Abstract
Objective
Randomized control trials are considered the highest level of evidence, yet the scalability and practicality of implementing randomized control trials in the thoracic surgical oncology space are not well described. The aim of this study is to understand what types of randomized control trials have been conducted in thoracic surgical oncology and ascertain their success rate in completing them as originally planned.
Methods
The ClinicalTrials.gov database was queried in April 2023 to identify registered randomized control trials performed in patients with lung cancer who underwent surgery (by any technique) as part of their treatment.
Results
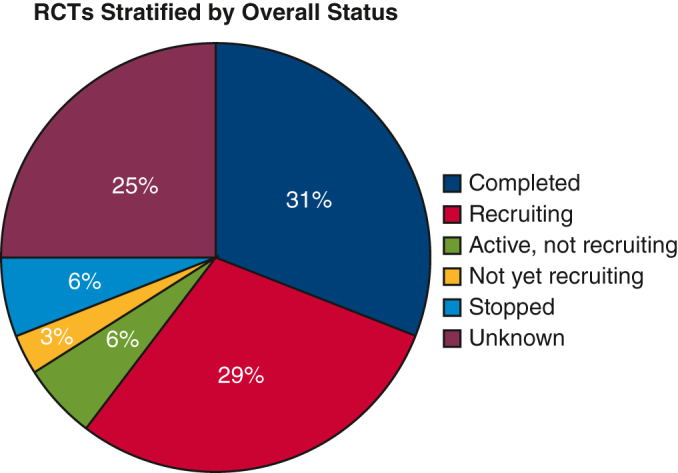
There were 68 eligible randomized control trials; 33 (48.5%) were intended to examine different perioperative patient management strategies (eg, analgesia, ventilation, drainage) or to examine different intraoperative technical aspects (eg, stapling, number of ports, port placement, ligation). The number of randomized control trials was relatively stable over time until a large increase in randomized control trials starting in 2016. Forty-four of the randomized control trials (64.7%) were open-label studies, 43 (63.2%) were conducted in a single facility, 66 (97.1%) had 2 arms, and the mean number of patients enrolled per randomized control trial was 236 (SD, 187). Of 21 completed randomized control trials (31%), the average time to complete accrual was 1605 days (4.4 years) and average time to complete primary/secondary outcomes and adverse events collection was 2125 days (5.82 years).
Conclusions
Given the immense investment of resources that randomized control trials require, these findings suggest the need to scrutinize future randomized control trial proposals to assess the likelihood of successful completion. Future study is needed to understand the various contributing factors to randomized control trial success or failure.
Key Words: lung cancer, randomized controlled trials, thoracic surgical oncology
Surgical-focused RCTs in lung cancer stratified by overall status.
Central Message.
Most surgical-focused lung cancer RCTs do not boast a high completion rate, and those that are completed do not consistently generate impactful clinical change.
Perspective.
There has been an increase in single-center, nonblinded RCTs in the surgical lung cancer world, which falls short of the true gold standard. Future study is needed to understand the various contributing factors to RCT success or failure, and alternative types of evidence generation should be sought, such as real-world evidence and pragmatic prospective observational studies.
Randomized controlled trials (RCTs) traditionally have been considered the gold standard for evidence-based change in practice in medicine and surgery. However, studies have shown that initiating clinical trials requires enormous effort, from securing large amounts of grant funding to assembling highly skilled teams to maintain research protocols until trial completion.1 A study by Wang-Gillam and colleagues2 compared the time to activate lung cancer trials between institutions in different continents and found that the administrative and regulatory processes are significantly lengthier and more laborious in US academic centers compared with European centers.
Nationally, an estimated less than 5% of adult patients with cancer are enrolled in a clinical trial, and of that small number, less than 10% are trials that involve surgical interventions.3 Influential entities like the National Cancer Institute recognize the need for more coordinated efforts to bolster clinical trial efforts in the surgical oncology space and have created working groups and integrated networks to reinvigorate these processes. Surgery-specific clinical trials present another dimension of challenges because of barriers to standardization of recruitment, blinding, interventions delivered, and variability in techniques for a given procedure.4 Consequently, trials often experience long lag times, failure to accrue, and poor cost-effectiveness. Early trial closures, or even completed trials that fail to affect clinical guidelines, contribute to inefficient use of resources and highlight the need to improve the pipeline from research to practice.5 Moreover, there is concern that once an RCT is successfully completed, the impact may not be material if the time to completion is long.
The concept of knowledge translation, or translation from research to clinical practice, has gained attention in the medical and surgical literature over the last decade, partially secondary to an influential report by the Institute of Medicine in 2001.6 Governmental entities, private foundations, and academic centers provide significant amounts of research-specific grant funding every year in hopes of driving more evidence-based care, but there have been few studies investigating the impact that research advances have had on both individual patient outcomes and overall public health benefit. Although there has been progress made in clinical outcomes in lung cancer during the last few decades, the impact of thoracic surgical oncology RCTs on this remains unclear.7 The aim of this study is to understand what types of surgical-based RCTs have been conducted in lung cancer and ascertain the successful rate of completion. We hypothesize that despite increased attention and funding in recent years, the execution and impact of surgical trials have not increased accordingly.5,6
Material and Methods
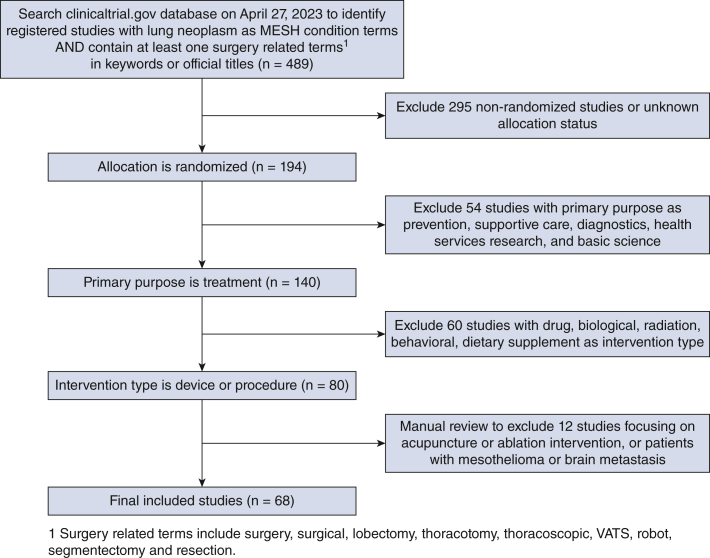
ClinicalTrials.gov is a website maintained by the National Library of Medicine that provides information on current clinical research studies in more than 200 countries.8 The database relies on study investigators to submit updated information during the course of their study and encourages public sharing of the research process and eventual outputs. The ClinicalTrials.gov database was queried to identify all registered RCTs in patients with lung cancer who underwent surgery (any technique) as part of their treatment performed until April 27, 2023. Inclusion criteria included “lung neoplasm” as a MESH condition term and contained at least one of the following terms in the keywords or official titles: “surgery,” “surgical,” “lobectomy,” “thoracotomy,” “thoracoscopic,” “VATS,” “robot,” “segmentectomy,” or “resection.” The query was limited to randomized trials only with treatment as the primary purpose and procedure or device as the intervention type (Figure 1). Exclusion criteria included nonrandomized trials and trials with basic science, diagnostic, health services, prevention, or supportive care as the primary purposes. Trials with medical-focused primary outcomes were excluded, even if they enrolled surgical patients. After initial selection based on title and abstract review, each study underwent full-text review to ensure inclusion criteria was uniformly met.
Figure 1.
Diagram of how RCTs were chosen for inclusion in the study. VATS, Video-assisted thoracic surgery.
The cohort was stratified by overall status, including active but not recruiting, completed, not yet recruiting, recruiting, stopped, and unknown. Characteristics of the studies were extracted, including start and completion dates, masking, number of participating facilities, lead sponsors, comparison types, number of arms, and numbers of actual and anticipated enrollment. Studies were also stratified by country and continent of origin based on overall status. A subgroup analysis was performed for the completed studies and time to trial completion and rate and timing of subsequent publication were collected. Additional analysis was performed to understand characteristics of trials with unknown status. Frequencies and percentages were calculated for categorical variables, and means (SD) were calculated for continuous variables. All analyses were performed using R 4.2.2.
Results
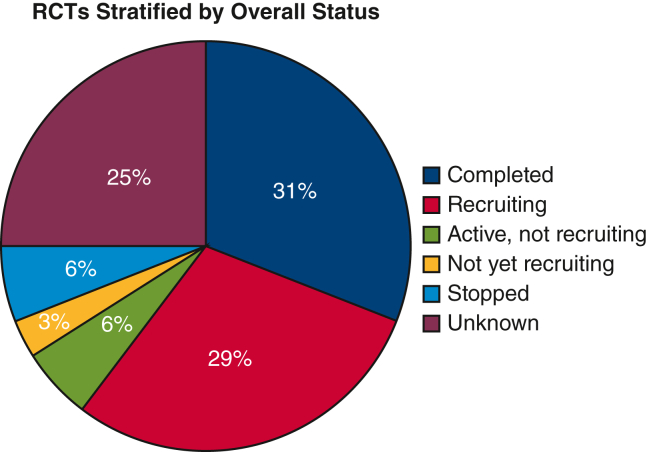
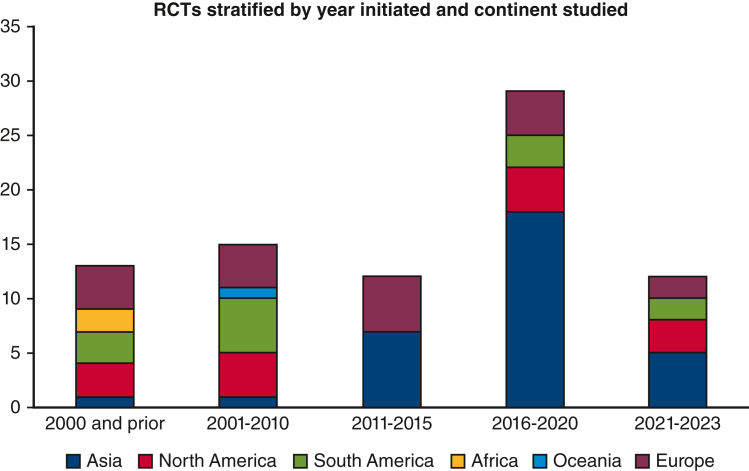
There were 68 studies that met the inclusion criteria, and of these, 21 (30.9%) had been completed, 20 (29.4%) were still recruiting, 17 (25%) were unknown, 4 (5.9%) were active but not recruiting, 4 (5.9%) were stopped, and 2 (2.9%) were not yet recruiting (Figure 2). The 4 studies labeled as “stopped” were all discontinued due to issues with enrollment and adequate patient accrual. Characteristics of the studies are shown in Tables 1 and E1. Overall, 17 (25%) of the studies were initiated from the year 2010 and prior, 44 (64.7%) were open label, 43 (63.2%) were performed in a single facility, 52 (76.5%) were funded institutionally, and 66 (97.1%) had 2 arms. Thirty-four trials (50%) were not stage-specific, whereas 25 trials (36.8%) enrolled patients with early-stage lung cancer, 13 trials (19.1%) enrolled patients with locally advanced disease, and 1 trial (1.5%) enrolled patients with late-stage disease. Figure 3 illustrates the initiation of RCTs by time period. The uptick in RCTs starting in 2016 is associated with the increase in single facility studies, with more than 72% of all RCTs recorded as single institution in the period from 2016 to 2023. More specifically, the majority (78.1%) of these single-facility RCTs were from Asia.
