Abstract
To test the effect of the physical proximity of two enzymes catalyzing sequential reactions, a bifunctional fusion enzyme, TPSP, was constructed by fusing the Escherichia coli genes for trehalose-6-phosphate (T6P) synthetase (TPS) and trehalose-6-phosphate phosphatase (TPP). TPSP catalyzes the sequential reaction in which T6P is formed and then dephosphorylated, leading to the synthesis of trehalose. The fused chimeric gene was overexpressed in E. coli and purified to near homogeneity; its molecular weight was 88,300, as expected. The Km values of the TPSP fusion enzyme for the sequential overall reaction from UDP-glucose and glucose 6-phosphate to trehalose were smaller than those of an equimolar mixture of TPS and TPP (TPS/TPP). However, the kcat values of TPSP were similar to those of TPS/TPP, resulting in a 3.5- to 4.0-fold increase in the catalytic efficiency (kcat/Km). The Km and kcat values of TPSP and TPP for the phosphatase reaction from T6P to trehalose were quite similar. This suggests that the increased catalytic efficiency results from the proximity of TPS and TPP in the TPSP fusion enzyme. The thermal stability of the TPSP fusion enzyme was quite similar to that of the TPS/TPP mixture, suggesting that the structure of each enzyme moiety in TPSP is unperturbed by intramolecular constraint. These results clearly demonstrate that the bifunctional fusion enzyme TPSP catalyzing sequential reactions has kinetic advantages over a mixture of both enzymes (TPS and TPP). These results are also supported by the in vivo accumulation of up to 0.48 mg of trehalose per g of cells after isopropyl-β-d-thiogalactopyranoside treatment of cells harboring the construct encoding TPSP.
The nonreducing disaccharide trehalose [α-d-glucopyranosyl-(1→1)-α-d-glucopyranose] has high water-holding activities, which maintain the fluidity of membranes under dry conditions (25). It also stabilizes enzymes, foods, cosmetics, and pharmaceuticals at high temperatures (8, 9, 38). Due to its desirable physical and chemical characteristics, commercial production of trehalose is anticipated. Escherichia coli synthesizes trehalose when exposed to high osmolarity (12, 20, 39, 41). In E. coli, trehalose is synthesized by two separate enzymes, trehalose-6-phosphate (T6P) synthetase (TPS) and trehalose-6-phosphate phosphatase (TPP), encoded by the genes otsA and otsB, respectively (15, 21). This is different from Saccharomyces cerevisiae, in which trehalose is synthesized by a large multisubunit complex with the catalytic activities of both TPS and TPP (6, 31, 43).
Overexpression of TPS and TPP might be one way to produce trehalose. We assumed that the physical proximity of two enzymes catalyzing sequential reactions might increase the reaction rate by facilitating transfer of the reaction intermediate when they are present in a complex. A variety of techniques have been applied to better understand the proximity effect of enzymes catalyzing sequential reactions, including cross-linking and coimmobilization (23, 32, 34). In many of these cases, the organized enzyme systems exhibited different kinetic or catalytic properties compared with their free counterparts in bulk solution. An attractive alternative approach to these systems is to fuse two enzymes by ligating their structural genes using recombinant DNA techniques. This procedure mimics the evolution of naturally occurring bifunctional enzymes, which might have evolved from smaller proteins through gene fusion (22). It also imitates naturally occurring multienzyme systems, such as the pyruvate dehydrogenase complex and fatty acyl coenzyme A synthase complex (18, 37). Fusion enzymes sometimes demonstrate their superiority over a mixture of individual native enzymes catalyzing the same multistep sequential reaction, but their effects have not been demonstrated definitively in kinetic parameters governing the reactions (5).
Here we describe the enzymatic properties of the recombinant fusion enzyme TPSP, which contains TPS and TPP. The catalytic efficiency of TPSP was 3.5 to 4.0 times higher than that of a mixture of individual enzymes, showing the kinetic advantage of the fusion enzyme. We also examined the internal trehalose accumulation of cells harboring the construct encoding the recombinant fusion protein after IPTG (isopropyl-β-d-thiogalactopyranoside) treatment.
MATERIALS AND METHODS
Bacterial strains.
All the plasmids constructed were propagated in E. coli MC1061. Plasmid pRSETB, based on the T7 RNA polymerase-driven pET system (24), was used as an expression vector and purchased from InVitrogen. For protein overexpression, plasmids were transformed in E. coli BL21(DE3)(pLysS) (hereafter called BL21) as described by Studier et al. (40) and obtained from InVitrogen.
Reagents and enzymes.
The restriction endonucleases, Klenow fragment, alkaline phosphatase, and T4 DNA ligase were purchased from New England Biolabs and Boehringer Mannheim. The Taq DNA polymerase and deoxynucleoside triphosphates were also from Boehringer Mannheim. Ni2+-nitrilotriacetic acid (NTA) agarose resin was from Qiagen. Trehalose, T6P, UDP-glucose (UDPG), glucose, and glucose 6-phosphate (G6P) were from Sigma Chemical Co., and the silica gel thin-layer chromatography (TLC) plate was from Aldrich.
Recombinant fusion gene construction.
