Abstract
Objective
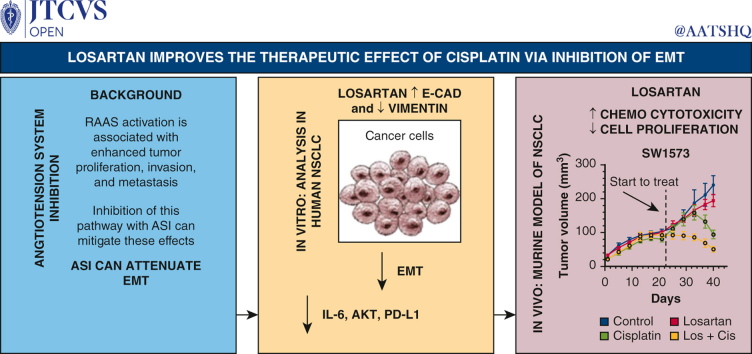
Previous studies have demonstrated synergistic antitumor effects of angiotensin system inhibition (ASI) combined with cisplatin therapy in pancreatic cancer. This study examines whether or not synergistic antitumor effects occur with combination ASI and cisplatin treatment in lung cancer, and whether or not ASI-induced changes in epithelial-mesenchymal transition play a role in the mechanism of this antitumor phenomenon.
Methods
A set of lung cancer cell lines representing a spectrum of epithelial to mesenchymal phenotypes were identified and characterized. Response of epithelial-mesenchymal transition markers to losartan was characterized. Cell culture models of lung cancer were next treated with losartan, cisplatin, or combination of both. Markers of epithelial-mesenchymal transition or surrogates of other signaling pathways (AKT, Stat3, and programmed death-ligand), and cell viability were quantified. Findings were confirmed in both allogenic and syngeneic in vivo murine flank tumor models.
Results
Losartan treatment significantly increased E-cadherin and reduced vimentin in human lung cancer cell lines. Combination treatment with losartan and cisplatin enhanced epithelial markers, reduced mesenchymal markers, inhibited promesenchymal signaling mediators, and reduced cell viability. Findings were confirmed in vivo in a murine flank tumor model with transition from mesenchymal to epithelial phenotype and reduced tumor size following combination losartan and cisplatin treatment.
Conclusions
Combination losartan and cisplatin treatment attenuates the epithelial-mesenchymal transition pathway and enhances the cytotoxic effect of chemotherapy with in vitro and in vivo models of non–small cell lung cancer. This study suggests an important role for ASI therapy in the treatment of lung cancer.
Key Words: losartan, lung cancer, epithelial-mesenchymal transition, cisplatin
Graphical Abstract
Improved antitumor efficacy of chemotherapy with losartan.
Central Message.
Losartan potentiates the therapeutic efficacy of cisplatin in a murine model of NSCLC via downregulation of epithelial to mesenchymal signaling pathways.
Perspective.
RAAS signaling influences tumorigenesis and treatment susceptibility in solid tumors. Through a series of experiments we found that losartan potentiates the antitumor efficacy of cisplatin in a murine model of NSCLC via attenuation of the EMT pathway. Angiotensin system inhibition should be further evaluated in a Phase 1 and 2 study when combined with neoadjuvant chemotherapy for locally advanced NSCLC.
See Discussion on page 322.
Lung cancer is among the most commonly diagnosed cancers and is the leading cause of cancer mortality worldwide. The role of angiotensin system inhibition (ASI) in modifying the risk of developing lung cancer has been an area of intense study, with important implications should this common class of drugs be found to promote lung cancer.1, 2, 3, 4 Comparatively little study has been done into the influence ASI may have on treatment of an established lung cancer. Several small retrospective studies have demonstrated a 40% increase in survival in patients incidentally receiving ASI versus those not exposed to ASI and a 47% increase in progression-free survival with ASI use, which was most pronounced with angiotensin receptor binders therapy.5, 6, 7, 8, 9 In each of these studies, chemotherapy included standard platinum-based therapy.
Significant effort has gone into understanding the effects of the renin angiotensin aldosterone system (RAAS) on the tumor microenvironment.10 Angiotensin II type 1 receptor (AT1R) overexpression has been associated with poor prognosis in patients with bladder cancer, operable breast cancer, ovarian cancer, and intestinal type gastric cancer, suggesting that local dysregulation of RAAS signaling may occur in or drive malignancy.11 Activation of the RAAS axis has been associated with enhanced tumor proliferation, invasion, and metastasis.12 RAAS signaling may create an immunosuppressive tumor microenvironment by activating profibrotic signaling pathways leading to a desmoplastic environment.13 Tumor angiogenesis and vascular permeability are enhanced by RAAS via vascular endothelial growth factor, promoting tumor growth.14 Inflammation and immune cell signaling are also influenced by RAAS in ways that can influence the response to malignancy.11 ASI appears to mitigate many of these effects, suggesting several mechanisms by which ASI could improve outcomes in cancer therapy.
Epithelial-mesenchymal transition (EMT) is a change from a polar and immobile epithelial cell to a mesenchymal cell, often accompanied by downregulation of E-cadherin and upregulation of vimentin.15 Tumor cells that develop EMT take on characteristics that can permit cellular escape from primary tumor, invade the microcirculation through the basement membrane, and form distant metastases.16 These cells also have increased proliferation and have been associated with drug resistance.17 This phenomenon is understood to be a significant component of tumor progression in many tumors, including lung cancer.18 The RAAS has been shown to drive EMT19 and ASI-inhibited metastasis in preliminary experiments.18
To further explore the interaction between the observed effects of both ASI and EMT on cancer treatment, we first developed an in vitro model of lung cancer featuring a range of cell types across the EMT spectrum and quantified the response of each cell type to ASI. We next established a series of tests to quantify malignant potential of the cells: cellular proliferation, proliferation markers, and cell migration. We then tested the response of cells across the EMT spectrum to cisplatin, the most common chemotherapeutic agent in lung cancer. Findings were confirmed in a murine model.
Method and Materials
In Vitro Studies
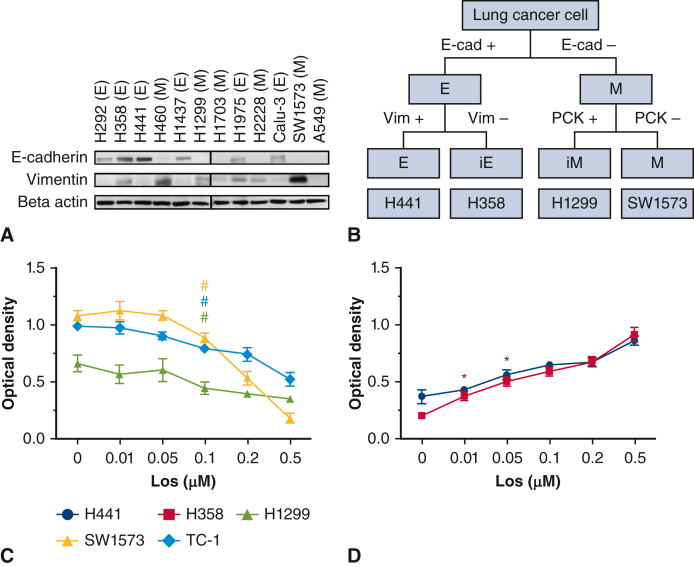
To create a library of cancer cell types representative of the EMT spectrum, we divided 12 candidate human lung cancer cell lines into 4 types based on surface expression of EMT markers: epithelial (E), intermediate E (iE), intermediate M (iM), and mesenchymal (M).20 The lung cancer cell lines H292, H358 (human squamous cell), H441, H460, H1437, H1299, H1703, H1975, H2228, Calu-3, SW1573, and A549, which are commonly used in lung cancer research, were initially classified by epithelial (E-cadherin+) and mesenchymal (vimentin+) phenotype. In both E-cadherin+ and vimentin+ cell lines, pan-cytokeratin expression was also assayed. The lung cancer cell lines were further divided into E, iE, iM, and M subtypes (Figure 1, A and B, and Figure E1, A).20 A representative lung cancer cell line was selected for each EMT classification for subsequent study (Figure 1, C and D): E, H441; iE, H358; iM, H1299; and M, SW1573. Human cell lines were originally obtained from the American Type Tissue Culture Collection in 2016. The mouse-derived lung cancer cell line, TC-1 (received as a gift from Sunil Singhal, MD, University of Pennsylvania) was selected for murine studies.21 All cell lines were cultured in a 37 °C cell culture incubator with RIPA-1640 medium, containing 10% fetal bovine serum and 1% penicillin, as previously described.22,23 All cell lines were tested for mycoplasma.
