Arabian population. E. faecalis was detected in 43 % of the 30 samples, notably prevalent in root-filled teeth with periradicular lesions. C. albicans was found in 30 % of the samples. Statistical analysis using SPSS software revealed a significant association between the presence of E. faecalis and C. albicans with retreatment failure. Logistic regression analysis indicated higher odds of retreatment failure in cases positive for E. faecalis (OR = 2.36) and C. albicans (OR = 1.92).
4. Discussion
The study revealed a significant presence of E. faecalis and C. albicans in endodontic retreatment cases. E. faecalis known for its ability to persist within dentinal tubules and was detected in a substantial proportion of cases, highlighting the challenges involved in eradicating this pathogen during retreatment procedures. E. faecalis is a gram-positive bacterium that is commonly found in the intestines. It can sometimes cause infections in other parts of the body, such as the urinary tract. E. faecalis often resistant to antibiotics, so it is important to identify the antibiotic susceptibility of the strain that is isolated in the patient's sample (Pinheiro et al., 2003). C. albicans, a fungal pathogen, was also commonly found in retreatment cases, suggesting its potential role in treatment failure (Hancock et al., 2001). C. albicans is a type of yeast that can also cause urinary tract infections. C. albicans is often resistant to antifungal medications, so it is also important to identify the antifungal susceptibility of the strain that is isolated in the patient's sample (Cheung et al (2001)).
The robust association between the presence of E. faecalis and retreatment failure emphasizes the imperative need for effective antimicrobial strategies tailored to combat these pathogens (Peciuliene et al., 2000). Conventional endodontic disinfection protocols, while beneficial, may not completely eliminate these microorganisms (Peciuliene et al., 2001). Hence, the study advocates for supplementary measures such as antimicrobial agents, intracranial medicaments, and biofilm-targeting regimens to enhance treatment outcomes.
The study highlighted that teeth with multiple root canals exhibited an increased likelihood of developing widened periodontal ligament space (PDL) compared to single-rooted teeth. The intricate anatomy and larger surface area of multi-rooted teeth make them more susceptible to bacterial infections, contributing to the elevated risk (Lima et al., 2001). The study also associated missed canals in multiple-rooted teeth with periapical radiolucency and widened PDL, further emphasizing the challenges in cleaning and disinfecting these complex root canal systems. Teeth with multiple root canals were more prone to symptomatic manifestations such as pain, swelling, and tenderness, with potential progression to a severe condition known as an abscess (Mann et al., 2011). Despite a relatively small sample size and the need for cautious interpretation, the study urges individuals facing dental issues to consult endodontists for comprehensive examinations and X-rays to assess potential problems.
The study extended its scope by identifying components of the typical oral microbiota, including Staphylococcus and Coliforms, in all sample tubes. Microorganisms considered to be part of the normal microbiota of the oral cavity were isolated in all sample tubes (Sheriff Maqbul et al., 2020). While we collected microbial samples under strict aseptic conditions, the presence of normal microbiota in our sample suggests that they may have been contaminated during collection process or processing of the samples. (Gouse et al., 2017). While aseptic conditions were rigorously applied during microbial sample collection, the presence of normal microbiota raises the possibility of contamination (Baumgartner et al., 2000). Nevertheless, the study maintains confidence in the consistent presence of normal microbiota across all samples (Rôças et al., 2009). The results do not alter the conclusion that many individuals in the study exhibited infections involving E. faecalis and C. albicans (Egan et al., 2002). In a broader context, the study positions these findings within the realm of endodontic infections, elucidating that these infections result from a combination of anaerobic microorganisms, including bacteria and fungi (Rocas et al., 2004). Periradicular lesions, stemming from inflammation around the root tip due to infections, are linked to microorganisms originating from root canals in teeth with necrotic pulps.
The study underscores the clinical significance of E. faecalis and C. albicans in endodontic retreatment cases, advocating for targeted antimicrobial interventions to enhance procedural success. Future research endeavors should explore larger and more diverse populations through multicenter studies to refine the understanding of microbial prevalence and devise more effective treatment strategies.
