Abstract
Background
Serum creatinine (Cr) and albumin (Alb) are important predictors of mortality in individuals with various diseases, including acute pancreatitis (AP). However, most previous studies have only examined the relationship between single Cr or Alb levels and the prognosis of patients with AP. To our knowledge, the association between short- and long-term all-cause mortality in patients with AP and the blood creatinine to albumin ratio (CAR) has not been investigated. Therefore, this study aimed to evaluate the short- and long-term relationships between CAR and all-cause mortality in patients with AP.
Methods
We conducted a retrospective study utilizing data from the Medical Information Market for Intensive Care (MIMIC-IV) database. The study involved analyzing various mortality variables and obtaining CAR values at the time of admission. The X-tile software was used to determine the optimal threshold for the CAR. Kaplan-Meier (K-M) survival curves and multivariate Cox proportional hazards regression models were used to assess the relationship between CAR and both short- and long-term all-cause mortality. The predictive power, sensitivity, specificity, and area under the curve (AUC) of CAR for short- and long-term mortality in patients with AP after hospital admission were investigated using Receiver Operating Characteristic analysis. Additionally, subgroup analyses were conducted.
Results
A total of 520 participants were included in this study. The CAR ideal threshold, determined by X-tile software, was 0.446. The Cox proportional hazards model revealed an independent association between CAR≥0.446 and all-cause mortality at 7-day (d), 14-d, 21-d, 28-d, 90-d, and 1-year (y) before and after adjustment for confounders. K-M survival curves showed that patients with CAR≥0.446 had lower survival rates at 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y. Additionally, CAR demonstrated superior performance, with higher AUC values than Cr, Alb, serum total calcium, Glasgow Coma Scale, Systemic Inflammatory Response Syndrome score, and Sepsis-related Organ Failure Assessment score at 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y intervals. Subgroup analyses showed that CAR did not interact with a majority of subgroups.
Conclusion
The CAR can serve as an independent predictor for short- and long-term all-cause mortality in patients with AP. This study enhances our understanding of the association between serum-based biomarkers and the prognosis of patients with AP.
Keywords: creatinine to albumin ratio, acute pancreatitis, all-cause mortality, MIMIC-IV, serum biomarker
1. Introduction
Acute pancreatitis (AP) is a prevalent disease of the digestive system, affecting 13-45 per 100,000 individuals annually, with gallstones, alcoholism, and triglyceridemia being the main causes (1, 2). Most patients are mildly symptomatic, presenting with epigastric or full abdominal pain, abdominal distension and vomiting, which can be controlled with supportive care and intravenous fluid therapy (3, 4). Approximately 20% of patients develop moderate or severe AP, with pancreatic necrosis and peripancreatic tissue necrosis, and even multi-organ failure, with a mortality rate of up to 30% in this group of patients (5–7). Therefore, early and accurate assessment of disease severity may help to reduce AP mortality.
The Ranson criteria (8), Balthazar grade (9), Sepsis-related Organ Failure Assessment (SOFA) (10), Acute Physiology and Chronic Health Evaluation II (APAChE-II) (8), and Bedside Index for Severity in AP (BISAP) (11) are now the most widely used approaches for assessing AP severity. However, these scoring systems are complex. Examples include the Ranson criteria, which includes 11 clinical and laboratory data, and APAChE-II, which includes age, chronic health status, and 12 acute physiological markers. Consequently, these scoring systems require time for data collection before concluding the condition assessment, which may cause a delay in determining the severity of the disease and miss the optimal window of opportunity for optimal treatment. Therefore, there is an urgent need for a simpler, quicker, highly repeatable, and sensitive indicator to gauge AP severity.
Previous studies have demonstrated a relationship between blood levels of serum creatinine (Cr), albumin (Alb), and C-reactive protein (CRP) with the severity and prognosis of AP (12–14). Combining these serum indicators, despite having low predictive value, effectively enhances predictive sensitivity (15). CRP and Cr levels are usually elevated in patients with AP; however, albumin levels frequently show varying degrees of decline. Previous studies have shown that CRP/Alb is a valuable tool for predicting the probability of death in patients with AP (16). According to Kaplan et al, in patients with AP, the risk of death increases 1.52-fold for every unit rise in CRP/Alb (16). Moreover, a CRP/Alb ratio >16.28, with 92.1% sensitivity and 58.0% specificity, is a significant predictor of mortality (16). Additionally, there is a strong link between Cr levels, prognosis, and the degree of AP (13). According to research by Lankisch et al. (13), blood Cr levels (≥ 2 mg/dl) upon admission, as well as 24 and 48 hours later, were useful in determining if the pancreas was necrotic. The creatinine-to-albumin ratio (CAR), according to Zhao et al. (17), is a practical, consistent, and easy-to-measure biochemical tool used to evaluate the effectiveness of pancreatitis debridement surgery.
However, the relationship between CAR and short- and long-term all-cause mortality in patients with AP remains unclear. Thus, we used the Medical Information Mart for Intensive Care IV version 2.2 [MIMIC-IV (v2.2)] database to collect data on the hospitalization status of patients with AP admitted between 2008 and 2019. The study aimed to examine the association between short- and long-term all-cause mortality and CAR in these patients.
2. Methods
2.1. Data source
The MIMIC-IV (v2.2) database, a large, openly available database created by the Massachusetts Institute of Technology’s Laboratory of Computational Physiology, provided all data used in this investigation (18). The official link is https://mimic.mit.edu/. MIMIC-IV is an invaluable resource for research in clinical decision support, predictive modeling, critical care outcomes, and related fields, owing to its extensive collection of Intensive Care Unit (ICU)-specific data. All patients admitted to the Beth Israel Deaconess Medical Center between 2008 and 2019 were included. All test results, prescription schedules, vital signs, length of hospital stay for each patient, and other specific information were recorded. To protect patient privacy, all personal data were de-identified, and patient identification was substituted with random numbers. Ethical approval and informed consent were waived.
2.2. Criteria for population selection and results
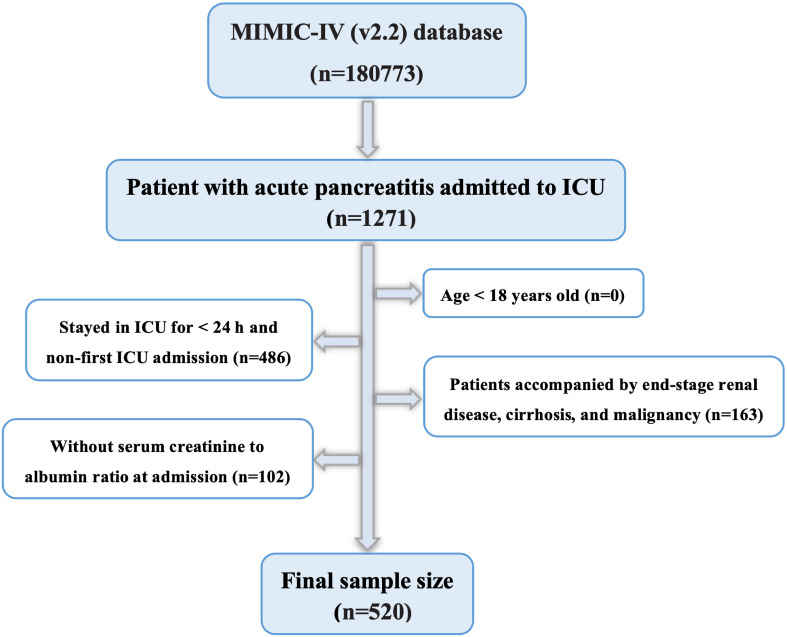
The MIMIC-IV database contains data on 180,733 individuals admitted to the ICU between 2012 and 2019. Hospital admission data for patients with AP were retrieved using the International Classification of Diseases, 9th Revision (ICD-9) code 577.0, and International Classification of Diseases, 10th Revision (ICD-10) codes K85-K85.92. A total of 5,894 patients with AP were included, of whom 1,271 were admitted to the ICU. Data regarding the initial ICU admissions of patients aged 18 years or older were collected for this study. The following patients were also excluded from this study: those who had end-stage renal disease, cirrhosis, malignancy, or recurrent admissions for acute pancreatitis, retaining only the initial admission data for this subgroup; those who spent less than 24 hours in the ICU; and those for whom Cr and Alb data were not recorded within 24 hours of admission. Finally, 520 patients were included in the study (Figure 1).