The TPS gene was obtained from E. coli genomic DNA by amplification by the Taq PCR with the primers PTPS1 (5′-CCGAATTCATGACTATGAGTCGTTTA-3′) and PTPS2 (5′-CCTACGCAAGCTTTGGAAAGG-3′), which contain the translation initiation and termination codons (bold) of the E. coli TPS gene (15, 21), respectively. EcoRI and HindIII restriction enzyme sites (underlined) were introduced into PTPS1 and PTPS2, respectively. The TPP gene was amplified in the same manner with the primers PTPP1 (5′-CCCCCCGGGATGACAGAACCGTTAACC-3′) and PTPP2 (5′-CCCCAAGCTTTTAGATACTACGACTAAA-3′), which contain the translation initiation and termination codons (bold) of the E. coli TPP gene (15, 21), respectively. SmaI and HindIII sites (underlined) were introduced into PTPP1 and PTPP2, respectively. The amplified DNA was ligated into EcoRI- and HindIII-digested pRSET-B (pRTPS for TPS) and SmaI- and HindIII-digested pRSET-C (pRTPP for TPP) and transformed into E. coli BL21.
To make an expression construct for the TPSP fusion enzyme, pRTPS was digested with HindIII, filled in with Klenow fragment, and then ligated to give pRTPS1, which contains a new NheI enzyme site. The plasmid pRTPP was digested with SmaI and HindIII, and the isolated TPP gene fragment was filled in with Klenow fragment and then ligated into pRTPS1, which was filled with Klenow and digested with NheI. This plasmid, named pRTPSP, was transformed into E. coli BL21.
Expression and purification of recombinant enzymes.
To express the recombinant enzymes, transformed E. coli BL21 was inoculated into 2 liters of Luria-Bertani (LB) medium containing ampicillin (50 μg/ml) and grown at 37°C. When the A590 reached 1.0, IPTG was added to a final concentration of 0.5 mM. Incubation was continued for another 5 h at 20°C, and the cells were harvested for purification.
The cell pellet was resuspended in 50 ml of lysis buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 10 mM imidazole) and sonicated for 10 cycles of 30 s each on ice. The crude extract of the recombinant enzyme was purified by Ni2+-NTA-agarose column chromatography and eluted with a 10 to 250 mM imidazole gradient (InVitrogen manual). The eluted fractions were analyzed by enzyme activity and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The peak fractions were pooled and purified further by Mono Q fast protein liquid chromatography (FPLC; Pharmacia). The product was dialyzed against Mono Q buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 2.5 mM MgCl2, and 10 mM β-mercaptoethanol and applied to a Mono Q HR 10/10 column. The enzyme was eluted at a flow rate of 0.5 ml/min with a linear gradient of NaCl (100 to 500 mM) in Mono Q buffer. The active fractions, which were eluted at a concentration of about 0.2 M NaCl, were combined and dialyzed against Mono Q buffer without NaCl. The enzymes were stored at 4°C and were stable for about 1 month. The protein concentration was determined by the method of Bradford (3) using bovine serum albumin as a standard. In the kinetic experiment, 10 pmol of enzyme was used.
Enzyme assay.
TPS activity was assayed by measuring the production of T6P from 7.5 mM UDPG and 15 mM G6P in 100 μl of the assay buffer of Cabib and Leloir (6) with a minor modification, containing 33 mM Tris-HCl (pH 7.5), 2.5 mM MgCl2, and 10 mM β-mercaptoethanol. TPP was assayed by measuring the production of trehalose from 10 mM T6P in the assay buffer. TPSP activity was assayed by measuring the production of trehalose from a mixture of substrates, UDPG and G6P, in the same assay buffer as used for the TPS assay. The enzyme reaction was carried out at 30°C for 60 min and terminated by incubating the reaction mixture in boiling water for 3 min. Trehalose and T6P were analyzed by high-pH ion-exchange chromatography (HPIC) and TLC, as described below.
The effect of temperature on enzyme activity was determined in the assay buffer at various temperatures between 10 and 60°C. The reaction mixtures were incubated at the indicated temperatures for 60 min and analyzed as described below. The heat stability of the enzyme was measured by preincubating 0.5 mg of enzyme solution per ml at 50°C in the assay buffer. After a specified length of time, the enzyme solutions were quenched for 5 min on ice, and the residual activities were determined.
Analysis of sugars.
Sugar analysis was carried out by TLC or HPIC. For TLC, the reaction mixture was spotted onto a silica gel TLC plate (F254; Merck) and developed twice in solution containing n-butanol, ethanol, and water (5:3:2) (2). Then 20% sulfuric acid was sprayed on the plate to char it for visualization (2). Quantitative analysis of sugar was carried out by HPIC with a Carbo-Pak PA1 column (4 by 250 mm) using the DX500 HPIC system (Dionex 500). Sugars were eluted in a continuous sodium acetate gradient of 0 to 250 mM in 150 mM NaOH solution over 30 min and monitored with an ED40 electrochemical detector (Dionex DC Amperometry).
Analysis of trehalose in E. coli.
E. coli BL21 transformed with recombinant plasmids was grown in LB medium at 37°C overnight, and 500 μl was inoculated into 50 ml of new LB medium. When the A590 reached 0.6, IPTG was added to a final concentration of 0.5 mM, and the incubation was continued for another 3 h at 37°C. One milliliter of the culture was taken and centrifuged for 15 min at 12,000 × g, and the supernatant was analyzed for trehalose by TLC and HPIC.
RESULTS
Expression and purification of the bifunctional fusion enzyme TPSP.
