Abstract
Parenting behaviors and neighborhood environment influence the development of adolescents’ brains and behaviors. Simultaneous trajectories of brain and behavior, however, are understudied, especially in these environmental contexts. In this four-wave study spanning 9–18 years of age (N=224 at baseline, N=138 at final assessment) we used longitudinal k-means clustering to identify clusters of participants with distinct trajectories of uncinate fasciculus (UF) fractional anisotropy (FA) and anxiety symptoms; we examined behavioral outcomes and identified environmental factors that predicted cluster membership. We identified three clusters of participants: 1) high UF FA and low symptoms (“low-risk”); 2) low UF FA and high symptoms (“high-risk”); and 3) low UF FA and low symptoms (“resilient”). Adolescents in disadvantaged neighborhoods were more likely to be in the resilient than high-risk cluster if they also experienced maternal warmth. Thus, neighborhood disadvantage may confer neural risk for psychopathology that can be buffered by maternal warmth, highlighting the importance of considering multiple environmental influences in understanding emotional and neural development in youth.
Keywords: Uncinate fasciculus, Neighborhood disadvantage, Parenting
Highlights
-
•
We longitudinally k-means clustered anxiety symptoms and uncinate fasciculus FA.
-
•
A resilient cluster of adolescents had high uncinate FA and low anxiety symptoms.
-
•
Resilient participants had low internalizing and high adaptability.
-
•
Resilient participants lived in disadvantaged neighborhoods, but had warm parents.
-
•
Disadvantage was characterized by socioeconomic status, pollutants, and health.
In early childhood, when the brain is most plastic and behavior is developing rapidly, developmental processes are strongly influenced by the environment, particularly the immediate home environment, including relationships with parental figures. In this context, parenting behaviors characterized by warmth and support have been found to be associated with healthier emotional functioning in children (Chen et al., 2019, Khaleque, 2013) and to promote resilience against the development of academic, social, and psychological problems related to experiencing adversity (Vazquez et al., 2023). In contrast, harsh and controlling parenting has been linked to increased levels of internalizing (Pinquart and Gerke, 2019), particularly anxiety (Liu and Wang, 2020), and externalizing symptoms (Pinquart, 2017). As Hyde et al. (2020) note, however, as children age, more distal environmental factors become increasingly influential. In particular, neighborhood socioeconomic disadvantage, which is often used to represent neighborhood quality, has been found to be associated with poor mental health in children, including anxiety, mood, and conduct disorders (Generaal et al., 2019, Sundquist et al., 2015). Researchers have also found that other aspects of “neighborhood,” including violence (Saxbe et al., 2018), air pollution (Manczak et al., 2022), and water contaminants (Manczak et al., 2020) are associated with negative mental health outcomes in children and adolescents. Conversely, proximity to parks (Reuben et al., 2020) and neighborhood social cohesion (Fletcher et al., 2019) have been found to promote psychological resilience, or positive mental health, despite facing adversity. Despite the likelihood that these neighborhood factors co-occur, however, few investigators have considered simultaneously multiple facets of neighborhood disadvantage in relation to child development, making it difficult to ascertain specific and cumulative effects of neighborhood-level factors on child functioning.
In understanding how context – both the proximal parenting environment and more distal neighborhood factors – are related to mental health, it is important to recognize the rapid development of the brain in childhood and adolescence and its sensitivity to external influences. Several studies suggest that parenting and envirionment, separately, are associated with alterations in regions implicated in emotional and cognitive functioning, including those within the prefrontal cortex, limbic regions (e.g., the amygdala, hippocampus), and the structural and functional connectivity between them. For example, aggressive parenting has been linked to altered structural development of frontal and limbic regions in adolescence (Whittle et al., 2011, Whittle et al., 2016). Further, low levels of parental warmth have been associated with altered fronto-limbic connectivity in youth (Miller et al., 2021) and with larger amygdala volume (Tottenham et al., 2010). Low parental warmth has also been associated with decreased fractional anisotropy (FA; i.e., a proxy measure for white matter structural organization) of the uncinate fasciculus (UF), a white matter tract that connects the frontal cortices with the limbic system (Eluvathingal et al., 2006). Similarly, more distal factors, such as neighborhood socioeconomic disadvantage, have been associated with thinner brain cortex (Miller et al., 2022, Rakesh et al., 2022), smaller cortical and subcortical volume (Hackman et al., 2021), alterations in brain network connectivity (Rakesh, Seguin, et al., 2021, 2021), limbic response to negative stimuli (Huggins et al., 2022), and anomalous white matter microstructure (Bell et al., 2021). Importantly, many of these neural alterations, in turn, have been linked to poor emotional and cognitive functioning in adolescence (Hackman et al., 2021, Miller et al., 2022, Rakesh et al., 2021). Other environmental factors have also been related to anomalous brain structure and function. For example, proximity to green spaces has been associated positively, and air pollution negatively, with alterations in brain volume (Beckwith et al., 2020, Dadvand et al., 2018), and community violence has been related to altered frontal-limbic volume and connectivity (Saxbe et al., 2018).