Figure 1.
Flowchart for participants from the MIMIC-IV (v 2.2).
2.3. Data extraction
CAR was chosen as the main study variable. Serum Cr and Alb levels were examined for the first time after admission to minimize treatment-related interference. All variables were extracted from the MIMIC-IV database using a Structured Query Language (SQL) with PostgreSQL. Demographic information, vital signs, clinical treatments, comorbidities, laboratory data, and treatment outcomes were the six main components of the extraction procedure. Table 1 presents a comprehensive list of the extracted variables.
Table 1.
Covariates extracted in detail from the MIMIC-IV database.
| Items | Composition |
|---|---|
| Demographic variables | Age, Gender, Race |
| Vital Signs | HR, SBP, DBP, MAP, RR, SpO2, Temperature |
| Clinical Treatments | Vasopressin, Octreotide, Statins, Betablockers, MV, CRRT, ERCP |
| Comorbidities | AKI, Sepsis, RF, HF, AF, Hypertension, Diabetes, Obesity |
| Laboratory variables | Neutrophil counts, Nymphocyte counts, Eosinophil counts, RBC, WBC, RDW, Plt, Hb, Lymphocyte percentage, HCT, Cr, Alb, BUN, TB, AST, ALT, Glucose, TC, TG, HDL-C, LDL-C, PT, INR, Blood amylase, Blood lipase, K, Na, TCa, AG, Lac |
| Clinical Outcomes | LOS ICU, LOS hospital, ICU mortality, In-hospital mortality, 7-day mortality, 14-day mortality, 21-day mortality, 28-day mortality, 90-day mortality, 1-year mortality |
MIMIC-IV, the Medical Information Mart for Intensive Care database; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; RR, respiratory rate; MV, mechanical ventilation; CRRT, continuous renal replacement therapy; ERCP, endoscopic retrograde cholangiopancreatography; AKI, acute kidney injury; RF, respiratory failure; HF, heart failure; AF, atrial fibrillation; RBC, red blood cell; WBC, white blood cell; RDW, erythrocyte distribution width; Plt, platelet; Hb, hemoglobin; HCT, hematocrit; Cr, creatinine; Alb, albumin; BUN, blood urea nitrogen; TB, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PT, prothrombin time; INR, international normalized ratio; K, serum potassium; Na, serum sodium; TCa, serum total calcium; AG, anion gap; Lac, lactate; CAR, serum creatinine to albumin ratio; LOS ICU, length of ICU stay; LOS hospital, length of hospital stay.
2.4. Endpoint events
The primary endpoint of the study was all-cause mortality within 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y of admission after hospital admission. Additionally, we also assessed the duration of stay in the ICU, the total length of stay, as well as ICU and hospital mortality during this period.
2.5. Statistical analysis
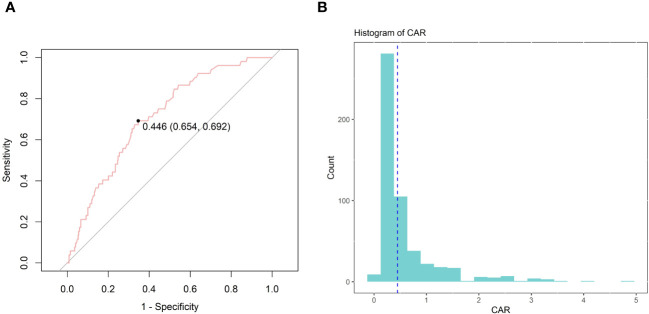
Continuous variables were expressed as mean ± standard deviation (SD) for normally distributed variables, median (IQR) for non-normally distributed continuous variables, and numbers (%) for categorical variables. The T-test or One-Way ANOVA was used to compare continuous variables, while Pearson’s χ2 test and Fisher’s test were used to compare categorical variables when examining baseline characteristics. The X-tile software (Version 3.6.1, Yale University School of Medicine) is commonly used to calculate optimal thresholds in survival analyses and continuous data from medical or epidemiologic studies. After determining the ideal CAR cutoff value based on whether death occurred on day 28 after admission, we divided the study patient population into two groups: low CAR and high CAR. The choice of the optimal cutoff point that maximized the risk ratio is presented in Figure 2, as well as the relationship of CAR≥0.446 and the distribution of CAR. Univariate and multivariate analyses of prognostic indicators were performed using the Cox proportional hazards model to identify independent predictors of death in patients with AP 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y after admission. HRs with 95% confidence intervals (CIs) are provided. Unadjusted survival curves were drawn using the Kaplan-Meier (K-M) method, and a log-rank test was used to compare the two sets of curves. The predictive power of CAR, Cr, Alb, and SOFA scores for mortality at 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y after admission was assessed using Receiver Operating Characteristic (ROC) analysis. The sensitivity and specificity for each indicator were determined, and the area under the curve (AUC) was calculated. Subgroup analyses were conducted to examine the potential impact of CAR on various parameters, such as age, sex, acute kidney injury (AKI), hypertension, and diabetes. The R statistical program (R version 4.2.2, R Foundation for Statistical Computing), SPSS Statistics 26 (IBM, Chicago, IL, USA), and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) were the software programs utilized for the studies.
Figure 2.
The choice of the optimal cutoff point maximized the risk ratio (A) and the relationship between CAR≥0.446 and the distribution of CAR (B).
3. Results
3.1. Baseline demographic and clinical characteristics
Ultimately, 520 patients with AP receiving treatment in the ICU were included in this study (Figure 1). Notably, 231 (44.4%) were women, and 289 were men (55.6%). Using X-tile software, the best CAR cutoff was determined based on whether the patient died on day 28 following admission. The results showed that 0.446 was the ideal CAR threshold (Figure 2). The study population was divided into two groups: those with a low CAR (<0.446) and those with a high CAR (≥ 0.446) and those with the high CAR group had lower mean arterial pressure, systolic and diastolic blood pressure, and body temperature, and greater rates of vasopressin and continuous renal replacement therapy. In addition, patients with a combination of AKI, sepsis, and respiratory failure (RF) were more likely to be in the high CAR group. Among the laboratory markers, patients in the high CAR group had elevated levels of lactate, anion gap, serum potassium, international normalized ratio, blood urea nitrogen, prothrombin time, and creatinine. Subsequent research revealed a greater risk of poor prognosis for patients in the high CAR group. They had higher ICU mortality (4.0% vs. 13.1%, P < 0.001), hospitalization mortality (6.2% vs. 19.2%, P < 0.001), 7-d mortality (1.6% vs. 6.1%, P = 0.011), 14-d mortality (3.7% vs. 12.1%, P < 0.001), 21-d mortality (4.7% vs. 16.2%, P < 0.001), 28-d mortality (5.0% vs. 18.2%, P < 0.001), 90-d mortality (10.2% vs. 27.8%, P < 0.001) and 1-y mortality (14.3% vs. 33.3%, P < 0.001). Furthermore, the high CAR group had longer ICU hospitalizations [(3.18 (1.86, 6.66) vs. 5.84 (2.37, 13.16), P < 0.001)] and longer hospital stays overall [11.33 (6.96, 21.34) vs. 16.71 (9.90, 28.76), P < 0.001)]. The detailed results are presented in Table 2.
Table 2.