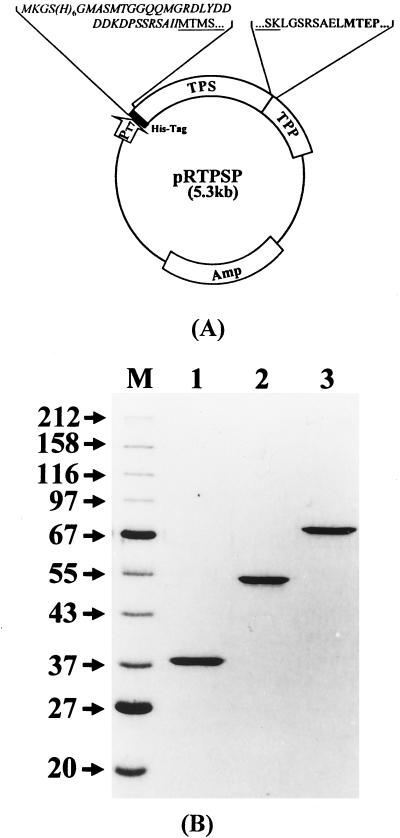
To test the effect of the physical proximity of two enzymes catalyzing sequential reactions, the E. coli genes encoding TPS and TPP were fused, and the recombinant fusion enzyme (TPSP) was expressed. Recombination was carried out so that the C terminus of TPS was fused with the N terminus of TPP in frame, as shown in Fig. 1A. The linker was designed to facilitate the recombinant DNA construction without deleting any amino acids of either protein moiety. This resulted in the insertion of 8 amino acids, LGSRSAEL, during construction. The expression vector also has a hexahistidine tag (His tag) at the N terminus of the recombinant enzyme that can be utilized for rapid purification by Ni2+-NTA chromatography (17, 19, 24). Recombinant TPS and TPP were also expressed and purified separately for comparison.
FIG. 1.
Purification of TPS, TPP, and the fusion enzyme TPSP. (A) Construct for recombinant TPSP expression. The recombinant DNA for the TPS-TPP fusion enzyme (TPSP) was inserted into pRSETB. The N terminus of the hybrid enzyme was fused to a hexahistidine peptide (His tag), shown as a solid box. The predicted amino acid sequences of the fusion boundaries are shown. The N- and C-terminal sequences of TPS are underlined, whereas the N-terminal sequence of TPP is shown in bold. (B) SDS-PAGE of the purified proteins TPP, TPS, and TPSP. The recombinant enzymes were purified by Ni2+-NTA column and Mono Q 10/20 column chromatography as described in Materials and Methods. The purified proteins were analyzed by SDS–12.5% PAGE and stained with Coomassie brilliant blue. Lane M, protein size markers. Lanes 1 to 3, 2 μg of purified TPP, TPS, and TPSP, respectively. The numbers on the left indicate the sizes of the markers (in kilodaltons).
Recombinant enzymes were purified by Ni2+-NTA column chromatography. Although they were relatively pure, the peak fractions eluted from the Ni2+-NTA-agarose column were pooled and purified further by Mono Q HR 10/10 column chromatography. The purified recombinant enzymes were near homogeneity, and the size of each enzyme in the SDS-PAGE analysis (Fig. 1B, lanes 1 to 3) was as expected. Taking into account the N-terminal His tag, the expected sizes of TPS, TPP, and TPSP were 54.5, 37.4, and 88.3 kDa, respectively (15, 21). The molecular weight of each recombinant enzyme was also confirmed by MALDI-TOF (matrix-assisted laser desorption ionization–time-of-flight) mass spectroscopy and gel filtration on an FPLC Superdex 200 HR 10/30 column. Gel filtration showed that the enzymes were monomeric (data not shown).
Trehalose synthesis by the bifunctional fusion enzyme TPSP.
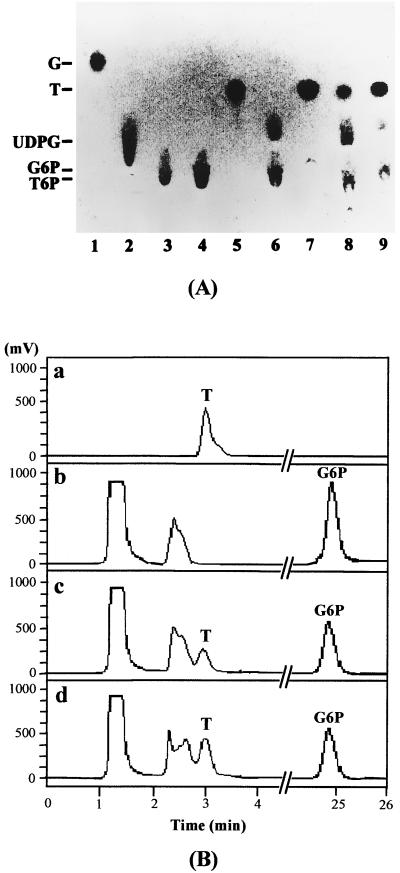
The trehalose synthesis activity of the purified bifunctional fusion enzyme TPSP was tested using a mixture of G6P and UDPG as the substrate. As shown in Fig. 2 (lane 9 in panel A and plate d in panel B), TPSP produced trehalose from this mixture. This demonstrated that TPSP was functional and catalyzed two sequential reactions, from G6P and UDPG to T6P and then to trehalose. This result is consistent with the activity of the individual recombinant enzymes catalyzing each reaction; the recombinant TPS catalyzed the synthesis of T6P from G6P and UDPG, as shown in Fig. 2A (lane 6), and the recombinant TPP catalyzed the hydrolysis of T6P to trehalose (Fig. 2A, lane 7). An equimolar mixture of these two enzymes produced trehalose from G6P and UDPG by catalyzing the sequential reactions (lane 8 in panel A and plate c in panel B of Fig. 2).
FIG. 2.