Figure 1.
A, Cell lines were characterized by expression levels of E-cadherin and vimentin. H441 and H358 expressed an epithelial phenotype–high E-cadherin and low vimentin. Lines H1299 and SW1573 expressed a mesenchymal phenotype–low E-cadherin and high vimentin. B, The lung cancer cell lines were separated into 4 subtypes: epithelial (E) (H441), intermediate epithelial (iE) (H358), intermediate mesenchymal (iM) (H1299), and mesenchymal (M) (SW1573). TC-1 was selected as a syngeneic cell line to balance differences of lung cancer cell phenotypes. Cell lines were exposed to losartan and significant decreases in vimentin (C) and E-cadherin (D) were observed (P < .05).
Figure E1.
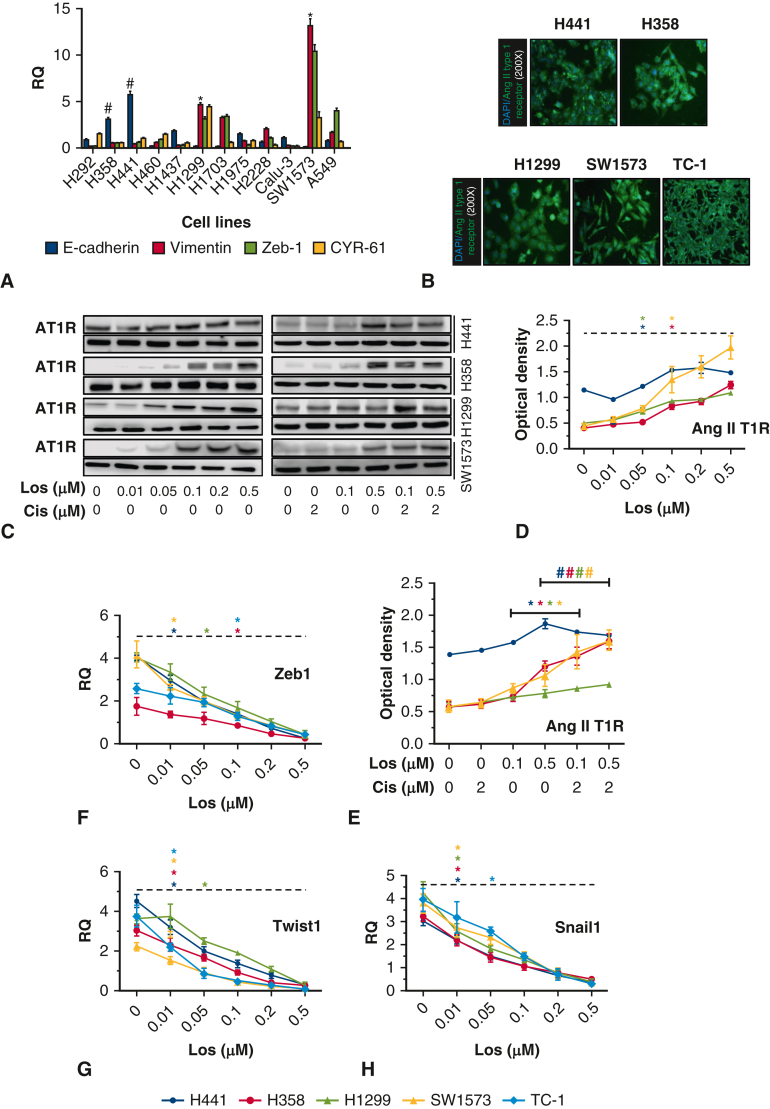
A, We assessed epithelial-mesenchymal transformation (EMT) makers (E-cadherin, vimentin, Zeb 1, and CYR-61) and identified primarily epithelial (H441, H358) and mesenchymal cell lines (H1299, SW1573). B, Angiotensin II type 1 receptor (AT1R) expression in all 5 lung cancer cell lines (magnification is 200 × ). C through E, AT1R expression in all 5 lung cancer cell lines treated with losartan alone or losartan combined with cisplatin. D, H441, ∗P = .001; H358, ∗P = .000; H1299, ∗P = .001; SW1573, ∗P = .003. E, H441, ∗P = .022, #P = .000; H358, ∗P = .000, #P = .013; H1299, ∗P = .043, #P = .029; SW1573, ∗P = .025, #P = .028. F, Zeb 1 expression with increasing losartan (H441, ∗P = .022; H358, ∗P = .014; H1299, ∗P = .001; SW1573, ∗P = .003; TC-1, ∗P = .001). G, Twist 1 expression with increasing losartan (H441, ∗P = .002; H358, ∗P = .024; H1299, ∗P = .020; SW1573, ∗P = .012; TC-1, ∗P = .001). H, Snail 1 expression with increasing losartan (H441, ∗P = .003; H358, ∗P = .001; H1299, ∗P = .001; SW1573, ∗P = .028; TC-1, ∗P = .024).
Before proceeding with AT1R inhibition experiments, all 5 cancer cell lines were found to have constitutive expression of AT1R (Figure E1, B). The cell lines used in this study were exquisitely sensitive to cisplatin, so we chose a <60% lethal dose to proceed with subsequent in vitro and in vivo experimentation (Figure 2, C).
Figure 2.
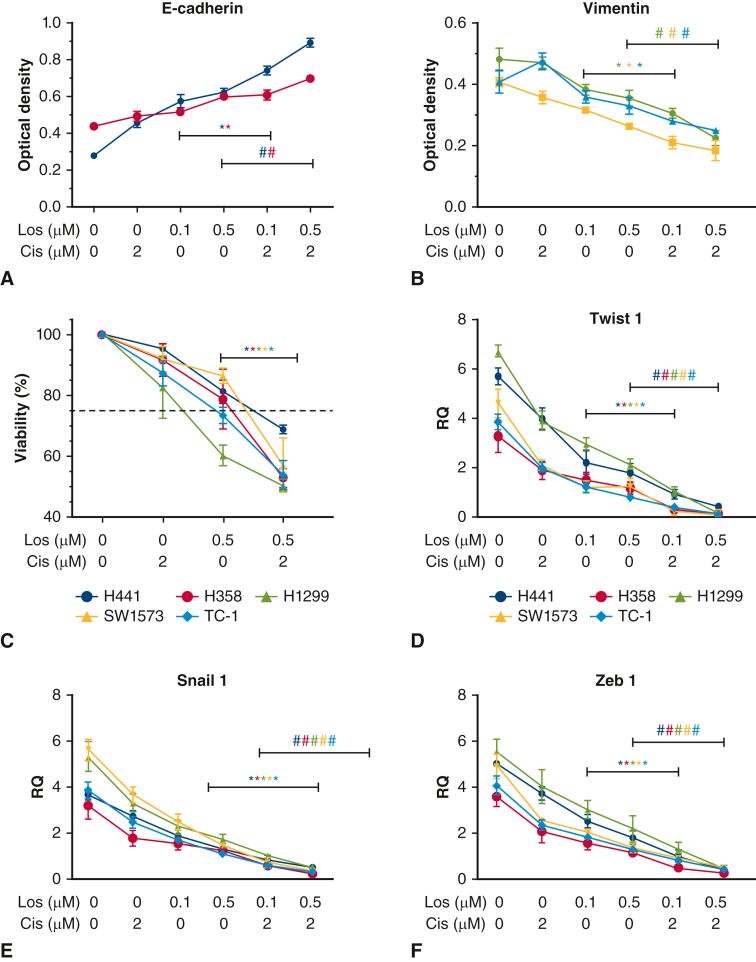
A, E-cadherin incrementally increased with cisplatin treatment, demonstrated dose-dependent increase with losartan, and significantly increased with combination losartan and cisplatin treatment over phosphate buffered saline (PBS) or single-drug treatment (P < .05). B, Vimentin decreased with cisplatin and showed a dose-dependent decrease with losartan. Combination losartan and cisplatin significantly reduced vimentin expression over PBS or single-drug treatment (P < .05). C, Cell viability was reduced with cisplatin and losartan individually, and combination treatment significantly reduced cell viability below single-drug treatment (P < .05). D through F, Epithelial-mesenchymal transition (EMT) mediators Twist 1, Snail 1, and Zeb 1 were significantly reduced with cisplatin and losartan monotreatment and demonstrated significant reduction with combination treatment below the single-agent treatment result (P < .05).