Baseline patient characteristics in patients with acute pancreatitis.
| Variable | Total (n=520) | CAR | P value | |
|---|---|---|---|---|
| <0.446 (n=322) | ≥0.446 (n=198) | |||
| Demographics | ||||
| Age | 59.00 (46.00, 74.00) | 55.00 (43.00, 70.00) | 65.00 (53.25, 76.00) | <0.001 |
| Gender | 0.057 | |||
| Male | 289 (55.6) | 168 (52.2) | 121 (61.1) | |
| Female | 231 (44.4) | 154 (47.8) | 77 (38.9) | |
| Race, n (%) | 0.399 | |||
| Asian | 20 (3.8) | 9 (2.8) | 11 (5.6) | |
| White | 324 (62.3) | 205 (63.7) | 119 (60.1) | |
| Black | 38 (7.3) | 22 (6.8) | 16 (8.1) | |
| Other | 138 (26.5) | 86 (26.7) | 52 (26.3) | |
| Vital Signs | ||||
| HR | 100.00 (84.00, 116.00) | 101.00 (85.00, 116.75) | 99.00 (83.00, 115.00) | 0.356 |
| SBP | 127.00 (109.75, 144.25) | 131.00 (114.00, 150.75) | 120.50 (101.00, 140.00) | <0.001 |
| DBP | 72.00 (60.00, 85.00) | 75.00 (63.00, 88.00) | 66.00 (56.00, 80.00) | <0.001 |
| MAP | 90.67 (78.00, 103.83) | 94.33 (80.42, 107.00) | 85.33 (73.42, 97.83) | <0.001 |
| Respiratory rate | 21.00 (17.00, 26.00) | 21.00 (18.00, 25.00) | 21.00 (17.00, 26.00) | 0.988 |
| Spo2 | 96.00 (94.00, 99.00) | 95.88 (94.00, 99.00) | 96.00 (94.00, 98.00) | 0.255 |
| Temperature | 36.89 (36.44, 37.39) | 36.94 (36.56, 37.56) | 36.67 (36.34, 37.22) | <0.001 |
| Clinical Treatment | ||||
| Vasopressin | 79 (15.2) | 23 (7.1) | 56 (28.3) | <0.001 |
| Octreotide | 29 (5.6) | 17 (5.3) | 12 (6.1) | 0.857 |
| Statins | 171 (32.9) | 99 (30.7) | 72 (36.4) | 0.219 |
| Betablockers | 308 (59.2) | 179 (55.6) | 129 (65.2) | 0.039 |
| MV | 460 (88.5) | 280 (87.0) | 180 (90.9) | 0.219 |
| CRRT | 61 (11.7) | 10 (3.1) | 51 (25.8) | <0.001 |
| ERCP | 31 (6.0) | 26 (8.1) | 5 (2.5) | 0.016 |
| Comorbidities | ||||
| AKI | 360 (69.2) | 195 (60.6) | 165 (83.3) | <0.001 |
| Sepsis | 381 (73.3) | 209 (64.9) | 172 (86.9) | <0.001 |
| RF | 234 (45.0) | 117 (36.3) | 117 (59.1) | <0.001 |
| HF | 93 (17.9) | 52 (16.1) | 41 (20.7) | 0.230 |
| AF | 115 (22.1) | 65 (20.2) | 50 (25.3) | 0.214 |
| Hypertension | 252 (48.5) | 181 (56.2) | 71 (35.9) | <0.001 |
| Diabetes | 164 (31.5) | 95 (29.5) | 69 (34.8) | 0.239 |
| Obesity | 69 (13.3) | 41 (12.7) | 28 (14.1) | 0.744 |
| Laboratory Indicators | ||||
| Neutrophil counts | 9.08 (5.27, 14.52) | 8.41 (4.92, 14.27) | 9.99 (6.46, 15.55) | 0.053 |
| Nymphocyte counts | 1.03 (0.59, 1.73) | 1.11 (0.63, 1.73) | 0.90 (0.55, 1.76) | 0.378 |
| Eosinophil counts | 0.01 (0.00, 0.11) | 0.01 (0.00, 0.10) | 0.01 (0.00, 0.12) | 0.714 |
| RBC | 3.71 (3.20, 4.23) | 3.76 (3.25, 4.22) | 3.59 (3.13, 4.23) | 0.256 |
| WBC | 13.50 (9.40, 18.70) | 13.30 (9.35, 18.55) | 13.80 (9.43, 19.17) | 0.656 |
| RDW | 14.50 (13.60, 15.70) | 14.30 (13.50, 15.50) | 14.70 (13.93, 15.97) | <0.001 |
| PLT | 194.00 (135.00, 275.00) | 205.00 (146.00, 287.00) | 176.50 (125.00, 239.50) | 0.002 |
| Hb | 11.30 (9.70, 13.00) | 11.40 (9.90, 12.93) | 11.00 (9.60, 13.17) | 0.226 |
| Lymphocyte percentage | 8.00 (4.00, 13.57) | 8.10 (4.32, 14.20) | 6.85 (3.68, 12.00) | 0.011 |
| HCT | 34.00 (29.60, 38.80) | 34.30 (29.98, 38.40) | 33.30 (29.40, 39.50) | 0.744 |
| Cr | 1.05 (0.70, 1.70) | 0.80 (0.60, 1.00) | 2.05 (1.50, 3.40) | <0.001 |
| Alb | 2.90 (2.50, 3.30) | 3.00 (2.62, 3.40) | 2.70 (2.30, 3.18) | <0.001 |
| BUN | 20.00 (12.00, 33.00) | 15.00 (10.00, 20.00) | 37.50 (25.25, 56.00) | <0.001 |
| TB | 1.00 (0.60, 2.60) | 0.90 (0.60, 2.32) | 1.10 (0.60, 2.90) | 0.672 |
| AST | 62.00 (33.00, 158.00) | 57.00 (32.00, 149.50) | 71.00 (38.00, 181.00) | 0.088 |
| ALT | 54.00 (24.00, 152.00) | 51.00 (24.00, 141.00) | 59.00 (24.75, 160.00) | 0.523 |
| Glucose | 134.00 (105.00, 174.50) | 130.00 (106.00, 164.75) | 137.00 (105.00, 188.00) | 0.105 |
| TC | 155.50 (116.00, 199.50) | 170.00 (127.75, 206.25) | 139.50 (107.00, 181.25) | 0.008 |
| TG | 183.00 (107.00, 326.00) | 163.50 (103.00, 293.75) | 201.00 (126.00, 366.00) | 0.101 |
| HDL-C | 26.00 (14.75, 35.25) | 30.00 (23.00, 38.00) | 21.00 (12.00, 32.00) | 0.018 |
| LDL-C | 58.50 (38.75, 89.75) | 66.00 (38.75, 98.25) | 52.00 (40.50, 84.25) | 0.317 |
| PT | 14.30 (12.90, 16.20) | 14.10 (12.70, 15.70) | 14.90 (13.00, 17.50) | <0.001 |
| INR | 1.30 (1.20, 1.50) | 1.30 (1.10, 1.40) | 1.30 (1.20, 1.60) | <0.001 |
| Blood amylase | 131.00 (53.00, 375.75) | 101.00 (50.00, 285.00) | 176.00 (63.00, 482.00) | 0.008 |
| Blood lipase | 223.00 (61.00, 1222.00) | 166.00 (50.50, 1010.00) | 356.50 (87.25, 1522.00) | 0.001 |
| K | 4.00 (3.60, 4.60) | 3.90 (3.60, 4.30) | 4.30 (3.82, 4.90) | <0.001 |
| Na | 138.00 (135.00, 142.00) | 138.00 (136.00, 141.00) | 139.00 (135.00, 142.75) | 0.199 |
| TCa | 7.90 (7.30, 8.40) | 8.00 (7.40, 8.50) | 7.70 (7.00, 8.28) | <0.001 |
| AG | 14.00 (12.00, 16.00) | 13.00 (11.00, 15.00) | 15.00 (12.00, 18.00) | <0.001 |
| Lac | 1.70 (1.20, 2.70) | 1.60 (1.10, 2.30) | 2.10 (1.30, 3.20) | <0.001 |
| CAR | 0.35 (0.25, 0.60) | 0.27 (0.21, 0.33) | 0.78 (0.53, 1.33) | <0.001 |
| Clinical Outcomes | ||||
| LOS ICU | 3.77 (1.96, 8.98) | 3.18 (1.86, 6.66) | 5.84 (2.37, 13.16) | <0.001 |
| LOS hospital | 13.71 (7.71, 23.76) | 11.33 (6.96, 21.34) | 16.71 (9.90, 28.76) | <0.001 |
| Hospital mortality | 58 (11.2) | 20 (6.2) | 38 (19.2) | <0.001 |
| ICU mortality | 39 (7.5) | 13 (4.0) | 26 (13.1) | <0.001 |
| 7-day mortality | 17 (3.3) | 5 (1.6) | 12 (6.1) | 0.011 |
| 14-day mortality | 36 (6.9) | 12 (3.7) | 24 (12.1) | <0.001 |
| 21-day mortality | 47 (9.0) | 15 (4.7) | 32 (16.2) | <0.001 |
| 28-day mortality | 52 (10.0) | 16 (5.0) | 36 (18.2) | <0.001 |
| 90-day mortality | 88 (16.9) | 33 (10.2) | 55 (27.8) | <0.001 |
| 1-year mortality | 112 (21.5) | 46 (14.3) | 66 (33.3) | <0.001 |
HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; RR, respiratory rate; MV, mechanical ventilation; CRRT, continuous renal replacement therapy; ERCP, endoscopic retrograde cholangiopancreatography; AKI, acute kidney injury; RF, respiratory failure; HF, heart failure; AF, atrial fibrillation; RBC, red blood cell; WBC, white blood cell; RDW, erythrocyte distribution width; Plt, platelet; Hb, hemoglobin; HCT, hematocrit; Cr, creatinine; Alb, albumin; BUN, blood urea nitrogen; TB, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PT, prothrombin time; INR, international normalized ratio; K, serum potassium; Na, serum sodium; TCa, serum total calcium; AG, anion gap; Lac, lactate; CAR, serum creatinine to albumin ratio; LOS ICU, length of ICU stay; LOS hospital, length of hospital stay.