Analysis of the reaction products of the fusion enzyme TPSP. The indicated purified recombinant enzymes (10 pmol each) were incubated for 60 min at 30°C in 100 μl of assay buffer containing 7.5 mM UDPG and 15 mM G6P or 10 mM T6P as substrates, and the reaction products were analyzed. (A) Silica gel TLC chromatogram. Lanes 1 to 5, 2 μg of standard glucose (G), UDPG, G6P, T6P, and trehalose (T), respectively. Lanes 6 to 9, 3 μl of the reaction products of TPS with G6P and UDPG, TPP with T6P, the TPS/TPP equimolar mixture with G6P and UDPG, and the TPSP fusion enzyme with G6P and UDPG, respectively. The identity of each spot is indicated on the left. (B) HPIC chromatogram. (a) A 52.5-ng amount of standard trehalose. (b and c) Control and reaction products of TPS/TPP mixture from G6P and UDPG after 0 and 60 min of incubation, respectively. (d) Reaction products of TPSP with G6P and UDPG after a 60-min incubation.
Kinetic parameters for the bifunctional fusion enzyme TPSP.
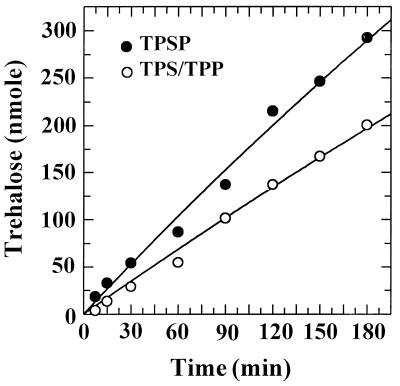
The time course and reaction rate of trehalose synthesis from UDPG and G6P by the recombinant fusion enzyme TPSP were investigated. The amount of trehalose synthesized from UDPG and G6P increased with time. The rate of trehalose synthesis by TPSP was at least 65% faster under the standard assay conditions than that by TPS/TPP, the equimolar mixture of the individual enzymes TPS and TPP, as shown in Fig. 3. The catalytic activity was proportional to the amount of enzyme and was not affected by adding bovine serum albumin to the assay mixture as an inert protein (data not shown).
FIG. 3.
Time course for trehalose production from UDPG and G6P by the recombinant enzyme. Ten picomoles of TPSP (●) or the TPS/TPP equimolar mixture (○) was incubated at 30°C in a final volume of 100 μl of assay buffer containing 7.5 mM UDPG and 15 mM G6P. Aliquots (10 μl) were withdrawn at the indicated times and quantified by HPIC.
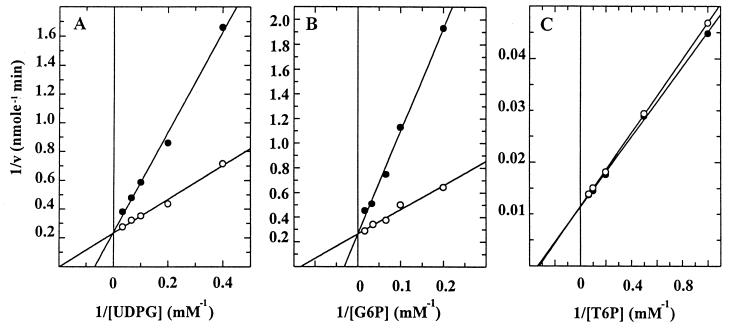
To characterize the enzymatic properties of the bifunctional fusion enzyme TPSP, the kinetic parameters shown in Table 1 were determined. These values were obtained from Lineweaver-Burk plots, which were linear within the experimental error (Fig. 4). Since it was impossible to separate the two sequential reactions catalyzed by TPSP, the kinetic parameters for the overall synthesis of trehalose from G6P and UDPG were determined. These results were compared with the results of a parallel assay with an equimolar mixture of TPS/TPP. The Km values of TPSP for UDPG and G6P were 69 and 76%, respectively, lower than those of TPS/TPP. The kcat values, the turnover number, of TPSP for UDPG and G6P were, however, similar to those of TPS/TPP. The catalytic efficiency of the bifunctional fusion enzyme, calculated as kcat/Km, was 3.5 to 4.0 times greater than that of TPS/TPP.
TABLE 1.
Kinetic parameters of recombinant enzymes TPS, TPP, TPS/TPP, and TPSP with different substratesa
| Enzyme | UDPG
|
G6P
|
T6P
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Km (mM) | kcat (s−1) | kcat/Km (mM−1 S−1) | Km (mM) | kcat (s−1) | kcat/Km (mM−1 S−1) | Km (mM) | kcat (s−1) | kcat/Km (mM−1 S−1) | |
| TPS | 8.6 ± 0.8 | 7.3 ± 0.8 | 0.8 ± 0.1 | 3.7 ± 1.3 | 6.7 ± 0.5 | 0.5 ± 0.1 | |||
| TPP | 2.5 ± 0.1 | 14.3 ± 0.6 | 5.8 ± 0.3 | ||||||
| TPSP | 2.6 ± 0.2 | 14.6 ± 1.4 | 5.6 ± 0.6 | ||||||
| TPSPb | 5.1 ± 0.6 | 3.6 ± 0.2 | 0.7 ± 0.1 | 7.5 ± 0.4 | 3.2 ± 0.2 | 0.4 ± 0.03 | |||
| TPS/TPPb | 16.7 ± 1.6 | 3.5 ± 0.3 | 0.2 ± 0.03 | 30.8 ± 2.2 | 3.2 ± 0.3 | 0.1 ± 0.01 | |||
Data are the mean values and standard deviations of three independent experiments. Experiment conditions are described in the legend to Fig. 4.
Parameters for overall sequential reaction from G6P and UDPG to trehalose.
FIG. 4.