5. Conclusion
This study provides valuable insights into the microbial landscape of endodontic retreatment cases, emphasizing the multifaceted challenges posed by E. faecalis and C. albicans. The nuanced findings pave the way for refined treatment approaches and underscore the dynamic interplay between microbial flora and treatment outcomes in endodontic scenarios.
CRediT authorship contribution statement
Siddiq Ahmed: Supervision. Sami Jehad Hassan: Supervision. Shaiq Gajdhar: Conceptualization, Methodology. Lama Saleh Alhazmi: Data. Rawan Yahya Khalifah: Literature collection. Juman Alhusain Alrifai: Conceptualization. Shymaa Salem Aljhdali: Conceptualization. Muazam Sheriff Maqbul: Study Design, Methodology, Interpretation of results.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are exceptionally grateful to the organization of Ibn Sina National College, Jeddah, KSA.
Funding
No funding.
Ethical Approval
The study was conducted under the title “ Prevalence of Enterococcus Faecalis and Candida albicans in Endodontic Retreatment Cases: A Comprehensive Study ” was approved by Ibn Sina National College Research Review Board Institutional Human Ethics Committee with ethical approval IRRB number H-38-15032018 along with the protocol identification number 015DP03012018.
Conflict of Interest
No Conflict of interest.
References
- Abraham S.B., al Marzooq F., Himratul-Aznita W.H., et al. Prevalence, virulence and antifungal activity of C. albicans isolated from infected root canals. BMC Oral Health. 2020;20:347. doi: 10.1186/s12903-020-01347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J.C., Watts C.M., Xia T. Occurrence of Candida albicans in infections of endodontic origin. J Endod. 2000;26:695–698. doi: 10.1097/00004770-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Chávez De Paz L.E., Dahlén G., Molander A., Möller A., Bergenholtz G. Bacteria recovered from teeth with apical periodontitis after antimicrobial endodontic treatment. Int Endod J. 2003;36(7):500–508. doi: 10.1046/j.1365-2591.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- Cheung G.S., Ho M.W. Microbial flora of root canal-treated teeth associated with asymptomatic periapical radiolucent lesions. Oral Microbiol Immunol. 2001;16:332–337. doi: 10.1034/j.1399-302x.2001.160603.x. [DOI] [PubMed] [Google Scholar]
- Egan M.W., Spratt D.A., Ng Y.L., Lam J.M., Moles D.R., Gulabivala K. Prevalence of yeasts in saliva and root canals of teeth associated with apical periodontitis. Int Endod J. 2002;35:321–329. doi: 10.1046/j.1365-2591.2002.00478.x. [DOI] [PubMed] [Google Scholar]
- Figdor D., Sundqvist G. A big role for the very small understanding the endodontic microbial flora. Australian Dental Journal. 2007;52:155–159. doi: 10.1111/j.1834-7819.2007.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Gouse B.S., Muazzam S.M., Gokul S.S., Ranjith M.S. Isolation and characterization of actinomycetes from soil of AI-Dawadmi, Saudi Arabia and screening their antibacterial activities. Int J Pharm Pharm Sci. 2017;9:267–279. [Google Scholar]
- Haapasalo M. Bacteroides spp. in dental root canal infections. Endod Dent Traumatol. 1989 Feb;5(1):1-10. doi: 10.1111/j.1600-9657. 1989.tb00330.x. [DOI] [PubMed]
- Hancock H.H., 3rd, Sigurdsson A., Trope M., Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:579–586. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- Lima K.C., Fava L.R., Siqueira J.F., Jr. Susceptibilities of Enterococcus faecalis biofilms to some antimicrobial medications. J Endod. 2001;27:616–619. doi: 10.1097/00004770-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Mann N.g.L., Gulabivala K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. International Endodontic Journal. 2011;44(7):583–609. doi: 10.1111/j.1365-2591.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- Maqbul M.S., Sarhan R.N., Alzubaidi F.A.S., Hejji A.T. The antimicrobial vulnerability testing of Linum flavum hydrocolloids against pediatric surgical MRSA isolates with qualitative bio-phytochemical analysis quantified by GC-MS-UV-Vis spectrophotometry. Medical Science. 2022;26:ms571e2688. doi: 10.54905/disssi/v26i130/ms571e2688. [DOI] [Google Scholar]