3.2. Univariate and multivariate Cox regression models of CAR with mortality in patients with AP
Univariate and multivariate Cox regression analyses were conducted to identify the potential relationship between the CAR and mortality in patients with AP. Elevated CAR (≥0.446) was substantially linked to an increased risk of mortality at various periods in the unadjusted initial model: 7-d (HR = 3.98, 95% CI: 1.40-11.28, P for trend = 0.010), 14-d (HR = 3.40, 95% CI: 1.70-6.80, P for trend = 0.001), 21-d (HR = 3.68, 95% CI: 1.99-6.79, P for trend < 0.001), 28-d (HR = 3.91, 95% CI: 2.17-7.05, P for trend < 0.001), 90-d (HR = 3.04, 95% CI: 1.97-4.68, P for trend < 0.001) and 1-y (HR = 2.70, 95% CI: 1.85-3.93, P for trend < 0.001). In the multivariate Model 1, the group of patients with CAR≥0.446 maintained a higher risk of mortality even after adjusting for confounding factors, including age and sex: 7-d (HR = 3.82, 95% CI: 1.33-10.94, P for trend = 0.013), 14-d (HR = 2.99, 95% CI: 1.48-6.04, P for trend = 0.002), 21-d (HR = 3.11, 95% CI: 1.67-5.81, P for trend < 0.001), 28-d (HR = 3.39, 95% CI: 1.86-6.16, P for trend < 0.001), 90-d (HR = 2.67, 95% CI: 1.72-4.15, P for trend < 0.001) and 1-y (HR = 2.33, 95% CI: 1.59-3.43, P for trend < 0.001). A subsequent multivariate model (Model 2) that included additional possible confounders also revealed an independent correlation between a higher CAR and an increased likelihood of mortality during the previously mentioned periods. The detailed results are presented in Table 3.
Table 3.
Univariate and multivariate Cox regression models of CAR with mortality in patients with AP.
| Outcome | Unadjusted, HR (95%CI) | Model 1, HR (95%CI) | Model 2, HR (95%CI) |
|---|---|---|---|
| 7-d | |||
| CAR<0.446 | 1 | 1 | 1 |
| CAR≥0.446 | 3.98 (1.40-11.28) | 3.82 (1.33-10.94) | 3.63 (1.19-11.04) |
| P for trend | 0.010 | 0.013 | 0.023 |
| 14-d | |||
| CAR<0.446 | 1 | 1 | 1 |
| CAR≥0.446 | 3.40 (1.70-6.80) | 2.99 (1.48-6.04) | 3.11 (1.52-6.36) |
| P for trend | 0.001 | 0.002 | 0.002 |
| 21-d | |||
| CAR<0.446 | 1 | 1 | 1 |
| CAR≥0.446 | 3.68 (1.99-6.79) | 3.11 (1.67-5.81) | 3.34 (1.77-6.30) |
| P for trend | <0.001 | <0.001 | <0.001 |
| 28-d | |||
| CAR<0.446 | 1 | 1 | 1 |
| CAR≥0.446 | 3.91 (2.17-7.05) | 3.39 (1.86-6.16) | 3.58 (1.95-6.59) |
| P for trend | <0.001 | <0.001 | <0.001 |
| 90-d | |||
| CAR<0.446 | 1 | 1 | 1 |
| CAR≥0.446 | 3.04 (1.97-4.68) | 2.67 (1.72-4.15) | 2.77 (1.77-4.34) |
| P for trend | <0.001 | <0.001 | <0.001 |
| 1-year | |||
| CAR<0.446 | 1 | 1 | 1 |
| CAR≥0.446 | 2.70 (1.85-3.93) | 2.33 (1.59-3.43) | 2.39 (1.61-3.54) |
| P for trend | <0.001 | <0.001 | <0.001 |
Model 1: Adjusted age and gender.
Model 2: Model 1+ Spo2+Temperature+Vasopressin+Betablockers+Plt+HCT.
3.3. Kaplan-Meier curve and ROC curve analysis
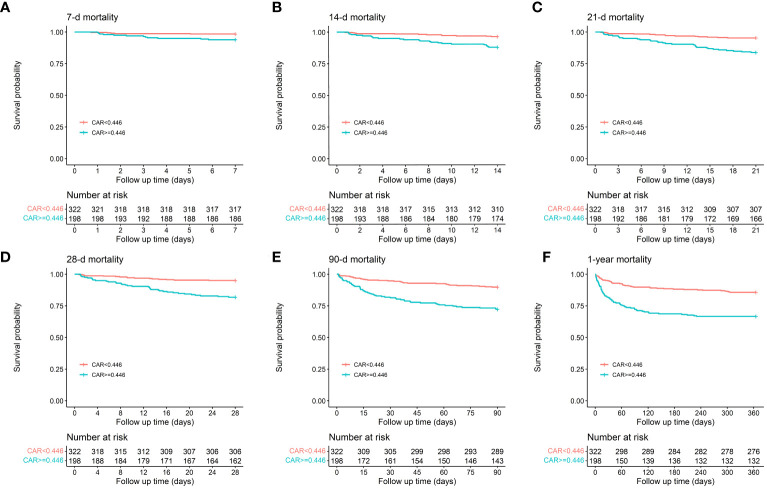
Patients with CAR≥0.446 had higher mortality at 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y intervals compared to those with CAR < 0.446, as demonstrated by the K-M survival curves (1.6% vs. 6.1%, P = 0.011; 3.7% vs. 12.1%, P < 0.001; 4.7% vs. 16.2%, P < 0.001; 5.0% vs. 18.2%, P < 0.001; 10.2% vs. 27.8%, P < 0.001; 14.3% vs. 33.3%, P < 0.001). Patients with CAR levels≥0.446 also had increased ICU and hospital mortality rates (4.0% vs. 13.1%, P < 0.001; 6.2% vs. 19.2%, P < 0.001). The results are presented in Figure 3.
Figure 3.
Kaplan-Meier survival analysis curves for all-cause mortality in patients with AP at 7-d (A), 14-d (B), 21-d (C), 28-d (D), 90-d (E), and 1-y (F) of hospital admission.
