Abstract
Objective
Pulmonary arterioplasty (PA plasty) at bidirectional cavopulmonary anastomosis (BDCA) is associated with increased morbidity, but outcomes to final stage palliation are unknown. We sought to determine the influence of PA plasty on pulmonary artery growth and hemodyamics at Fontan.
Methods
We retrospectively reviewed clinical data and outcomes for BDCA patients from 2006 to 2018. PA plasty was categorized by extent (type 1-4), as previously described. Outcomes included pulmonary artery reintervention and mortality before final palliation.
Results
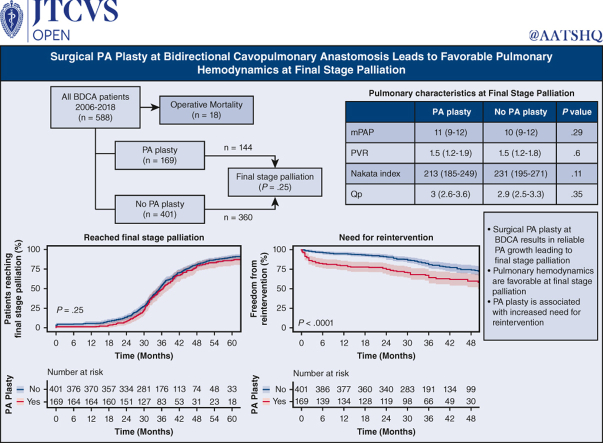
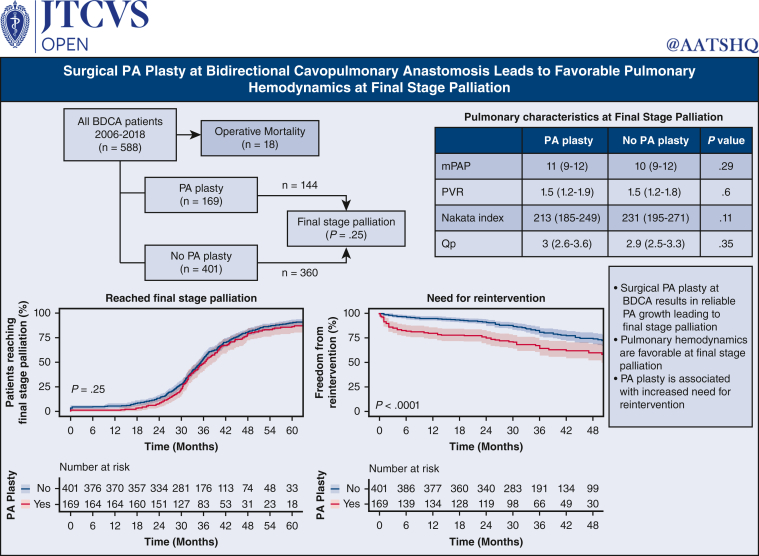
Five hundred eighty-eight patients underwent BDCA. One hundred seventy-nine patients (30.0%) underwent concomitant PA plasty. Five hundred seventy (97%) patients (169 [94%] PA plasty) survived to BDCA discharge. One hundred forty out of 570 survivors (25%) required PA/Glenn reintervention before final stage palliation (59 out of 169 [35%]) PA plasty; 81 out of 401 (20%) non-PA plasty; P < .001). Twelve-, 24-, and 36-month freedom from reintervention after BDCA was 80% (95% CI, 74-86%), 75% (95% CI, 69-82%), and 64% (95% CI, 57-73%) for PA plasty, and 95% (95% CI, 93-97%), 91% (95% CI, 88-94%), and 81% (95% CI, 76-85%) for non-PA plasty (P < .001). Prefinal stage mortality was 37 (6.3%) (14 out of 169 PA plasty; 23 out of 401 non-PA plasty; P = .4). Five hundred four (144 PA plasty and 360 non-PA plasty) patients reached final stage palliation (471 Fontan, 26 1.5-ventricle, and 7 2-ventricular repair). Pre-Fontan PA pressure and pulmonary vascular resistance were 10 mm Hg (range, 9-12 mm Hg) and 1.6 mm Hg (range, 1.3-1.9 mm Hg) in PA plasty and 10 mm Hg (range, 8-12 mm Hg) and 1.5 mm Hg (range, 1.3-1.9 mm Hg) in non-PA plasty patients, respectively (P = .29, .6). Fontan hospital mortality, length of stay, and morbidity were similar.
Conclusions
PA plasty at BDCA does not confer additional mortality risk leading to final palliation. Despite increased pulmonary artery reintervention, there was reliable pulmonary artery growth and favorable pulmonary hemodynamics at final stage palliation.
Key Words: single ventricle, Fontan, bidirectional cavopulmonary anastomosis, pulmonary arterioplasty
Graphical Abstract
Cumulative incidence function of reaching final palliation by need for PA plasty at BDCA.
Central Message.
Pulmonary arterioplasty at stage 2 palliation results in reliable pulmonary artery growth and favorable pulmonary artery hemodynamics leading up to final stage palliation.
Perspective.
Not infrequently, surgical augmentation of the pulmonary arteries is required at stage 2 palliation. We previously showed that this increases hospital morbidity and mortality, but outcomes to final stage palliation are unknown. Amongst hospital survivors, PA plasty does not confer additional mortality risk leading to final palliation. Reliable growth and favorable PA hemodynamics are seen.
Surgical outcomes of single ventricle (SV) palliation continue to improve with patients surviving to older ages. Classically, SV palliation is performed in 3 staged surgeries. Total passive systemic venous return to the pulmonary arteries (PAs) is achieved resulting in Fontan circulation. Optimal PA architecture and low pulmonary vascular resistance (PVR) are critically important to accommodate passive blood flow and achieve successful SV palliation.
The second stage of palliation diverts venous blood from the superior vena cava directly into the PA and is most frequently accomplished with a bidirectional cavopulmonary anastomosis (BDCA).1, 2, 3 Concomitant procedures to address anatomic and physiologic lesions may be required during BDCA to optimize hemodynamics for final stage palliation. Pulmonary arterioplasty (PA plasty) is among the more common concomitant procedures. We have previously shown that the need for PA plasty at the time of BDCA is associated with increased hospital morbidity and mortality.4 However, the influence of PA plasty on outcomes and pulmonary hemodynamics through completion of final stage palliation has not been reported. We therefore focused our analysis on PA growth and progression toward final stage palliation in patients who required PA plasty at the time of BDCA.