In this context, investigators have focused on the UF as a particularly important brain structure in emotional development. This white matter tract has been implicated in emotion regulation, learning, and memory (Olson et al., 2015). FA is a measure of the degree to which water in the tract diffuses in one direction, as opposed to equally in all directions; this metric has been used as a proxy for the microstructural structural organization of white matter tracts (Mori, 2007). Further, FA has been found to be sensitive to age-related changes in white matter tracts, making it a particularly salient metric when examining developmental questions in samples of youth (Guo et al., 2022). The UF is relatively slow to mature. Findings from cross-sectional studies suggest that UF FA values increase across childhood and adolescence, peak in adulthood, and then decline (Lebel et al., 2008, Lebel et al., 2012). In contrast, tracts such as the corpus callosum (CC) seem to mature earlier, in adolescence (Lebel et al., 2012). Therefore, the UF may therefore be more susceptible to disruptive experiences and environmental factors throughout childhood and adolescence than are areas and tracts of the brain that develop more quickly. Decreased FA of the UF has been associated with symptoms of both anxiety and depression (Hanson et al., 2015, Xu et al., 2023) and with general risk for the development of psychopathology (Versace et al., 2018). In particular, the UF has been implicated in the development of anxiety disorders (Lee and Lee, 2020) and, in fact, has been posited to be a possible target for intervention and prevention of anxiety disorders (Linke, 2019). We know little, however, about how the UF and symptoms of anxiety develop concurrently. Notably, the UF has been found to be sensitive to context and experience; specifically, adolescents who have experienced greater exposure to early life stress have been found to have poorer UF integrity (Hanson et al., 2015, Ho et al., 2017), which in turn was associated with more anxiety symptoms (Ho et al., 2017). Further, high levels of air pollutants have recently been linked with poorer UF integrity in children (Lubczyńska et al., 2020). Two recent studies have now reported associations between neighborhood socioeconomic disadvantage and poorer UF integrity: whereas Bell et al. (2021) did not find specificity in these effects with respect to community violence, Kulla et al., (2023) found specific effects of neighborhood poverty on UF integrity. Neither of these studies, however, examined the effects of parenting behaviors or neighborhood levels of pollution on brain structure; further, they relied on one timepoint of UF integrity.
As noted above, it is important to recognize that parenting and neighborhood influences do not occur in isolation. Neighborhood socioeconomic status (SES) is generally associated with family SES, which can influence parenting behaviors through difficulties in parental mental health (see Conger et al., 2010 for a review). Interestingly, investigators have not found a strong association between parenting behaviors and neighborhood disadvantage (e.g., Demidenko et al., 2021; Roos et al., 2021). In fact, family SES and positive parenting behaviors have been posited to buffer the negative effects of neighborhood disadvantage on child outcomes and promote the ability to adapt to difficult circumstances. For example, associations between neighborhood disadvantage and both decreased cortical thickness (Rakesh et al., 2022) and alterations in network connectivity (Rakesh, Zalesky, et al., 2021) were attenuated in youth with higher family SES. Further, the associations of neighborhood disadvantage on frontolimbic structural development (Whittle et al., 2017), poor health (Mrug et al., 2022), and levels of internalizing and externalizing symptoms (Flouri et al., 2015) have been found to be buffered by positive parenting behaviors, including affection, support, and warmth. Collectively, these findings highlight the importance of considering multiple proximal and distal environmental factors in the development of child and adolescent emotional development.