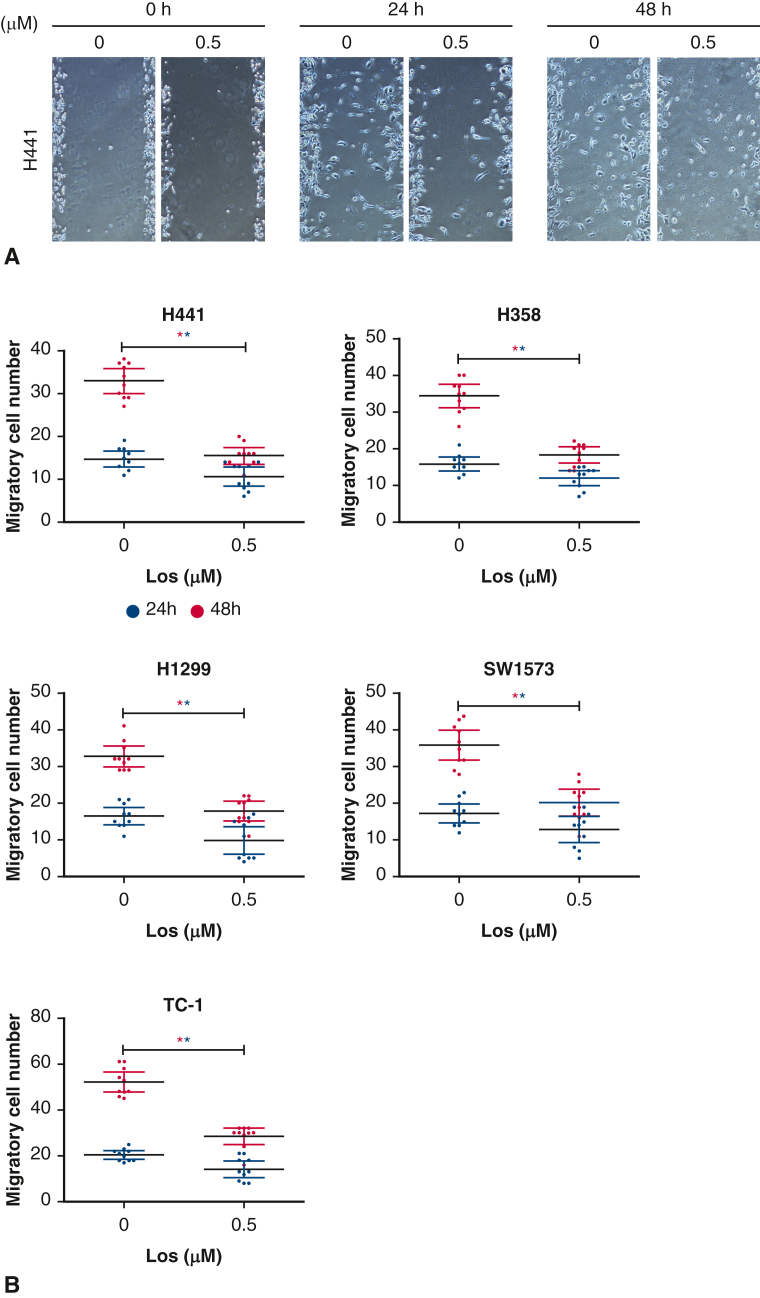
To test cell migration as a proxy for metastatic potential, a scratch assay was performed as previously described24; briefly, cell lines were seeded into 6-well plates (2 × 106 cells/mL) after being serum starved for 12 hours. Five scratch lines per well were created before application of either phosphate buffered saline (PBS) control or losartan 0.5 μM. Wells were photographed at 0, 24, and 48 hours after seeding. The number of migratory cells was quantified and compared among groups (Figure 3, A and B).
Figure 3.
Representative image of cell migration assessed via scratch assay in the presence of losartan for each cell line (A), quantification of migratory cell number (B). At 24 hours, there was no significant difference in migratory cell number between losartan treated and control cells. At 48 hours, losartan treatment had significantly reduced the migratory cell number for each cell type (P < .05).
Cell proliferation assay, immunofluorescence, immunohistochemistry, Western blotting, reverse transcription qualitative polymerase chain reaction, and screening for AT1R expression are described in Appendix E1.
In Vivo Studies
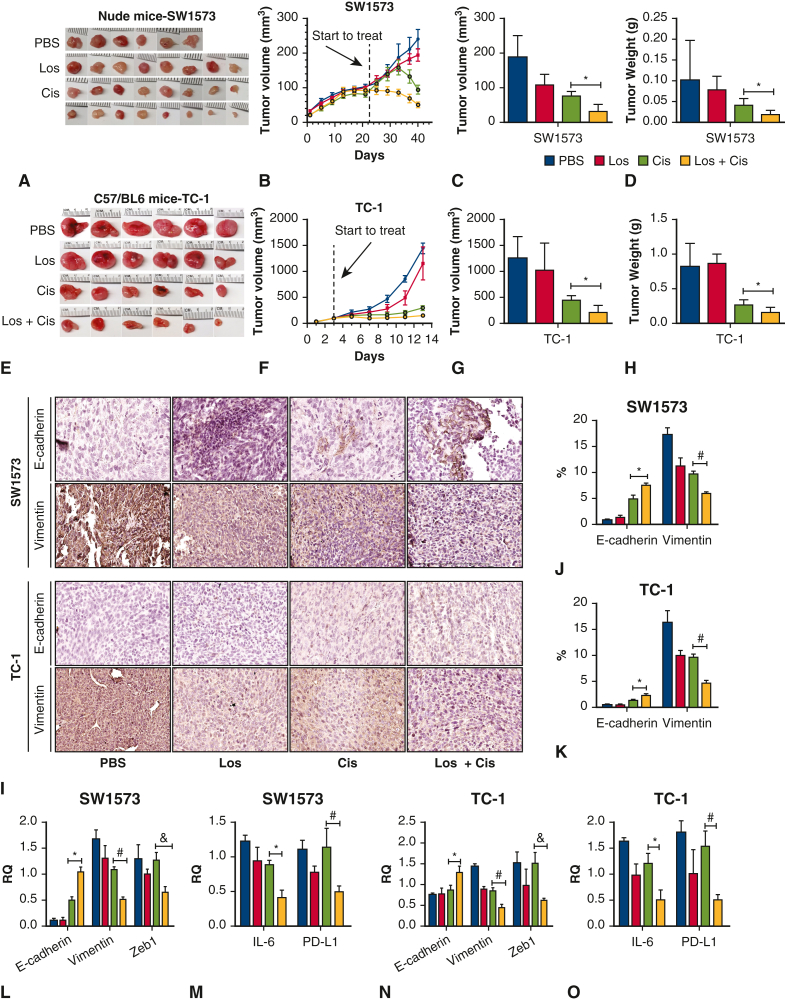
All animal experiments were performed in compliance with Institutional Animal Care and Use Committee--approved protocols at Massachusetts General Hospital (protocol No. 2010N000005; November 11, 2018). Nude mice and C57BL/6 (male, aged 8-12 weeks) (25 g), were purchased from the Jackson Laboratory and kept in a pathogen-free facility. Mice were anesthetized (ketamine, AH01VGG; Henry Schein Putney, Xylazine, 061716A; AKORN Animal Health) and injected with 100 μL (5 million cells) SW1573 (nude mice) or TC-1 (C57BL/6) cell line preparation (mixed with Matrigel in a ratio of 1:1) on both flanks. Mouse body weight and tumor volume changes were recorded every 3 days. After 4 weeks, mice were randomly divided into 4 treatment groups: PBS control (100 μL via daily gavage), losartan (30 mg/kg/d via daily gavage), cisplatin (5 mg/kg in 125 μL volume via intraperitoneal injection every 3 days for 5 total doses), or losartan plus cisplatin. Flank tumors were harvested, and tumor weight and volume were obtained (Figure 4). The tumors were then divided in half, with one-half fixed in formalin for histology and the other half flash frozen in liquid nitrogen and then stored at −80 °C for biochemical analysis.