Lineweaver-Burk plots for trehalose synthesis catalyzed by TPSP or TPS/TPP in the presence of UDPG and G6P: effect of UDPG and G6P concentrations on enzyme activity. (A) Double-reciprocal plot of the initial velocity at various concentrations of UDPG against 30 mM G6P. Reactions involving the TPSP fusion enzyme (●) and TPS/TPP mixture (○) are shown. (B) Double-reciprocal plot of initial velocity at various concentrations of G6P against 15 mM UDPG. (C) Double-reciprocal plot of the initial velocity at various concentrations of T6P. Each point represents the mean of three determinations. Linear least-squares analyses were used to determine the slopes and intercepts in the double-reciprocal plots. Ten picomoles of TPSP, TPS, or TPP was used, and the reaction velocity (v) is expressed as nanomoles of trehalose formed per minute.
The kinetic parameters for the TPP activity of TPSP and TPP were also measured at various concentrations of T6P. The Km and kcat values for T6P hydrolysis of TPSP were similar to those of TPP. The catalytic efficiency (kcat/Km) of TPSP for TPP activity was also similar to that of TPP, implying that the fusion of the two enzymes did not affect the kinetic properties of the TPP activity of TPSP (Table 1). This suggests that the kinetic properties of the TPS activity of TPSP are similar to those of TPS, because the kcat values of TPSP and TPS/TPP were similar.
Temperature dependence of TPSP activity.
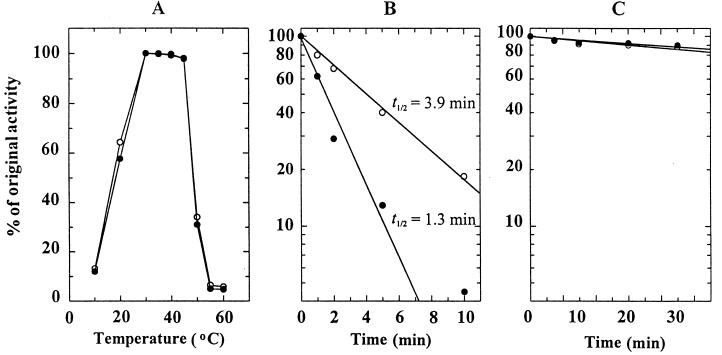
To examine the structural disturbance of each enzyme moiety in the fusion protein, the effect of temperature on enzyme activity was determined at various temperatures between 10 and 60°C. As shown in Fig. 5A, the highest activity was observed at 30 to 40°C for both the TPSP fusion enzyme and the TPS/TPP mixture. Although the absolute activities of trehalose synthesis by TPSP and TPS/TPP were different, there was not much difference in their temperature dependence.
FIG. 5.
Trehalose synthesis activity of TPSP and TPS/TPP. (A) Temperature dependence. Ten picomoles of TPSP (●) or the TPS/TPP equimolar mixture (○) was incubated for 60 min at various temperatures between 10 and 60°C in the standard assay mixture. After boiling for 3 min at 100°C, trehalose was analyzed as described in Materials and Methods. (B) Heat stability. One hundred picomoles of TPSP (●) or the TPS/TPP equimolar mixture (○) was incubated for the indicated lengths of time at 50°C in a final volume of 100 μl of a reaction mixture containing 33 mM Tris-HCl (pH 7.4) and 2.5 mM MgCl2. (C) Heat stability of T6P phosphatase activity. One hundred picomoles of TPSP (●) or TPP (○) was incubated for the indicated lengths of time at 50°C in a final volume of 100 μl of a reaction mixture containing 33 mM Tris-HCl (pH 7.4) and 2.5 mM MgCl2. After quenching on ice for 5 min, 7.5 mM UDPG and 15 mM G6P (B) or 10 mM T6P (C) was added to the reaction mixture. The remaining activity is expressed as a percentage of the original activity.
To determine differences in their stability against thermal denaturation, both the fusion enzyme TPSP and the equimolar mixture TPS/TPP were incubated for a specified time at 50°C. As shown in Fig. 5B, TPSP and TPS/TPP retained 11 and 3% of their original activity, respectively, after incubation for 30 min. The half-life of the trehalose synthesis activity of TPSP was calculated to be 3.9 min at 50°C, while that of TPS/TPP was 1.3 min. Both TPSP and TPP retained about 90% of their original T6P hydrolysis activities after incubation for 30 min at 50°C (Fig. 5C). These results suggest that fusion of the two enzymes did not cause any significant structural perturbation.
Trehalose biosynthesis in recombinant E. coli.
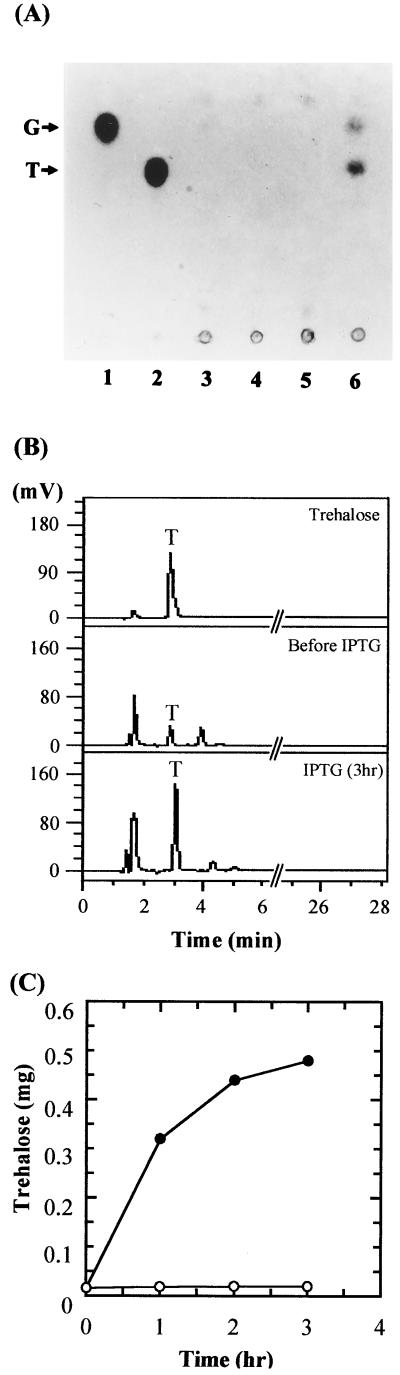
To test the enzyme activity of the bifunctional fusion enzyme in vivo, trehalose was quantified in the transformed strain of E. coli. No trehalose was detected in cells transformed with pRSETB, pRTPS, or pRTPP, even after the cells were induced with IPTG. However, in the recombinant E. coli strain transformed with pRTPSP, in which the bifunctional fusion enzyme TPSP accounted for up to 10% of the total cellular protein, up to approximately 0.48 mg of trehalose accumulated per g of cultured cells after 3 h of induction (Fig. 6).