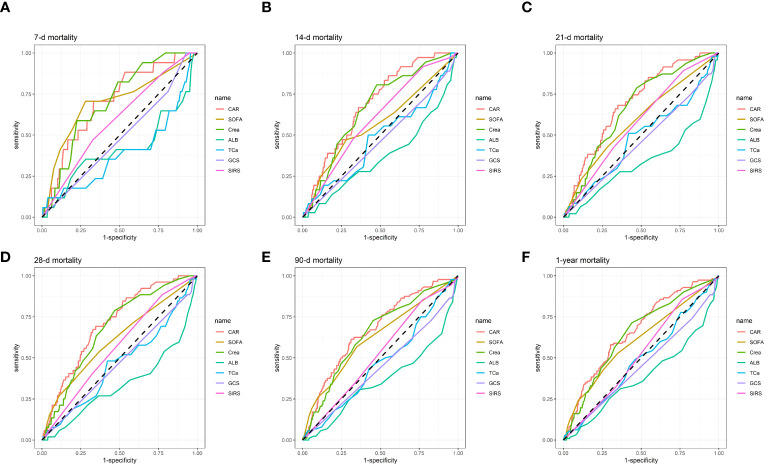
ROC curves were generated for predicting all-cause death in patients at 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y following admission using seven measures: Cr, Alb, serum total calcium (TCa), Glasgow Coma Scale (CGS), Systemic Inflammatory Response Syndrome (SIRS) score, and SOFA score (Figure 4). For specific details regarding Figure 4, please refer to Table 4. Our results revealed that, in terms of AUC values, CAR was superior to Cr, Alb, TCa, CGS, SIRS score, and SOFA score at 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y intervals. For example, compared to Cr [67.91% (95% CI: 60.98%-74.84%)], Alb [34.08% (95% CI: 25.34%-42.83%)], TCa [46.59% (95% CI: 37.99-55.19)], CGS [47.43% (95% CI: 41.21-53.65)], SIRS [56.89% (95% CI: 49.74-64.04)], and SOFA [61.06% (95% CI: 52.68%-69.44%)], CAR had a considerably improved AUC on day 28 of admission [70.98% (95% CI: 64.26%-77.69%)]. Similarly, the AUC for CAR was considerably higher on day 90 of admission [67.23% (95% CI: 61.17%-73.30%)] than for Alb [36.33% (95% CI: 29.41%-43.24%), Cr [64.77% (95% CI: 58.53%-71.01%), TCa [46.81% (95% CI: 40.21-53.41)], CGS [44.56% (95% CI: 39.39-49.72)], SIRS [52.78% (95% CI: 46.85-58.70)], and SOFA [62.47% (95% CI: 55.91%-69.03%)]. The detailed results are presented in Table 4. Consequently, our findings highlight the significant predictive benefits of the CAR.
Figure 4.
ROC curves for predicting all-cause mortality in patients with AP at 7-d (A), 14-d (B), 21-d (C), 28-d (D), 90-d (E), and 1-y (F) after admission.
Table 4.
Information of ROC curves in Figure 4.
| Variables | AUC (%) | 95%CI (%) | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|
| 7-d | |||||
| CAR | 70.68 | 58.77-82.59 | 0.486 | 0.706 | 0.668 |
| SOFA | 69.90 | 55.35-84.44 | 2.500 | 0.706 | 0.720 |
| Cr | 70.49 | 59.81-81.18 | 1.750 | 0.588 | 0.775 |
| Alb | 41.54 | 24.52-58.56 | 3.250 | 0.353 | 0.718 |
| TCa | 39.14 | 23.15-55.12 | 9.350 | 0.118 | 0.962 |
| GCS | 48.52 | 38.69-58.35 | 13.500 | 1.000 | 0.072 |
| SIRS | 59.26 | 46.87-71.64 | 3.500 | 0.471 | 0.672 |
| 14-d | |||||
| CAR | 69.13 | 61.33-76.94 | 0.317 | 0.861 | 0.446 |
| SOFA | 57.19 | 46.86-67.53 | 2.500 | 0.472 | 0.719 |
| Cr | 66.74 | 58.37-75.11 | 1.050 | 0.806 | 0.523 |
| Alb | 37.41 | 27.01-47.81 | 1.450 | 1.000 | 0.006 |
| TCa | 48.13 | 37.59-58.66 | 8.050 | 0.500 | 0.578 |
| GCS | 46.85 | 39.05-54.12 | 8.500 | 1.000 | 0.019 |
| SIRS | 60.51 | 52.19-68.84 | 3.500 | 0.472 | 0.678 |
| 21-d | |||||
| CAR | 69.65 | 62.53-76.77 | 0.446 | 0.681 | 0.649 |
| SOFA | 59.81 | 50.53-67.82 | 2.500 | 0.426 | 0.719 |
| Cr | 66.71 | 59.42-74.00 | 1.050 | 0.787 | 0.529 |
| Alb | 34.57 | 25.28-43.86 | 1.450 | 1.000 | 0.006 |
| TCa | 48.35 | 39.14-57.56 | 8.050 | 0.511 | 0.581 |
| GCS | 46.07 | 39.34-52.80 | 8.500 | 1.000 | 0.019 |
| SIRS | 58.04 | 50.57-65.50 | 2.500 | 0.894 | 0.226 |
| 28-d | |||||
| CAR | 70.98 | 64.26-77.69 | 0.446 | 0.692 | 0.654 |
| SOFA | 61.06 | 52.68-69.44 | 1.500 | 0.538 | 0.630 |
| Cr | 67.91 | 60.98-74.84 | 1.050 | 0.788 | 0.532 |
| Alb | 34.08 | 25.34-42.83 | 1.450 | 1.000 | 0.006 |
| TCa | 46.59 | 37.99-55.19 | 8.050 | 0.481 | 0.578 |
| GCS | 47.43 | 41.21-53.65 | 8.500 | 1.000 | 0.019 |
| SIRS | 56.89 | 49.74-64.04 | 2.500 | 0.885 | 0.226 |
| 90-d | |||||
| CAR | 67.23 | 61.17-73.30 | 0.465 | 0.614 | 0.688 |
| SOFA | 62.47 | 55.91-69.03 | 1.500 | 0.568 | 0.650 |
| Cr | 64.77 | 58.53-71.01 | 1.050 | 0.727 | 0.546 |
| Alb | 36.33 | 29.41-43.24 | 1.250 | 1.000 | 0.002 |
| TCa | 46.81 | 40.21-53.41 | 5.800 | 0.977 | 0.046 |
| GCS | 44.56 | 39.39-49.72 | 3.500 | 0.989 | 0.012 |
| SIRS | 52.78 | 46.85-58.70 | 2.500 | 0.852 | 0.229 |
| 1-year | |||||
| CAR | 66.39 | 60.85-71.93 | 0.465 | 0.580 | 0.696 |
| SOFA | 60.53 | 54.53-66.53 | 1.500 | 0.527 | 0.652 |
| Cr | 64.65 | 58.98-70.32 | 1.050 | 0.714 | 0.559 |
| Alb | 38.70 | 32.42-44.99 | 1.250 | 1.000 | 0.002 |
| TCa | 48.97 | 42.92-55.02 | 7.950 | 0.491 | 0.538 |
| GCS | 45.32 | 40.76-49.88 | 3.500 | 0.991 | 0.012 |
| SIRS | 52.33 | 47.01-57.65 | 2.500 | 0.857 | 0.235 |
ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval; CAR, serum creatinine to albumin ratio; Cr, creatinine; Alb, albumin; TCa, serum total calcium; SOFA, Sepsis-related Organ Failure Assessment score; GCS, Glasgow Coma Scale; SIRS, Systemic Inflammatory Response Syndrome score.
3.4. Subgroup analyses for the CAR on clinical outcomes in patients with AP
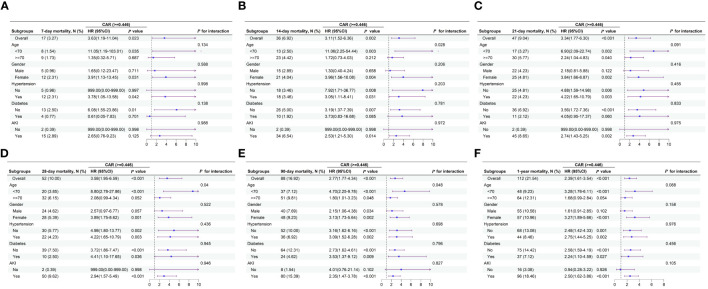
Figure 5 illustrates the presence of the relationship between the 7-d, 14-d, 21-d, 28-d, 90-d, and 1-y CAR and all-cause mortality in different subgroups of patients with AP. When stratified analyses were performed for age, sex, AKI, hypertension, and diabetes, the forest plots showed no significant interaction between CAR and most subgroups (P > 0.05), except at 14-d, 28-d, and 90-d, where a small interaction between CAR and age subgroups was observed (P for interaction=0.028, 0.04, and 0.048). The results are shown in Figure 5.