Figure 4.
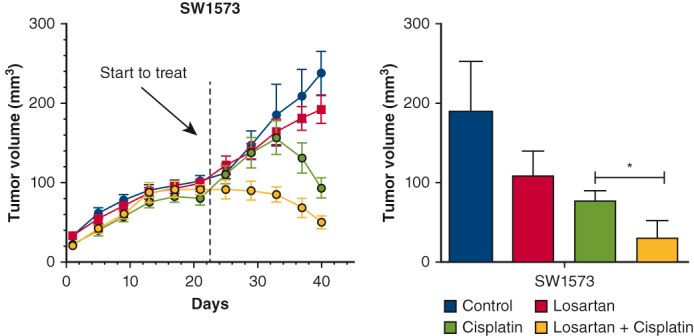
A through D, Allogeneic flank tumor model: Human SW1573 cells were injected into the flanks of nude mice on the day indicated by the red arrow. E through H, Syngeneic flank tumor model: Murine TC-1 cells were injected into the flank of C57BL/6 mice. In both flank tumor models, losartan did not influence tumor volume or weight. Cisplatin significantly reduced tumor weight and volume in both models.∗ Combination treatment with losartan and cisplatin significantly reduced tumor size and volume below that of phosphate buffered saline control, losartan alone, or cisplatin alone.∗I through K, Immunohistochemical quantification of epithelial-mesenchymal transition markers E-cadherin and vimentin in allogeneic and syngeneic flank tumor models. Losartan and cisplatin each increase E-cadherin separately, and the combination of losartan and cisplatin is associated with an increase in E-cadherin over individual treatment.∗ Vimentin is reduced by losartan and cisplatin individually, and combination treatment reduced vimentin below either individual treatment.∗L and N, E-cadherin and vimentin expression changes confirmed via polymerase chain reaction test. L through O, Zeb 1, programmed death-ligand (PDL-1), and interleukin 6 (IL-6) expression was unchanged by losartan or cisplatin alone but were significantly reduced with combination treatment.∗ ∗P < .05.
Drug Dosing
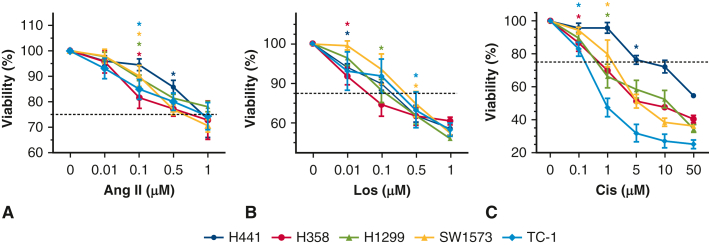
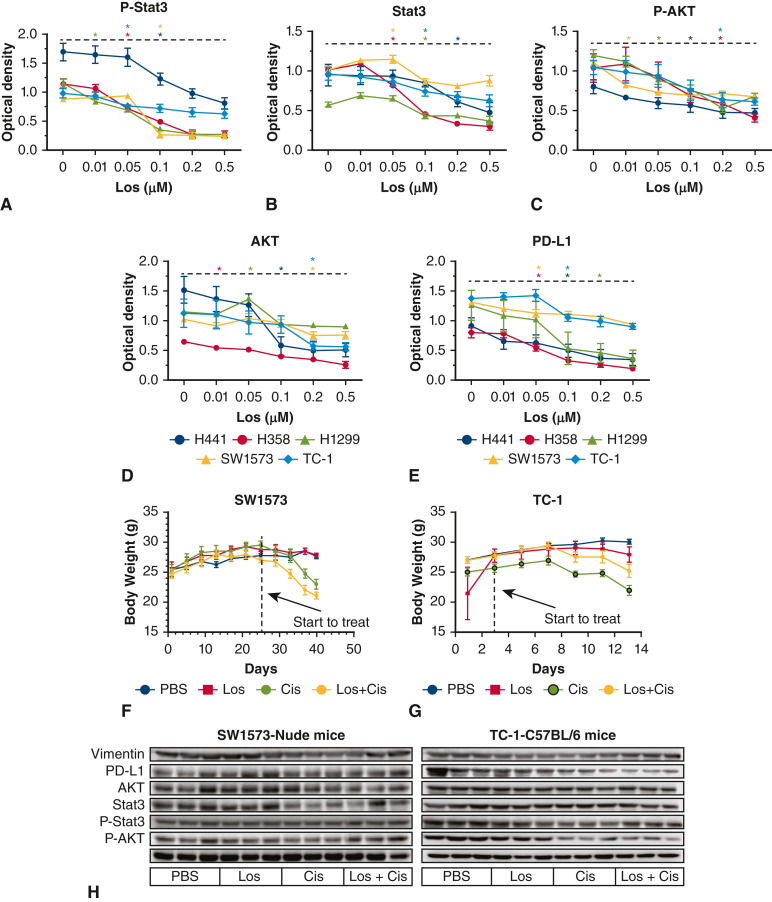
In vitro losartan dosing was determined by dose ranging study (Figure E2, B). The maximum dose of losartan that produced ≥80% cell viability was determined to be 0.1 μM, which was then used for in vitro experiments. An angiotensin II dose-ranging study was also performed in a similar manner (Figure E2, A). Both losartan and angiotensin II dosing was consistent with previous studies.25 In vivo dosing was based on previous work.26 In human beings, a chronic dose of 100 mg losartan daily produced trough serum drug levels of 0.82 μmol/L and peak levels of 1.88 μmol/L—a concentration range that is similar to the exposure dosing in this study.27 Cisplatin dose ranging was performed (Figure E2, C) and 2 μM was the maximum dose that produced >60% cell viability was used for in vitro experiments. In vivo dosing of cisplatin (5 mg/kg in 125 μL volume via intraperitoneal injection every 3 days for 5 total doses) was based on previous studies.28
Figure E2.
Dose ranging studies: The 5 lung cancer cell lines were treated with increasing doses of angiotensin II (A) and losartan (B) with cell viability measured. The first statistically significant point on the cell survival curve was shown by ∗. H441 ∗P = .014; H358 ∗P = .012; H1299 ∗P = .000; SW1573 ∗P = .019; TC-1 ∗P = .012. (H) H441 ∗P = .018; H358 ∗P = .010; H1299 ∗P = .000; SW1573 ∗P = .000; TC-1 ∗P = .005. C, Cell viability with increasing doses of cisplatin. The first statistically significant point on the cell survival curve was shown by ∗. H441, ∗P = .000; H358, ∗P = .002; H1299, ∗P = .000; SW1573, ∗P = .003; TC-1, ∗P = .008. Differences observed in the cell viability following cisplatin treatment between Figure E2, C, and Figure 2, C, were likely due to normal experimental variation because these experiments were conducted at different times.