FIG. 6.
In vivo synthesis of trehalose by overexpression of the bifunctional fusion enzyme TPSP. E. coli harboring plasmid pRSETB, pRTPS, pRTPP, or pRTPSP was induced with 0.5 mM IPTG for 3 h and lysed by sonication. (A) Each supernatant (5 μl) was spotted onto a TLC plate and analyzed as described in Materials and Methods. Lane 1, glucose; lane 2, trehalose; lane 3, pRSETB; lane 4, pRTPS; lane 5, pRTPP; lane 6, pRTPSP. (B) HPIC chromatogram. Trehalose, 110 ng of standard trehalose; before IPTG, sample before IPTG treatment; and IPTG (3 h), sample after IPTG treatment for 3 h. Five microliters of the cultured cells was analyzed. (C) Supernatants were analyzed by HPIC. Symbols are pRTPSP (●) and pRTPSP, no induction (○).
DISCUSSION
We constructed a bifunctional TPSP fusion enzyme by in-frame fusion of the structural genes for TPS and T6P. The catalytic efficiency, kcat/Km, of TPSP for both sequential reactions was 3.5 to 4.0 times more efficient than that of TPS/TPP due to the reduced Km value. Since the kinetic properties of the TPP moiety of TPSP were similar to those of TPP protein, the kinetic properties of the TPS moiety of TPSP can be similar to those of TPS. Therefore, the reduction in the Km value of TPSP for the overall sequential reaction results from the physical proximity of each enzyme, TPS and TPP, in the fusion protein. It was previously shown that differences in the Km values of native enzymes and fusion proteins reflect the presence of specific interactions resulting from the fusion of the enzymes (5, 28). A proximity effect or substrate channeling operates in a fusion enzyme and increases the overall catalytic activity of the reaction (4, 13, 14, 26, 27, 33, 35, 42). In this process, the reaction product of the first enzyme is transferred directly to the active site of the second enzyme in the sequential enzyme complex or fusion enzyme, without diffusing freely in solution. This has been explained by the presence of an electrostatic channel on the surface of the protein that connects the two active sites (13, 26). In our system, a negatively charged intermediate, such as T6P, may interact with such a channel electrostatically and increase the substrate transfer efficiency, as occurred in TPSP.
However, it is possible that the His tag at the N terminus affects the catalytic properties of recombinant enzymes. Therefore, we compared the properties of the recombinant fusion enzyme with those of an equimolar mixture of the separate recombinant enzymes, which have the His tag in common. The Km and kcat values for T6P hydrolysis of the recombinant TPSP were similar to those of the recombinant TPP, as shown in Table 1. This suggests that the His tag does not significantly affect the catalytic properties of recombinant enzymes, such as recombinant TPS and TPSP. Some studies found that the behavior of the recombinant His-tagged protein was indistinguishable from that of the native protein (28, 36).
Thermal denaturation experiments revealed that there were no intrinsic differences in the structures of each enzyme moiety in the fusion enzyme and the individual enzymes, even though TPSP was slightly more stable than the TPS/TPP mixture. Still, some studies showed that the fusion enzyme was more stable against thermal denaturation than the individual enzymes were (16, 30).
To facilitate independent folding of each enzyme moiety in the fusion protein, any structural perturbation caused by intramolecular strain in the complex should be minimized and the linker should accommodate conformational flexibility. The effects of linker peptides in fusion proteins are variable (1, 10, 11). In a β-galactosidase/galactose dehydrogenase fusion enzyme, the specific activity of the galactose dehydrogenase moiety increased with a longer linker (3 versus 9 or 13 amino acids) and the sequential reaction was carried out more efficiently (7). Although a longer linker might release steric perturbation, the longer linker would be more accessible to degrading enzymes, resulting in lower stability (5). We tested a linker 17 amino acids long, S(GGGGS)3V, but this resulted in fast degradation of TPSP (data not shown).
The fusion of structural genes in frame to produce a fusion enzyme catalyzing sequential reactions can increase the efficiency of the enzyme, as shown in this study. It is also advantageous to control the expression of two genes as a single unit and to purify the recombinant fusion protein in one process. Recombinant DNA technologies for creating and overexpressing a gene fusion can be used to improve the productivity of enzyme technology.
ACKNOWLEDGMENTS
This investigation was supported by a grant from the Genetic Engineering Program of the Ministry of Education. H.S.S. is the recipient of a Ph.D. fellowship from the Ministry of Education through the Graduate School of Agricultural Biotechnology of Seoul National University.