Methods
Patients
We retrospectively reviewed the records of all patients who underwent BDCA at Children's Hospital Los Angeles between 2006 and 2018. Under institutional review board approved protocols (CHLA-22-00246; approved August 16, 2022), patient records were identified from our surgical database. Patient demographic characteristics, preoperative investigations, operative characteristics, follow-up, and outcomes data were collected. See Figure 1 for a graphical abstract of the study. Patients were selected to receive a PA plasty based on pre-BDCA catheterization as previously described.4 We elect to perform surgical PA plasty at the time of BDCA in the setting of >50% reduction in diameter of the PA, compared with the hilar PA diameter, or for any stenosis that results in significant pressure gradient in the PA.
Figure 1.
Graphical abstract. Surgical pulmonary arterioplasty at bidirectional cavopulmonary anastomosis (BDCA) results in reliable pulmonary artery growth and favorable pulmonary artery hemodynamics leading up to final stage palliation. PA plasty, Pulmonary arterioplasty.
Study Period
For the purposes of this analysis, patients were followed from the time of BDCA discharge. Follow-up was truncated at successful final stage palliation (1.5- or 2-ventricle repair or Fontan) or transplant. For patients not reaching final stage palliation, follow-up was until mortality or last clinic visit awaiting completion Fontan (alive awaiting Fontan). Follow-up was achieved via chart review and telephone interview.
Data Collection
Data were retrospectively collected from patient medical records and from institutional Society of Thoracic Surgeons’ (STS) Congenital Heart Surgery Database (CHSD) forms. STS-CHSD criteria were used to define genetic syndromes, chromosomal anomalies, diagnosis, and preoperative demographic variables. Surveillance echocardiograms were used to classify atrioventricular valve regurgitation and ventricular function. Atrioventricular valve regurgitation was dichotomized as mild or less and moderate or more severe, and systemic ventricular function as mild or less and moderate or more severe reduction. Our institutional preference is to undertake routine preoperative cardiac catheterization before BDCA and final stage palliation. Data collected from catheterization included hemodynamic measurements, PVR, and mean PA pressure (MPAP).
Surgical reports were reviewed to collect operative data. We preferentially perform BDCA and Fontan palliation under cardiopulmonary bypass with a beating heart. Aortic crossclamping or deep hypothermic circulatory arrest is used when needed for concomitant procedures. Extent of PA plasty at the time of BDCA was recorded based on our previously defined classification4—no arterioplasty, patch closure of the PA confluence after dividing the main PA,1 plasty of the central PA extending into the proximal portion of 1 or both branch PAs,2 unilateral augmentation of the PA extending to the hilum,3 and bilateral hilum to hilum arterioplasty.4 An extracardiac (usually 18 mm) polytetrafluoroethylene graft is used to construct the Fontan circuit and fenestration is used sparingly, usually in the setting of significantly elevated PVR. Patients whose anatomy was amenable underwent 1.5- or 2-ventricle repair characterized by ventricular and/or atrial septation and establishment of right ventricle to PA continuity.
Outcome Measures
Surgical morbidity was defined per STS-CHSD criteria.5 Primary outcomes were achieving final stage palliation and reintervention on the PA. Patients were considered to have reached final stage palliation if they were discharged alive following completion Fontan or 1.5-ventricle or 2-ventricular repair. Need for heart transplant was not included in this end point. Cardiac catheterization was undertaken when indicated clinically or when noninvasive imaging modalities suggested anatomic lesions in the Glenn and/or PA. Reintervention was undertaken if a pressure gradient >2 mm Hg or a significant narrowing (>50% reduction in diameter of Glenn/PA anastomosis regardless of gradient) was encountered at catheterization. Type of reintervention (angioplasty, stent, or surgery) was determined jointly by the interventional cardiologist and cardiac surgeon. For the sake of these analyses, any intervention on the superior vena cava (Glenn) or PA was classified as reintervention.
Statistical Analysis
Statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc). Categorical data are presented as percentage and continuous non-normally distributed data are presented as median and interquartile range (IQR). Normally distributed continuous variables were compared by 2-tailed Student t tests or analysis of variance for multiple comparisons, and non-normally distributed variables were compared by Wilcoxon test. Categorical data were compared by χ2 tests. Durability of reconstructed PA was evaluated by Kaplan-Meier estimates for reintervention and compared by log-rank test between groups. Competing risk analyses were undertaken with death/transplant, alive awaiting Fontan, and reached final stage of palliation (Fontan or 1.5- or 2-ventricle repair) as outcomes. Cumulative incidence function analysis with Gray's test for significance was used to compare the outcome of reached final stage between the groups.
Results
Patient Demographics
Five hundred eighty-eight patients (248 girls [42%]) underwent BDCA at our institution over the 12-year period. One hundred seventy-nine patients (30%) required PA plasty at the time of BDCA. Five hundred seventy patients, including 169 (95%) who underwent PA plasty, survived to discharge following BDCA and constitute the cohort in the current analysis. Baseline characteristics of these patients are shown in Table 1. PA plasty patients were slightly younger, with significantly more dominant right ventricles and more likely to be male patients. Patch materials used included homograft (88 [49.2%]), polytetrafluoroethylene (32 [17.9%]), bovine pericardium (31 [17.3%]), CoreMatrix (CoreMatrix) (9 [5.0%]), autologous pericardium (7 [3.9%]), and Contegra (Medtronic) (3 [1.7%]). Median pre-Glenn Nakata index was 162 mm2/m2 (IQR, 118.5-219.5 mm2/m2) in the PA plasty group and 218 (IQR, 174-249) in the non-PA plasty group.
Table 1.
Demographic variables of hospital survivors of bidirectional cavopulmonary anastomosis (BDCA)
| Variable | Entire cohort | PA plasty | No PA plasty | P value |
|---|---|---|---|---|
| No of patients | 570 (100) | 169 | 401 | |
| Age (mo) | 6.9 (5.5-8.8) | 6.6 (5.5-8.7) | 7.2 (5.7-9.0) | .04 |
| Weight (kg) | 6.7 (5.9-7.8) | 6.8 (5.8-7.6) | 6.7 (5.9-7.8) | .77 |
| Female sex | 241 (42) | 57 (34) | 184 (46) | .01 |
| Dominant ventricle | .005 | |||
| Right | 280 (49) | 100 (59) | 180 (45) | |
| Left | 238 (42) | 62 (37) | 176 (44) | |
| Biventricle | 47 (8) | 6 (4) | 41 (10) | |
| Undetermined | 5 (1) | 1 (1) | 4 (1) | |
| Heterotaxy | 78 (14) | 28 (17) | 50 (13) | .24 |
| Genetic syndrome | 40 (7) | 13 (8) | 27 (7) | .82 |
| Prematurity | 94 (16) | 32 (19) | 62 (16) | .37 |
| Pre-BDCA cath data | ||||
| MPAP (mm Hg) | 13 (11-15) | 13.5 (12-16) | 13 (11-15) | .08 |
| iPVR (Woods units × m2) | 1.7 (1.3-2.2) | 1.7 (1.3-2.3) | 1.7 (1.3-2.1) | .74 |
| Pre-BDCA echo data | ||||
| Moderate or more depressed ventricular function | 59 (10) | 15 (9) | 44 (11) | .55 |
Values are presented as median (interquartile range) or n (%). PA plasty, Pulmonary arterioplasty; cath, cardiac catheterization; MPAP, mean pulmonary artery pressure; iPVR, indexed pulmonary vascular resistance; echo, echocardiography.