Figure 5.
Forest plots of subgroup analysis of the relationship between all-cause mortality and CAR in patients with AP admitted 7-d (A), 14-d (B), 21-d (C), 28-d (D), 90-d (E), and 1-y (F).
4. Discussion
Numerous recent studies have examined serum biomarkers, such as the neutrophil/lymphocyte ratio (19, 20), red blood cell distribution width/platelet ratio (21), blood glucose/lymphocyte ratio (22), and CRP/Alb ratio (16), to determine the prognosis of AP. However, no research has been published on the use of CAR to predict the prognosis of patients with AP. Our results suggest that among patients with AP, CAR is an independent factor influencing both short- and long-term all-cause mortality. The short- and long-term all-cause mortality was significantly higher in AP patients with CAR≥0.446 than in patients with CAR < 0.446, according to K-M survival analysis plots. Our findings demonstrated that for both short and long-term intervals, CAR outperformed Cr, Alb, TCa, CGS, SIRS score, and SOFA score in terms of AUC values. Subgroup analyses suggested that CAR did not significantly interact with the most of the subgroups.
Numerous studies have demonstrated that elevated Cr is an adverse prognostic indicator of AP (13, 19, 23, 24). Long-established markers of renal function, such as creatinine and blood urea nitrogen (BUN), have variable accuracy in predicting the severity of pancreatitis. Cr is a reasonably priced, easily accessible, and highly dependable blood marker that strongly correlates with renal function. Furthermore, Cr is less sensitive to minor changes in intravascular blood volume and more indicative of internal organ damage (24). Talamini et al. (23) reported that in 192 patients with new-onset pancreatitis, Cr levels > 2 mg/dl were a significant predictor of mortality. Muddana et al. (24) found that elevated Cr was a significant predictor of necrosis in AP based on a study of 185 patients. AKI is a common complication of AP (25, 26). Devani et al. (27) reported that 7.9% of hospitalized patients with AP experienced AKI. Furthermore, older age, male sex, sepsis, RF, and chronic kidney disease (CKD) are associated with an increased risk of concomitant AKI in patients with AP (27).
Nevertheless, the pathophysiologic mechanism behind AKI in individuals with AP remain incompletely understood (28). However, the key pathophysiological mechanism is the early activation of pancreatic enzymes in acinar cells, which results in autodigestion of the pancreas and surrounding tissues (29). This sets off a series of events that ultimately lead to AKI (29). An increase in abdominal pressure, hypovolemia, hypotension, severe vasoconstriction of the renal vasculature, hypercoagulability, and fibrin deposition in the glomeruli are consequences of activated enzymes and proteases released into the circulatory system and damage to the vascular endothelium (30). Moreover, glomerular damage caused by autodigestion induces the release of cytokines and the production of oxygen-free radicals (31). Animal studies have shown that hypovolemia is essential in the early phases of AP and leads to AKI (32). In comparison with the control group, the glomerular filtration rate (GFR) decreased by 40% and the plasma volume by 26% four hours after the initiation of pancreatitis. Plasma infusion successfully reversed this decline in the GFR (32). Substances released by the necrotic pancreas, such as trypsin, chymotrypsin, bradykinin, histamine, prostaglandin E, endotoxins, and bacteria, have also been shown to be associated with AKI development (32). For example, histamine in pancreatic exudates may cause increased vascular permeability, hypovolemia, and hypotension (33). Peritoneal lavage increases urine output in patients with AP and AKI, suggesting that dialysis removes the substances responsible for causing AKI (33). Cytokines may be responsible for renal impairment. For instance, TNF-α can act directly on tubular and glomerular capillaries, causing tubular necrosis and ischemia. TNF-α can also cause endothelial cells to produce IL-1β, IL-8, and IL-6, which can cause thrombosis, renal ischemia, and the production of oxygen-free radicals (30). The urine N-acetyl-β-glucosidase to Cr ratio and early serum PLA2 activity are positively correlated in patients with AP, indicating that PLA2 may hydrolyze renal tubular epithelial cell membranes and result in AKI (33). Mucosal permeability is increased by inflammatory mediators that cause endotoxins and bacteria to move out of the colon. Endotoxins increase endothelin levels, which can induce tubular necrosis, reduced renal blood flow, and vasoconstriction, all of which can result in AKI (34). Ventricular compartment syndrome is an uncommon AP that can compress and impede renal blood flow in the venous and arterial veins. This compression can lead to increased renal pressure, reduced venous blood flow, decreased perfusion pressure, and increased venous pressure, all of which contribute to AKI (35). Hence, with the onset of AP, several pathophysiological pathways may result in AKI, which may increase blood Cr levels.
The primary protein in plasma, Alb, is produced by the liver and is crucial for preserving the osmolality of plasma colloids (36). Alb, a negative acute-phase reactant, is correlated with the degree of inflammation, prognosis, and mortality of several illnesses (4). Among its many biological activities, albumin may act as an antioxidant, prevent platelet activation and aggregation, and serve as a transporter for a variety of physiologically active substances (37). According to Shannon et al. (38), individuals with low Alb levels often have a poor prognosis. Serum Alb levels are typically low in critically ill patients with a variety of diseases. This decrease may be attributed to the role of Alb in increasing the production of several anti-inflammatory substances (such as lipoxins, hemolysins, and protective proteins) during oxidative stress to facilitate recovery from the disease (39). This process requires a significant amount of albumin. For instance, the Alb level has been shown to be a reliable indicator of death in older individuals with sepsis (40). Additionally, previous studies have shown that serum Alb levels may predict the prognosis of AP to some degree (12, 14). Nevertheless, in terms of forecasting short- and long-term all-cause mortality in AP, Alb performed worse than CAR, according to our results. Chronic inflammation or a patient’s nutritional state also influences Alb levels. Thus, utilizing Alb levels alone to predict the prognosis of AP has limitations. Therefore, we calculated the serum Cr: Alb ratio to predict the prognosis of patients with AP more accurately by decreasing the effect of individual variables on the regulatory systems via inverse changes generated by two separate processes.
The results of our study found that CAR was a stronger predictor of short- and long-term prognosis in AP compared with other control indicators. Alb and TCa are important indicators of a patient’s nutritional status and electrolyte balance. However, in AP, due to the inflammatory response and tissue damage, decreases in Alb and TCa may not be specific indicators because they are influenced by multiple factors, including the release of inflammatory mediators and an increase in endogenous damage molecules (41–43). Therefore, the use of Alb and TCa alone to predict mortality in patients with AP may be influenced by other factors, resulting in inadequate predictive power. The SOFA score, GCS and SIRS score are commonly used to assess the severity of disease and systemic inflammatory response in critically ill patients (8–11). However, these scores may have some limitations in predicting short- and long-term mortality in patients. For example, the SOFA score mainly focuses on the function of multiple organ systems and does not specifically address the pathological and physiological characteristics of pancreatitis itself, thus may lack sensitivity or specificity (4, 10). Similarly, although the GCS score and SIRS score reflect the patient’s level of consciousness and systemic inflammatory response, they may not fully consider the specific conditions of pancreatic tissue damage and inflammation in the prognosis assessment of pancreatitis (44, 45). CAR takes Cr and Alb levels into account and reflects a combination of renal function and nutritional status. In AP, elevated Cr levels may reflect renal dysfunction or systemic metabolic changes, while decreased Alb levels may indicate deterioration in nutritional status. Therefore, CAR, as a comprehensive indicator, can more comprehensively reflect the metabolism and nutritional status of patients, and is more closely related to the pathological and physiological characteristics of AP. Compared with other control indicators, CAR may have higher specificity and sensitivity and more accurately predict short- and long-term mortality in patients with AP.