REFERENCES
- 1.Argos P. An investigation of oligopeptides linking domains in protein tertiary structures and possible candidates for general gene fusion. J Mol Biol. 1990;211:943–958. doi: 10.1016/0022-2836(90)90085-Z. [DOI] [PubMed] [Google Scholar]
- 2.Boos W, Ehmann U, Forkl H, Klein W, Rimmele M, Postma P. Trehalose transport and metabolism in Escherichia coli. J Bacteriol. 1990;172:3450–3461. doi: 10.1128/jb.172.6.3450-3461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Bülow L. Characterization of an artificial bifunctional enzyme, β-galactosidase/galactokinase, prepared by gene fusion. Eur J Biochem. 1987;163:443–448. doi: 10.1111/j.1432-1033.1987.tb10889.x. [DOI] [PubMed] [Google Scholar]
- 5.Bülow L, Mosbach K. Multienzyme systems obtained by gene fusion. Trends Biotechnol. 1991;9:226–231. doi: 10.1016/0167-7799(91)90075-s. [DOI] [PubMed] [Google Scholar]
- 6.Cabib E, Leloir L F. The biosynthesis of trehalose phosphate. J Biol Chem. 1958;231:259–275. [PubMed] [Google Scholar]
- 7.Carlsson H, Ljung S, Bülow L. Physical and kinetic effects on introduction of various linker regions in β-galactosidase/galactose dehydrogenase fusion enzymes. Biochim Biophys Acta. 1996;1293:154–160. doi: 10.1016/0167-4838(95)00240-5. [DOI] [PubMed] [Google Scholar]
- 8.Carninci P, Nishiyama Y, Westover A, Itoh M, Nagaoka S, Sasaki N, Okazaki Y, Muramatsu M, Hayashizaki Y. Thermostabilization and thermoactivation of thermolabile enzymes by trehalose and its application for the synthesis of full length cDNA. Proc Natl Acad Sci USA. 1988;95:520–524. doi: 10.1073/pnas.95.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colaco C, Sen S, Thangavelu M, Pinder S, Roser B. Extraordinary stability of enzymes dried in trehalose: simplified molecular biology. Biotechnology. 1992;10:1007–1011. doi: 10.1038/nbt0992-1007. [DOI] [PubMed] [Google Scholar]
- 10.Crawford I P, Clarke M, van Cleemput M, Yanofsky C. Crucial role of the connecting region joining the two functional domains of yeast tryptophan synthetase. J Biol Chem. 1987;262:239–244. [PubMed] [Google Scholar]
- 11.Curtis B M, Williams D E, Broxmeyer H E, Dunn J, Farrah T, Jeffery E, Clevenger W, deRoos P, Martin U, Friend D. Enhanced hematopoietic activity of a human granulocyte/macrophage colony-stimulating factor-interleukin 3 fusion protein. Proc Natl Acad Sci USA. 1991;88:5809–5813. doi: 10.1073/pnas.88.13.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinnbier U, Limpinsel E, Schmid R, Bakker E P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol. 1988;150:348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- 13.Elcock A, Huber G A, McCammon J A. Electrostatic channeling of substrate between enzyme active sites: comparison of simulation and experiment. Biochemistry. 1997;36:16049–16058. doi: 10.1021/bi971709u. [DOI] [PubMed] [Google Scholar]
- 14.Elcock A, McCammon A. Evidence for electrostatic channeling in a fusion protein of malate dehydrogenase and citrate synthase. Biochemistry. 1996;35:12652–12658. doi: 10.1021/bi9614747. [DOI] [PubMed] [Google Scholar]
- 15.Giaever H M, Styrvold O B, Kassen I, Strøm A R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988;170:2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossmann M, Wong R, Szkudlinski M W, Weintraub B D. Human thyroid-stimulating hormone (hTSH) subunit gene fusion produces hTSH with increased stability and serum half-life and compensates for mutagenesis-induced defects in subunit association. J Biol Chem. 1997;272:21312–21316. doi: 10.1074/jbc.272.34.21312. [DOI] [PubMed] [Google Scholar]
- 17.Gu J, Stephenson C G, Iadarola M J. Recombinant proteins attached to a nickel-NTA column: use in affinity purification of antibodies. Biotechniques. 1994;17:257. [PubMed] [Google Scholar]
- 18.Harwood J L. Fatty acid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:101–138. [Google Scholar]
- 19.Janknecht R, de Martynoff G, Lou J, Hipskind R A, Nordheim A, Stunnenberg H G. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci USA. 1991;88:8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaasen I, Falkenberg P, Styrbold O B, Strøm A R. Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by KatF (AppR) J Bacteriol. 1992;174:889–898. doi: 10.1128/jb.174.3.889-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaasen I, Mcdougall J, Strøm A R. Analysis of the otsBA operon for osmoregulatory trehalose synthesis in Escherichia coli and homology of the OtsA and OtsB proteins to the yeast trehalose-6-phosphate synthase/phosphatase complex. Gene. 1994;145:9–15. doi: 10.1016/0378-1119(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 22.Kisher K, Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- 23.Koch-Schmidt A C, Mattiasson B, Mosbach K. Aspects of microenvironmental compartmentation: an evaluation of the influence of restricted diffusion, exclusion effects, and enzyme proximity on the overall efficiency of the sequential two-enzyme system malate dehydrogenase-citrate synthase in its soluble and immobilized form. Eur J Biochem. 1977;81:71–78. doi: 10.1111/j.1432-1033.1977.tb11928.x. [DOI] [PubMed] [Google Scholar]