Outcomes Following BDCA Discharge
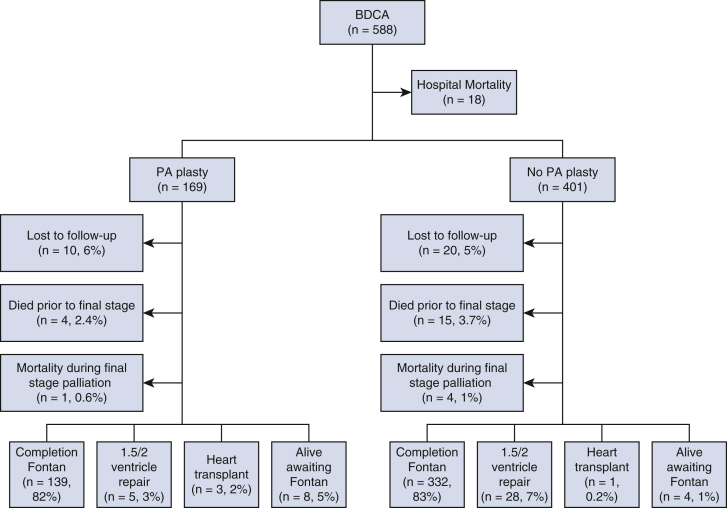
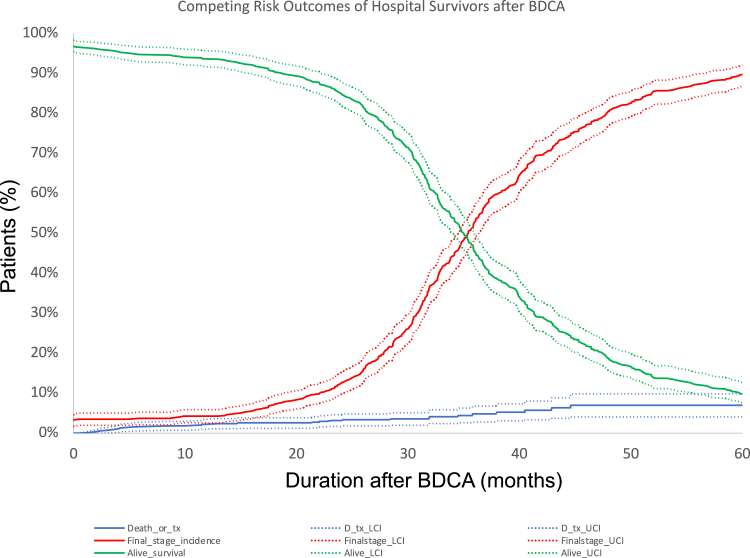
Median follow-up for the entire cohort is 34.8 (IQR, 28.9-44.5) months following BDCA discharge. Figure 2 demonstrates final palliation status at last follow-up. Thirty patients (5.1%) were lost to follow-up. Follow-up data are complete for the rest of 558 (95%) patients. Thirty-seven (6.3%) patients died before final stage palliation, 18 (3%) during Glenn hospitalization and 19 (3%) after Glenn discharge. Of these, 14 (2.3%) were in the PA plasty group and 23 (4%) in the non-PA plasty group (P = .3). Five (2%) patients died at the time of Fontan (1 in the PA plasty and 4 in the non-PA plasty groups). Four patients underwent orthotopic heart transplant after BDCA before reaching final stage palliation. Five hundred four patients (86%) have completed final stage palliation at a median age of 3.4 years (IQR, 3-4.1 years). Of these, 471 underwent Fontan, 26 underwent 1.5-ventricle repair, and 7 underwent 2-ventricle repair. Outside of those lost to follow-up, 12 patients (2.1%) are alive awaiting completion Fontan (8 in PA plasty and 4 in non-PA plasty groups; P = .3). Figure 3, A, shows the competing risk curves for these outcomes as a function of time following BDCA discharge.
Figure 2.
Palliation status of 588 bidirectional cavopulmonary anastomosis (BDCA) patients at last follow-up. PA plasty, Pulmonary arterioplasty.
Figure 3.
Competing risk outcomes of 570 hospital survivors of bidirectional cavopulmonary anastomosis (BDCA) as a function of time following BDCA discharge.
Fontan Outcomes
We compared outcomes in the 471 patients who reached Fontan stratified by need for PA plasty at BDCA. One hundred thirty-nine PA plasty patients underwent Fontan, and 332 non-PA plasty patients underwent Fontan. Preoperative variables leading to completion Fontan are shown in Table 2. Fontan was performed at median 34 months (IQR, 28.7-40.5 months) after BDCA. Significantly more patients in the PA plasty group were on home oxygen pre-Fontan (P = .03). At pre-Fontan catheterization, the PA Nakata index was comparable between the PA plasty and non-pA plasty groups, indicating continued PA growth following PA plasty. This resulted in comparable pulmonary blood flow between the 2 groups. Physiologic parameters, including MPAP and indexed PVR were similar between groups. Operative data and outcomes are shown in Table 3. All patients underwent extracardiac Fontan and a fenestration was created in 57 (12%) patients (17 [12%] in the PA plasty and 40 [12%] in the non-PA plasty groups). The most frequently utilized conduit was an 18-mm polytetrafluoroethylene graft (433 [92%]), followed by a 16-mm polytetrafluoroethylene graft (28 [6%]). There were 104 patients with concomitant procedures, including 15 atrioventricular valve interventions, 46 patients with Glenn revision or PA plasty, and 12 with aortic interventions. There was no difference between groups in terms of any of these parameters.
Table 2.
