Abstract
Metabolite-based T-cell immunity is emerging as a major player in antimicrobial immunity, autoimmunity, and cancer. Here, small-molecule metabolites were identified to be captured and presented by the major histocompatibility complex class-I-related molecule (MR1) to T cells, namely mucosal-associated invariant T cells (MAIT) and diverse MR1-restricted T cells. Both MR1 and MAIT are evolutionarily conserved in many mammals, suggesting important roles in host immunity. Rational chemical modifications of these naturally occurring metabolites, termed altered metabolite ligands (AMLs), have advanced our understanding of the molecular correlates of MAIT T cell receptor (TCR)-MR1 recognition. This review provides a generalized framework for metabolite recognition and modulation of MAIT cells.
Introduction
Mucosal-associated invariant T (MAIT) cells are a unique subset of innate-like T cells that are evolutionarily conserved across mammalian species [1–3]. Typically, human MAIT cells express a semi-invariant αβ T-cell receptor (TCR), TRAV1–2–TRAJ33/20/12, paired with a limited TCR β-chain repertoire, including TRBV6 or TRBV20 genes [4–10]. During microbial infection, the major histocompatibility complex (MHC) class-I-related protein (MR1) binds to vitamin-B2 derivatives, originating from the biosynthesis pathway of riboflavin, and presents them on the surface of the antigen (Ag)- presenting cells [8,11]. These MR1–Ag complexes are recognized by MAIT TCRs and subsequently stimulate MAIT cells [12–14]. Consequently, MAIT cells are considered to play protective roles in antibacterial immunity [15,16], and have been implicated in auto-immunity and cancer [17–19], making them potentially attractive therapeutic targets [20–22].
During the last few years, several research groups have synthesized and functionally investigated analogs of MR1-binding ligands [23–28] (recent advances in the chemical synthesis of MR1-binding ligands are recently reviewed) [29]. For example, a systematic study was undertaken into how modifications of small-molecule heterocyclic metabolites can impact on T-cell function [23]. Namely, twenty synthetic altered metabolite ligands (AMLs) were investigated, in an analogous manner to altered peptide ligands (APLs) and MHC-restricted immunity [23]. Indeed, APLs have proven to be a useful approach to understand the concepts of MHC-restricted T-cell immunity, such as TCR signaling by agonist and antagonist APL [30], and we affirm that the AMLs approach will have crucial implications for future investigations of MAIT cell biology. Here, we review recent advances in the understanding of the MAIT TCR–MR1 axis.
MR1 presents diverse small-molecule metabolites
MR1 is a highly conserved Ag-presenting molecule that is ubiquitously expressed in all cells. However, MR1 upregulation at the cell surface correlates with Ag availability [31–37]. Initially, two classes of vitamin-B-derived compounds were identified as ligands able to be captured and presented by MR1 [11]. The first class included the photodegradation product of folic acid (vitamin B9), 6-formylpterin (6-FP), and its synthetic derivative, acetyl-6-formylpterin (Ac-6-FP). These metabolites have a bicyclic pterin-based structure with a chemically reactive formyl group (Figure 1). They upregulate MR1 to the cell surface, but do not stimulate the majority of MAIT cells. Both 6-FP and Ac-6-FP inhibit MAIT cell activity, but Ac-6-FP has a 100-fold greater inhibitor potency than 6-FP [6].
Figure 1.
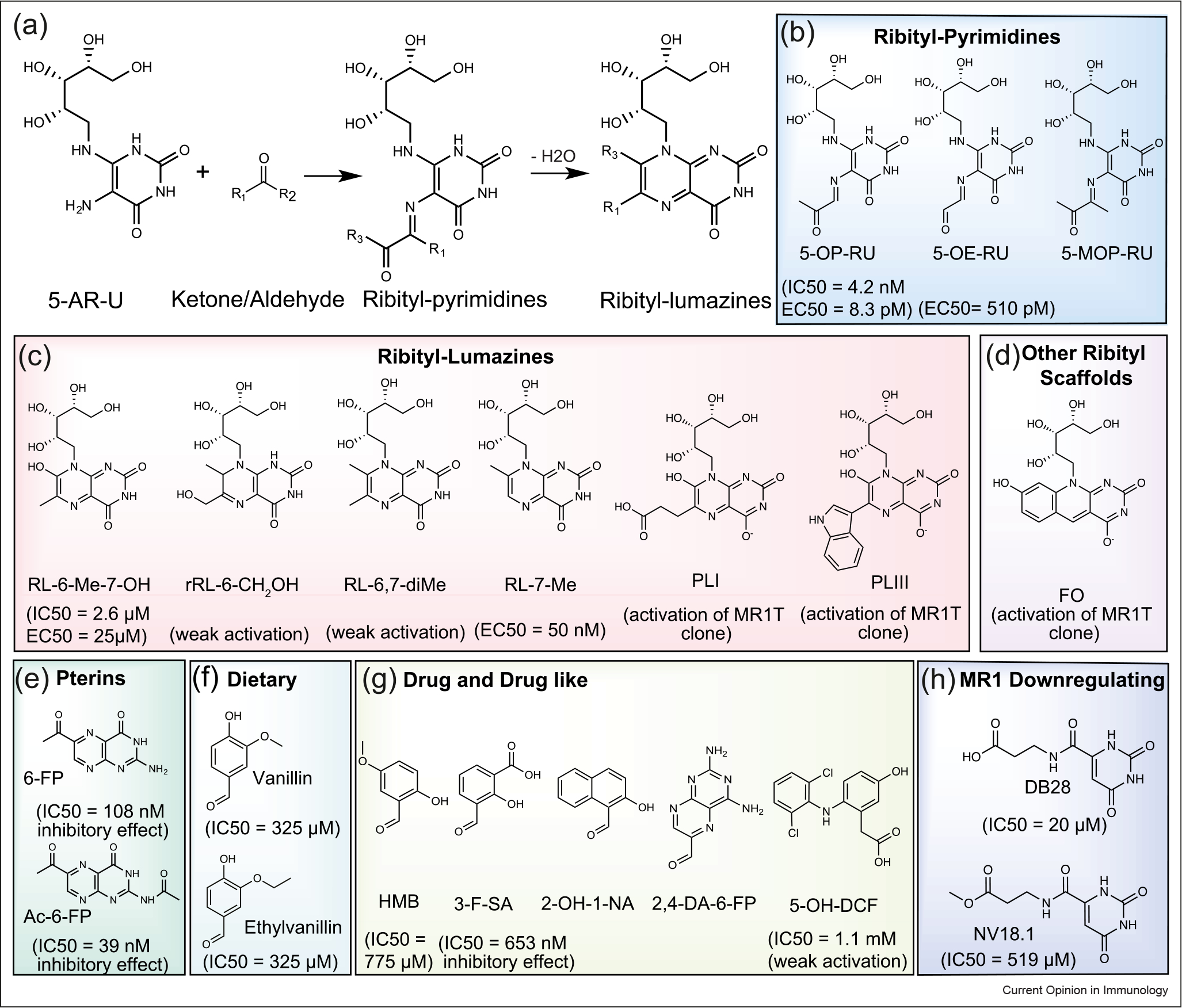