Lactated Ringer’s solution is important in the management of patients with AP. Hypocalcemia is a common complication of AP and is closely related to impaired renal, pulmonary and cardiovascular functions (5). Lactated Ringer’s solution contains 3 mEq/l of calcium ions, which helps to increase serum calcium concentration and maintain electrolyte stability, thus preventing early organ failure (46). Wu et al. found that lactated Ringer’s solution reduces systemic inflammatory response and serum C-reactive protein in patients with AP better than normal saline (47). The systemic inflammatory response typically depletes large amounts of albumin, and Ringer’s lactate helps maintain albumin levels at normal levels (48). In addition, with the addition of sodium lactate to Ringer’s solution, lactic acid can be metabolized to bicarbonate in the body, enhancing the buffering effect in the body, which is important for metabolic acidosis associated with AP patients (49, 50).
The primary benefit of this study is its extensive real-world dataset, demonstrating that CAR can accurately predict the mortality of patients with AP across a wide range of periods. Nevertheless, this study has some limitations. First, our study was a single-center retrospective; therefore, multicenter prospective investigations will be needed to validate our results. To make our conclusions more reliable, we will conduct a multicenter study in China in the future. Second, the MIMIC-IV provided demographic information for our study, which included patients hospitalized from 2008 to 2019. However, such an extended timeframe does not ensure the consistency of treatment plans for patients with AP, considering the advancements in medical technology and therapeutic approaches. Consequently, it is plausible that the inconsistent treatment plans may have affected the findings of this study. Third, this study did not include participants under the age of 18 years; thus, further research is needed to determine whether the findings apply to people under that age. Additionally, due to the limitations of the database, we were unable to obtain the specific cause of death for each patient. This limitation prevented us from providing information on the impact of CAR on AP-specific mortality. Finally, the study cohort consisted of critically ill patients receiving ICU care; therefore, patients with typical mild pancreatitis may not have benefitted from the results.
5. Conclusion
In patients with AP, CAR may serve as an independent predictor of both short- and long-term all-cause mortality. The findings from our study may equip healthcare providers with additional tools for promptly diagnosing patients with AP, assessing disease severity, and treating individuals with poor prognoses. To support the prognostic utility of CAR in patients with AP, large-sample multicenter prospective studies are required.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
JW: Writing – review & editing, Writing – original draft, Resources, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. HaL: Writing – original draft, Methodology, Data curation, Conceptualization. HWL: Writing – original draft, Project administration, Data curation, Conceptualization. RS: Writing – review & editing, Software, Investigation. SC: Writing – review & editing, Methodology, Formal analysis. JH: Writing – review & editing, Investigation, Funding acquisition. HuL: Writing – review & editing, Resources, Project administration, Methodology. PY: Writing – review & editing, Resources, Investigation, Conceptualization. XC: Writing – original draft, Methodology, Formal analysis. YW: Writing – review & editing, Funding acquisition, Data curation. XZ: Writing – review & editing, Resources, Project administration. DW: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Formal analysis.
Acknowledgments
We are grateful to the MIMIV-IV participants and staff. We appreciate all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by NHC Key Laboratory of Nuclear Technology Medical Transformation (Mianyang Central Hospital) (Grant No.2023HYX032), Medical Research Youth Innovation Project of Sichuan Province, China (grant no. Q23046), the Medical research Project of Sichuan Province (grant no. S23060), and the Scientific Research Project of Mianyang Health Commission (grant no. 202341).
Abbreviations
CAR, serum creatinine to albumin ratio; Cr, serum creatinine; Alb, albumin; AP, acute pancreatitis; MIMIC-IV, medical Information Market for Intensive Care (MIMIC-IV) database; AUC, area under the curve; ROC, receiver operating characteristic; SOFA, sepsis-related organ failure assessment; APAChE-II, acute physiology and chronic health evaluation II, CRP, C-reactive protein; ICU, intensive care unit, ICD, international classification of diseases; SQL, structured query language, AKI, acute kidney injury; BISAP, bedside index for severity in AP; CI, confidence interval.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Goyal H, Awad H, Hu ZD. Prognostic value of admission red blood cell distribution width in acute pancreatitis: a systematic review. Ann Transl Med. (2017) 5:342. doi: 10.21037/atm.2017.06.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Szatmary P, Grammatikopoulos T, Cai W, Huang W, Mukherjee R, Halloran C, et al. Acute pancreatitis: diagnosis and treatment. Drugs. (2022) 82:1251–76. doi: 10.1007/s40265-022-01766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiriyama S, Gabata T, Takada T, Hirata K, Yoshida M, Mayumi T, et al. New diagnostic criteria of acute pancreatitis. J Hepatobil Pancreat Sci. (2010) 17:24–36. doi: 10.1007/s00534-009-0214-3 [DOI] [PubMed] [Google Scholar]
- 4. Liu Q, Zheng HL, Wu MM, Wang QZ, Yan SJ, Wang M, et al. Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with acute pancreatitis: A retrospective analysis of the MIMIC-IV database. Front Immunol. (2022) 13:1076121. doi: 10.3389/fimmu.2022.1076121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. (2019) 156:2008–23. doi: 10.1053/j.gastro.2018.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo Y, Li Z, Ge P, Guo H, Li L, Zhang G, et al. Comprehensive mechanism, novel markers and multidisciplinary treatment of severe acute pancreatitis-associated cardiac injury - A narrative review. J Inflammation Res. (2021) 14:3145–69. doi: 10.2147/JIR.S310990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang J, Xia Y, Cao Y, Cai X, Jiang S, Liao Y, et al. Evaluating the efficacy and timing of blood purification modalities in early-stage hyperlipidemic acute pancreatitis treatment. Lipids Health Dis. (2023) 22:208. doi: 10.1186/s12944-023-01968-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Staubli SM, Oertli D, Nebiker CA. Assessing the severity of acute pancreatitis (ASAP) in Switzerland: A nationwide survey on severity assessment in daily clinical practice. Pancreatology. (2017) 17:356–63. doi: 10.1016/j.pan.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 9. Xiao B, Xu HB, Jiang ZQ, Zhang J, Zhang XM. Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging. Quant Imaging Med Surg. (2019) 9:1973–85. doi: 10.21037/qims.2019.11.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 11. Arif A, Jaleel F, Rashid K. Accuracy of BISAP score in prediction of severe acute pancreatitis. Pak J Med Sci. (2019) 35:1008–12. doi: 10.12669/pjms.35.4.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walker H, Melling J, Jones M, Melling CV. C-reactive protein accurately predicts severity of acute pancreatitis in children. J Pediatr Surg. (2022) 57:759–64. doi: 10.1016/j.jpedsurg.2021.08.007 [DOI] [PubMed] [Google Scholar]
- 13. Lankisch PG, Weber-Dany B, Maisonneuve P, Lowenfels AB. High serum creatinine in acute pancreatitis: a marker for pancreatic necrosis? Am J Gastroenterol. (2010) 105:1196–200. doi: 10.1038/ajg.2009.688 [DOI] [PubMed] [Google Scholar]