Demographics and preoperative variables at Fontan
| Total | PA plasty | No PA plasty | P value | |
|---|---|---|---|---|
| No of patients | 471 | 139 | 332 | |
| Demographics | ||||
| Age (y) | 3.5 (3.1-4.1) | 3.4 (3.1-4.1) | 3.5 (3.1-4.1) | .95 |
| Weight (kg) | 14 (13-16) | 14 (13-16) | 14 (13-16) | .29 |
| Female sex | 196 (42) | 45 (32) | 151 (46) | .09 |
| Dominant ventricle | .09 | |||
| Right | 235 (50) | 79 (57) | 156 (47) | |
| Left | 200 (43) | 55 (40) | 145 (44) | |
| Biventricle | 31 (7) | 4 (3) | 27 (8) | |
| Undetermined | 5 (1) | 1 (1) | 4 (1) | |
| Heterotaxy | 63 (13) | 22 (16) | 41 (12) | .39 |
| Genetic syndrome | 27 (6) | 9 (7) | 18 (5) | .82 |
| Prematurity | 71 (15) | 24 (17) | 47 (14) | .47 |
| Pre-Fontan medications | ||||
| Digoxin | 33 (7) | 12 (9) | 21 (6) | .49 |
| Sildenafil | 47 (10) | 19 (14) | 28 (8) | .12 |
| Enalapril | 203 (43) | 62 (45) | 141 (43) | .75 |
| Oxygen | 28 (5.9) | 14 (10.1) | 14 (4.2) | .03 |
| Pre-Fontan cath data | ||||
| MPAP (mm Hg) | 11 (9-12) | 11 (9-12) | 10 (9-12) | .29 |
| iPVR (Woods units × m2) | 1.5 (1.2-1.8) | 1.5 (1.2-1.9) | 1.5 (1.2-1.8) | .6 |
| Pulmonary blood flow (L/min/m2) | 2.9 (2.5-3.5) | 3 (2.6-3.6) | 2.9 (2.5-3.3) | .35 |
| Nakata index (mm2/m2) | 226 (188-264) | 213 (185-249) | 231 (195-271) | .11 |
| Pre-Fontan echo data | ||||
| Moderate or worse AV valve regurgitation | 37 (8) | 15 (11) | 22 (7) | .18 |
| Moderate or more depressed ventricular function | 15 (3) | 4(3) | 11 (3) | 1.0 |
Values are presented as median (interquartile range) or n (%). PA plasty, Pulmonary arterioplasty; cath, cardiac catheterization; MPAP, mean pulmonary artery pressure; iPVR, indexed pulmonary vascular resistance; echo, echocardiography; AV, atrioventricular.
Table 3.
Operative data and outcomes of completion Fontan
| Total | PA plasty | No PA plasty | P value | |
|---|---|---|---|---|
| No of patients | 471 | 139 | 332 | |
| Duration of CPB (min) | 47 (36-63) | 45 (35-64) | 48 (38-62) | .54 |
| Aortic crossclamp | ||||
| Utilized | 117 (25) | 26 (19) | 91 (27) | .06 |
| Duration in 79 patients (min) | 28 (15-45) | 32 (2.3-50) | 26 (16-40) | .92 |
| DHCA | ||||
| Utilized | 6 (1.3) | 3 (2) | 3 (1) | .51 |
| Duration in 4 patients (min) | 16 (10-23) | 21 (16-23) | 10 (8-19) | .66 |
| Fontan characteristic | ||||
| Fenestrated | 57 (12) | 17 (12) | 40 (12) | 1 |
| 18-mm graft | 433 (92) | 133 (96) | 300 (90) | .15 |
| Patients with concomitant | ||||
| Procedures∗ | 104 (22) | 25 (18) | 79 (24) | .07 |
| PA Plasty/Glenn intervention | 46 (10) | 11 (8) | 35 (11) | .48 |
| AV valve intervention | 15 (3) | 6 (4) | 9 (3) | |
| Aortic intervention | 12 (3) | 4 (3) | 8 (2) | |
| Postop ICU LOS (d) | 3 (2-5) | 3 (2-6) | 3 (2-5) | .09 |
| Postop iNO use | 74 (16) | 23 (17) | 51 (15) | .85 |
| Chest tube duration (d) | 5 (4-7) | 5 (4-8) | 5 (4-7) | .24 |
| Mortality | 5 (1) | 1 (1) | 4 (1) | 1 |
| Major morbidity∗ | 83 (17.6) | 29 (21) | 54 (16) | .29 |
| Unplanned reoperation | 52 (11) | 18 (13) | 34 (10) | .49 |
| Phrenic nerve injury | 23 (5) | 10 (7) | 13 (4) | .20 |
| Sternal wound infection | 12 (3) | 4 (3) | 8 (2) | .64 |
| Neurologic complication | 10 (2) | 1 (1) | 9 (3) | .31 |
| Permanent pacemaker | 8 (2) | 3 (2) | 5 (2) | .91 |
| ECMO | 2 (1) | 1 (1) | 1 (0.5) | 1 |
| Medications at discharge | ||||
| Digoxin | 15 (3) | 6 (4) | 9 (3) | .54 |
| Sildenafil | 66 (14) | 29 (21) | 37 (11) | <.001 |
| Oxygen | 13 (3) | 6 (4) | 7 (2) | .31 |
Values are presented as median (interquartile range) or n (%). PA, Pulmonary artery; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; AV, atrioventricular; ICU, intensive care unit; LOS, length of stay; iNO, inhaled nitric oxide; ECMO, extracorporeal membrane oxygenation.
Data not mutually exclusive.
There were 5 (1%) surgical Fontan mortalities. Eighty-three patients experienced major morbidity events (29 [21%] of PA plasty group patients and 54 [16%] of non-PA plasty group patients; P = .29) with no differences in specific complications. There was no difference between the 2 groups in postoperative outcomes. More patients who had PA plasty were discharged on sildenafil compared with those without PA plasty (P = .03).
Outcomes for 1.5-Ventricle and 2-Ventricular Repair
Twenty-six (5.2%) patients underwent a 1.5-ventricle repair (2 in the PA plasty and 24 in the non-PA plasty groups) and 7 (1%) underwent biventricular repair (3 PA plasty and 4 non-PA plasty patients). Demographics and outcomes for this subgroup is shown in Table E1. There were no differences in preoperative age, weight, or anatomy. There were also no differences in bypass or crossclamp times and postoperative outcomes were similar between the 2 groups. Of the 2 patients with major morbidities in the PA plasty group, both patients required reoperations and 1 required a pacemaker. Of the 3 patients with major morbidities in the non-PA plasty group, 2 required reoperation and extracorporeal membrane oxygenation, and 2 required pacemakers. There was no in-hospital mortality.