Figure 2.
RCTs stratified by overall status. RCTs, Randomized controlled trials.
Table 1.
Characteristics of randomized controlled trials stratified by overall status
| Characteristic | Overall, N = 68∗ | Completed, N = 21∗ | Recruiting, N = 20∗ | Active, not recruiting, N = 4∗ | Not yet recruiting, N = 2∗ | Stopped, N = 4∗ | Unknown, N = 17∗ |
|---|---|---|---|---|---|---|---|
| Periods | |||||||
| Year 2000 and earlier | 9 (13.2%) | 8 (38.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.9%) |
| Year 2001-2010 | 8 (11.8%) | 2 (9.5%) | 1 (5.0%) | 1 (25.0%) | 0 (0.0%) | 3 (75.0%) | 1 (5.9%) |
| Year 2011-2015 | 12 (17.6%) | 4 (19.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 8 (47.1%) |
| Year 2016-2020 | 28 (41.2%) | 6 (28.6%) | 12 (60.0%) | 2 (50.0%) | 0 (0.0%) | 1 (25.0%) | 7 (41.2%) |
| Year 2021-2023 | 11 (16.2%) | 1 (4.8%) | 7 (35.0%) | 1 (25.0%) | 2 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| Masking | |||||||
| Open label | 44 (64.7%) | 10 (47.6%) | 16 (80.0%) | 4 (100.0%) | 0 (0.0%) | 3 (75.0%) | 11 (64.7%) |
| Single blinded | 9 (13.2%) | 3 (14.3%) | 1 (5.0%) | 0 (0.0%) | 1 (50.0%) | 0 (0.0%) | 4 (23.5%) |
| Double blinded | 7 (10.3%) | 5 (23.8%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) |
| Triple blinded | 5 (7.4%) | 1 (4.8%) | 1 (5.0%) | 0 (0.0%) | 1 (50.0%) | 0 (0.0%) | 2 (11.8%) |
| Quadruple blinded | 1 (1.5%) | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| NULL | 2 (2.9%) | 2 (9.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Single facility | |||||||
| True | 43 (63.2%) | 11 (52.4%) | 16 (80.0%) | 1 (25.0%) | 1 (50.0%) | 2 (50.0%) | 12 (70.6%) |
| Lead sponsor category | |||||||
| Federal | 3 (4.4%) | 0 (0.0%) | 1 (5.0%) | 2 (50.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Industry | 4 (5.9%) | 2 (9.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (25.0%) | 1 (5.9%) |
| Institutional | 52 (76.5%) | 13 (61.9%) | 18 (90.0%) | 1 (25.0%) | 2 (100.0%) | 2 (50.0%) | 16 (94.1%) |
| Network | 9 (13.2%) | 6 (28.6%) | 1 (5.0%) | 1 (25.0%) | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) |
| Comparison types | |||||||
| Intraoperative technical management | 16 (23.5%) | 6 (28.6%) | 5 (25.0%) | 0 (0.0%) | 1 (50.0%) | 0 (0.0%) | 4 (23.5%) |
| Modalities of lobectomy | 9 (13.2%) | 2 (9.5%) | 4 (20.0%) | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 2 (11.8%) |
| Patient management strategies | 17 (25.0%) | 5 (23.8%) | 4 (20.0%) | 0 (0.0%) | 1 (50.0%) | 1 (25.0%) | 6 (35.3%) |
| Types of lung resection | 8 (11.8%) | 0 (0.0%) | 4 (20.0%) | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 3 (17.6%) |
| Surgery + adjunct treatments | 10 (14.7%) | 6 (28.6%) | 1 (5.0%) | 1 (25.0%) | 0 (0.0%) | 1 (25.0%) | 1 (5.9%) |
| Surgery vs nonsurgical treatments | 8 (11.8%) | 2 (9.5%) | 2 (10.0%) | 1 (25.0%) | 0 (0.0%) | 2 (50.0%) | 1 (5.9%) |
| No. of arms | |||||||
| 2 | 66 (97.1%) | 21 (100.0%) | 19 (95.0%) | 4 (100.0%) | 2 (100.0%) | 4 (100.0%) | 16 (94.1%) |
| 3 | 2 (2.9%) | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.9%) |
| Actual enrollment | |||||||
| Mean (SD) | 236 (187) | 256 (146) | 510 (269) | 21 (12) | |||
| Median (IQR) | 216 (57-346) | 246 (162-360) | 510 (415-606) | 20 (16-26) | |||
| Unknown | 46 | 5 | 20 | 2 | 2 | 0 | 17 |
| Estimated/target enrollment | |||||||
| Mean (SD) | 372 (330) | 349 (224) | 443 (389) | 640 (564) | 81 (44) | 664 (432) | 216 (173) |
| Median (IQR) | 286 (100-588) | 300 (165-555) | 300 (175-618) | 602 (240-1002) | 81 (66-96) | 767 (453-978) | 120 (82-339) |
| Unknown | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
SD, Standard deviation; IQR, interquartile range.
n (%) for categorical variables; mean (SD) and median (IQR) for continuous variables.
Figure 3.
RCTs stratified by year initiated and continent studied.∗ ∗Total numbers may be higher than reported in Table 1 because some RCTs were performed across several continents. RCTs, Randomized controlled trials.
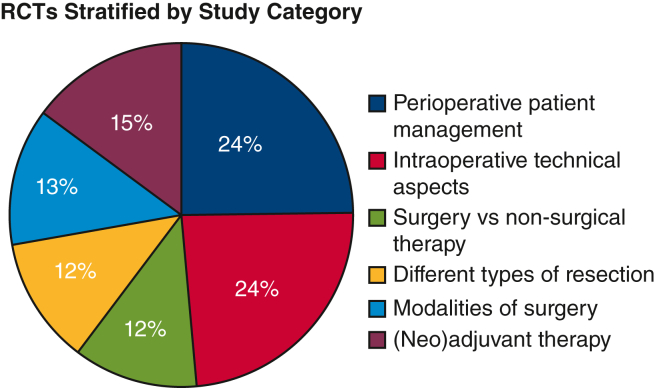
Figure 4 shows the different categories of study of the 68 eligible RCTs. Thirty-three trials (48.5%) were intended to examine different perioperative patient management strategies (eg, analgesia, ventilation, drainage) or to examine different intraoperative technical aspects (eg, stapling, number of ports, port placement, ligation) (Table E2). The remaining 35 studies examined modalities of lobectomy (eg, open surgery, video-assisted thoracoscopic surgery, robot-assisted), extent of lung resection, surgery with adjunct treatments, and surgery versus nonsurgical treatment. In the year 2000 and earlier, most of the studies (66.7%) focused on surgery and adjunct treatments. However, in more recent years, specifically from 2016 to the present day, study topics have shifted to include more patient management strategies and intraoperative technical management (Table E3).
Figure 4.
Comparison types of the 68 eligible RCTs. RCTs, Randomized controlled trials.
Although some RCTs were performed in multiple continents, the highest number of studies were initiated in Asia (n = 34) (Table E4). The remaining studies were relatively evenly split among Europe, North, and South America, with limited studies performed in Africa and Oceania. Europe had the highest rate of completed studies at 59%, especially compared with North and South America (28.6% and 30.8%, respectively), which all have a similar distribution of RCTs in each time period. On the other hand, Asia has the lowest rate of RCT completion to date (14.7%). This may be associated with the higher number of studies initiated in Asia from the year 2016 and beyond.
In a subgroup analysis focusing on the 21 completed studies alone, 10 (47.6%) were initiated before the year 2010, 10 (47.6%) were open-label studies, and 11 (52.4%) were conducted in a single facility. Of the 16 studies that reported enrollment numbers, the mean actual enrollment was lower than the anticipated enrollment at 256 patients (SD, 146) versus 349 patients (SD, 224), respectively. Specifically, there was on average 93 less patients enrolled in RCTs than originally anticipated, or a 26% mean reduction in actual enrollment. We calculated the time from start to completion of trials to investigate potential lags during the research process (Table E5). The median primary completion date, or time at which the last data point for the primary outcome was collected from the last enrolled patient, was 1370 days (SD, 677, 2572), or 3.8 years. On the other hand, the median study completion date, defined as the last data point for all remaining outcome measures, was 1554 days (SD, 704, 3346), or 4.3 years. Of the 21 completed studies, 14 (66.7%) successfully published articles from results of their study and the average time from trial completion to publication was 2.9 years. Average journal impact factor from the year of publication of the published studies was 28.1 (range, 0.2-176.1; median, 6.2). This equates to an average of 8.7 years from trial registration to publication. Of the 2 broad categories as described above, RCTs that studied surgery in a more traditional fashion (open surgery vs video-assisted thoracoscopic surgery vs robot-assisted, types of lung resection, surgery with adjunct treatments, and surgery vs nonsurgical treatment) were published in higher-impact journals than RCTs that studied perioperative and intraoperative strategies (median impact factor 49.7 vs 7.6, respectively).