Broad range of MR1 Ags. (a) Condensation reactions of bacterially produced riboflavin intermediate 5-A-RU with reactive ketones/aldehydes producing pyrimidine-based Ags, which subsequently dehydrate to ribityllumazine compounds if not captured by MR1. (b–h) Chemical structures of different categories of MR1-binding Ags. (b–d) Riboflavin-based Ags. The MR1 ligand affinities (IC50) as measured by the fluorescence-based polarization assay and the activation potency (EC50) of MAIT cells are included. (b) Ribityl-pyrimidines: 5-OP-RU, 5-OE-RU, and 5-(1-methyl-2-oxopropylideneamino)-6-d-ribitylaminouracil (5-MOP-RU). (c) Ribityl-lumazines: RL-6-Me-7-OH, reduced 6-hydroxymethyl-8-d-ribityllumazine (rRL-6-CH2OH), 6,7-dimethyl-8-d-ribityllumazine (RL-6,7-diMe), and RL-7-Me, 6-(2-carboxyethyl)-7-hydroxy-8-ribityllumazine (PLI), and 6-(1H-indol-3-yl)-7-hydroxy-8-ribityllumazine (PLIII). (d) Other ribityl scaffolds: FO. (e) Folate-based metabolites, including 6-FP and Ac-6-FP. (f–h) Various classes of small-molecule scaffolds aside from vitamin-B derivatives, including (f) dietary compounds∷ Vallin, and Ethylvanillin; (g) drug-related compounds: HMB, 3-F-SA, 2-OH-1-NA, 2,4-DA-6-FP, and DCF; and (h) MR1 downregulated compounds: DB28 and NV18.1.
The second class of MR1 ligands was derived through a biosynthetic intermediate of riboflavin (vitamin B2). These metabolites are formed during infection from a plethora of distinct riboflavin-producing microbes, which can potently activate MAIT cells [8,11,38–40]. Vitamin B2 cannot be synthesized by mammals, thus, the riboflavin-based ligands are defined as a ‘molecular signature’ of infection. The microbially derived riboflavin intermediate, 5-amino-6-d-ribitylaminouracil (5-A-RU), can nonenzymatically react with ketones/aldehydes, such as methylglyoxal (pyruvaldehyde) and glyoxal, derived from bacterial metabolism or mammalian glycolysis to produce pyrimidine-based metabolites (Figure 1a–c). These highly potent and unstable ribityl-pyrimidines, such as 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil (5-OP-RU) and 5-(2-oxoethylideneamino)-6-d-ribitylaminouracil (5-OE-RU), can be captured and stabilized by MR1, via formation of a covalent Schiff base with Lys43 of MR1. However, they are also extremely unstable in water (5-OP-RU has half-life time of 88 min of physiological conditions [23]) and undergo cyclization to thermodynamically more stable ribityl-lumazines, such as 7-hydroxy-6-methyl-8-d-ribityllumazine (RL-6-Me-7-OH) and 7-methyl-ribityllumazine (RL-7-Me) [8]. Moreover, Harrif et al. identified new ribityl-lumazines as weak MAIT agonists, 6-(2-carboxyethyl)-7-hydroxy-8-ribityllumazine (photolumazine I (PLI)) and 6-(1H-indol-3-yl)-7-hydroxy-8-ribityllumazine (photolumazine III (PLIII)), as well as a new ribityl-based scaffold of 7,8-didemethyl-8-hydroxy-5-deazariboflavin (FO) (Figure 1c–d) [39•].
Apart from vitamin-B-related MR1 ligands, Keller et al. described a panel of drug and drug-like molecules with broad chemical structures that can be displayed by MR1 (Figure 1g) [41]. These included a diverse panel of mono- and bicyclic–chemical structures, such as 2-hydroxy-5-methoxybenzaldehyde (HMB), aspirin analogs (e.g. 3-formyl-salicylic acid (3-F-SA)), a photodegradation product of the chemotherapeutic aminopterin (2,4-diamino-6-formylpterdine (2,4-DA-6-FP)), the sirtinol inhibitor (2-hydroxy-1-naphthaldehyde (2-OH-1-NA)), and the anti-inflammatory drug diclofenac (DCF) and its metabolite 5-hydroxy-diclofenac (5-OH-DCF). Most pharmaceutical metabolites have so far not activated MAIT cells, but DCF metabolites weakly activated some MAIT TCRs. Further, in silico screening revealed two uracil-based compounds, 3-[(2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl) formamido] propanoic acid (DB28), and its ester analog, methyl 3-[(2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl) formamido] propanoate (NV18.1), that can bind the MR1 pocket (Figure 1h) [42•]. Most recently, having established a fluorescence-based polarization assay to measure affinity for MR1 ligands in vitro, two diet-derived MR1-binding molecules were identified, vanillin and ethylvanillin (Figure 1f) [43]. Interestingly, a recent report suggested that MR1 recognizes and presents nonmicrobial tumor-cell-derived metabolites, although the ligand identity is yet to be identified [18••].