Fate of the PA during Follow-up
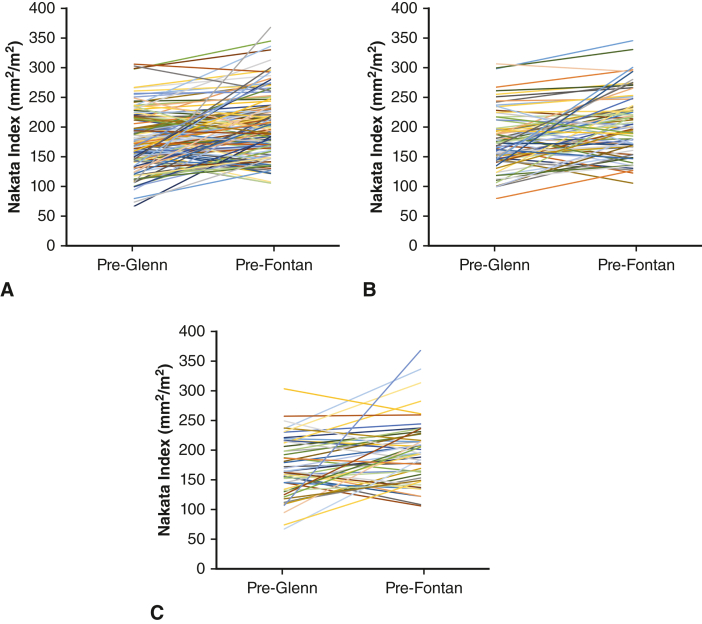
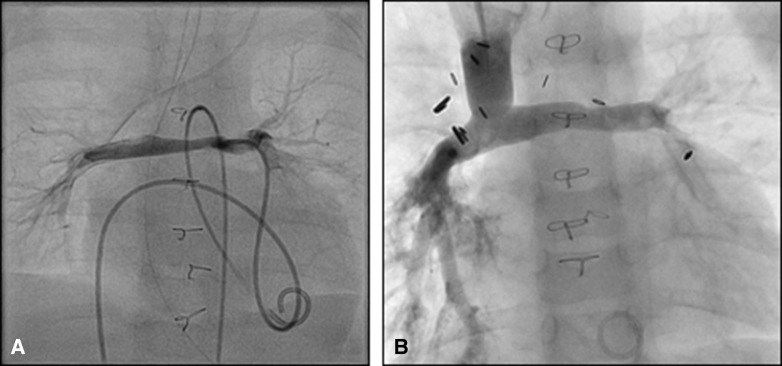
We used Nakata index at pre-Fontan catheterization to assess PA size at final stage palliation. Overall, the Nakata index was comparable at final stage palliation in children who did and did not undergo PA plasty at BDCA (Table 2 and Table E2). We combined any reintervention on the BDCA or reconstructed PA as a PA reintervention for the purposes of this study. When stratified by those who required PA reintervention before final stage palliation, Nakata index was lower in the subgroup that had PA plasty at BDCA. However, in the subgroup that did not require reintervention, the Nakata index was comparable between those who did and did not undergo PA plasty at BDCA. We compared Nakata indices (indexed to body surface area) pre-BDCA and pre-Fontan in 134 patients for whom both data were available (Figure E1). Nakata index pre-Fontan was 197 mm2/m2 (IQR, 166-228 mm2/m2) compared with 171 mm2/m2 (IQR, 145-204 mm2/m2) pre-BDCA (P < .0001), indicating continued growth of PA following PA plasty. The corresponding values in those that did not require PA reintervention were 198 mm2/m2 (IQR, 168-232 mm2/m2) compared with 174 mm2/m2 (IQR, 148-198 mm2/m2) (P < .0001), and in those that required PA reintervention were 195 mm2/m2 (IQR, 160-224 mm2/m2) compared with 165 mm2/m2 (IQR, 137-211 mm2/m2) (P < .0001). The subgroup that did not require reintervention demonstrates excellent PA size pre-Fontan even in patients who had small PA at BDCA (Figure E1, B). We interpret these data to indicate that following adequate PA plasty at BDCA, reliable PA growth commensurate with somatic growth can be expected in a subset of patients, similar to the growth seen in patients who did not require PA plasty at BDCA. Figure 4 shows representative catheterization images of a child with Norwood/Sano reconstruction pre-BDCA with bilateral PA stenosis. He underwent Type 4 PA plasty at BDCA and his pre-Fontan catheterization (Figure 4, B) shows a well-developed PA.
Figure E1.
Nakata index on catheterization pre-Glenn and pre-Fontan in 134 patients who required pulmonary arterioplasty (PA plasty) at bidirectional cavopulmonary anastomosis (BDCA) for whom both data are available (A), stratified by the 81 who did not (B) and 53 who did (C) require reintervention before final stage palliation.
Figure 4.
Representative pre-bidirectional cavopulmonary anastomosis (BDCA) (A) catheterization image of a child with Norwood/Sano reconstruction for hypoplastic left heart syndrome demonstrating significant bilateral pulmonary artery stenosis. He underwent hilum-to-hilum pulmonary arterioplasty at BDCA and his pre-Fontan catheterization (B) shows well developed pulmonary arteries. He has successfully reached Fontan circulation.
In all, 140 (26%) patients required reintervention before final stage palliation. Table 4 demonstrates reinterventions stratified by PA plasty at BDCA and by PA plasty type in the PA plasty group. Eighty-eight (15%) patients had PA reinterventions distal to BDCA anastomosis and 52 (9%) at the BDCA anastomosis. Of the 59 PA plasty patients who required PA or Glenn reintervention, there were 36 stents placed, 48 angioplasties performed, and 11 surgical reinterventions performed. Of the 81 non-PA plasty group patients who required reintervention, there were 23 stents placed, 38 angioplasties performed, and 29 surgical reinterventions. Forty-one surgical reinterventions were in the form of PA plasty at the time of Fontan, 9 of those in the PA plasty group (type 1: n = 1, type 2: n = 3, type 3: n = 4, and type 4: n = 1) and 32 of those in the non-PA plasty group (type 1: n = 9, type 2: n = 9, type 3: n = 9, and type 4: n = 5). There was 1 PA stent placed during surgery in a PA plasty patient, and there were 2 surgical Glenn reinterventions at the time of Fontan, both in PA plasty patients.
Table 4.
Reintervention on bidirectional cavopulmonary anastomosis (BDCA)/pulmonary artery (PA) following BDCA discharge
| Entire cohort | No PA plasty | PA plasty | PA plasty type |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| No of patients | 570 | 401 | 169 | 18 | 62 | 46 | 43 |
| Patients requiring re-intervention | 140 (26) | 81 (20) | 59 (35) | 2 (11) | 20 (33) | 18 (39) | 19 (46) |
| All interventions | |||||||
| Stent | 23 | 36 | 2 | 12 | 10 | 12 | |
| Angioplasty | 38 | 48 | 1 | 11 | 20 | 16 | |
| Surgical | 29 | 11 | 0 | 5 | 3 | 3 | |
Values are presented as n (%) or n. PA, Pulmonary artery.