There were 17 of the 68 RCTs with their overall status recorded as “unknown” in the database. Of these 17 studies, 10 (59%) were from 2015 or earlier, 11 (65%) were open label, 12 (71%) were from a single facility, and 16 (94%) had 2 arms (Table E6). The last updates of the overall status unknown RCTs were recruiting (11, 64.7%), not yet recruiting (4, 23.5%), active not recruiting (1, 5.9%), and enrolling by invitation (1, 5.9%). The median time from trial start to last update was 134 days (SD, −2, 972), and median time since trial start to the present data query in ClinicalTrials.gov in April 2023 was 2827 days (SD, 2461, 3891), or 7.7 years.
Discussion
Lung cancer remains the leading cause of cancer mortality around the world, and RCTs have historically been touted as the most important way to generate evidence to evolve clinical care. Our study assessed all surgical-related RCTs conducted in lung cancer and found 68 studies that met criteria over 30 years that covered a heterogenous range of topics. The number of RCTs was relatively stable over time until a large increase in RCTs starting in 2016, but this may be partially due to policy changes from entities such as the Food and Drug Administration and the International Committee of Medical Journal Editors to improve reporting into the ClinicalTrials.gov database. Nevertheless, we found that this increase in RCTs correlated with an increase in single-center trials after 2016 performed mostly in Asia. Of the 68 RCTs, the overall status was reported as completed in only 21 (30.9%), still recruiting in 20 (29.4%), and unknown in 17 (25.0%). Within the group of completed studies, time from trial start to completion was long at an average of 4 to 6 years, with an additional 1 to 5 years before an associated publication is identified. Within the group of “unknown” status studies, the median time from trial initiation to date of data extraction in April 2023 was 7.7 years, which suggests that these 17 studies have likely been abandoned or discontinued as a prior study using the ClinicalTrials.gov database demonstrated that the median time to study discontinuation is 2.2 years.9
One of the motivations for the study we undertook was to scrutinize the practicality of the long-held belief that RCTs are the absolute gold standard for guiding clinical decision-making and affecting national and international recommendations in lung cancer. However, it is likely that RCTs studying differences in medical management are easier to conduct than surgical RCTs. Studies have suggested that pharmaceutical trials have a higher proportion of completed trials than device or procedural interventions.10,11 In general, RCTs focused on chemotherapy, radiotherapy, and more recently immunotherapy for lung cancer are more common than surgery.12 Consequently, there has been more scrutiny in increasing the value and efficacy of these trials over the last 2 decades. For example, the National Cancer Institute collaborates with civil societies to hold national symposiums to address issues such as trial redundancies and delays, but the majority of best practices developed are not applicable to surgical trials.13,14 In the thoracic surgical space, efforts by societies such as the American Association for Thoracic Surgery are attempting to place surgeons in the center of the RCT discussion by creating a formal collaborative working group such as the Thoracic Surgical Oncology Group, which holds multiple levels of stakeholders accountable for successful completion of lung and esophageal cancer RCTs. By screening participating sites for adoption of best practices and confirming sites have experience in clinical research, it is hoped that trials within Thoracic Surgical Oncology Group will be higher quality. On a smaller scale, academic cancer centers such as MD Anderson have analyzed their institutional data on trial efficacy and success, and have found that cancer therapeutics trials fare better than nondrug trials.15,16 Surgical-related trials present different challenges compared with medical and drug trials for reasons such as the rarity of certain surgical conditions, time constraints for operating schedules, patient preferences for surgical modalities and specific surgeons, and the variability of surgical proficiency and techniques.17 In the United States specifically, insurance status likely also plays a role, because it often dictates access to and quality of surgical care.6,18 Even so, lung cancer surgical RCTs, which we found to have a completion rate of only 30.9%, seem to lag far behind success rates of other surgical specialties, as illustrated by completion rates of 70.7% in head and neck cancer RCTs and 59.9% in orthopedic-focused RCTs in other ClinicalTrials.gov studies.10,19,20 Another study that grouped surgical RCTs as a whole found a completion rate of 78.5% of the 2542 included trials, which is a stark contrast to our finding and further highlights the scope of the problem.9 A majority of the trials we examined were open-label and single-center, falling short of the true gold standard of a blinded multicenter RCT, further supporting the need to improve on current processes.
Existing literature has shown that over-optimism in timelines and expected enrollment in RCTs is an active problem and that there are techniques to balance for it. Initiating a pilot study before launching a full-scale RCT imbued with a massive amount of resources, both personnel and infrastructure, is an excellent way to gauge feasibility and temper expectations. These pilots can either be part of the larger RCT or external to it and can contribute to building a research network across institutions, which will increase actual enrollment and lend generalizability to the study cohort. We also identified enrollment and accrual as a major barrier in the evidence to practice pipeline. The 21 completed studies in our analysis reported that the number of actual enrolled patients was approximately 74% of what was anticipated. In addition, the most common reason that RCTs were discontinued was secondary to accrual issues. A cross-sectional study by Shadbolt and colleagues9 found that of 2542 surgical RCTs performed in 2010 to 2014, only 45.9% met their prespecified enrollment target, and those that did not met their target were shy by approximately 31% of the planned study sample. This is highly concerning because power analyses done during RCT design specify a minimum number of subjects to avoid a type II error, and approximately half of trials do not meet this benchmark. One of the ways to ameliorate low RCT accrual is to include more centers and more physicians. However, surgical-focused RCTs depend on all the included hospitals and surgeons to have the same level of technical abilities and resources. Moreover, for RCTs that study various surgical modalities such as open, video-assisted, and robotic-assisted surgery, which in our search comprised 13% of all lung cancer RCTs, the surgeon learning curve is challenging to control for and can significantly skew outcomes. One solution to combat poor accrual proposed by Halpern21 is to intervene preemptively by the use of a prospective preference assessment to gather information about participants' incentives and concerns for trial participation to refine trial design and identify unique cohort characteristics that may limit generalizability. Likewise, Kaur and colleagues22 developed a survey tool to highlight common facilitators and barriers to recruitment in clinical trials and stratified these variables by patient-level, clinician-level, trial-level, and site-level factors. The authors recommended using this survey as a flexible framework for ongoing RCTs that are facing enrollment issues to enable real-time troubleshooting and increase the rate of trial completions. Addressing accrual challenges from different perspectives will shed further light on the main downfall of current trials: long lag time throughout the RCT process.
The literature on delays in the research to practice continuum has not been studied widely due to complexities in defining and measuring time lag. Published studies have measured lag as various permutations of time between ethical approval and trial start to publication and guideline change.23, 24, 25 Fiteni and colleagues26 evaluated 34 RCTs on operable non–small cell lung cancer and found that the majority of articles failed to report time-to-event end points, which complicates the ability to measure time lag. Our results found that the average study completion time for surgical lung cancer RCTs was 5.8 years and then 2.9 years from trial completion to publication, with only 67% of completed studies having published a manuscript available on PubMed or Google Scholar. Although a certain amount of lag is necessary to vet new interventions and ensure safety and efficacy, optimizing unnecessary delays is beneficial to individual physicians and policymakers alike, because it could lead to quicker clinical impact and improved cost-effectiveness in the public health sector.1 Morris and colleagues1 published an article in 2011 gathering evidence about the consensus in medicine that the average time lag from “bench to bedside” is 17 years.18,27,28 This statistic is concerning because the original clinical question at stake may no longer be relevant by the time results are circulated and publicly available. Interventions to minimize lags can be organized by pretrial, during trial, and post-trial time periods. In the pretrial setting, regulatory processes can be streamlined to enable quicker activation to trial start.2 In ongoing trials, rapid early accrual, which we have discussed as a current barrier, has been shown to be an independent predictor of both study completion (hazard ratio, 1.4; P = .004) and successful publication (hazard ratio, 1.09; P = .011).29 Last, after trial completion, addressing publication bias will enable quicker dissemination. Urrútia and colleagues30 showed that trials with positive results are significantly more likely to publish not only faster but also in a higher impact factor journal. The more objectively we can quantify time lags in research, the better we will be equipped to implement changes to close the evidence to practice gap.
The long-lasting impacts of RCTs occur not only from publication alone but also from broad and high-yield dissemination of key takeaways.31,32 The ability of RCTs to change clinical practice is more nuanced in surgery compared with other specialties. In medical specialties, RCT findings can streamline the development of guidelines that inform recommendations for specific clinical interventions. This process involves stakeholders such as physicians, researchers, and patient advocates, and the guidelines are disseminated through channels such as continuing education courses, journal articles, and online resources.33 On the other hand, in surgical specialties, the process of incorporating new evidence into clinical practice is more complex because of the increased invasiveness, risk, and severity of possible complications. In addition, studies have shown that surgeons greatly value clinical autonomy, which likely plays a role in reluctance to change practices based on the results of a single RCT.34,35 Decision science studies that evaluate physician behavior have found that decontextualized knowledge does not lead to incorporation into daily practice.E1 RCTs are typically conducted in highly controlled settings that may not mirror real-world experiences and diverse patient demographics. Consequently, surgeons carry an additional weight of contextualizing RCT results through a local lens to assess if their patient population and hospital resources are appropriate for the proposed practice change. There have been several successes in the field that can serve as examples for designing future trials, including those that popularized video-assisted thoracoscopic surgery over open surgery as the mainstay of thoracic surgery.E2,E3 In fact, this phenomenon is now repeating itself as robotic-surgery gains more traction and is becoming a major player in the treatment of all stages of lung cancer.E4, E5, E6 Two other examples of highly successful and impactful RCTs (JCOG0802 and CALGB 140503) have received significant international attention and bolstered the use of segmentectomy for small, early-stage non–small cell lung cancers.E7,E8 Additionally, another key research area in lung cancer that has seen surgeon participation is the use of immunotherapy and targeted therapy in treatment of lung cancer. Although these are medical intervention trials and were not included in this analysis, results of these pharmaceutical trials are changing surgical practice.E9,E10 Although these are only a few examples, effective use of implementation science principles will help structure the integration of academic output into practice without taking away from personalized shared decision-making between surgeons and patients.E11
Study Limitations
Our study has several limitations due to the lack of granularity and consistency in conducting retrospective database studies. This study was not meant to be an overarching review on all lung cancer RCTs, but a hypothesis-driven query to gauge completion and publication rates of surgical-focused RCTs in lung cancer historically up until April 2023. An important caveat to note is that there are many landmark trials regarding neoadjuvant and adjuvant therapy in lung cancer management that are not included in our analysis. Additionally, multiple platforms exist to register RCTs, and even for RCTs registered in clincialtrials.gov, the trials may contain incomplete information or record information in a different format leading to missing data.E12 Several key trials, such as JCOG 0802/WJOG4607L, were not included in this analysis because RCTs from Japan use different registration databases that capture data fields dissimilarly and may not provide researchers uniform access for analysis. The lack of a standardized, international repository of RCTs is problematic and should prompt efforts to prioritize better data organization and compilation. This study aimed to perform a pulse check on the status of surgical lung cancer RCTs with the hopes of identifying areas of quality improvement for future trials. Our objective was not to summarize the content of individual RCTs and should not be used as a comprehensive resource, but rather as a tool that demonstrates the broad landscape of trends that have occurred in lung cancer surgical research stratified by time period and region of the world. Although our current study might not capture all international RCTs on this topic, it is, to our knowledge, the only one that synthesizes data from RCTs in the surgical lung cancer sphere to examine current effectiveness in translational research.