- 14. Jiang Z, An X, Li Y, Xu C, Meng H, Qu Y. Construction and validation of a risk assessment model for acute kidney injury in patients with acute pancreatitis in the intensive care unit. BMC Nephrol. (2023) 24:315. doi: 10.1186/s12882-023-03369-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Z, Dong L, Zhang Y, Yang C, Gou S, Li Y, et al. Prediction of severe acute pancreatitis using a decision tree model based on the revised atlanta classification of acute pancreatitis. PloS One. (2015) 10:e0143486. doi: 10.1371/journal.pone.0143486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaplan M, Ates I, Akpinar MY, Yuksel M, Kuzu UB, Kacar S, et al. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobil Pancreat Dis Int. (2017) 16:424–30. doi: 10.1016/S1499-3872(17)60007-9 [DOI] [PubMed] [Google Scholar]
- 17. Zhao Z, Yu Y, Xie R, Yang K, Xu D, Li L, et al. Prognostic value of the creatinine-albumin ratio in acute pancreatitis debridement. BMC Surg. (2020) 20:322. doi: 10.1186/s12893-020-00991-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crosignani A, Spina S, Marrazzo F, Cimbanassi S, Malbrain MLNG, Van Regenmortel N, et al. Intravenous fluid therapy in patients with severe acute pancreatitis admitted to the intensive care unit: a narrative review. Ann Intensive Care. (2022) 12:98. doi: 10.1186/s13613-022-01072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kong W, He Y, Bao H, Zhang W, Wang X. Diagnostic value of neutrophil-lymphocyte ratio for predicting the severity of acute pancreatitis: A meta-analysis. Dis Markers. (2020) 2020:9731854. doi: 10.1155/2020/9731854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cetinkaya E, Senol K, Saylam B, Tez M. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol. (2014) 20:14450–4. doi: 10.3748/wjg.v20.i39.14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Tang S, Wang Y. Prognostic value of glucose-to-lymphocyte ratio in critically ill patients with acute pancreatitis. Int J Gen Med. (2021) 14:5449–60. doi: 10.2147/IJGM.S327123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Talamini G, Bassi C, Falconi M, Sartori N, Frulloni L, Di Francesco V, et al. Risk of death from acute pancreatitis. Role of early, simple “routine” data. Int J Pancreatol. (1996) 19:15–24. doi: 10.1007/BF02788371 [DOI] [PubMed] [Google Scholar]
- 24. Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. (2009) 104:164–70. doi: 10.1038/ajg.2008.66 [DOI] [PubMed] [Google Scholar]
- 25. Li H, Qian Z, Liu Z, Liu X, Han X, Kang H. Risk factors and outcome of acute renal failure in patients with severe acute pancreatitis. J Crit Care. (2010) 25:225–9. doi: 10.1016/j.jcrc.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 26. Zhou J, Li Y, Tang Y, Liu F, Yu S, Zhang L, et al. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrol (Carlton). (2015) 20:485–91. doi: 10.1111/nep.12439 [DOI] [PubMed] [Google Scholar]
- 27. Devani K, Charilaou P, Radadiya D, Brahmbhatt B, Young M, Reddy C. Acute pancreatitis: Trends in outcomes and the role of acute kidney injury in mortality- A propensity-matched analysis. Pancreatology. (2018) 18:870–7. doi: 10.1016/j.pan.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 28. Nassar TI, Qunibi WY. AKI associated with acute pancreatitis. Clin J Am Soc Nephrol. (2019) 14:1106–15. doi: 10.2215/CJN.13191118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. (2007) 132:1127–51. doi: 10.1053/j.gastro.2007.01.055 [DOI] [PubMed] [Google Scholar]
- 30. Zhang XP, Wang L, Zhou YF. The pathogenic mechanism of severe acute pancreatitis complicated with renal injury: a review of current knowledge. Dig Dis Sci. (2008) 53:297–306. doi: 10.1007/s10620-007-9866-5 [DOI] [PubMed] [Google Scholar]
- 31. Levy M, Geller R, Hymovitch S. Renal failure in dogs with experimental acute pancreatitis: role of hypovolemia. Am J Physiol. (1986) 251:F969–77. doi: 10.1152/ajprenal.1986.251.6.F969 [DOI] [PubMed] [Google Scholar]
- 32. Satake K, Kanazawa G, Hiura A, Nishiwaki H, Ha SS, Chung YS, et al. Renal function in experimentally induced acute pancreatitis in dogs: how it is affected by the nephrotoxic substance in pancreatic exudate from ascitic fluid. Jpn J Surg. (1991) 21:88–95. doi: 10.1007/BF02470871 [DOI] [PubMed] [Google Scholar]
- 33. Ofstad E, Amundsen E, Hagen PO. Experimental acute pancreatitis in dogs. II. Histamine release induced by pancreatic exudate. Scand J Gastroenterol. (1969) 4:75–9. doi: 10.3109/00365526909180153 [DOI] [PubMed] [Google Scholar]
- 34. Bose SM, Verma GR, Mazumdar A, Giridhar M, Ganguly NK. Significance of serum endotoxin and antiendotoxin antibody levels in predicting the severity of acute pancreatitis. Surg Today. (2002) 32:602–7. doi: 10.1007/s005950200109 [DOI] [PubMed] [Google Scholar]
- 35. Patel DM, Connor MJ, Jr. Intra-abdominal hypertension and abdominal compartment syndrome: an underappreciated cause of acute kidney injury. Adv Chronic Kidney Dis. (2016) 23:160–6. doi: 10.1053/j.ackd.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 36. Attieh RM, Wadei HM. Acute kidney injury in liver cirrhosis. Diagn (Basel). (2023) 13:2361. doi: 10.3390/diagnostics13142361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xia M, Zhang C, Gu J, Chen J, Wang LC, Lu Y, et al. Impact of serum albumin levels on long-term all-cause, cardiovascular, and cardiac mortality in patients with first-onset acute myocardial infarction. Clin Chim Acta. (2018) 477:89–93. doi: 10.1016/j.cca.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 38. Shannon CM, Ballew SH, Daya N, Zhou L, Chang AR, Sang Y, et al. Serum albumin and risks of hospitalization and death: Findings from the Atherosclerosis Risk in Communities study. J Am Geriatr Soc. (2021) 69:2865–76. doi: 10.1111/jgs.17313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu Y, Guo H, Chen X, Zhang Q. Association between lactate/albumin ratio and all-cause mortality in patients with acute respiratory failure: A retrospective analysis. PloS One. (2021) 16:e0255744. doi: 10.1371/journal.pone.0255744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arnau-Barrés I, Güerri-Fernández R, Luque S, Sorli L, Vázquez O, Miralles R. Serum albumin is a strong predictor of sepsis outcome in elderly patients. Eur J Clin Microbiol Infect Dis. (2019) 38:743–6. doi: 10.1007/s10096-019-03478-2 [DOI] [PubMed] [Google Scholar]
- 41. Ni T, Wen Y, Wang Y, Jiang W, Sheng H, Chen E, et al. Association between albumin or prealbumin levels at different stages and prognosis in severe acute pancreatitis: a 5-year retrospective study. Sci Rep. (2022) 12:16792. doi: 10.1038/s41598-022-21278-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petersen OH, Gerasimenko JV, Gerasimenko OV, Gryshchenko O, Peng S. The roles of calcium and ATP in the physiology and pathology of the exocrine pancreas. Physiol Rev. (2021) 101:1691–744. doi: 10.1152/physrev.00003.2021 [DOI] [PubMed] [Google Scholar]
- 43. Sutton R. Parenchymal pressure injury Ca2+ entry mechanism in pancreatitis. Cell Calcium. (2020) 88:102208. doi: 10.1016/j.ceca.2020.102208 [DOI] [PubMed] [Google Scholar]
- 44. Feng A, Ao X, Zhou N, Huang T, Li L, Zeng M, et al. A novel risk-prediction scoring system for sepsis among patients with acute pancreatitis: A retrospective analysis of a large clinical database. Int J Clin Pract. (2022) 2022:5435656. doi: 10.1155/2022/5435656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu F, Yao J, Liu C, Shou S. Construction and validation of machine learning models for sepsis prediction in patients with acute pancreatitis. BMC Surg. (2023) 23:267. doi: 10.1186/s12893-023-02151-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khatua B, Yaron JR, El-Kurdi B, Kostenko S, Papachristou GI, Singh VP. Ringer’s lactate prevents early organ failure by providing extracellular calcium. J Clin Med. (2020) 9:263. doi: 10.3390/jcm9010263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. (2011) 9:710–717.e1. doi: 10.1016/j.cgh.2011.04.026 [DOI] [PubMed] [Google Scholar]
- 48. Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. (2000) 85:599–610. doi: 10.1093/bja/85.4.599 [DOI] [PubMed] [Google Scholar]
- 49. Kayhan S, Selcan Akyol B, Ergul M, Baysan C. The effect of type of fluid on disease severity in acute pancreatitis treatment. Eur Rev Med Pharmacol Sci. (2021) 25:7460–7. doi: 10.26355/eurrev_202112_27443 [DOI] [PubMed] [Google Scholar]
- 50. Aggarwal A, Manrai M, Kochhar R. Fluid resuscitation in acute pancreatitis. World J Gastroenterol. (2014) 20:18092–103. doi: 10.3748/wjg.v20.i48.18092 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.