Geometric features of MR1 binding pocket
X-ray crystal structures of the ternary TCR–MR1–Ags complexes have been published during the last few years, providing some molecular insights into the functions of MR1, MR1-binding ligands, and Ag recognition by the MAIT TCRs [5,6,8,11,23,38,41,44–46]. The MR1–β2m heterodimer complex possesses an Ag-binding cleft (~750Å3) composed of A′ and F′ pockets that are formed between the α1- and α2-helices and sitting atop an anti-parallel β-sheet. All previously recognized ligands were found to bind in the A′ pocket of MR1, where an ‘aromatic cradle’ is formed by aromatic and charged amino acid residues (Figure 2a–c). An unusual feature of the MR1-binding pocket is the ability of the epsilon–amino group of MR1–Lys43 to form a Schiff base with the carbonyl group of a small molecule Ag. MR1–Lys43 is located at the base of MR1-binding cleft and thus the associated ligands are buried deep within the relatively large MR1 cavity. Consequently, all identified MR1-binding ligands to date are not exposed very much on the MR1 surface to T-cell receptors. Intriguingly, some MR1 ligands such as the ribityl-lumazines cannot form a Schiff-base interaction with MR1–Lys43 due to the lack of a reactive carbonyl group and exhibit very low capacity to upregulate MR1 to the surface of Ag-presenting cells [8,11,23]. In the next sections, we will review the capability of the MR1 molecule to capture and display diverse classes of small-molecule metabolites and their recognition by MAIT TCRs.
Figure 2.
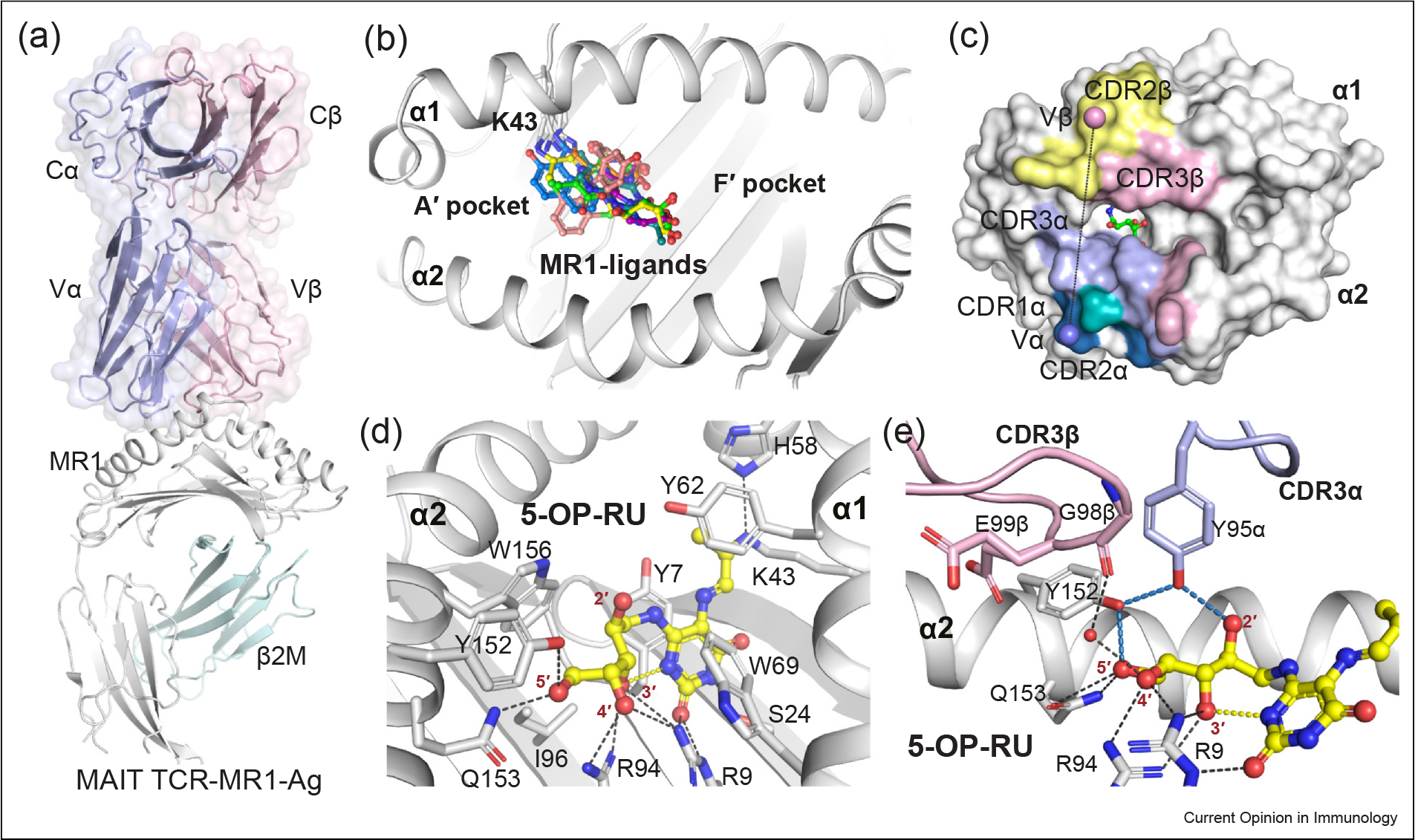
MAIT TCR recognition of MR1–pyrimidine-based metabolites. (a) Cartoon representation of the crystal structure of the ternary complex of the typical MAIT TRAV1–2+ A-F7 TCR–MR1–5-OP-RU (PDB: 6PUC). The constant (C) and variable (V) domains of the TCRα and TCRβ chains are shown as light-blue and light-pink surfaces, respectively. The MR1 and β2-microglobulin (β2M) molecules are shown as white and pale-cyan cartoons, respectively. (b) Surface representation of the MR1–Ag- binding A′ and F′ pockets that are formed between MR1 α1 and α2-helices. All recognized MR1 ligands (represented as colored sticks), to date, dock in the A′ pocket. (c) The footprint of the MAIT A-F7 TCR on the surface of MR1–5-OP-RU. The atomic footprint is colored according to the TCR segment making contact via its complementarity-determining region (CDR) loops colored as follows: CDR1α, teal; CDR2α, sky-blue; CDR3α, light-blue; frameworks of α-chain, dark-green; CDR1β, roseberry; CDR2β, pink; CDR3β, yellow-orange; and frameworks of β-chain, dark-gray. (d) Interactions of 5-OP-RU (yellow sticks) within the MR1-binding groove. MR1 α-helices and β-sheets are presented as white cartoon. (e) Interactions between the CDR3α and CDR3β loops of the MAIT A-F7 TCR and MR1–5-OP-RU. The relevant intramolecular H-bonds of 5-OP-RU are colored yellow. The MR1 pocket H-bonds are colored black, the TCR-related H-bonds are colored sky-blue. The CDR3α and CDR3β loop are colored as light-blue and light-pink, respectively. Waters are shown as red spheres. The structural illustrations in Figures 2, 3 and 4 were prepared using PyMOL Molecular Graphics System, Version 2.0, Schrodinger.