Conclusions
Given the immense investment in resources that RCTs require, these findings suggest the need for future RCT proposals to be scrutinized for the chances of successful completion. Those RCTs that are completed are most often single center and nonblinded, falling short of the true gold standard of a multicenter, single-blind trial. This study also highlights that not all RCTs have equal impact potential, and that single-facility studies on minor surgical differences could lead to misappropriation of limited resources and ultimately could be a detriment toward advancing lung cancer care. Alternatively, other types of evidence generation should be sought, such as real-world evidence and pragmatic prospective observational studies. With the amount of observational data constantly generated in the surgical oncology world, we may already have a sufficient amount of evidence on certain lung cancer topics, even without RCTs, that shows large effects relative to the background noise.E13,E14 In addition to challenging the impact of surgical RCTs, we must also be more willing to use large observational data when honing practice guidelines and perhaps deemphasize the “level of evidence” paradigm that places so much weight on RCT-generated data. Future study is needed to understand the various contributing factors to RCT success or failure, which will hopefully increase high-yield evidence generation that can be translated to improved clinical guidelines.
Conflict of Interest Statement
Y.L. is a full-time employee at Intuitive Surgical. D.S.O. is a part-time medical advisor at Intuitive Surgical. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Discussed at the International Thoracic Surgical Oncology Summit, September 22-23, 2023, New York, NY.
Appendix E1
Table E1.
Detailed list of all 68 included studies sorted by start year
| National clinical trial ID | Official title | Comparison types | No. of arms | Masking | Single facility | Start year | Overall status | Lead sponsor |
|---|---|---|---|---|---|---|---|---|
| NCT00002623E15 | Randomized Trial of Surgery vs Radiotherapy in Patients with Stage IIIa Non-Small Cell Lung Cancer After a Response to Induction-Chemotherapy | Surgery vs Nonsurgical Treatments | 2 | NULL | False | 1994 | Completed | European Organisation for Research and Treatment of Cancer (EORTC) |
| NCT00002550E16 | A Phase III Comparison Between Concurrent Chemotherapy Plus Radiotherapy and Concurrent Chemotherapy Plus Radiotherapy Followed by Surgical Resection for Stage IIIA (N2) Non-Small Cell Lung Cancer | Surgery vs Nonsurgical Treatments | 2 | Open Label | False | 1994 | Completed | Radiation Therapy Oncology Group |
| NCT00176137E17 | Cisplatin/Etoposide Followed by Twice-Daily Chemoradiation (hfRT/CT) vs Cisplatin/Etoposide Alone Before Surgery in Stage III Non-Small Cell Lung Cancer. A Phase III Trial of the German Lung Cancer Cooperative Group (GLCCG) | Surgery + Adjunct Treatments | 2 | Open Label | False | 1995 | Completed | Heidelberg University |
| NCT00003159 | Randomized Trial of Surgical Resection with or without Pre-Operative Chemotherapy in Patients with Operable Non-Small Cell Lung Cancer (NSCLC) of Any Stage | Surgery + Adjunct Treatments | 2 | NULL | False | 1997 | Completed | Medical Research Council |
| NCT00003317 | A Phase III Study of Surgical Resection and Chemotherapy (Paclitaxel and Carboplatin) with or without Adjuvant Radiotherapy for Resected Stage IIIA Non-Small Cell Lung Cancer | Surgery + Adjunct Treatments | 2 | Open Label | False | 1998 | Completed | Alliance for Clinical Trials in Oncology |
| NCT00273494 | Scandinavian Neoadjuvant Phase III Study of Induction Chemotherapy Followed by Irradiation Alone or Surgery Plus Irradiation in NSCLC Stage IIIA/N2 (T1N2, T2N2, T3/N2). | Surgery vs Nonsurgical Treatments | 2 | Open Label | True | 1998 | Unknown | Rigshospitalet, Denmark |
| NCT00913705 | Randomized Trial of Surgery with or without Paclitaxel Plus Carboplatin as Neoadjuvant or Adjuvant Chemotherapy in Patients with Operable, Non-small-cell Lung Cancer | Surgery + Adjunct Treatments | 2 | Open Label | False | 1999 | Completed | Spanish Lung Cancer Group |
| NCT00004011E18 | A Randomized Phase III Trial of Surgery Alone or Surgery Plus Preoperative Paclitaxel/Carboplatin in Clinical Stage IB (T2N0), II (T1-2N1, T3N0) and Selected IIIA (T3N1) Non-Small Cell Lung Cancer (NSCLC) | Surgery + Adjunct Treatments | 2 | Open Label | False | 1999 | Completed | SWOG Cancer Research Network |
| NCT00191126 | Randomized Phase III Trial of Surgery Alone or Surgery Plus Preoperative Gemcitabine-Cisplatin in Clinical Early Stages(T2N0, T1 - 2N1, T3N0 AND T3N1) Non-Small Cell Lung Cancer (NSCLC) | Surgery + Adjunct Treatments | 2 | Open Label | False | 2000 | Completed | Eli Lilly and Company |
| NCT00113386 | Phase III Randomized Trial of Preoperative Chemotherapy vs Preoperative Concurrent Chemotherapy and Thoracic Radiotherapy Followed by Surgical Resection and Consolidation Chemotherapy in Favorable Prognosis Patients With Stage IIIA (N2) Non-Small Cell Lung Cancer | Surgery + Adjunct Treatments | 2 | Open Label | False | 2005 | Stopped | Radiation Therapy Oncology Group |
| NCT00591552 | Use of Harmonic Scalpel to Decrease Lymphatic and Chest Tube Drainage After Lymph Node Dissection With Lobectomy. A Single Center Prospective Randomized Controlled Study | Different Patient Management Strategies | 2 | Open Label | True | 2007 | Recruiting | Sentara Cardiovascular Research Institute |
| NCT00499330E7 | A Phase III Randomized Trial of Lobectomy vs Sublobar Resection for Small (≤2 cm) Peripheral Non-Small Cell Lung Cancer | Different Types of Lung Resection | 2 | Open Label | False | 2007 | Active, not recruiting | Alliance for Clinical Trials in Oncology |
| NCT00841750 | The NoTube Study: Evaluation of the Necessity of a Chest Tube After a Video-assisted Thoracoscopic Surgery Pulmonary Wedge Resection | Different Patient Management Strategies | 2 | Single | False | 2008 | Unknown | Fundación Oftalmológica de Santander Clínica Carlos Ardila Lulle |
| NCT01278888E19 | Minimally Invasive or Open Surgery for Lung Cancer: Pain, Quality of Life and Economics | Different Modalities of Lobectomy | 2 | Double | True | 2008 | Completed | Odense University Hospital |
| NCT00840749E20 | International Randomized Study to Compare CyberKnife® Stereotactic Radiotherapy with Surgical Resection in Stage I Non-small Cell Lung Cancer | Surgery vs Nonsurgical Treatments | 2 | Open Label | False | 2008 | Stopped | Accuracy Incorporated |
| NCT00687986E20 | A Randomized Clinical Trial of Surgery vs Radiosurgery (Stereotactic Radiotherapy) in Patients with Stage IA NSCLC Who Are Fit to Undergo Primary Resection | Surgery vs Nonsurgical Treatments | 2 | Open Label | True | 2008 | Stopped | Amsterdam UMC, location VUmc |
| NCT00925444E21 | Medical and Economic Evaluation of FORESEAL vs the Current Therapeutic Approach (Stapling Alone or Associated with Tissue Sealant) in Terms of Air Leakage Duration After Lung Resection for Cancer. | Different Intraoperative Technical Management | 2 | Open Label | True | 2009 | Completed | Assistance Publique, Hôpitaux de Paris |
| NCT01575314E22 | Cost-consequence Analysis of Parenchymal Stapling Device vs Hand-sewing for Pulmonary Lobectomy in Lung Disease: A Randomized Controlled Trial | Different Intraoperative Technical Management | 2 | Double | True | 2011 | Completed | Chiang Mai University |
| NCT01368601 | Effects of Intraoperative Continuous Airway Pressure (CPAP) on the Inflammatory Response of the Lung with Cancer Undergoing Lobectomy. A Randomised Placebo-controlled Trial | Different Patient Management Strategies | 2 | Triple | True | 2011 | Unknown | Parc de Salut Mar |
| NCT01533233 | Safety and Results of Thoracoscopic Lobectomy Using Nonintubated Anesthesia vs Intubated General Anesthesia for Lung Cancer Patients | Different Patient Management Strategies | 2 | Open Label | True | 2011 | Unknown | National Taiwan University Hospital |
| NCT01574729E23 | Phase II Study of Surgery Combined with Recombinant Adenoviral Human p53 Gene Therapy in Treatment Advanced Non-small-cell Carcinoma | Surgery + Adjunct Treatments | 2 | Open Label | True | 2012 | Unknown | Shenzhen SiBiono GeneTech Co, Ltd |
| NCT01621698E24 | Early vs Late Paravertebral Block for Analgesia in Video Assisted Thoracoscopic Lung Resection | Different Patient Management Strategies | 2 | Triple | True | 2012 | Completed | University Hospitals Bristol and Weston NHS Foundation Trust |
| NCT01685580E25 | Evaluation of Non-Invasive Ventilation Preoperative Lung Resection Surgery | Different Patient Management Strategies | 2 | Open Label | False | 2012 | Completed | University Hospital, Brest |
| NCT01933828 | Thoracoscopic vs Open Lobectomy for Early Stage Lung Cancer: a Randomized Prospective Trial | Different Modalities of Lobectomy | 3 | Single | True | 2013 | Unknown | Radboud University Medical Center |