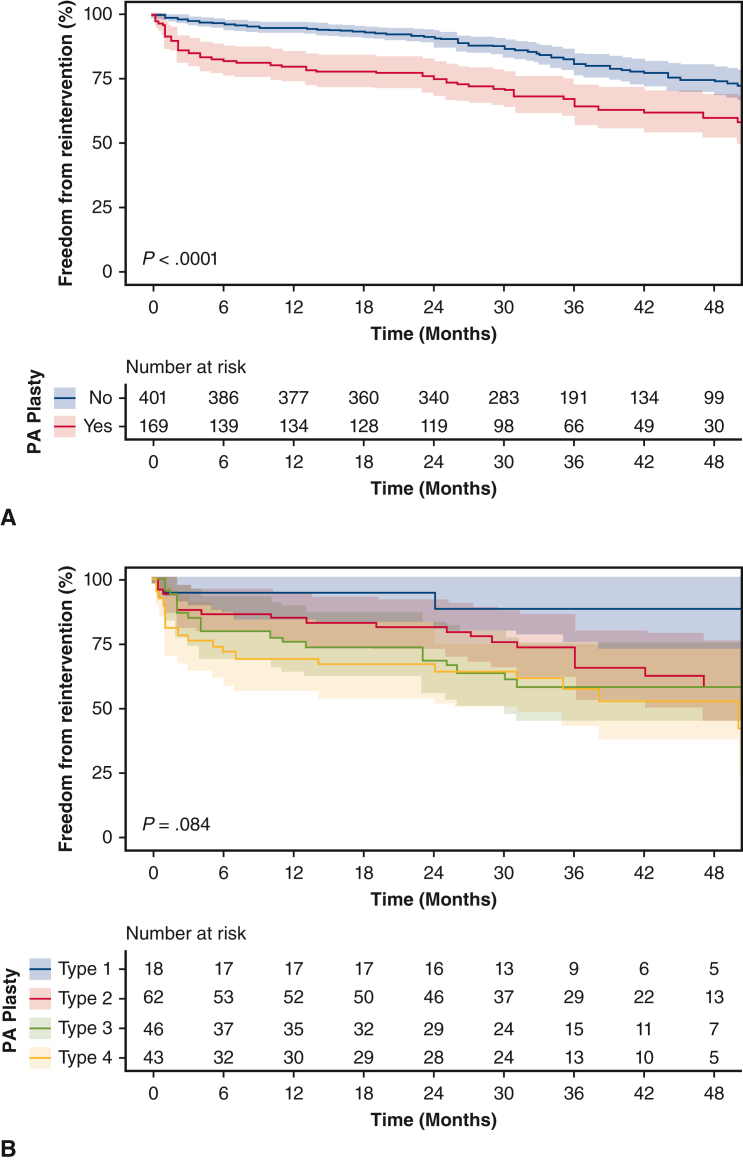
There were significantly more PA reinterventions in the PA plasty group compared with the non-PA plasty group, (35% vs 20%) with an odds ratio of 2.1 (95% CI, 1.5-3.2; P < .001). Figure 5, A, shows freedom from PA reintervention following BDCA discharge in the patients who did and did not require PA plasty. Amongst those with PA plasty at BDCA, freedom from reintervention at 12, 24, and 36 months were 80% (95% CI, 74-86%), 75% (95% CI, 69-82%), and 64% (95% CI, 57-73%), respectively. Amongst those who did not require PA plasty at BDCA, freedom from reintervention was 95% (95% CI, 93-97%), 91% (95% CI, 88-94%) and 81% (95% CI, 76-85%) (P < .001). We studied need for reintervention in PA plasty patients based on type of PA plasty at the time of BDCA: type 1 (2 out of 18 [11%]), type 2 (20 out of 62 [33%]), type 3 (18 out of 46 [39%]), and type 4 (19 out of 43 [46%]). Of the PA plasty patients who required a stent or angioplasty reintervention, 16 had both a localized stenosis and diffuse hypoplasia requiring both stent and angioplasty, 21 had only localized stenosis requiring only angioplasty, 18 had only diffuse hypoplasia requiring only stenting. Of the no-PA plasty patients, 9 had both localized stenosis and diffuse hypoplasia requiring stenting and angioplasty, 29 had localized stenosis requiring only angioplasty, and 23 had diffuse hypoplasia requiring stenting. Time-varying need for re-intervention on the PA stratified by type of PA plasty in these 140 patients is shown in Figure 5, B. This was not significantly different between types of PA plasty (P = .08). On multiple regression analysis, in addition to PA plasty at BDCA, prior use of Sano was independently associated with need for reintervention (P < .001).
Figure 5.
Kaplan-Meier analysis of freedom from re-intervention on the pulmonary arteries (PA) following discharge from bidirectional cavopulmonary anastomosis (BDCA) stratified by pulmonary arterioplasty (PA plasty) (A) and type of PA plasty at BDCA (B) with 95% CIs shown.
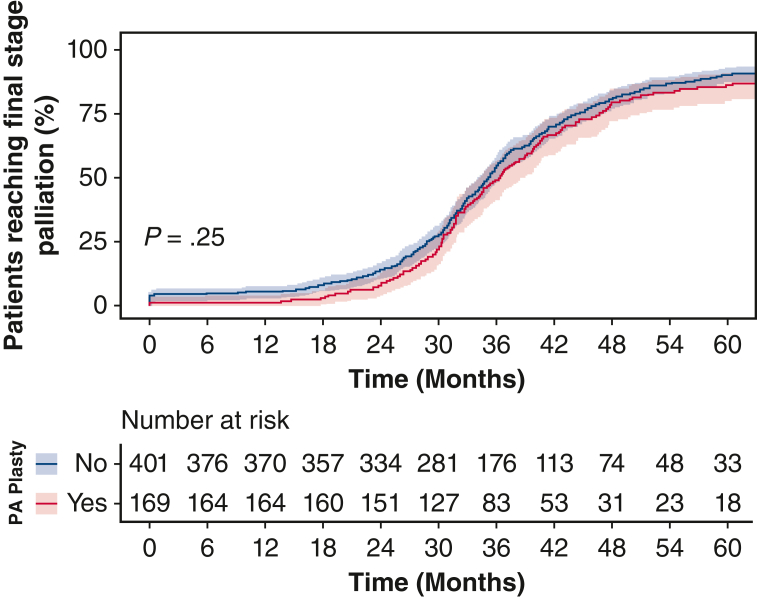
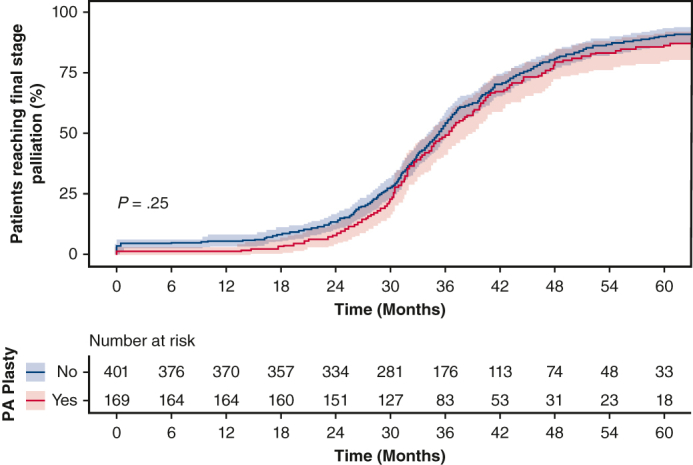
Despite increased PA reinterventions in the PA plasty group, the MPAP and indexed PVR were not significantly different between the 2 groups at pre-Fontan catheterization (Table 2), further indicating that the pulmonary vasculature showed favorable remodeling and satisfactory hemodynamics for successful completion of palliation. We performed cumulative incidence function analysis of successfully reaching final stage palliation with death or transplant as competing risks (Figure 3, B). There was no statistically significant difference in incidence of achieving final stage palliation between PA plasty and no PA plasty groups (P = .25).