Mucosal-associated invariant T TCR recognition of ribityl-pyrimidine Ags
The most potent MAIT agonist identified to date is 5-OP-RU, where its α-imminocarbonyl forms a Schiff-base covalent bond with MR1–Lys43 [8]. The amine and carbonyl groups of the 5-OP-RU uracil ring form H-bond with Ser24 and Arg9, respectively, whereby the entire ring is sandwiched between MR1–Tyr7 and Tyr62 (Figure 2c). The ribityl moiety of 5-OP-RU is stabilized by H-bonding with MR1–Arg9, Arg94, Tyr152, and Gln153. In addition, the ribityl 3′-OH group interacts with the amine group of the uracil ring that together limits the movement of the ribityl moiety within the MR1-binding cleft.
The crystal structure of the typical MAIT TCR–MR1–5-OP-RU complexes shows that the TCR docks centrally atop the A′ pocket of MR1, and the α- and β-chains of the TCR sit over the α2- and α1-helices of MR1, respectively (Figure 2a). The buried surface area (BSA) at the interface between MAIT TCRs and MR1 varied between 1050 and 1200 Å2, with the TCR α- and β-chains contributing almost equally to the BSA of MAIT TCR–MR1 interfaces (Figure 2c). Variations in TRBV utilization within the human MAIT TCR repertoire, such as TRBV6–1, TRBV6–4, and TRBV20, are shown to modestly impact on TCR recognition of MR1. Indeed, most of the previously investigated MAIT TCRs exhibited similar affinities (KD 2–8 μM) to MR1–5-OP-RU protein as measured by surface plasmon resonance affinity-based binding studies [6]. Moreover, mutagenesis studies to date on MAIT TCRs established that no distinct TCRβ residue was essential for MAIT cell activation [7]. Altogether, this revealed a fine-tuning role of the MAIT TCR β-chain on the MAIT TCR–MR1 recognition axis, whereas the semi-invariant TRAV1–2 α-chain plays a prominent role in MAIT stimulation.
In the crystal structure of MAIT TRAV1–2+ TCR–MR1–5-OP-RU complexes, 5-OP-RU makes a very small contribution (less than 1%) to the exposed surface area of the MR1–5-OP-RU binary complex for TCR engagement (Figure 2c–e). The ribityl 2′-OH group forms a single H-bond interaction with the evolutionary conserved (TRAJ33/12/20-encoded) Tyr95α from the CDR3α loop of the TRAV1–2 α-chain of MAIT TCRs (Figure 2e). This raised important questions regarding whether the interaction of the Tyr95α of MAIT TCR with the ligand is the sole regulating factor for MAIT activation. If so, why is 5-OP-RU the most potent MAIT agonist, compared with ribityl-lumazines that also carry the same ribityl chain? To answer these questions, we recently investigated how twenty closely related synthetic analogs of 5-OP-RU (or AMLs), impact on the MAIT TCR–MR1 recognition and MAIT stimulation [23,25].
This large panel of AMLs along with intensive biochemical, MR1 upregulation, and MAIT activation assays allowed some general observations to be made on the MAIT TCR–MR1 axis: (1) the precise chemical structure of the Ag ligand and its capacity to form a Schiff-base adduct with MR1 has a profound effect on MAIT-activating potency and propensity to upregulate MR1 cell surface expression. (2) The ribityl moiety of MR1 ligands is required for potent activation of MAIT cells but it is not essential to MR1 upregulation. (3) Removal of the 2′-OH or 3′-OH positions of the ribityl moiety significantly reduced MAIT TCR recognition, whereas removing either the ribityl 4′- or 5′-hydroxyl moieties could be compensated by the others for stimulating MAIT cells. (4) Indeed, no apparent correlation was found between ligand propensity to upregulate MR1 cell surface expression and ligand potency in activating MAIT cells (5).
The structural studies on MAIT TCR–MR1–AML complexes reveal a network of H-bond interactions, featuring an ‘interaction triad’ structural motif that is formed between the Tyr95α from CDR3α loop of the MAIT TCRs, the ribityl moiety, and Tyr152 of MR1 [6,23,38]. This interaction triad is a limiting molecular feature for MAIT stimulation, with its partial or complete disruption resulting in moderate or nonstimulatory MAIT Ags, respectively. Further, site-directed mutagenesis of Tyr95α significantly impacted the MAIT recognition and activation [38]. This suggested that conservation of the ‘interaction triad’ is a critical molecular feature for MAIT stimulation. In conclusion, MAIT cell activation potency is orchestrated by a network of dynamic compensatory interactions within, and nearby, this MAIT TCR–MR1–Ag ‘interaction triad’. New AML analogs have also been described with modifications in the rings of the ligands [24]. It would be interesting to solve the structures of these AMLs to see how they impact the MAIT TCR recognition and the activation triad.
Mucosal-associated invariant T TCR recognition of noncovalent agonists
MAIT-stimulating ligands with moderate potency include the microbial ribityl-lumazines and pharmaceutical DCF metabolites (Figure 1c and g). Structural data for RL-6-Me-7-OH and 5-OH-DCF bound to MR1 revealed that neither formed a Schiff-base adduct with MR1–Lys43 [11,41]. However, Lys43 maintained a similar orientation within the MR1 cleft as when covalently bound to Schiff-based forming ligands (Figure 3). The ribityl chain of 5-OP-RU and of lumazine derivative RL-6-Me-7-OH was in identical positions in the Ag-binding pocket of MR1, with a conserved interaction triad network. Nevertheless, the lumazines were orders- of-magnitude less-potent MAIT agonists than the ribityluracil adducts (Figure 3b). This is likely due to the poor propensity of ribityl-lumazines to upregulate surface MR1 levels, attributable to their inability to form a covalent Schiff base with MR1.
Figure 3.