| NCT02011997 | Comparison of Video-Assisted Thoracoscopic Segmentectomy vs Lobectomy for Lung Adenocarcinoma in Situ and with Microinvasion | Different Types of Lung Resection | 2 | Open Label | False | 2013 | Unknown | The First Affiliated Hospital of Guangzhou Medical University |
| NCT02481661 | A Phase III Randomized Trial of Anatomical Segmentectomy vs Lobectomy by Minimal Incision for Stage IA Peripheral Non-Small Cell Lung Cancer (≤2 cm) | Different Types of Lung Resection | 2 | Open Label | True | 2015 | Unknown | Zhejiang Cancer Hospital |
| NCT02462356E26 | A Randomized Controlled Study: the Effect of Multiple-portal VATS vs Conventional VATS Lobectomy for NSCLC | Different Intraoperative Technical Management | 2 | Single | True | 2015 | Unknown | Second Affiliated Hospital, School of Medicine, Zhejiang University |
| NCT03521375 | Video Assisted Thoracoscopic Lobectomy vs Conventional Open Lobectomy for Lung Cancer, a Multi-centre Randomised Controlled Trial With an Internal Pilot | Different Modalities of Lobectomy | 2 | Double | False | 2015 | Completed | University of Bristol |
| NCT02933294 | Uniportal vs Triportal Thoracoscopic Lobectomy and Sublobectomy for Early-Stage Lung Cancer: a Multicenter Randomized Controlled Trial | Different Intraoperative Technical Management | 2 | Open Label | False | 2015 | Unknown | Cancer Institute and Hospital, Chinese Academy of Medical Sciences |
| NCT02595944 | Adjuvant Nivolumab in Resected Lung Cancers (ANVIL)-A Randomized Phase III Study of Nivolumab After Surgical Resection and Adjuvant Chemotherapy in Non-Small Cell Lung Cancers | Surgery vs Nonsurgical Treatments | 2 | Open Label | False | 2016 | Active, not recruiting | National Cancer Institute (NCI) |
| NCT02817048E27 | Thoracoscopic Surgery Without Chest Tube Placement in Patients with Peripheral Lung Nodules: A Prospective Randomized Trial | Different Patient Management Strategies | 2 | Open Label | False | 2016 | Unknown | National Taiwan University Hospital |
| NCT02702921E28 | A Prospective, Randomized, Controlled, Multi-Center Evaluation of a Powered Vascular Stapler in VAT Lobectomies | Different Intraoperative Technical Management | 2 | Open Label | True | 2016 | Completed | Ethicon Endo-Surgery |
| NCT02617186 | Robotic Lobectomy vs Thoracoscopic Lobectomy for Early-Stage Lung Cancer: A Randomized Controlled Trial | Different Modalities of Lobectomy | 2 | Single | True | 2016 | Recruiting | St Joseph's Healthcare Hamilton |
| NCT03436329E29 | Reduced Dissemination of Tumor Cells with Primary Ligation of the Vein in Patients With Lung Cancer: A Multi-center Randomized Controlled Trial | Different Intraoperative Technical Management | 2 | Triple | True | 2016 | Unknown | West China Hospital |
| NCT02911259E30 | Suction on Post-Operative Chest Tubes After Video-Assisted Thoracoscopic Surgery Lobectomy for Presumed or Confirmed Primary Lung Cancer - At Which Level? | Different Patient Management Strategies | 2 | Single | True | 2016 | Completed | Rigshospitalet, Denmark |
| NCT02992353 | Single-port vs Two-port vs Three-port Video Assisted Thoracoscopic Pulmonary Resection on Non-small Cell Lung Cancer: a Prospective Randomised Controlled Trial | Different Intraoperative Technical Management | 2 | Open Label | True | 2016 | Unknown | Cancer Institute and Hospital, Chinese Academy of Medical Sciences |
| NCT02360761 | Surgical Treatment of Elderly Patients with Early Stage Non-small Cell Lung Cancer (STEPS): Comparison Between Sublobar Resection and Lobectomy - an Open, Multicenter, Randomized Phase III Clinical Trial | Different Types of Lung Resection | 2 | Open Label | False | 2016 | Unknown | Peking University People's Hospital |
| NCT03108560 | A Multi-center, Randomized-controlled, Open-label Clinical Trial: Sublobar Resection vs Lobectomy for cT1N0M0 Non-small-cell Lung Cancer | Different Types of Lung Resection | 2 | Open Label | True | 2017 | Recruiting | Shanghai Zhongshan Hospital |
| NCT03134534E5 | The Study of Robotic-assisted Thoracoscopic Surgery vs Video-assisted Thoracoscopic Surgery Lobectomy for Non-small Cell Lung Cancer on Short-term and Long-term Outcomes (RVlob) | Different Modalities of Lobectomy | 2 | Open Label | True | 2017 | Active, not recruiting | Ruijin Hospital |
| NCT03331588E31 | Comparison Study of Post-operative Pain and Quality of Life Between Subxiphoid and Intercostal Video-assisted Thoracic Surgery for Radical Lung Cancer Resection | Different Intraoperative Technical Management | 2 | Double | True | 2017 | Completed | Shanghai Pulmonary Hospital, Shanghai, China |
| NCT03351842 | A Phase II Study of Adjuvant Chemotherapy After Surgery for Stage I Lung Adenocarcinoma Patients with Micropapillary Component ≥20% | Surgery + Adjunct Treatments | 2 | Open Label | True | 2017 | Recruiting | Shanghai Pulmonary Hospital, Shanghai, China |
| NCT02718365 | A Multi-center, Prospective, Randomized Controlled Clinical Trial: Comparison Between Wedge Resection and Segmentectomy in the Surgical Treatment of Ground Glass Opacity-dominant Stage IA Non-small Cell Lung Cancer | Different Types of Lung Resection | 2 | Open Label | True | 2017 | Recruiting | West China Hospital |
| NCT02984761 | CSP #2005 - Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy Trial (VALOR) | Surgery vs Nonsurgical Treatments | 2 | Open Label | False | 2017 | Recruiting | Veterans Affairs Office of Research and Development |
| NCT02804893E6 | Prospective, Randomized, Multicentric Study on Videothoracoscopic (Vats) vs Robotic Approach For Lobectomy Or Anatomical Segmentectomy In Patients Affected By Early Lung Cancer (ROMAN) | Different Modalities of Lobectomy | 2 | Open Label | True | 2017 | Unknown | Istituto Clinico Humanitas |
| NCT03379350E32 | The Impacts of Intermittent Chest Tube Clamping on Chest Tube Drainage Duration and Postoperative Hospital Stay After Lung Cancer Surgery: A Prospective Study | Different Patient Management Strategies | 2 | Open Label | True | 2017 | Completed | Peking University Cancer Hospital & Institute |
| NCT03432637 | Thoracoscopic Lobectomy Under Spontaneous Ventilating Anesthesia or Intubated Anesthesia: A Prospective, Multicentre, Open-label, Randomized Control Trial | Different Patient Management Strategies | 2 | Open Label | False | 2018 | Recruiting | Guangzhou Institute of Respiratory Disease |
| NCT03925103E33 | Ergonomical Assessment of Three-Dimensional vs Two-Dimensional Thoracoscopic Lobectomy for Lung Cancer | Different Intraoperative Technical Management | 2 | Single | True | 2018 | Completed | University of Rome Tor Vergata |
| NCT03523468E34 | Curative Effect and Quality of Life Between Uniportal Sleeve Lobectomy and Open-chest Sleeve Lobectomy for Central Type Lung Cancer: a Prospective Randomized Controlled Study | Different Modalities of Lobectomy | 2 | Triple | True | 2018 | Recruiting | Shanghai Pulmonary Hospital |
| NCT03523234 | Neoadjuvant Therapy Combined with Radical Surgery for the Treatment of Small Cell Lung Cancer (SCLC) in II and IIIA Stage | Surgery vs Nonsurgical Treatments | 2 | Open Label | True | 2018 | Recruiting | Shanghai Pulmonary Hospital |
| NCT03439696 | Needlescopic-assisted Uniportal VATS vs Conventional Uniportal VATS. A Randomized Prospective Noninferiority Study | Different Intraoperative Technical Management | 2 | Single | True | 2018 | Completed | Institutional Review Board of National Taiwan University Hospital Hsin-Chu Branch |
| NCT03471884 | Effects of Nonintubated Thoracoscopic Lobectomy on Lung Protection: A Randomized Controlled Trial | Different Patient Management Strategies | 2 | Single | True | 2018 | Unknown | National Taiwan University Hospital |
| NCT03137576 | Pain Blocks in Awake Thoracic Surgery: A Randomized Prospective Trial to Test the Non-inferiority of Erector Spinae Plane Block (ESPB) in Comparison with Paravertebral Block During Non-intubate, Thoracoscopic Lung Resection. | Different Patient Management Strategies | 2 | Double | True | 2019 | Stopped | IRCCS Sacro Cuore Don Calabria di Negrar |
| NCT04009915 | Comparison of Curative Effect and Postoperative Survival Rate Between Video-assisted Thoracoscopic Surgery and Open Thoracic Surgery for Stage II - III Lung Cancer, A Prospective, Randomized, Controlled Trial: (The VOLCANO Study) | Different Modalities of Lobectomy | 2 | Double | True | 2019 | Recruiting | Shanghai Pulmonary Hospital, Shanghai, China |
| NCT04212481 | Randomized Controlled Trial of the Comparison of Uniport VATS and Non-Uniport VATS for Lung Cancer | Different Intraoperative Technical Management | 3 | Quadruple | True | 2019 | Recruiting | The Second Hospital of Shandong University |
| NCT03997799 | Comparison of Two Techniques of Video Assisted Thoracic Surgery (VATS) Uniportal Lobectomies Through the Transcervical and Standard Intercostal Approaches for Clinical Stage I Non-Small Cell Lung Cancer (NSCLC) in the Prospective Randomized Single-institutional Trial | Different Intraoperative Technical Management | 2 | Open Label | True | 2019 | Recruiting | Pulmonary Hospital Zakopane |