Discussion
Successful SV palliation depends on adequate pulmonary vascular compliance and well-developed PA architecture. Ensuring optimal PA growth and architecture has been an area of increasing interest in the field as SV palliation outcomes have improved. PA stenosis is frequently encountered in this patient population from developmental PA defects (such as ductal origin of PA), the technique of initial palliation (Blalock-Taussig shunt vs Sano during Norwood procedure6, 7, 8) or technical factors during surgical palliation stages. Our group has previously shown4 that PA plasty at BDCA is independently associated with increased surgical mortality and morbidity at BDCA. More patients require supplemental oxygen and pulmonary vasodilatory therapy at discharge following PA plasty. However, our prior analysis did not study the influence of PA plasty after BDCA discharge, and on final stage palliation. To that end, our current study demonstrates that PA plasty at BDCA does not adversely influence ultimate success of final stage palliation. Cumulative incidence function analysis shows that, regardless of the need for PA plasty at BDCA, equal proportions of patients reach final stage palliation over similar time-intervals (Figure 6). There are no significant differences in pre-Fontan MPAP and indexed PVR between patients who required a PA plasty at the time of BDCA and those that did not. This leads us to conclude that the pulmonary vascular bed can appropriately remodel after PA plasty to allow for adequate and favorable hemodynamics for successful Fontan circulation. Although mortality at BDCA hospitalization was increased in PA plasty patients in our prior study, these patients do not carry this risk because they proceed to and through subsequent palliation.
Figure 6.
Cumulative incidence function of reaching final stage palliation amongst hospital survivors of bidirectional cavopulmonary anastomosis (BDCA) stratified by pulmonary arterioplasty (PA plasty) at BDCA, with 95% CIs shown.
This physiologic benefit in PA hemodynamics is associated with an anatomically favorable PA. Using Nakata indices pre-BDCA and pre-Fontan, we demonstrate that PA continue to grow commensurate with somatic growth following PA plasty at BDCA. This was evident even in patients with particularly small PA at BDCA. This would imply that the residual native tissue in the augmented PA continues to grow comparable to the growth seen in patients who did not require PA plasty at BDCA. It has been suggested that pulsatile blood flow is required to grow hypoplastic PA. Our results would indicate that with an adequate PA plasty, PA growth can be expected even in the setting of passive venous flow. These adequate-sized PA allow for sufficient pulmonary blood flow comparable to that observed in children who did not require PA plasty at BDCA. More patients in the PA plasty group were discharged home on sildenafil, although the use of sildenafil was not standardized and was at the discretion of the bedside provider. Whether or not this observation has clinical relevance remains to be seen.
Prior PA plasty is associated with an increase in reintervention on the PA. In patients who did not require PA plasty at BDCA, 20% still required a PA intervention before Fontan, but there were significantly more interventions (35%) in those patients who had a PA plasty at BDCA (P < .001). This could be interpreted to imply that poor PA growth following first stage palliation represents an inherently abnormal vascular architecture that leads to need for repeat interventions in a subset of patients. Regardless, it is imperative to ensure that neonatal surgical palliation establishes optimal conditions for PA remodeling and growth. To this end, evaluating factors associated with the initial need for PA plasty may have merit in identifying this patient cohort and potentially changing their treatment algorithms. Several studies have demonstrated that a Sano shunt from the right ventricle to the PA is associated with increased PA interventions over long term follow-up.6 Our multiple regression analysis confirms that prior Sano is an independent predictor of PA reintervention, implying that the risk of PA intervention associated with Sano extends beyond the initial PA plasty. The extent of PA plasty was not associated with need for reintervention, despite a strong trend (11% in type 1, and 46% in type 4; P = .08). This may be a function of small numbers in the subgroups analyses.
When more extensive PA plasty is required, the amount of native PA capable of growth is reduced, which would result in restricted PA growth and the increased need for reintervention. Cresalia and colleagues9 studied 135 2-ventricle patients who had undergone surgical PA plasty and found a 33% reintervention rate over a median of 4 years with a 10-year rate of 54%. They found that reintervention was significantly associated with bilateral PA stenosis, and thus more extensive PA plasty. Wilder and colleagues10 studied 434 tetralogy of Fallot repairs and found that extensive hilum to hilum PA plasty was associated with significantly reduced freedom from reintervention out to 10 years compared with proximal PA plasty or unaugmented PAs. Other factors such as concomitant aortic intervention at BDCA or need for deep hypothermic circulatory arrest during BDCA, which is a surrogate for more complex reconstruction,4 were not associated with PA reintervention in this analysis.
Given that the goal of this study was to determine whether or not PA plasty at BDCA influences final stage success, we censored our data at final stage palliation. This limits our analysis to a median 3 years after BDCA. Longer follow-up time would be needed to investigate whether PA growth mirrors somatic growth and favorable PA hemodynamics continue past final stage palliation. In addition, our study has limitations inherent to a single center retrospective analysis. The indication for PA plasty is not standardized in clinical practice in the entire field, and, as such, our study represents contemporary practice patterns. Lastly, our group has a strong bias toward surgical arterioplasty of hypoplastic branch PA at the time of BDCA because we believe that this is more durable than transcatheter interventions. It is possible that a percutaneous approach to pulmonary interventions would provide different results, but our institutional bias prevents us from investigating this. Future studies with more patients from multiple centers would be most effective to answer this question. That said, we submit that the surgical outcomes reported here could serve as a benchmark for PA outcomes in this cohort of patients. In particular, we demonstrate that nearly two-thirds of the patients with hypoplastic PA at BDCA have adequate PA growth following surgical PA plasty, comparable to that in control population without any further reintervention on the PA. This will need to factor into the consideration when surgical versus transcatheter therapy is contemplated in single ventricle patients with hypoplastic PA.