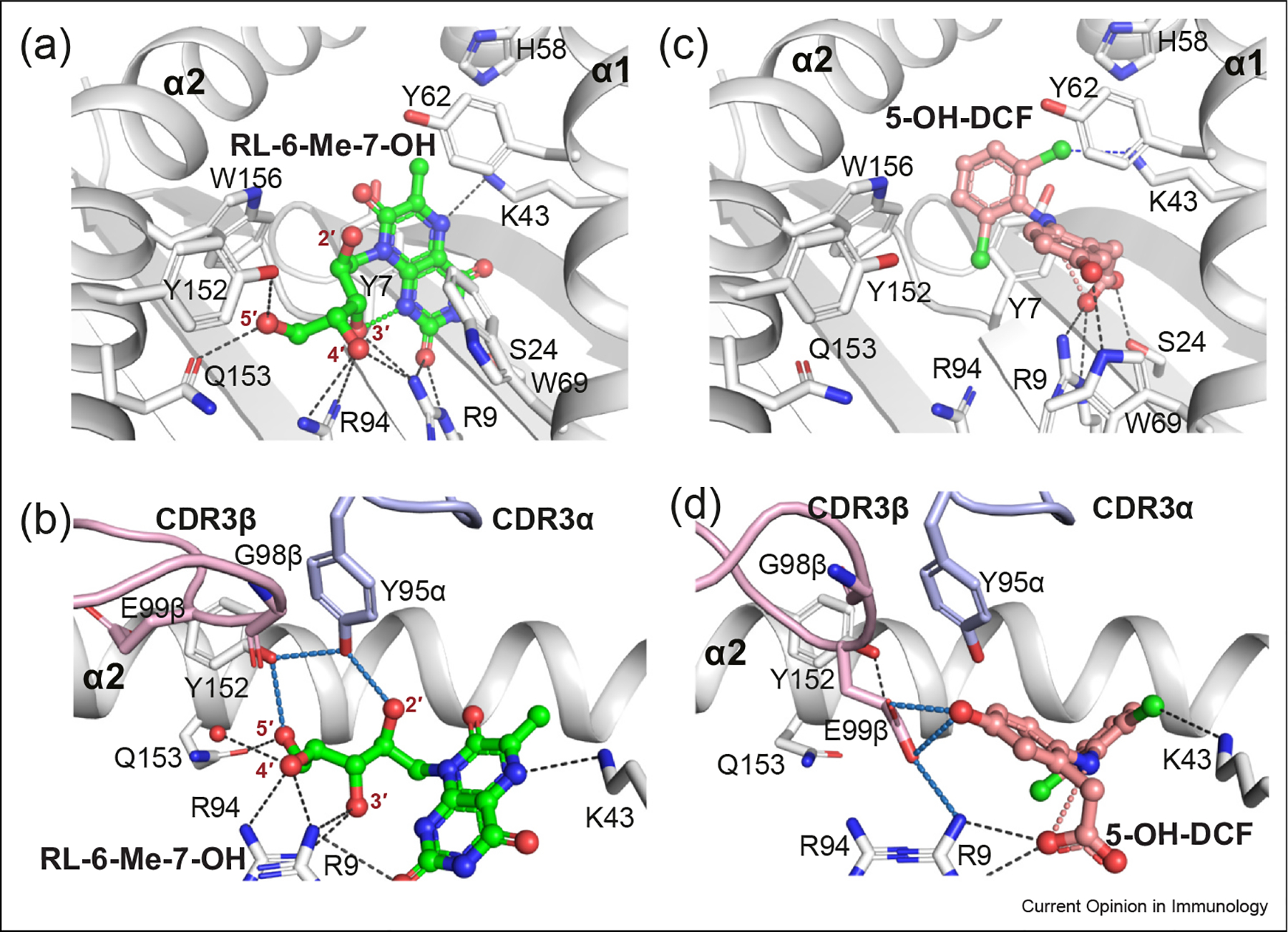
MR1 presentation of noncovalent MAIT agonists. The RL-6-Me-7-OH (a–b) and 5-OH-DCF (c–d) metabolites are shown as colored sticks in the MR1-binding pocket (upper panels) and their interactions with A-F7 MAIT TCR (bottom panels) (PDB: 4L4V and 5U72, respectively). RL-6-Me-7-OH and 5-OH-DCF ligands and their intramolecular H-bonds are colored green and salmon, respectively. Halogen bonds are colored in blue. H-bonds, CDR loops are colored as in Figure 2.
The crystal structure of MAIT A-F7 TCR–MR1–5-OH-DCF shows that the binding mode and bonding contacts of 5-OH-DCF with MR1 differ from the interactions with the riboflavin-based metabolites, where Tyr7 and Trp69 residues had undergone conformational changes to accommodate the ligand (Figure 3c–d). Regarding the TCR contacts, the structural data show a deformation of the interaction triad network at the TCR–MR1 interface, with the 5-OH-DCF forming polar interactions with the CDR3β loop, but not with either TCR CDR3α or MR1–Tyr152, resulting in modest MAIT activation (Figure 3d). This is consistent with variations in the activation potency 5-OH-DCF in diverse Jurkat. MAIT TCR cell lines [41].
MR1 presentation of MAIT non-stimulating metabolites
Through in silico screening and in vitro functional assays, diverse sets of MAIT nonstimulating, and even antagonist metabolites have been identified [6,11,41,42]. These include pterin-related compounds, such as 6-FP, Ac-6-FP, and 2,4-DA-6-FP, as well as drug and drug-related metabolites, such as 3-F-SA, 5-F-SA, 2-OH-1-NA, HMB, and the diet-derived natural products, vanillin and ethylvanillin (Figure 1e–g), which can up-regulate MR1 cell surface expression without activating MAIT cells. The crystal structures of their complexes with MAIT TCR–MR1 revealed their ability to covalently bind MR1–Lys43 (Figure 4). These distinct compounds were captured within the A′ cleft of MR1, are wedged through hydrophobic interactions between Tyr7 and Tyr62 that characteristically form the aromatic cradle. By contrast, the crystal structures of MAIT–MR1–DB28 and NV18.1 show a lack of covalent MR1–Lys43 bond, which accentuates the importance of forming a Schiff-base adduct to refold MR1 that promotes MR1 trafficking to the cell surface (Figures 1h and 4f). This demonstrated that the MR1 pocket was sufficiently adaptable to fit diverse chemical scaffolds of mono- and biheterocyclic ligands.
Figure 4.