- Molander A., Reit C., Dahlén G., Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- Muazzam S.M., Yumna A.B., Samaher G.B., Shaden N.A., Bashair M.M., Khan A.A., et al. A comparative study of different types of thyme essential oils against Streptococcus pyogenes to determine their biochemical and antimicrobial properties. Orient J Chem. 2020;36:220–228. [Google Scholar]
- Ørstavik D. In: Essential Endodontology. 2nd edition. Ørstavik D., editor. Wiley Blackwell; Oxford, UK: 2020. Apical periodontitis: microbial infection and host responses; pp. 1–10. [Google Scholar]
- Peciuliene V., Balciuniene I., Eriksen H.M., Haapasalo M. Isolation of Enterococcus faecalis in previously root-filled canals in aLithuanian population. J Endod. 2000;26:593–595. doi: 10.1097/00004770-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Peciuliene V., Reynaud A.H., Balciuniene I., Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J. 2001;34:429–434. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro E.T., Gomes B.P., Ferraz C.C., Sousa E.L., Teixeira F.B., Souza-Filho F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- Poptani B., Sharaff M., Archana G., Parekh V. Detection of Enterococcus faecalis and Candida albicans in previously root-filled teeth in a population of Gujarat with polymerase chain reaction. Contemp Clin Dent. 2013;4:62–66. doi: 10.4103/0976-237X.111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rôças I.N. Distinctive features of the microbiota associated with different forms of apical periodontitis. Journal of Oral Microbiology. 2009;1 doi: 10.3402/jom.v1i0.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocas I.N., Young Jung I.I., Chan-YL S.JF. Polymerase Chain reaction identification of microorganisms in previously root filled teeth in a south Korean population. J Endod. 2004;30:504–508. [PubMed] [Google Scholar]
- Samaranayake L. Elsevier; Amsterdam: 2018. Essential microbiology for Dentistry-E-Book. [Google Scholar]
- Segura-Egea J.J., Castellanos-Cosano L., Machuca G., López-López J., Martín-González J., Velasco-Ortega E., Sánchez-Domínguez B., López-Frías F.J. Diabetes mellitus, periapical inflammation, and endodontic treatment outcome. Med Oral Patol Oral Cir Bucal. 2012;17(2):e356–e361. doi: 10.4317/medoral.17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff Maqbul M., A Khan A., Mohammed T., Shakeel Iqubal S., Rahman Ikbal A., Ahmed Shaikh I., Muddapur U., Shahid Hussain M., K Singh S. The Efficacy of Cinnamomum Tamala as a Potential Antimicrobial Substance against the Multi-Drug Resistant Enterococcus Faecalis from Clinical Isolates. Advanced. Materials Letters. 2020;11(1):1–4. doi: 10.5185/amlett.2020.011464. [DOI] [Google Scholar]
- Siqueira J.F., Jr. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34(1):1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Stuart C.H., Schwartz S.A., Beeson T.J., Owatz C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Sundqvist G., Figdor D., Persson S., Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- Torabinejad M., Corr R., Handysides R., Shabahang S. Outcomes of Nonsurgical Retreatment and Endodontic Surgery: A Systematic Review. J Endod. 2009;35(7):930–937. doi: 10.1016/j.joen.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Zoletti G.O., Siqueira J.F., Santos K.R.N. Identification of Enterococcus faecalis in Root-filled Teeth with or without Periradicular Lesions by Culture-dependent and—Independent Approaches. Journal of Endodontics. 2006;32(8):722–726. doi: 10.1016/j.joen.2006.02.001. [DOI] [PubMed] [Google Scholar]
Further reading
- Sheriff Maqbul M., A Khan A., Mohammed T., Shakeel Iqubal S., Rahman Ikbal A., Ahmed Shaikh I., Muddapur U., Shahid Hussain M., K Singh S. Antifungal Activity of Salvia jordanii Against the Oral Thrush Caused by the Cosmopolitan Yeast Candida albicans among Elderly Diabetic Type 2 patients. Advanced. Materials Letters. 2020;11(3):1–4. doi: 10.5185/amlett.2020b.031493. [DOI] [Google Scholar]