Statistical Analysis
For in vitro and animal in vivo experiments, 1-way analysis of variance was performed to evaluate intergroup differences.
Results
Losartan-Attenuated EMT in Lung Cancer Cell Lines
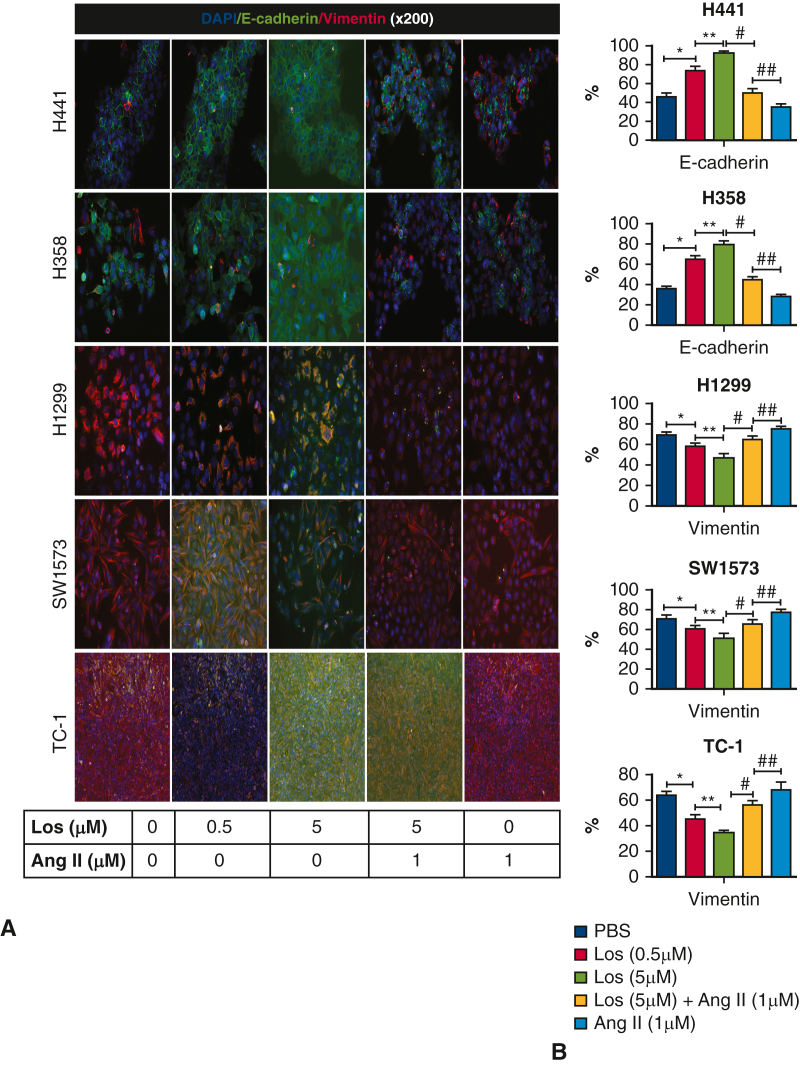
Following establishment of the cell library, we sought to test whether or not previously reported effects of RAAS signaling on EMT also occurred in lung cancer. We tested the effect of losartan on EMT markers in lung cancer cell lines. Losartan exposure resulted in a dose-dependent reduction in vimentin in mesenchymal cell lines, with a significant reduction in vimentin detected at the 0.1 μM dose (P < .05) (Figure 1, C). Losartan exposure resulted in a dose-dependent increase in E-cadherin in epithelial cell lines with a significant increase in E-cadherin detected at the 0.01 μM dose with H358 and the 0.05 μM dose for H441 (P < .05) (Figure 1, D). These findings were confirmed with immunofluorescence (Figure 5), demonstrating a dose-dependent reduction in vimentin in mesenchymal cell lines. An increase in E-cadherin in epithelial lines was also observed. Co-exposure with losartan and angiotensin II negated this effect and returned levels of vimentin and E-cadherin to baseline levels. The effect of losartan treatment on cell motility was assessed via scratch assay (Figure 3). The migratory cell number was not significantly changed by losartan treatment at 24 hours, but at 48 hours losartan treatment significantly reduced migratory cell number in all cell lines. In total, these findings suggest that the effect of losartan on EMT previously observed with other cancer types also occurs in lung cancer.
Figure 5.
A, Representative images of immunofluorescence staining for E-cadherin and vimentin in each cell line. Cells were treated with losartan and angiotensin II (200×). B, Quantification of staining with image analysis. Losartan treatment significantly increased E-cadherin or reduced vimentin in each cell line (P < .05). Treatment with combination losartan and angiotensin II returned vimentin and E-cadherin levels to baseline.
Combined Treatment With Losartan and Cisplatin Attenuated EMT Response
To model the interaction between ASI and platinum-based chemotherapy observed on outcomes in patients with lung cancer, we exposed lung cancer cell lines to either losartan, cisplatin, or a combination of the 2. In epithelial cells exposed to combination treatment, an increase in E-cadherin was observed with cisplatin and with losartan; however, combination treatment resulted in a significant increase in E-cadherin over that of losartan or cisplatin treatment alone (P < .05) (Figure 2, A). In mesenchymal cell lines vimentin was reduced by cisplatin and losartan, and combination treatment resulted in a significant decrease in vimentin over losartan or cisplatin treatment alone (P < .05) (Figure 2, B). The antitumor effect of combined losartan and cisplatin treatment was assessed and resulted in significantly reduced cancer cell viability than treatment with either losartan or cisplatin alone (P < .05) (Figure 2, C).
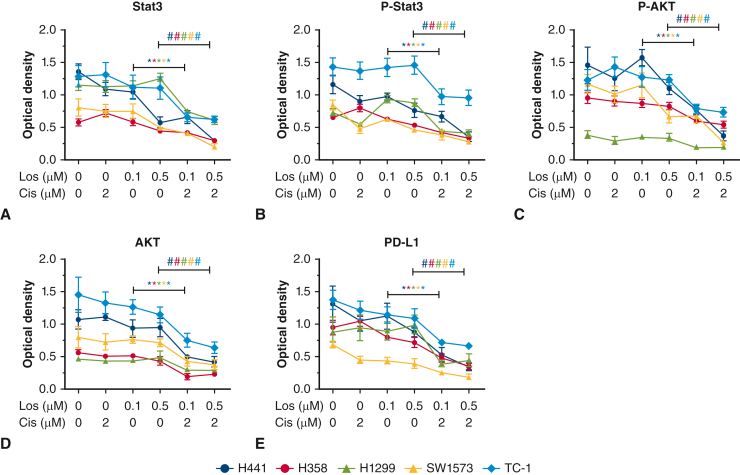
The effect of combined losartan and cisplatin treatment on established EMT mediators Zeb1 (Figure 3, C), Twist 1(Figure 3, D), and Snail 1 (Figure 3, E) was then evaluated. Treatment of either losartan or cisplatin reduced expression of all 3 mediators. Combined treatment with losartan and cisplatin reduced expression levels of all 3 mediators below that of cells treated with losartan or cisplatin alone, irrespective of cell line phenotype. AKT and Stat3 are signaling mediators with important cell cycle functions that can drive tumor replication and inhibit apoptosis. Both AKT and Stat3 levels were not significantly changed by either losartan or cisplatin alone; however, combination treatment resulted in significant reduction of AKT and Stat3 and their phosphorylated states (P < .05) (Figure 6). The effect of combination therapy resulted in almost complete suppression of these mediators and may point to a mechanism for the effects of losartan and cisplatin on EMT.
Figure 6.