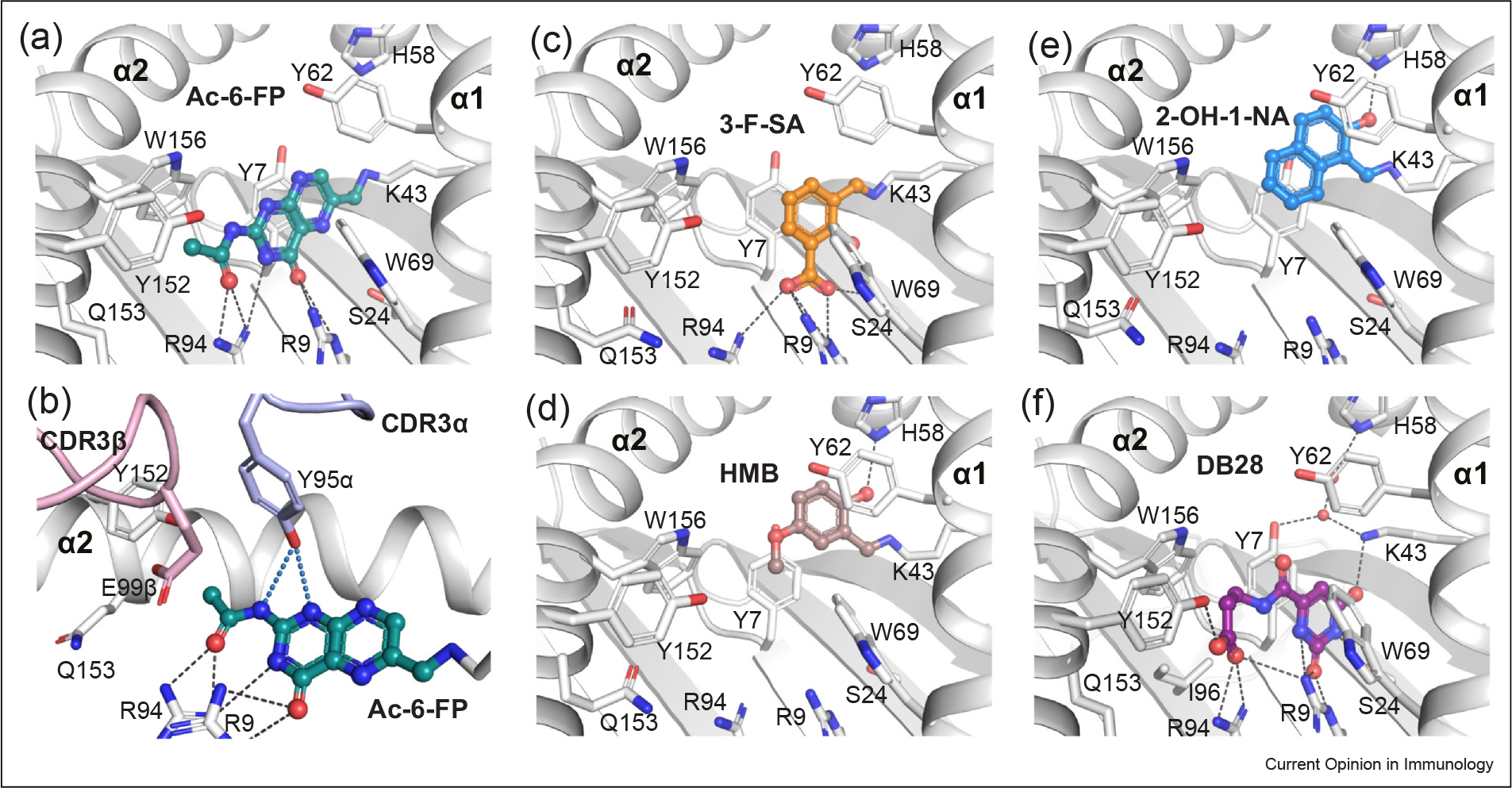
MR1 presentation of small-molecule MAIT nonstimulating ligands. (a–b) Capture of Ac-6-FP ligand (deep-teal sticks) within the MR1-binding cleft (a) and its interaction with the CDR loops of MAIT TCR (b). (c–f) Docking of diverse chemical identities aside from vitamin-B derivatives within the MR1 groove: (c) 3-F-SA (orange), (d) HMB (brown), (e) 2-OH-1-NA (marine), and (f) DB28 (purple). Coloring as in Figure 3 2. Crystal structures of MAIT–MR1 ligands: Ac-6-FP (PDB: 4PJ5), 3-F-SA (PDB: 5U6Q), HMB (PDB: 5U2V), 2-OH-1-NA (PDB: 5U16), DB28 (PDB: 6PVC), and NV18.1 (PDB: 6PVD), used in figure.
Within the MAIT TCR–MR1-Ac-6-FP crystal structure, Ac-6-FP forms a H-bond with TCR–Tyr95α, but no interactions were observed between MR1–Tyr152α and either the ligand or TCR–Tyr95α and therefore the TCR–MR1 interaction triad was distorted (Figure 4b). Similarly, all of these MAIT nonstimulating compounds completely disrupted the TCR–MR1 interaction triad, resulting in the absence of MAIT activation by these ligands.
Concluding remarks
The repertoire of the MR1 ligandome is broader than we previously thought, with the MR1-binding cleft displaying enough plasticity to accommodate a wide array of vitamin metabolites and small heterocyclic molecules. In this review, we summarized key factors relating to Ag presentation by MR1 to MAIT cells. Ongoing chemical, structural, and functional studies will provide the rationale for designing MAIT cell activating and inhibiting ligands for potential therapy of immunological and other disorders.
Acknowledgements
This work is supported by grants from the National Health and Medical Research Council of Australia (NHMRC 1125493, 1113293), and the National Institutes of Health (NIH) RO1 AI148407-01A1. W.A. is supported by an Australian ARC Discovery Early Career Researcher Award (DECRA) fellowship (DE220101491). D.P.F. is supported by CE200100012. D.P.F and J.R. are supported by Investigator Grants from the NHMRC (2009551, 2008981) and J.R. is supported by an ARC Discovery Project (DP220102401).
Footnotes
Declaration of Competing Interest
J.M., D.P.F., and J.R. are inventors on patents describing MR1 ligands and MR1-tetramer reagents.