| NCT04937283E35 | Comparison of Segmentectomy vs Lobectomy for Lung Adenocarcinoma ≤ 2 cm With Micropapillary and Solid Subtype Negative by Intraoperative Frozen Sections: A Prospective and Multi-center Randomized Controlled Trial Study | Different Types of Lung Resection | 2 | Open Label | False | 2019 | Recruiting | Shanghai Pulmonary Hospital, Shanghai, China |
| NCT04309955 | Randomized Clinical Trial of Modified vs Traditional Thoracic Drainage After Thoracoscopic Surgery for Lung Cancer | Different Patient Management Strategies | 2 | Open Label | True | 2019 | Unknown | Daping Hospital and the Research Institute of Surgery of the Third Military Medical University |
| NCT04665531 | Postoperative Analgesia with a Catheter Under the Erector Spinae Muscle for Video-thoracoscopic Lung Surgery | Different Patient Management Strategies | 2 | Double | True | 2020 | Completed | Surgery Bitenc |
| NCT04944563 | Comparison of Segmentectomy vs Lobectomy for Early-stage Non-small Cell Lung Cancer ≤ 2 cm in the Middle Third of the Lung Field: A Prospective and Multi-center RCT Study | Different Types of Lung Resection | 2 | Open Label | True | 2021 | Recruiting | The First Affiliated Hospital with Nanjing Medical University |
| NCT05202249 | Effect of Muscle and Skin Fixation of Thoracic Drainage Tube on Postoperative Pain in Patients Undergoing Uniport Thoracoscopic Pulmonary Resection | Different Patient Management Strategies | 2 | Open Label | True | 2021 | Recruiting | Daping Hospital and the Research Institute of Surgery of the Third Military Medical University |
| NCT04923412 | Prospective Randomized Controlled Study on the Effects of Vagus Nerve Pulmonary Branch Preservation During Video-assisted Thoracic Surgery Lobectomy in Non-small Cell Lung Cancer: Can it Decrease Postoperative Cough and Pulmonary Complications | Different Intraoperative Technical Management | 2 | Open Label | True | 2021 | Recruiting | Seoul National University Bundang Hospital |
| NCT04989283 | NASSIST (Neoadjuvant Chemoradiation +/− Immunotherapy Before Surgery for Superior Sulcus Tumors): A Randomized Phase II Trial of Trimodality +/− Atezolizumab in Resectable Superior Sulcus Non-Small Cell Lung Cancer | Surgery + Adjunct Treatments | 2 | Open Label | False | 2021 | Active, not recruiting | National Cancer Institute (NCI) |
| NCT05502523 | The Impact of Surgical Technique on Circulating Tumor DNA in Early-Stage Non-Small Cell Lung Cancer | Different Intraoperative Technical Management | 2 | Open Label | True | 2022 | Recruiting | Thomas Jefferson University |
| NCT05358158 | Efficacy of Avoiding Chest Drain After Video-assisted Thoracoscopic Surgery Wedge Resection | Different Patient Management Strategies | 2 | Open Label | False | 2022 | Recruiting | Rigshospitalet, Denmark |
| NCT05453721 | Effect and Long-Term Outcomes of Indocyanine Green Fluorescence Imaging Method vs Modified Inflation-Deflation Method in Identification of Intersegmental Plane: A Multicenter, Prospective, Randomized Controlled Trial | Different Intraoperative Technical Management | 2 | Open Label | True | 2022 | Recruiting | The First Affiliated Hospital of Nanchang University |
| NCT03786003 | Lobectomy for Clinical Stage T1N0M0 Solid NSCLC by VATS vs Thoracotomy | Different Modalities of Lobectomy | 2 | Open Label | True | 2022 | Recruiting | Fudan University |
| NCT05666908 | High-flow Nasal Oxygenation Improves Blood Oxygen Saturation During Asphyxia During Pulmonary Surgery with Double-lumen Endotracheal Intubation: a Randomized Controlled Study | Different Patient Management Strategies | 2 | Triple | False | 2023 | Not yet recruiting | Shenzhen Second People's Hospital |
| NCT05727735 | The Feasibility of Assessing Economic Costs of the Signia Stapler vs Vessel Sealer Extend Energy Device with SureForm Stapling in Robotic-Assisted Segmentectomy for Lung Cancer | Different Intraoperative Technical Management | 2 | Single | True | 2023 | Not yet recruiting | St Joseph's Healthcare Hamilton |
SWOG, Southwest Oncology Group; IRCCS, Scientific Institute for Research, Hospitalization, and Healthcare.
Table E2.
Studies investigating perioperative strategies or intraoperative techniques
| Variable studied | No. of RCTs (n = 33) | Study descriptions |
|---|---|---|
| Perioperative strategies | ||
| Analgesia | 8 | Pain and quality of life, paravertebral/erector spinae blocks, ergonomics, vagus nerve preservation |
| Ventilation | 7 | Intraoperative CPAP, nonintubated anesthesia, noninvasive/spontaneous ventilation, high-flow nasal cannula use |
| Drainage | 6 | Harmonic scalpel use, necessity of chest tube, suction, tube clamping |
| Intraoperative techniques | ||
| Stapling | 4 | Use of tissue sealant, stapling costs, powered vascular stapler |
| No. of ports | 6 | Multiple vs uniport |
| Port placement | 1 | Uniportal VATS techniques |
| Ligation | 1 | Primary vein ligation |
RCT, Randomized controlled trial; CPAP, continuous positive airway pressure; VATS, video-assisted thoracoscopic surgery.
Table E3.
Included studies stratified by time period initiated and topic of interest
| Comparison types | Year 2000 and earlier, N = 9, n (%) | Year 2001-2010, N = 8, n (%) | Year 2011-2015, N = 12, n (%) | Year 2016-2020, N = 28, n (%) | Year 2021-2023, N = 11, n (%) |
|---|---|---|---|---|---|
| Patient management strategies | 0 (0.0%) | 2 (25.0%) | 4 (33.3%) | 7 (25.0%) | 4 (36.4%) |
| Intraoperative technical management | 0 (0.0%) | 1 (12.5%) | 3 (25.0%) | 8 (28.6%) | 4 (36.4%) |
| Surgery + adjunct treatments | 6 (66.7%) | 1 (12.5%) | 1 (8.3%) | 1 (3.6%) | 1 (9.1%) |
| Modalities of lobectomy | 0 (0.0%) | 1 (12.5%) | 2 (16.7%) | 5 (17.9%) | 1 (9.1%) |
| Types of lung resection | 0 (0.0%) | 1 (12.5%) | 2 (16.7%) | 4 (14.3%) | 1 (9.1%) |
| Surgery vs nonsurgical treatments | 3 (33.3%) | 2 (25.0%) | 0 (0.0%) | 3 (10.7%) | 0 (0.0%) |
Table E4.
Characteristics of included randomized controlled trials stratified by continents
| Characteristic | Asia |
Europe |
North America |
South America |
Africa |
Oceania |
|---|---|---|---|---|---|---|
| N = 34∗ | N = 22∗ | N = 14∗ | N = 13∗ | N = 2∗ | N = 1∗ | |
| Overall status group | ||||||
| Completed | 5 (14.7%) | 13 (59.1%) | 4 (28.6%) | 4 (30.8%) | 2 (100.0%) | |
| Recruiting | 14 (41.2%) | 2 (9.1%) | 4 (28.6%) | 3 (23.1%) | ||
| Active, not recruiting | 1 (2.9%) | 3 (21.4%) | 3 (23.1%) | 1 (100.0%) | ||
| Not yet recruiting | 1 (2.9%) | 1 (7.1%) | ||||
| Stopped | 1 (2.9%) | 3 (13.6%) | 2 (14.3%) | 2 (15.4%) | ||
| Unknown | 12 (35.3%) | 4 (18.2%) | 1 (7.7%) | |||
| Periods | ||||||
| Year 2000 and earlier | 1 (2.9%) | 6 (27.3%) | 3 (21.4%) | 3 (23.1%) | 2 (100.0%) | |
| Year 2001-2010 | 1 (2.9%) | 4 (18.2%) | 4 (28.6%) | 5 (38.5%) | 1 (100.0%) | |
| Year 2011-2015 | 7 (20.6%) | 5 (22.7%) | ||||
| Year 2016-2020 | 19 (55.9%) | 5 (22.7%) | 4 (28.6%) | 3 (23.1%) | ||
| Year 2021-2023 | 6 (17.6%) | 2 (9.1%) | 3 (21.4%) | 2 (15.4%) | ||
| Masking | ||||||
| Open label | 24 (70.6%) | 11 (50.0%) | 12 (85.7%) | 12 (92.3%) | 2 (100.0%) | 1 (100.0%) |
| Single blinded | 3 (8.8%) | 3 (13.6%) | 2 (14.3%) | 1 (7.7%) | ||
| Double blinded | 3 (8.8%) | 4 (18.2%) | ||||
| Triple blinded | 3 (8.8%) | 2 (9.1%) | ||||
| Quadruple blinded | 1 (2.9%) | |||||
| NULL | 2 (9.1%) | |||||
| Single facility | ||||||
| True | 25 (73.5%) | 13 (59.1%) | 5 (35.7%) | 3 (23.1%) | ||
| Lead sponsor category | ||||||
| Federal | 3 (21.4%) | 3 (23.1%) | ||||
| Industry | 3 (8.8%) | 2 (9.1%) | 2 (14.3%) | 2 (15.4%) | 1 (50.0%) | |
| Institutional | 31 (91.2%) | 17 (77.3%) | 3 (21.4%) | 2 (15.4%) | ||
| Network | 3 (13.6%) | 6 (42.9%) | 6 (46.2%) | 1 (50.0%) | 1 (100.0%) | |
| Comparison types | ||||||
| Different intraoperative technical management | 10 (29.4%) | 3 (13.6%) | 3 (21.4%) | 2 (15.4%) | ||
| Different modalities of lobectomy | 4 (11.8%) | 4 (18.2%) | 1 (7.1%) | |||
| Different patient management strategies | 8 (23.5%) | 7 (31.8%) | 1 (7.1%) | 2 (15.4%) | ||
| Different types of lung resection | 7 (20.6%) | 1 (7.1%) | 1 (7.7%) | 1 (100.0%) | ||
| Surgery + adjunct treatments | 3 (8.8%) | 4 (18.2%) | 4 (28.6%) | 4 (30.8%) | 1 (50.0%) | |
| Surgery vs nonsurgical treatments | 2 (5.9%) | 4 (18.2%) | 4 (28.6%) | 4 (30.8%) | 1 (50.0%) | |
| No. of arms | ||||||
| 2 | 33 (97.1%) | 21 (95.5%) | 14 (100.0%) | 13 (100.0%) | 2 (100.0%) | 1 (100.0%) |
| 3 | 1 (2.9%) | 1 (4.5%) | ||||
| Actual enrollment | ||||||
| Mean (SD) | 166 (106) | 196 (168) | 317 (248) | 317 (248) | 346 (117) | 701 (NA) |
| Median (IQR) | 180 (82-232) | 230 (43-297) | 354 (118-454) | 354 (118-454) | 346 (304-388) | 701 (701-701) |
| Unknown | 27 | 11 | 7 | 6 | ||
| Estimated/target enrollment | ||||||
| Mean (SD) | 375 (345) | 373 (302) | 510 (406) | 508 (419) | 605 (134) | 1297 (NA) |
| Median (IQR) | 252 (114-490) | 300 (94-600) | 574 (100-670) | 542 (100-728) | 605 (558-652) | 1297 (1297-1297) |
| Unknown | 1 | 1 | 1 |
SD, Standard deviation; IQR, interquartile range; NA, not available.
n (%) for categorical variables; mean (SD) and median (IQR) for continuous variables.