A through D, Expression of proliferation markers AKT and Stat3 and their phosphorylated molecules in all 5 lung cancer cell lines treated with losartan, cisplatin, or combined losartan plus cisplatin. No treatment effect on AKT, AKT-p, Stat3, or Stat3-p levels were observed with single agent cisplatin or losartan. Combination losartan and cisplatin significantly reduced AKT, AKT-p, Stat3, and Stat3-p in all cell lines (∗P < .05). Programmed death-ligand (E) was not significantly influenced by either losartan or cisplatin, but combination treatment significantly reduced programmed death-ligand (PDL-1) in all cell lines (P < .05).
Treatment With Losartan and Cisplatin Reduced Tumor Size and Attenuated EMT
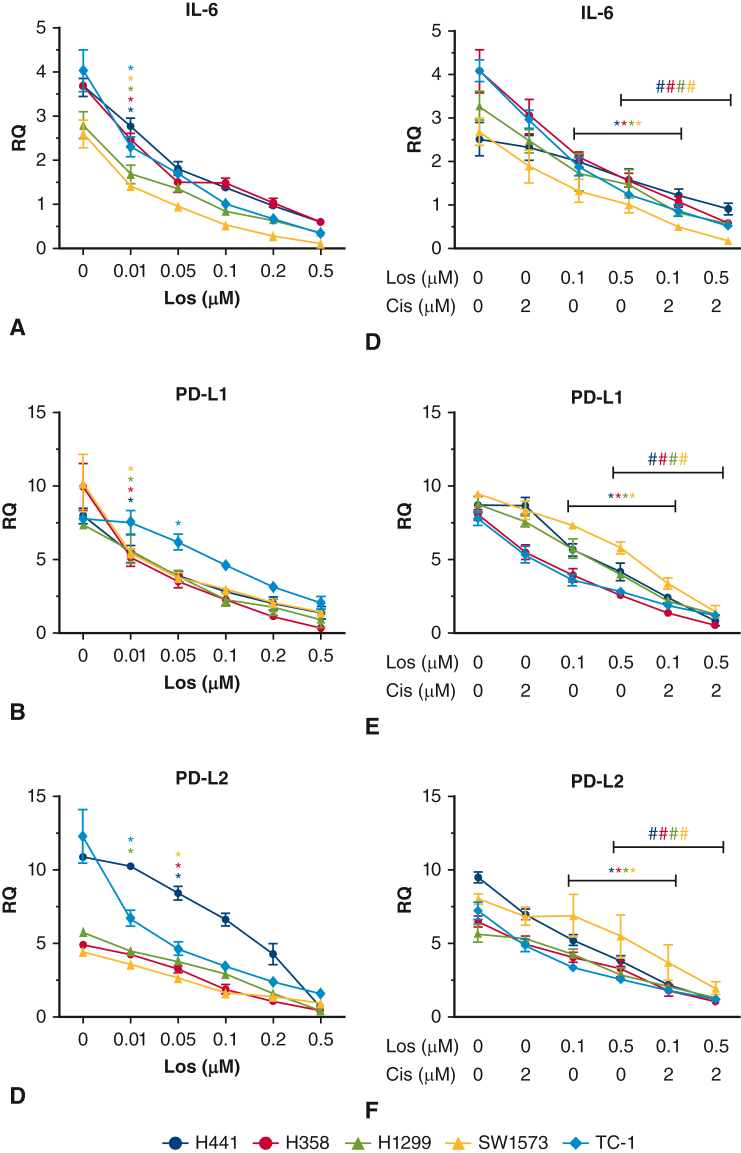
To test the effects of combined losartan and cisplatin treatment on a human lung cancer cell line in vivo, we utilized an allogenic tumor model. Nude mice received bilateral subcutaneous flank injections of SW1573 (100 μL, 5 million cells [n = 4]) (Figure 4, A). Combination losartan and cisplatin treatment reduced tumor volume and tumor weight significantly compared with control or single-drug therapy (Figure 4, A-D). EMT markers were assessed via immunohistochemistry and polymerase chain reaction (Figure 4, I-L) and demonstrated a significant elevation of E-cadherin and decrease in vimentin with combined treatment versus PBS control and single-drug therapy (P < .05), demonstrating the transformation of the mesenchymal tumor phenotype typically expressed by SW1573 into a more epithelial phenotype. Zinc finger E-box binding homeobox 1 (ZEB1), interleukin 6, and programmed death-ligand were all significantly reduced only with combination treatment (P < .05) (Figure 4, L and M). This relationship can also be seen across all cell lines in Figure E4. We also tested the interaction between losartan and cisplatin in a syngeneic flank model in C57BL/6 with TC-1 (Figure 4, E-H, K, N, and O, and Figure E3, G), and the results were consistent with the observations made with the allogenic model above.
Figure E4.
A, Interleukin 6 (IL-6) expression with increasing losartan (H441, ∗P = .001; H358, ∗P = .000; H1299, ∗P = .000; SW1573, ∗P = .000; TC-1, ∗P = .000). B, Program death ligand 1 (PD-L1) expression with increasing losartan (H441, ∗P = .000; H358, ∗P = .000; H1299, ∗P = .043; SW1573, ∗P = .000; TC-1, ∗P = .020). C, Program death ligand 2 (PD-L2) expression with increasing losartan (H441, ∗P = .012; H358, ∗P = .000; H1299, ∗P = .001; SW1573, ∗P = .002; TC-1, ∗P = .000). D, IL-6 expression with increasing losartan and losartan combined cisplatin (H441, ∗P = .032, #P = .0.044; H358, ∗P = .021, #P = .025; H1299, ∗P = .042, #P = .042; SW1573, ∗P = .033, #P = .036; TC-1, ∗P = .001, #P = .013). E, PD-L1 expression with increasing losartan and combined cisplatin (H441, ∗P = .000, #P = .000; H358, ∗P = .000, #P = .023; H1299, ∗P = .000, #P = .000; SW1573, ∗P = .000, #P = .000; TC-1, ∗P = .005, #P = .007). F, PD-L2 expression with increasing losartan and combined with cisplatin (H441, ∗P = .000, #P = .000; H358, ∗P = .002, #P = .002; H1299, ∗P = .001, #P = .008; SW1573, ∗P = .046, #P = .033; TC-1, ∗P = .004, #P = .011).
Figure E3.
A, P-stat3 expression with increasing losartan (H441, ∗P = .024; H358, ∗P = .000; H1299, ∗P = .000; SW1573, ∗P = .000; TC-1, ∗P = .032). B, Stat3 expression with increasing losartan (H441, ∗P = .030; H358, ∗P = .032; H1299, ∗P = .015; SW1573, ∗P = .040; TC-1, ∗P = .036). C, P-protein kinase B (AKT) expression with increasing losartan (H441, ∗P = .033; H358, ∗P = .047; H1299, ∗P = .002; SW1573, ∗P = .000; TC-1, ∗P = .024). D, AKT expression with increasing losartan (H441, ∗P = .003; H358, ∗P = .032; H1299, ∗P = .000; SW1573, ∗P = .005; TC-1, ∗P = .043). E, program death ligand 1 (PD-L1) expression with increasing losartan (H441, ∗P = .034; H358, ∗P = .005; H1299, ∗P = .038; SW1573, ∗P = .005; TC-1, ∗P = .005). F, Body weight of nude mice with SW1573. G, Body weight of C57BL/6 mice with TC-1. H, Vimentin, P-stat3, Stat3, P-AKT, AKT, and PD-L1 expression in mice with lung cancer.
Discussion
In this study, we created a library of cancer cell lines based on their epithelial versus mesenchymal characteristics and quantified the effects of losartan treatment on these cell lines. We identified a synergistic effect of combined losartan and cisplatin exposure on EMT phenotype, which was associated with enhanced cytotoxicity. The synergistic effect of co-treatment with losartan and cisplatin was evaluated in vivo with both allogenic and syngeneic models with significant tumor volume reduction.