Data Availability
No data were used for the research described in the article.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Tilloy F, Treiner E, Park S-H, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O: An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib–restricted α/β T cell subpopulation in mammals. J Exp Med 1999, 189:1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O: Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003, 422:164–169. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Koay HF, McCluskey J, Gherardin NA: The biology and functional importance of MAIT cells. Nat Immunol 2019, 20:1110–1128. [DOI] [PubMed] [Google Scholar]
- 4.Eckle SB, Birkinshaw RW, Kostenko L, Corbett AJ, McWilliam HE, Reantragoon R, Chen Z, Gherardin NA, Beddoe T, Liu L, et al. : A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J Exp Med 2014, 211:1585–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gherardin NA, Keller AN, Woolley RE, Le Nours J, Ritchie DS, Neeson PJ, Birkinshaw RW, Eckle SB, Waddington JN, Liu L, et al. : Diversity of T cells restricted by the MHC class I-related molecule MR1 facilitates differential antigen recognition. Immunity 2016, 44:32–45. [DOI] [PubMed] [Google Scholar]
- 6.Eckle SBG, Birkinshaw RW, Kostenko L, Corbett AJ, McWilliam HEG, Reantragoon R, Chen Z, Gherardin NA, Beddoe T, Liu L, et al. : A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J Exp Med 2014, 211:1585–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SBG, Uldrich AP, Birkinshaw RW, Patel O, et al. : Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 2013, 210:2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ••.Corbett AJ, Eckle SBG, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, et al. : T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014, 509:361–365. [DOI] [PubMed] [Google Scholar]; Described the microbial MAIT agonists that derived from riboflavin biosynthesis pathway in the riboflavin producing microbes.
- 9.Gold MC, Lewinsohn DM: Co-dependents: MR1-restricted MAIT cells and their antimicrobial function. Nat Rev Micro 2013, 11:14–19. [DOI] [PubMed] [Google Scholar]
- 10.Lepore M, Kalinichenko A, Calogero S, Kumar P, Paleja B, Schmaler M, Narang V, Zolezzi F, Poidinger M, Mori L, et al. : Functionally diverse human T cells recognize non-microbial antigens presented by MR1. eLife 2017, 6:e24476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, et al. : MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491:717–723. [DOI] [PubMed] [Google Scholar]
- 12.Awad W, Le Nours J, Kjer-Nielsen L, McCluskey J, Rossjohn J: Mucosal-associated invariant T cell receptor recognition of small molecules presented by MR1. Immunol Cell Biol 2018, 96:588–597. [DOI] [PubMed] [Google Scholar]
- 13.Eckle SBG, Corbett AJ, Keller A, Chen Z, Godfrey DI, Liu L, Mak JYM, Fairlie DP, Rossjohn J, McCluskey J: Recognition of Vitamin B precursors and byproducts by Mucosal Associated Invariant T cells. J Biol Chem 2015, 290:30204–30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souter MNT, Awad W, Li S, Pediongco TJ, Meehan BS, Meehan LJ, Tian Z, Zhao Z, Wang H, Nelson A, et al. : CD8 coreceptor engagement of MR1 enhances antigen responsiveness by human MAIT and other MR1-reactive T cells. J Exp Med 2022, 219:e20210828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howson LJ, Salio M, Cerundolo V: MR1-restricted mucosal-associated invariant T cells and their activation during infectious diseases. Front Immunol 2015, 6:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meermeier EW, Harriff MJ, Karamooz E, Lewinsohn DM: MAIT cells and microbial immunity. Immunol Cell Biol 2018, 96:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouxel O, Da silva J, Beaudoin L, Nel I, Tard C, Cagninacci L, Kiaf B, Oshima M, Diedisheim M, Salou M, et al. : Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat Immunol 2017, 18:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. ••.Crowther MD, Dolton G, Legut M, Caillaud ME, Lloyd A, Attaf M, Galloway SAE, Rius C, Farrell CP, Szomolay B, et al. : Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat Immunol 2020, 21:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]; Described the role of MR1 restricted T cells in caner cells and suggests that MR1 may present some cancer specific ligands.
- 19.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, et al. : The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinks TSC, Marchi E, Jabeen M, Olshansky M, Kurioka A, Pediongco TJ, Meehan BS, Kostenko L, Turner SJ, Corbett AJ, et al. : Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep 2019, 28:3249–3262 e3245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Provine NM, Klenerman P: MAIT cells in health and disease. Annu Rev Immunol 2020, 38:203–228. [DOI] [PubMed] [Google Scholar]
- 22.Constantinides MG, Link VM, Tamoutounour S, Wong AC, Perez-Chaparro PJ, Han S-J, Chen YE, Li K, Farhat S, Weckel A, et al. : MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 2019, 366:eaax6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ••.Awad W, Ler GJM, Xu W, Keller AN, Mak JYW, Lim XY, Liu L, Eckle SBG, Le Nours J, McCluskey J, et al. : The molecular basis underpinning the potency and specificity of MAIT cell antigens. Nat Immunol 2020, 21:400–411. [DOI] [PubMed] [Google Scholar]; Unearthed general principles that underpin metabolite recognition by MR1 and activation of MAIT cells.