- 24.Kroll D J, Abdel-Malek Abdel-Hafiz H, Simson S, Chen C Y, Gutierrez-Hartmann A, Lustbader J W, Hoeffler J P. A multifunctional prokaryotic protein expression system: overproduction, affinity purification, and selective detection. DNA Cell Biol. 1993;12:441–453. doi: 10.1089/dna.1993.12.441. [DOI] [PubMed] [Google Scholar]
- 25.Leslie S B, Israeli E, Lighthart B, Crowe J H, Crowe L M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61:3592–3597. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang P-H, Anderson K S. Substrate channeling and domain-domain interactions in bifunctional thymidylate synthase-dihydrofolate reductase. Biochemistry. 1998;37:12195–12205. doi: 10.1021/bi9803168. [DOI] [PubMed] [Google Scholar]
- 27.Liang P-H, Anderson K S. Kinetic reaction scheme for the dihydrofolate reductase domain of the bifunctional thymidylate synthase-dihydrofolate reductase from Leishmania major. Biochemistry. 1998;37:12206–12212. doi: 10.1021/bi9803170. [DOI] [PubMed] [Google Scholar]
- 28.Lin Q, Yu N-J, Spremulli L L. Expression and functional analysis of Euglena gracilis chloroplast initiation factor 3. Plant Mol Biol. 1996;32:937–945. doi: 10.1007/BF00020490. [DOI] [PubMed] [Google Scholar]
- 29.Lindbladh C, Rault M, Hagglund C, Small W C, Mosbach K, Bülow L, Evans C, Srere P A. Preparation and kinetic characterization of a fusion protein of yeast mitochondrial citrate synthase and malate dehydrogenase. Biochemistry. 1994;33:11692–11698. doi: 10.1021/bi00205a004. [DOI] [PubMed] [Google Scholar]
- 30.Ljungcrantz P, Carlsson H, Mansson M-O, Buckel P, Mosbach K, Bülow L. Construction of an artificial bifunctional enzyme, β-galactosidase/galactose dehydrogenase, exhibiting efficient galactose channeling. Biochemistry. 1989;28:8786–8792. doi: 10.1021/bi00448a016. [DOI] [PubMed] [Google Scholar]
- 31.Londesborough J, Vuorio O. Trehalose-6-phosphate synthase/phosphatase complex from baker's yeast: purification of a proteolytically activated form. J Gen Microbiol. 1991;137:323–330. doi: 10.1099/00221287-137-2-323. [DOI] [PubMed] [Google Scholar]
- 32.Manson M-O, Siegbahn N, Mosbach K. Site-to-site directed immobilization of enzymes with bis-NAD analogues. Proc Natl Acad Sci USA. 1983;80:1487–1491. doi: 10.1073/pnas.80.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meek T D, Garvey E P, Santi D V. Purification and characterization of the bifunctional thymidylate synthetase-dihydrofolate reductase from methotrexate-resistent Leishmania tropica. Biochemistry. 1985;24:678–686. doi: 10.1021/bi00324a021. [DOI] [PubMed] [Google Scholar]
- 34.Mosbach K, Mattiasson B. Matrix-bound enzymes. II. Studies on a matrix-bound two-enzyme system. Acta Chem Scand. 1970;24:2093–2100. doi: 10.3891/acta.chem.scand.24-2093. [DOI] [PubMed] [Google Scholar]
- 35.Pan P, Woehl E, Dunn M F. Protein architecture, dynamics and allostery in tryptophan synthase channeling. Trends Biochem Sci. 1997;22:22–27. doi: 10.1016/s0968-0004(96)10066-9. [DOI] [PubMed] [Google Scholar]
- 36.Popp B, Deborah A C, Benz R, Neupert W, Lill R. The role of the N and C termini of recombinant Neurospora mitochondria porin in channel formation and voltage-dependent gating. J Biol Chem. 1996;271:13593–13599. doi: 10.1074/jbc.271.23.13593. [DOI] [PubMed] [Google Scholar]
- 37.Reed L J, Pettit F H, Hamilton L, Collins J H, Oliver R M. Reconstitution of the Escherichia coli pyruvate dehydrogenase complex. Proc Natl Acad Sci USA. 1975;72:3068–3072. doi: 10.1073/pnas.72.8.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roser B. Trehalose, a new approach to premium dried foods. Trends Food Sci Technol. 1991;2:166–169. [Google Scholar]
- 39.Strøm A R, Falkenverg P, Landfald B. Genetics of osmoregulation in Escherichia coli: uptake and biosynthesis of organic osmolytes. FEMS Microbiol Rev. 1986;39:79–86. [Google Scholar]
- 40.Studier F W, Rosenberg A H, Dunn J J, Dubendorff L W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 41.Styrbold O B, Strøm A R. Synthesis, accumulation, and excretion of trehalose in osmotically stressed Escherichia coli K-12 strains: influence of amber suppressors and function of the periplasmic trehalase. J Bacteriol. 1991;173:1187–1192. doi: 10.1128/jb.173.3.1187-1192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamada Y, Swanson B A, Arabshahi A, Frey P A. Preparation and characterization of a bifunctional fusion enzyme composed of UDP-galactose 4-epimerase and galactose-1-P uridylyltransferase. Bioconjug Chem. 1994;5:660–665. doi: 10.1021/bc00030a023. [DOI] [PubMed] [Google Scholar]
- 43.Vandercammen A, Francois J, Hers H G. Characterization of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase of Saccharomyces cerevisiae. Eur J Biochem. 1989;182:613–620. doi: 10.1111/j.1432-1033.1989.tb14870.x. [DOI] [PubMed] [Google Scholar]