This study reports the novel finding that combination therapy with losartan and cisplatin results in enhanced tumor toxicity—a finding that was confirmed in both in vitro and in vivo models. This finding is consistent with observational studies in human beings reporting increased response to chemotherapy with ASI in lung cancer.3 In these studies, cisplatin was a consistent element of therapy. Subgroup analysis of bevacizumab, a vascular endothelial growth factor inhibitor, did not show an interaction with concurrent ASI therapy.6 Although testing the interaction between additional classes of chemotherapeutic agents was beyond the scope of this study, establishing an explicit link between cisplatin and ASI offers an exciting new direction in the investigation of the antitumor effects of ASI therapy. The 40% increase in survival reported by observational studies evaluating ASI and therapeutic regimens including cisplatin suggest that patients could realize significant benefit if this synergistic effect can be harnessed and implemented.3
Classically, loss of an epithelial cellular phenotype and adoption of an mesenchymal phenotype has been associated with enhanced tumorigenesis. We utilized a set of cancer cell types across the EMT spectrum and confirmed that ASI treatment drives cells toward the E phenotype with increased E-cadherin and decreased vimentin. EMT has previously been shown to be induced by RAAS signaling in a model of colorectal cancer,19 where ASI had similar effects—increasing E-cadherin and reducing vimentin. No studies have previously explored the relationship between EMT and ASI in lung cancer.
Surprisingly, cells exposed to both losartan and cisplatin demonstrated an exaggerated shift toward an E phenotype with mediators of EMT (Twist 1, Snail 1, and Zeb 1) all highly suppressed. This finding was observed both in vitro and in vivo models. Previous consideration of the role of RAAS in potentiating the effect of chemotherapy was centered around RAAS effects on the tumor microenvironment—altering blood flow, potentiating local immune function, or altering local fibrosis. Although the antitumor effects of ASI likely involve these mechanisms, the current findings suggest that there may also be an intracellular interaction between losartan and cisplatin that is capable of influencing the phenotype of cancer cell and directly altering the cytotoxic effects of cisplatin.
This study has several important limitations. This study used representative cell lines across the EMT spectrum, and these select cell lines may not represent the average lung cancer. Although it appears that increased losartan doses correspond with enhanced epithelialization of tumor phenotype, the losartan dosing was not fully optimized and limited by toxicity in the in vivo experiments. Additional studies are needed to find the optimal dosing regimen to balance drug toxicity with antitumor effects. In this study, work was done in cell culture and murine models. The effects of ASI on the tumor microenvironment are complex, necessitating confirmation with in vivo human studies. Identifying the specific mechanism by which losartan and cisplatin interact to enhance cytotoxicity is beyond the scope of this article. Additional studies are required to further explore this finding. Finally, whereas combination therapy was seen to have effects that were beyond individual therapy conditions, more work will be needed to determine whether or not the observed effect is synergistic or additive.
Conclusions
We confirm the dose-dependent cytotoxic effect of losartan in lung cancer models and report a novel synergistic effect of combination losartan and cisplatin treatment that appears to be due to modulation of the EMT phenotype by losartan. More research is needed to elucidate the mechanism of this drug interaction and to further explore its application in clinical medicine.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/angiotensin-system-inhibitors-are-associated-with-improved-survival-in-locally-advanced-nsclc-and-exhibit-anti-tumor-synergism-with-chemotherapy-in-a-murine-model-of-nsclc.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Supported by Thoracic Surgery Departmental Funding.
Drs Tang and Abston contributed equally to this article.
Appendix E1. Supplementary Methods
Cell Proliferation Assay
Cell proliferation assay was performed as previously described. Briefly, H441, H358, H1299, SW1573, TC-1 cell lines were maintained as above. Cells were seeded onto 24-well plates and were cultured to an exponential growth level prepared about 2 × 104 cells/mL, 0.5 mL per well, each group repeated with 4 wells. Cells underwent serum starvation for 12 hours, and then wells were assigned to receive 48 hours of treatment in 1 of the following groups: phosphate buffered saline (PBS) control; angiotensin II (A9525; Sigma) at 0.01, 0.1, 0.5, and 1 μM doses; losartan (180220; American Health) at 0.01, 0.1, 0.5, and 1 μM doses; cisplatin (NDC 16729-288-38; Accord Healthcare) at 0.1, 1, 5, 10, and 50 μM doses28; and cisplatin 2 μM combined with losartan 0.5 μM.
After 48 hours of treatment, the culture medium was aspirated, and 0.5 mL MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) (C18H16BrN5S [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] (298-93-1; Sigma) was added to each well. After 3 hours, the supernatant was aspirated and discarded. After adding 200 μL dimethyl sulfoxide, the solution was thoroughly shaken and dissolved. 100 μL of each well was added to 96-well plates, and then analyzed in a spectrophotometer at 540 nm (Emax Precision Microplate Reader; Molecular Devices).
Immunofluorescence
H441, H358, H1299, SW1573, and TC-1 cell lines were seeded into 24-well plates at 6 × 104 cells/mL. Cells underwent serum starvation for 12 hours, and then wells were assigned to receive 48 hours of treatment in 1 of the following groups: PBS control, losartan 0.5 μM, losartan 5 μM, angriotensin II (Ang II), or losartan 5 μM + Ang II 1 μM. Next, primary antibody was applied (E-cadherin, 1:400, ab76055; Abcam, vimentin 1:400, 5741S; Cell Signaling Technology), followed by second antibody (Cy3-AffiniPure Donkey Anti-Mouse IgG, 1:500, 715-165-150; Jackson ImmunoResearch; Donkey Anti-Rabbit IgG, 1:1000, ab150073; Abcam), and DAPI (1:500, ab104139; Abcam).
Immunohistochemistry
Human lung adenocarcinoma cancer tissue and healthy lung tissue sections were obtained as above. Tissue was processed as previously described. The primary antibody (E-cadherin, 1:300, 3195S; Cell Signaling Technology, vimentin, 1:300, 5741S; Cell Signaling Technology, and angiotensin II type 1 receptor, 1:200, ab124505; Abcam) was added dropwise. The secondary antibody (2668240; M IHC Select) was then added per protocol. The above-mentioned steps were also performed on mouse tumor tissue.
Western Blotting
H441, H358, H1299, SW1573, and TC-1 cell lines were seeded into 6-well plates at 2 × 105 cells/mL. Cells underwent serum starvation for 12 hours, and then wells were assigned to receive 48 hours of treatment in 1 of the following groups: PBS control; losartan 0.01, 0.05, 0.1, 0.2, or 0.5 μM; cisplatin 2 μM and losartan 0.1 μM; or cisplatin 2 μM and losartan 0.5 μM. The primary antibodies (E-cadherin, 1:1000, 3195S; Cell Signaling Technology, vimentin, 1:1000, 5741S; Cell Signaling Technology, Stat3, 1:1000, 9132; Cell Signaling Technology, AKT, 1:1000, 9272; Cell Signaling Technology, P-Stat3 1:1000, 9145, Cell Signaling Technology, P-AKT 1:1000, 13038; Cell Signaling Technology, angiotensin II type 1 receptor, 1:1500, ab124505; Abcam, program death ligand 1 (PD-L1), 1:1000, ab233482; Abcam) and secondary anti-rabbit antibodies (7074s; Cell Signaling Technology) were applied.
Reverse Transcription Qualitative Polymerase Chain Reaction
H441, H358, H1299, SW1573, and TC-1 cell lines were prepared using the same treatment groups as described above in the Western blot section. RNA was extracted, quantification and copy-DNA prepared via standard protocol. All primers (18S Hs03003631_g1, IL-6 Hs00174131_m1, PD-L1 Hs00204257_m1, PD-L2 Hs00228839_m1, Zeb1 Hs01566408_m1, Twist1 Hs01675818_s1, and Snail1 Hs00195591_m1) were purchased from Thermo Fisher, and processed per the company's official website instructions.