Conclusions
We show that amongst BDCA survivors, the need for PA plasty at the time of BDCA does not lead to increased mortality risk through final stage palliation. There is reliable PA growth following PA plasty without any reintervention in nearly two-thirds of patients. Although there is an overall increased need for PA reintervention after PA plasty, optimal pulmonary hemodynamics at final stage palliation and acceptable outcomes after final stage palliation can be expected.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
IRB: CHLA-22-00246; approved August 16, 2022.
Read at the 49th Annual Meeting of the Western Thoracic Surgical Association, Coeur d’Alene, Idaho, June 21-24, 2023.
Current affiliations: Dr Gray is now at the University of Alabama; Dr Bowdish is at Cedars Sinai Medical Center; and Dr Kumar is now at Omaha, Neb.
Appendix E1
Table E1.
Operative data and outcomes for 1.5-ventricle and 2-ventricle repair
| Total | PA plasty | No PA plasty | P value | |
|---|---|---|---|---|
| No of patients | 33 | 5 | 28 | |
| Age (y) | 0.96 (0.6-2.2) | 0.75 (0.6-9.9) | 0.97 (0.6-2.2) | .58 |
| Weight (kg) | 8.5 (6.6-11.8) | 8.4 (7.1-8.8) | 8.9 (6.2-11.8) | .96 |
| Diagnosis | ||||
| PA/IVS | 11 (33) | 0 | 11 (39) | .23 |
| TGA/DORV | 5 (15) | 2 (40) | 3 (11) | .32 |
| Unbalanced AV canal | 4 (12) | 0 | 4 (14) | .88 |
| Duration of CPB (min) | 101 (81-155) | 99 (92-115) | 116 (47-187) | .76 |
| Aortic crossclamp | ||||
| Duration (min) | 85 (61-116) | 85 (79-91) | 93 (48-151) | .93 |
| Postop ICU LOS (d) | 5 (3-9) | 5 (4-6) | 5 (3-15) | .58 |
| Chest tube duration (d) | 4 (3-7) | 4 (3-5) | 4 (3-7) | .93 |
| Postop hospital LOS (d) | 8 (6-11) | 7.5 (7-9) | 9 (6-12) | .88 |
| Mortality | 0 | 0 | 0 | |
| Major morbidity∗ | 5 (15) | 2 (40) | 3 (11) | .32 |
Values are presented as median (interquartile range) or n (%). PA plasty, Pulmonary arterioplasty; PA/IVS, pulmonary atresia/intact ventricular septum; TGA/DORV, transposition of great arteries/double outlet right ventricle; AV, atrioventricular; CPB, cardiopulmonary bypass; ICU, intensive care unit; LOS, length of stay.
Data not mutually exclusive.
Table E2.
Pulmonary artery Nakata index at final stage palliation stratified by need for pulmonary arterioplasty (PA plasty) at bidirectional cavopulmonary anastomosis (BDCA) and need for pulmonary artery (PA) or Glenn intervention prior to at final stage palliation
| PA/Glenn intervention before or at final palliation | PA plasty at BDCA |
||
|---|---|---|---|
| Yes (n = 169) | No (n = 401) | P value | |
| Yes (n = 140) |
198 (182-229) (n = 59) |
233 (180-276) (n = 81) |
.01 |
| No (n = 430) |
223 (188-258) (n = 110) |
230 (195-265) (n = 320) |
.24 |
| Total (N = 570) |
213 (185-249) (n = 169) |
231 (195-271) (n = 401) |
.11 |
Values are presented as median (interquartile range).
References
- 1.Pridjian A.K., Mendelsohn A.M., Lupinetti F.M., et al. Usefulness of the bidirectional Glenn procedure as staged reconstruction for the functional single ventricle. Am J Cardiol. 1993;71:959–962. doi: 10.1016/0002-9149(93)90914-x. [DOI] [PubMed] [Google Scholar]
- 2.Jonas R.A. Indications and timing for the bidirectional Glenn shunt versus the fenestrated Fontan circulation. J Thorac Cardiovasc Surg. 1994;108:522–524. [PubMed] [Google Scholar]
- 3.Lai L., Laussen P.C., Cua C.L., et al. Outcomes after bidirectional Glenn operation: Blalock-Taussig shunt versus right ventricle-to-pulmonary artery conduit. Ann Thorac Surg. 2007;83:1768–1773. doi: 10.1016/j.athoracsur.2006.11.076. [DOI] [PubMed] [Google Scholar]
- 4.Cleveland J.D., Tran S., Takao C., et al. Need for pulmonary arterioplasty during Glenn independently predicts poor surgical outcome. Ann Thorac Surg. 2018;106:156–164. doi: 10.1016/j.athoracsur.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs M.L., O’Brien S.M., Jacobs J.P., et al. An empirically based tool for analyzing morbidity associated with operations for congenital heart disease. J Thorac Cardiovasc Surg. 2013;145:1046–1057. doi: 10.1016/j.jtcvs.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gist K.M., Barrett C.S., Graham D.A., et al. Pulmonary artery interventions after Norwood procedure: does type or position of shunt predict need for intervention? J Thorac Cardiovasc Surg. 2013;145(6):1485–1492. doi: 10.1016/j.jtcvs.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Barron D.J., Brooks A., Stickley J., et al. The Norwood procedure using a right ventricle-pulmonary artery conduit: comparison of the right-sided versus left-sided conduit position. J Thorac Cardiovasc Surg. 2009;138:528–537. doi: 10.1016/j.jtcvs.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Pruetz J.D., Badran S., Dorey F., et al. Differential branch pulmonary artery growth after the Norwood procedure with right ventricle-pulmonary artery conduit versus modified Blalock-Taussig shunt in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2009;137:1342–1348. doi: 10.1016/j.jtcvs.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Cresalia N.M., Armstrong A.K., Romano J.C., et al. Long-term outcomes after surgical pulmonary arterioplasty and risk factors for reintervention. Ann Thorac Surg. 2018;105(2):622–628. doi: 10.1016/j.athoracsur.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Wilder T.J., Van Arsdell G.S., Pham-Hung E., et al. Aggressive patch augmentation may reduce growth potential of hypoplastic branch pulmonary arteries after tetralogy of Fallot repair. Ann Thorac Surg. 2016;101(3):996–1004. doi: 10.1016/j.athoracsur.2015.11.040. [DOI] [PubMed] [Google Scholar]