Table E5.
Time for completion of completed lung cancer surgical randomized controlled trials
| Characteristic | Completed RCTs, N = 21∗ |
|---|---|
| Periods | |
| Year 2000 and earlier | 8 (38.1%) |
| Year 2001-2010 | 2 (9.5%) |
| Year 2011-2015 | 4 (19.0%) |
| Year 2016-2020 | 6 (28.6%) |
| Masking | |
| Open label | 10 (47.6%) |
| Single blinded | 3 (14.3%) |
| Double blinded | 5 (23.8%) |
| Triple blinded | 1 (4.8%) |
| NULL | 2 (9.5%) |
| Single facility | |
| True | 11 (52.4%) |
| No. of arms | |
| 2 | 21 (100.0%) |
| Actual enrollment | |
| Mean (SD) | 256 (146) |
| Median (IQR) | 246 (162-360) |
| Unknown | 5 |
| Estimated/target enrollment | |
| Mean (SD) | 349 (224) |
| Median (IQR) | 300 (165-555) |
| Unknown | 2 |
| Days of completion† | |
| Mean (SD) | 2125 (1785) |
| Median (IQR) | 1554 (704-3346) |
| Days of primary completion‡ | |
| Mean (SD) | 1605 (1119) |
| Median (IQR) | 1370 (677-2572) |
RCT, Randomized controlled trial; SD, standard deviation; IQR, interquartile range.
n (%) for categorical variables; mean (SD) and median (IQR) for continuous variables.
From study start date to the date on which the last participant was examined or received an intervention/treatment to collect final data for the primary outcome measures, secondary outcome measures, and adverse events (ie, the last participant's last visit).
From study start date to the date on which the last participant was examined or received an intervention to collect final data for the primary outcome measure.
Table E6.
Characteristics and last known status for randomized controlled trials with unknown overall status
| Characteristic | RCTs with overall status unknown, N = 17∗ |
|---|---|
| Periods | |
| Year 2000 and earlier | 1 (5.9%) |
| Year 2001-2010 | 1 (5.9%) |
| Year 2011-2015 | 8 (47%) |
| Year 2016-2020 | 7 (41%) |
| Masking | |
| Open label | 11 (65%) |
| Single blinded | 4 (24%) |
| Triple blinded | 2 (12%) |
| Single facility | |
| True | 12 (71%) |
| No. of arms | |
| 2 | 16 (94%) |
| 3 | 1 (5.9%) |
| Estimated/target enrollment | |
| Mean (SD) | 216 (173) |
| Median (IQR) | 120 (82-339) |
| Days from start to last update | |
| Mean (SD) | 694 (1090) |
| Median (IQR) | 134 (−2 to 972) |
| Days from start to present | |
| Mean (SD) | 3408 (1819) |
| Median (IQR) | 2827 (2461-3891) |
| Last known status | |
| Recruiting | 12 (70.6%) |
| Not yet recruiting | 4 (23.5%) |
| Active, not recruiting | 1 (5.9%) |
RCT, Randomized controlled trial; SD, standard deviation; IQR, interquartile range.
n (%) for categorical variables; mean (SD) and median (IQR) for continuous variables.
References
- 1.Morris Z.S., Wooding S., Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang-Gillam A., Williams K., Novello S., Gao F., Scagliotti G.V., Govindan R. Time to activate lung cancer clinical trials and patient enrollment: a representative comparison study between two academic centers across the Atlantic. J Clin Oncol. 2010;28(24):3803–3807. doi: 10.1200/JCO.2010.28.1824. [DOI] [PubMed] [Google Scholar]
- 3.Menezes A.S., Barnes A., Scheer A.S., et al. Clinical research in surgical oncology: an analysis of ClinicalTrials.gov. Ann Surg Oncol. 2013;20(12):3725–3731. doi: 10.1245/s10434-013-3054-y. [DOI] [PubMed] [Google Scholar]
- 4.Catto J.W.F., Blazeby J.M., Holmberg L., Hamdy F.C., Brown J. In defense of randomized clinical trials in surgery: let us not forget Archie Cochrane's legacy. Eur Urol. 2017;71(5):820–821. doi: 10.1016/j.eururo.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Naredi P., La Quaglia M.P. The future of trials in surgical oncology. Nat Rev Clin Oncol. 2015;12(7):425–431. doi: 10.1038/nrclinonc.2015.72. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine (US) Committee on Quality of Health Care in America Crossing the quality chasm: a new health system for the 21st century. National Academies Press (US) 2001. http://www.ncbi.nlm.nih.gov/books/NBK222274/ [PubMed]
- 7.National Cancer Institute: Lung cancer statistics. https://seer.cancer.gov/statfacts/html/lungb.html
- 8.ClinicalTrials.gov. https://clinicaltrials.gov/about-site/about-ctg
- 9.Shadbolt C., Naufal E., Bunzli S., et al. Analysis of rates of completion, delays, and participant recruitment in randomized clinical trials in surgery. JAMA Netw Open. 2023;6(1):e2250996. doi: 10.1001/jamanetworkopen.2022.50996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson A.L., Fladie I., Anderson J.M., Lewis D.M., Mons B.R., Vassar M. Rates of discontinuation and non-publication of head and neck cancer randomized clinical trials. JAMA Otolaryngol Neck Surg. 2020;146(2):176. doi: 10.1001/jamaoto.2019.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman S.J., Shelton B., Mahmood H., Fitzgerald J.E., Harrison E.M., Bhangu A. Discontinuation and non-publication of surgical randomised controlled trials: observational study. BMJ. 2014;349:g6870. doi: 10.1136/bmj.g6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian J., Regenbogen T., Nagaraj G., et al. Review of ongoing clinical trials in non-small-cell lung cancer: a status report for 2012 from the ClinicalTrials.gov Web site. J Thorac Oncol. 2013;8(7):860–865. doi: 10.1097/JTO.0b013e318287c562. [DOI] [PubMed] [Google Scholar]
- 13.Szczepanek C.M., Hurley P., Good M.J., et al. Feasibility of a centralized clinical trials coverage analysis: a joint initiative of the American Society of Clinical Oncology and the National Cancer Institute. J Oncol Pract. 2017;13(6):395–400. doi: 10.1200/JOP.2016.020313. [DOI] [PubMed] [Google Scholar]
- 14.Denicoff A.M., McCaskill-Stevens W., Grubbs S.S., et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267–276. doi: 10.1200/JOP.2013.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang C., Sherman S.I., Price M., et al. Clinical trial characteristics and barriers to participant accrual: the MD Anderson cancer Center experience over 30 years, a historical foundation for trial improvement. Clin Cancer Res. 2017;23(6):1414–1421. doi: 10.1158/1078-0432.CCR-16-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang C., Hess K.R., Sanders D., et al. Modifying the clinical research infrastructure at a dedicated clinical trials unit: assessment of trial development, activation, and participant accrual. Clin Cancer Res. 2017;23(6):1407–1413. doi: 10.1158/1078-0432.CCR-16-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fung E.K., Loré J.M. Randomized controlled trials for evaluating surgical questions. Arch Otolaryngol Head Neck Surg. 2002;128(6):631–634. doi: 10.1001/archotol.128.6.631. [DOI] [PubMed] [Google Scholar]
- 18.Grant J., Green L., Mason B. The Health Economics Research Group; 2010. From Bedside to Bench: Comroe and Dripps Revisited. [Google Scholar]
- 19.Shepard S., Anderson J.M., Heigle B., et al. Rates of discontinuation and non-publication of upper and lower extremity fracture clinical trials. J Orthop Surg. 2023;18(1):256. doi: 10.1186/s13018-023-03698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenthal R., Kasenda B., Dell-Kuster S., et al. Completion and publication rates of randomized controlled trials in surgery: an empirical study. Ann Surg. 2015;262(1):68–73. doi: 10.1097/SLA.0000000000000810. [DOI] [PubMed] [Google Scholar]
- 21.Halpern S.D. Prospective preference assessment: a method to enhance the ethics and efficiency of randomized controlled trials. Control Clin Trials. 2002;23(3):274–288. doi: 10.1016/s0197-2456(02)00191-5. [DOI] [PubMed] [Google Scholar]
- 22.Kaur G., Smyth R.L., Williamson P. Developing a survey of barriers and facilitators to recruitment in randomized controlled trials. Trials. 2012;13:218. doi: 10.1186/1745-6215-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decullier E., Lhéritier V., Chapuis F. Fate of biomedical research protocols and publication bias in France: retrospective cohort study. BMJ. 2005;331(7507):19. doi: 10.1136/bmj.38488.385995.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ioannidis J.P. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279(4):281–286. doi: 10.1001/jama.279.4.281. [DOI] [PubMed] [Google Scholar]
- 25.Health Economics Research Group. Office of Health Economics. RAND Europe . Evaluation Forum; 2008. Medical Research: What's it Worth? Estimating the Economic Benefits from Medical Research in the UK. [Google Scholar]
- 26.Fiteni F., Paillard M.J., Westeel V., Bonnetain F. Time-to-event endpoints in operable non-small-cell lung cancer randomized clinical trials. Expert Rev Anticancer Ther. 2017;17(2):167–173. doi: 10.1080/14737140.2016.1271718. [DOI] [PubMed] [Google Scholar]
- 27.Green L.W., Ottoson J.M., García C., Hiatt R.A. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health. 2009;30:151–174. doi: 10.1146/annurev.publhealth.031308.100049. [DOI] [PubMed] [Google Scholar]
- 28.Balas E.A., Boren S.A. Managing clinical knowledge for health care improvement. Yearb Med Inform. 2000;(1):65–70. [PubMed] [Google Scholar]
- 29.Haidich A.B., Ioannidis J.P. Effect of early patient enrollment on the time to completion and publication of randomized controlled trials. Am J Epidemiol. 2001;154(9):873–880. doi: 10.1093/aje/154.9.873. [DOI] [PubMed] [Google Scholar]