Animal Experiments
Twenty-three male nude mice aged 10 weeks (25 g) were purchased from the Jackson Laboratory and kept in a pathogen-free facility in accordance with an approved animal Institutional Animal Care and Use Committee (protocol No. 2010N000005). All mice were housed at the Animal Laboratory at the Massachusetts General Hospital. Mice were anesthetized (ketamine, AH01VGG; Henry Schein Putney, Xylazine, 061716A; AKORN Animal Health) and injected with 100 μL (5 million cells) of SW1573 cell line preparation (mixed with Matrigel in a ratio of 1:1) on both flanks. Mouse body weight and tumor volume changes were recorded every 3 days. After 4 weeks, mice were randomly divided into 4 treatment groups: PBS control (100 μL via daily gavage), losartan (30 mg/kg/d via daily gavage), cisplatin (125 μL via intraperitoneal injection every 3 days for 5 total doses), and losartan plus cisplatin. The above procedure was repeated in C57BL/6 (male, age 8-12 weeks, 25 g, Jackson Laboratory) using TC-1 cells. Treatment was initiated 3 days after TC-1 cell injection. Following 2 weeks of treatment, the mice were killed via cervical dislocation. Flank tumors were harvested and tumor weight and volume were obtained. The tumors were then divided in half, with one-half fixed in formalin for histology and the other half flash frozen in liquid nitrogen and then stored at −80 °C for biochemical analysis.
References
- 1.Batais M., Almigbal T., Alotaibi K., et al. Angiotensin converting enzyme inhibitors and risk of lung cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2021;100(17) doi: 10.1097/MD.0000000000025714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachow T., Schiffl H., Lang S.M. Risk of lung cancer and renin-angiotensin blockade: a concise review. J Cancer Res Clin Oncol. 2021;147(1):195–204. doi: 10.1007/s00432-020-03445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z., Wei L., Yin C., Li W., Wan B. Angiotensin receptor blocker associated with a decreased risk of lung cancer: an updated meta-analysis. J Pers Med. 2023;13(2):243. doi: 10.3390/jpm13020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., Yao T., Wang Z., et al. Association between angiotensin-converting enzyme inhibitors and the risk of lung cancer: a systematic review and meta-analysis. Br J Cancer. 2023;128(2):168–176. doi: 10.1038/s41416-022-02029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aydiner A., Ciftci R., Sen F. Renin-angiotensin system blockers may prolong survival of metastatic non–small cell lung cancer patients receiving erlotinib. Medicine (Baltimore) 2015;94(22) doi: 10.1097/MD.0000000000000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menter A.R., Carroll N.M., Sakoda L.C., et al. Effect of angiotensin system inhibitors on survival in patients receiving chemotherapy for advanced non-small-cell lung cancer. Clin Lung Cancer. 2017;18(2):189–197.e3. doi: 10.1016/j.cllc.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao L., Chen W., Zhou L., Wan H., Gao B., Feng Y. Impact of angiotensin i-converting enzyme inhibitors and angiotensin II type-1 receptor blockers on survival of patients with NSCLC. Sci Rep. 2016;6 doi: 10.1038/srep21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J., Zhou Z., Xu Z., et al. Retrospective clinical study of renin-angiotensin system blockers in lung cancer patients with hypertension. PeerJ. 2019;7 doi: 10.7717/peerj.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilop S., von Hobe S., Crysandt M., Esser A., Osieka R., Jost E. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non–small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009;135(10):1429–1435. doi: 10.1007/s00432-009-0587-3. [DOI] [PubMed] [Google Scholar]
- 10.Pinter M., Jain R.K. Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci Transl Med. 2017;9(410) doi: 10.1126/scitranslmed.aan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George A.J., Thomas W.G., Hannan R.D. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10(11):745–759. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
- 12.Hassani B., Attar Z., Firouzabadi N. The renin-angiotensin-aldosterone system (RAAS) signaling pathways and cancer: foes versus allies. Cancer Cell Int. 2023;23(1):254. doi: 10.1186/s12935-023-03080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diop-Frimpong B., Chauhan V.P., Krane S., Boucher Y., Jain R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108(7):2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita M., Hayashi I., Yamashina S., Fukamizu A., Itoman M., Majima M. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26(2):271–279. doi: 10.1093/carcin/bgh324. [DOI] [PubMed] [Google Scholar]
- 15.Terry S., Savagner P., Ortiz-Cuaran S., et al. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11(7):824–846. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcucci F., Stassi G., De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016;15(5):311–325. doi: 10.1038/nrd.2015.13. [DOI] [PubMed] [Google Scholar]
- 17.Kajiyama H., Shibata K., Terauchi M., et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31(2):277–283. [PubMed] [Google Scholar]
- 18.Qian Y.R., Guo Y., Wan H.Y., et al. Angiotensin-converting enzyme 2 attenuates the metastasis of non–small cell lung cancer through inhibition of epithelial-mesenchymal transition. Oncol Rep. 2013;29(6):2408–2414. doi: 10.3892/or.2013.2370. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen L., Ager E.I., Neo J., Christophi C. Regulation of colorectal cancer cell epithelial to mesenchymal transition by the renin angiotensin system. J Gastroenterol Hepatol. 2016;31(10):1773–1782. doi: 10.1111/jgh.13307. [DOI] [PubMed] [Google Scholar]
- 20.Huang R.Y., Wong M.K., Tan T.Z., et al. An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530) Cell Death Dis. 2013;4(11):e915. doi: 10.1038/cddis.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khosravi A.R., Alheidary S., Nikaein D., Asghari N. Aspergillus fumigatus conidia stimulate lung epithelial cells (TC-1 JHU-1) to produce IL-12, IFNgamma, IL-13 and IL-17 cytokines: modulatory effect of propolis extract. J Mycol Med. 2018;28(4):594–598. doi: 10.1016/j.mycmed.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Tang H., Bai Y., Pan G., et al. Interleukin-6 and insulin-like growth factor-1 synergistically promote the progression of NSCLC. Autoimmunity. 2018;51(8):399–407. doi: 10.1080/08916934.2018.1550079. [DOI] [PubMed] [Google Scholar]
- 23.Tang H., Bai Y., Shen W., et al. Clinical significance of combined detection of interleukin-6 and tumour markers in lung cancer. Autoimmunity. 2018;51(4):191–198. doi: 10.1080/08916934.2018.1477133. [DOI] [PubMed] [Google Scholar]
- 24.Vang Mouritzen M., Jenssen H. Optimized scratch assay for in vitro testing of cell migration with an automated optical camera. J Vis Exp. 2018;(138) doi: 10.3791/57691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olschewski D.N., Hofschroer V., Nielsen N., Seidler D.G., Schwab A., Stock C. The angiotensin II type 1 receptor antagonist losartan affects NHE1-dependent melanoma cell behavior. Cell Physiol Biochem. 2018;45(6):2560–2576. doi: 10.1159/000488274. [DOI] [PubMed] [Google Scholar]
- 26.Xiao C., Zhou Q., Li X., et al. Losartan and dexamethasone may inhibit chemotaxis to reduce the infiltration of Th22 cells in IgA nephropathy. Int Immunopharmacol. 2017;42:203–208. doi: 10.1016/j.intimp.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Kappert K., Tsuprykov O., Kaufmann J., et al. Chronic treatment with losartan results in sufficient serum levels of the metabolite EXP3179 for PPARgamma activation. Hypertension. 2009;54(4):738–743. doi: 10.1161/HYPERTENSIONAHA.109.132886. [DOI] [PubMed] [Google Scholar]
- 28.Perše M. Cisplatin mouse models: treatment, toxicity and translatability. Biomedicines. 2021;9(10):1406. doi: 10.3390/biomedicines9101406. [DOI] [PMC free article] [PubMed] [Google Scholar]