- 24.Braganza CD, Shibata K, Fujiwara A, Motozono C, Sonoda KH, Yamasaki S, Stocker BL, Timmer MSM: The effect of MR1 ligand glyco-analogues on mucosal-associated invariant T (MAIT) cell activation. Org Biomol Chem 2019, 17: 8992–9000. [DOI] [PubMed] [Google Scholar]
- 25.Mak JYW, Xu W, Reid RC, Corbett AJ, Meehan BS, Wang H, Chen Z, Rossjohn J, McCluskey J, Liu L, et al. : Stabilizing short-lived Schiff base derivatives of 5-aminouracils that activate mucosal-associated invariant T cells. Nat Commun 2017, 8:14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braganza CD, Motozono C, Sonoda K-h, Yamasaki S, Shibata K, Timmer MSM, Stocker BL: Agonistic or antagonistic mucosal-associated invariant T (MAIT) cell activity is determined by the 6-alkylamino substituent on uracil MR1 ligands. Chem Commun 2020, 56:5291–5294. [DOI] [PubMed] [Google Scholar]
- 27.Lange J, Anderson RJ, Marshall AJ, Chan STS, Bilbrough TS, Gasser O, Gonzalez-Lopez C, Salio M, Cerundolo V, Hermans IF, et al. : The chemical synthesis, stability, and activity of MAIT cell prodrug agonists that access MR1 in recycling endosomes. ACS Chem Biol 2020, 15:437–445. [DOI] [PubMed] [Google Scholar]
- 28.Ler GJM, Xu W, Mak JYW, Liu L, Bernhardt PV, Fairlie DP: Computer modelling and synthesis of deoxy and monohydroxy analogues of a ribitylaminouracil bacterial metabolite that potently activates human T cells. Chemistry 2019, 25:15594–15608. [DOI] [PubMed] [Google Scholar]
- 29.Mak JYW, Liu L, Fairlie DP: Chemical modulators of mucosal associated invariant T cells. Acc Chem Res 2021, 54: 3462–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloan-Lancaster J, Allen PM: Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol 1996, 14:1–27. [DOI] [PubMed] [Google Scholar]
- 31.McWilliam HEG, Eckle SBG, Theodossis A, Liu L, Chen Z, Wubben JM, Fairlie DP, Strugnell RA, Mintern JD, McCluskey J, et al. : The intracellular pathway for the presentation of vitamin B-related antigens by the antigen-presenting molecule MR1. Nat Immunol 2016, 17:531–537. [DOI] [PubMed] [Google Scholar]
- 32.Harriff MJ, Karamooz E, Burr A, Grant WF, Canfield ET, Sorensen ML, Moita LF, Lewinsohn DM: Endosomal MR1 trafficking plays a key role in presentation of Mycobacterium tuberculosis ligands to MAIT cells. PLoS Pathog 2016, 12:e1005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang S, Gilfillan S, Kim S, Thompson B, Wang X, Sant AJ, Fremont DH, Lantz O, Hansen TH: MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J Exp Med 2008, 205:1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howson LJ, Awad W, von Borstel A, Lim HJ, McWilliam HEG, Sandoval-Romero ML, Majumdar S, Hamzeh AR, Andrews TD, McDermott DH, et al. : Absence of mucosal-associated invariant T cells in a person with a homozygous point mutation in MR1. Sci Immunol 2020, 5:eabc9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozemuller E, Eckle SBG, McLaughlin I, Penning M, Mulder W, de Bruin H, van Wageningen S: MR1 encompasses at least six allele groups with coding region alterations. HLA 2021, 98:509–516. [DOI] [PubMed] [Google Scholar]
- 36.McShan AC, Devlin CA, Papadaki GF, Sun Y, Green AI, Morozov GI, Burslem GM, Procko E, Sgourakis NG: TAPBPR employs a ligand-independent docking mechanism to chaperone MR1 molecules. Nat Chem Biol 2022, 18:859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McWilliam HEG, Mak JYW, Awad W, Zorkau M, Cruz-Gomez S, Lim HJ, Yan Y, Wormald S, Dagley LF, Eckle SBG, et al. : Endoplasmic reticulum chaperones stabilize ligand-receptive MR1 molecules for efficient presentation of metabolite antigens. Proc Natl Acad Sci USA 2020, 117:24974–24985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel O, Kjer-Nielsen L, Le Nours J, Eckle SBG, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, et al. : Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun 2013, 4:2142. [DOI] [PubMed] [Google Scholar]
- 39. •.Harriff MJ, McMurtrey C, Froyd CA, Jin H, Cansler M, Null M, Worley A, Meermeier EW, Swarbrick G, Nilsen A, et al. : MR1 displays the microbial metabolome driving selective MR1-restricted T cell receptor usage. Sci Immunol 2018, 3:eaao2556. [DOI] [PMC free article] [PubMed] [Google Scholar]; Expanded our knowledge on the broad MR1 ligandome of microbial Ags that could impact MAIT biology.
- 40.Soudais C, Samassa F, Sarkis M, Le Bourhis L, Bessoles S, Blanot D, Hervé M, Schmidt F, Mengin-Lecreulx D, Lantz O: In vitro and in vivo analysis of the gram-negative bacteria–derived riboflavin precursor derivatives activating mouse MAIT cells. J Immunol 2015, 194:4641–4649. [DOI] [PubMed] [Google Scholar]
- 41. ••.Keller AN, Eckle SBG, Xu W, Liu L, Hughes VA, Mak JYW, Meehan BS, Pediongco T, Birkinshaw RW, Chen Z, et al. : Drugs and drug-like molecules can modulate the function of mucosal-associated invariant T cells. Nat Immunol 2017, 18:402–411. [DOI] [PubMed] [Google Scholar]; Discovered drugs and drug-metabolites that can modulate MAIT functions.
- 42. •.Salio M, Awad W, Veerapen N, Gonzalez-Lopez C, Kulicke C, Waithe D, Martens AWJ, Lewinsohn DM, Hobrath JV, Cox LR, et al. : Ligand-dependent downregulation of MR1 cell surface expression. Proc Natl Acad Sci 2020, 117:10465–10475. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovered the first non-microbial ligand which retains MR1 in the ER in an immature ligand-receptive form and competitively inhibits stimulatory ligands.
- 43.Wang CJH, Awad W, Liu L, Mak JYW, Veerapen N, Illing PT, Purcell AW, Eckle SBG, McCluskey J, Besra GS, et al. : Quantitative affinity measurement of small molecule ligand binding to Major Histocompatibility Complex class-I related protein 1 MR1. J Biol Chem 2022, 298:102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Sagaseta J, Dulberger CL, McFedries A, Cushman M, Saghatelian A, Adams EJ: MAIT recognition of a stimulatory bacterial antigen bound to MR1. J Immunol 2013, 191:5268–5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López-Sagaseta J, Dulberger CL, Crooks JE, Parks CD, Luoma AM, McFedries A, Van Rhijn I, Saghatelian A, Adams EJ: The molecular basis for Mucosal-Associated Invariant T cell recognition of MR1 proteins. Proc Natl Acad Sci USA 2013, 110:E1771–E1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Nours J, Gherardin NA, Ramarathinam SH, Awad W, Wiede F, Gully BS, Khandokar Y, Praveena T, Wubben JM, Sandow JJ, et al. : A class of gammadelta T cell receptors recognize the underside of the antigen-presenting molecule MR1. Science 2019, 366:1522–1527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.