- 30.Urrútia G., Ballesteros M., Djulbegovic B., Gich I., Roqué M., Bonfill X. Cancer randomized trials showed that dissemination bias is still a problem to be solved. J Clin Epidemiol. 2016;77:84–90. doi: 10.1016/j.jclinepi.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 31.To M.J., Jones J., Emara M., Jadad A.R. Are reports of randomized controlled trials improving over time? A systematic review of 284 articles published in high-impact general and specialized medical journals. PLoS One. 2013;8(12):e84779. doi: 10.1371/journal.pone.0084779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergeat D., Lombard N., Gasmi A., Le Floch B., Naudet F. Data sharing and reanalyses among randomized clinical trials published in surgical journals before and after adoption of a data availability and reproducibility policy. JAMA Netw Open. 2022;5(6):e2215209. doi: 10.1001/jamanetworkopen.2022.15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rangachari P., Rissing P., Rethemeyer K. Awareness of evidence-based practices alone does not translate to implementation: insights from implementation research. Qual Manag Health Care. 2013;22(2):117–125. doi: 10.1097/QMH.0b013e31828bc21d. [DOI] [PubMed] [Google Scholar]
- 34.Steffensen F.H., Sørensen H.T., Olesen F. Impact of local evidence-based clinical guidelines–a Danish intervention study. Fam Pract. 1997;14(3):209–215. doi: 10.1093/fampra/14.3.209. [DOI] [PubMed] [Google Scholar]
- 35.Cabana M.D., Rand C.S., Powe N.R., et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
E-References
- Mohan D., Rosengart M.R., Farris C., Fischhoff B., Angus D.C., Barnato A.E. Sources of noncompliance with clinical practice guidelines in trauma triage: a decision science study. Implement Sci. 2012;7:103. doi: 10.1186/1748-5908-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent M.S., Hartwig M.G., Vallières E., et al. Pulmonary Open, Robotic, and Thoracoscopic Lobectomy (PORTaL) study: an analysis of 5721 cases. Ann Surg. 2023;277(3):528–533. doi: 10.1097/SLA.0000000000005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiolfi A., Nosotti M., Micheletto G., et al. Pulmonary lobectomy for cancer: systematic review and network meta-analysis comparing open, video-assisted thoracic surgery, and robotic approach. Surgery. 2021;169(2):436–446. doi: 10.1016/j.surg.2020.09.010. [DOI] [PubMed] [Google Scholar]
- Patel Y.S., Baste J.M., Shargall Y., et al. Robotic lobectomy is cost-effective and provides comparable health utility scores to video-assisted lobectomy: early results of the RAVAL Trial. Ann Surg. 2023;278(6):841–849. doi: 10.1097/SLA.0000000000006073. [DOI] [PubMed] [Google Scholar]
- Jin R., Zheng Y., Yuan Y., et al. Robotic-assisted versus video-assisted thoracoscopic lobectomy: short-term results of a randomized clinical trial (RVlob Trial) Ann Surg. 2022;275(2):295–302. doi: 10.1097/SLA.0000000000004922. [DOI] [PubMed] [Google Scholar]
- Veronesi G., Abbas A.E.S., Muriana P., et al. Perioperative outcome of robotic approach versus manual videothoracoscopic major resection in patients affected by early lung cancer: results of a randomized multicentric study (ROMAN Study) Front Oncol. 2021;11 doi: 10.3389/fonc.2021.726408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altorki N., Wang X., Kozono D., et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med. 2023;388(6):489–498. doi: 10.1056/NEJMoa2212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji H., Okada M., Tsuboi M., et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, noninferiority trial. Lancet Lond Engl. 2022;399(10335):1607–1617. doi: 10.1016/S0140-6736(21)02333-3. [DOI] [PubMed] [Google Scholar]
- Li Y., Juergens R.A., Finley C., Swaminath A. Current and future treatment options in the management of stage III NSCLC. J Thorac Oncol. 2023;18(11):1478–1491. doi: 10.1016/j.jtho.2023.08.011. [DOI] [PubMed] [Google Scholar]
- Spigel D.R., Faivre-Finn C., Gray J.E., et al. Five-year survival outcomes from the PACIFIC Trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen N., Nymann C., Konradsen H. Implementing research results in clinical practice–the experiences of healthcare professionals. BMC Health Serv Res. 2015;16(1):48. doi: 10.1186/s12913-016-1292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse T., Fain K.M., Zarin D.A. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ. 2018;361:k1452. doi: 10.1136/bmj.k1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasziou P., Chalmers I., Rawlins M., McCulloch P. When are randomised trials unnecessary? Picking signal from noise. BMJ. 2007;334(7589):349–351. doi: 10.1136/bmj.39070.527986.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petticrew M., Chalabi Z., Jones D.R. To RCT or not to RCT: deciding when “more evidence is needed” for public health policy and practice. J Epidemiol Community Health. 2012;66(5):391–396. doi: 10.1136/jech.2010.116483. [DOI] [PubMed] [Google Scholar]
- Van Meerbeeck J.P., Kramer G.W.P.M., Van Schil P.E.Y., et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99(6):442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- Albain K.S., Rusch V.W., Crowley J.J., et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- Thomas M., Rübe C., Hoffknecht P., et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9(7):636–648. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- Pisters K.M.W., Vallières E., Crowley J.J., et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol. 2010;28(11):1843–1849. doi: 10.1200/JCO.2009.26.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendixen M., Jørgensen O.D., Kronborg C., Andersen C., Licht P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- Chang J.Y., Senan S., Paul M.A., et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alifano M., Jayle C., Bertin F., et al. Medical and economic evaluation of FOREseal bioabsorbable reinforcement sleeves compared with current standard of care for reducing air leakage duration after lung resection for malignancy: a randomized trial. Ann Surg. 2017;265(1):45–53. doi: 10.1097/SLA.0000000000001687. [DOI] [PubMed] [Google Scholar]
- Tantraworasin A., Seateang S., Bunchungmongkol N. Staplers versus hand-sewing for pulmonary lobectomy: randomized controlled trial. Asian Cardiovasc Thorac Ann. 2014;22(3):309–314. doi: 10.1177/0218492313491754. [DOI] [PubMed] [Google Scholar]
- Deng B., Sun T., Tang B., et al. Surgery combined with adenoviral p53 gene therapy for treatment of non-small cell lung cancer: a phase II study. Oncotarget. 2017;8(63):107089–107095. doi: 10.18632/oncotarget.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalanathan K., Knight T., Rasburn N., Joshi N., Molyneux M. Early versus late paravertebral block for analgesia in video-assisted thoracoscopic lung resection. A double-blind, randomized, placebo-controlled trial. J Cardiothorac Vasc Anesth. 2019;33(2):453–459. doi: 10.1053/j.jvca.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Paleiron N., Grassin F., Lancelin C., et al. Assessment of preoperative noninvasive ventilation before lung cancer surgery: The preOVNI randomized controlled study. J Thorac Cardiovasc Surg. 2020;160(4):1050–1059.e3. doi: 10.1016/j.jtcvs.2019.09.193. [DOI] [PubMed] [Google Scholar]
- Yao J., Chang Z., Zhu L., Fan J. Uniportal versus multiportal thoracoscopic lobectomy: Ergonomic evaluation and perioperative outcomes from a randomized and controlled trial. Medicine (Baltimore) 2020;99(42):e22719. doi: 10.1097/MD.0000000000022719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H.C., Yang S.M., Hung M.H., Cheng Y.J., Hsu H.H., Chen J.S. Thoracoscopic surgery without drainage tube placement for peripheral lung nodules. Ann Thorac Surg. 2020;109(3):887–893. doi: 10.1016/j.athoracsur.2019.10.048. [DOI] [PubMed] [Google Scholar]
- Molins L., Lanuti M., Force S., et al. Evaluation of a powered vascular stapler in video-assisted thoracic surgery lobectomy. J Surg Res. 2020;253:26–33. doi: 10.1016/j.jss.2020.03.023. [DOI] [PubMed] [Google Scholar]
- Wei S., Guo C., He J., et al. Effect of vein-first vs artery-first surgical technique on circulating tumor cells and survival in patients with non-small cell lung cancer: a randomized clinical trial and registry-based propensity score matching analysis. JAMA Surg. 2019;154(7):e190972. doi: 10.1001/jamasurg.2019.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbek B.L., Christensen M., Hansen H.J., Kehlet H., Petersen R.H. The effects of low suction on digital drainage devices after lobectomy using video-assisted thoracoscopic surgery: a randomized controlled trial. Eur J Cardiothorac Surg. 2019;55(4):673–681. doi: 10.1093/ejcts/ezy361. [DOI] [PubMed] [Google Scholar]
- Chen Z., Jiang L., Zheng H., Zhang W., Lv X., Abdellateef A. Early postoperative pain after subxiphoid uniportal thoracoscopic major lung resection: a prospective, single- blinded, randomized controlled trial. Interact Cardiovasc Thorac Surg. 2022;35(1):ivac133. doi: 10.1093/icvts/ivac133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Pei Y., Lv C., Wang Y., et al. Intermittent chest tube clamping decreases chest tube duration time and drainage volume after lung cancer surgery in patients without air leak: an open-label, randomized controlled trial. Transl Lung Cancer Res. 2022;11(3):357–365. doi: 10.21037/tlcr-22-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhouly A.G., Sorge R., Rogliani P., et al. Ergonomical assessment of three-dimensional versus two-dimensional thoracoscopic lobectomy. Semin Thorac Cardiovasc Surg. 2020;32(4):1089–1096. doi: 10.1053/j.semtcvs.2020.05.018. [DOI] [PubMed] [Google Scholar]
- Qu J.C., Soultanis K.M., Jiang L. Surgical techniques and outcome analysis of uniportal video-assisted thoracic surgery complex sleeve lung resection: a 20 case-series study. J Thorac Dis. 2021;13(4):2255–2263. doi: 10.21037/jtd-20-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Yao J., Si H., et al. Frozen sections accurately predict the IASLC proposed grading system and prognosis in patients with invasive lung adenocarcinomas. Lung Cancer. 2023;178:123–130. doi: 10.1016/j.lungcan.2023.02.010. [DOI] [PubMed] [Google Scholar]