The present study was designed to examine the associations between the UF, symptoms of anxiety, which research consistently links with UF integrity, and neighborhood and family environments. Specifically, our goals were to 1) delineate the concurrent development of UF fractional anisotropy (FA) and anxiety symptoms across three time points in adolescence; 2) examine the nature of associations among UF FA, anxiety, parenting, and neighborhood disadvantage; and 3) explore which aspects of neighborhood disadvantage in particular are associated with the development of the UF FA and anxiety symptoms. We used longitudinal k-means clustering, a data-driven approach that allows us to group participants based on simultaneous trajectories of multiple variables. We clustered participants into groups that have similar trajectories of UF microstructure and anxiety symptoms. We then tested whether cluster membership was associated with late adolescent symptoms of internalizing, externalizing, and adaptability, or the ability to adjust and cope with difficult circumstances. We also conducted multinomial logistic regression analyses to examine differences in these participant clusters as a function of neighborhood disadvantage and parenting warmth. We hypothesized that we will identify a “low-risk” cluster of participants with high UF FA and low anxiety symptoms, and, further, that these participants will be characterized by having experienced high maternal warmth and living in more advantaged communities. In addition, we hypothesized that we will also identify a “high-risk” cluster of participants with low UF FA and high anxiety symptoms, characterized by having experienced low maternal warmth and living in more disadvantaged neighborhoods. We also hypothesized that maternal warmth will interact with neighborhood disadvantage, such that participants living in disadvantaged neighborhoods who also report high maternal warmth will be more likely to be in the low-risk cluster than the high-risk cluster. We included race as a covariate in analyses predicting cluster membership. Historical inequities resulting in racial residential segregation may not be entirely captured by census tract-level data. As reviewed by Riley (2018), physical distance encapsulated in the bounds of a census tract does not equate to access to resources or lived experiences of social and structural racism, which are often linked to health disparities. Based on these factors, we hypothesized that participants who identify as belonging to historically marginalized racial groups (i.e., Black, Hispanic, and Asian) will be more likely to belong to a “high-risk” cluster. Finally, we also used least absolute shrinkage and selection operator “LASSO” regressions to identify which facets of neighborhood disadvantage are associated with cluster membership.
1. Methods
1.1. Participants
Participants in this study were drawn from an ongoing longitudinal study of early life stress in the California San Francisco Bay Area; they were recruited with flyers and local media advertisements. Participants, ages 9–13 years at study entry, were matched on pubertal stage using self-reported Tanner Staging (Morris and Udry, 1980). Exclusion criteria for participation included post-pubertal status, non-fluency in English, neurological disorder, major medical illness, and inability to participate in magnetic resonance imaging (MRI) due to contraindications, such as braces, metal in the body, claustrophobia, etc. Participants and their legal guardians gave informed written assent and consent, respectively, and received financial compensation for their participation. All study procedures were approved by the Stanford University Institutional Review Board and were conducted in accordance with the Declaration of Helsinki.
Participants were invited for assessments approximately every two years over eight years. At Time 1 (T1, June 2015-April 2017), 224 participants (57.3% female) with a mean age of 11.36 (SD=1.05) completed the baseline assessment; at Time 2 (T2) 168 participants (56.5% female) with a mean age of 13.40 (SD=1.10) completed the assessment; at Time 3 (T3) 164 participants (58.1%) with a mean age of 15.56 (SD=1.20) completed the assessment; and at Time 4 (T4) 138 participants (58.5% female) with a mean age of 17.58 (SD=1.36) completed the assessment. See Table 1 for full details on participant sex, race, socioeconomic status, and other demographic details.
Table 1.
Demographic and descriptive statistics.
| %/M | SD | Range | |
|---|---|---|---|
| Female % | 57.3% | ||
| Race/Ethnicity % | |||
| White | 44.0% | ||
| Black | 8.7% | ||
| Hispanic | 8.7% | ||
| Asian | 11.0% | ||
| Multiracial | 21.1% | ||
| Other | 6.4% | ||
| Age at Baseline | 11.36 | 1.05 | 9.11–13.98 |
| Income-to-Needs Ratio | 1.28 | 0.56 | 0–2 |
| Maternal Warmth | 43.92 | 7.29 | 18–55 |
| Neighborhood | 16.84 | 11.84 | 2.44–71.30 |
| MASC T1 | 19.71 | 11.08 | 0–54 |
| MASC T2 | 19.71 | 12.91 | 0–52 |
| MASC T3 | 25.80 | 9.66 | 0–57 |
| UF FA T1 | 0.457 | 0.03 | 0.35–0.55 |
| UF FA T2 | 0.441 | 0.03 | 0.34–0.53 |
| UF FA T3 | 0.435 | 0.03 | 0.38–0.51 |
| Internalizing T4 | 14.68 | 10.72 | 0–50 |
| Externalizing T4 | 10.38 | 8.12 | 0–35 |
| Adaptability T4 | 25.31 | 7.63 | 9–40 |
Note: Neighborhood=neighborhood disadvantage score; MASC=Multidimensional Anxiety Scale for Children; UF FA=uncinate fasciculus fractional anisotropy; Internalizing=internalizing symptoms; Externalizing=externalizing symptoms; T1=Time 1; T2=Time 2; T3=Time 3; T4=Time4.
1.2. Measures
Parenting. At baseline, adolescents completed the 11-item Parenting Styles and Dimensions - Warmth questionnaire (Robinson et al., 2001). Adolescents were instructed to complete the questionnaire with respect to the parent or guardian who accompanied them to the assessment. This measure assesses the frequency with which the parent shows affection to and support for the adolescent (e.g., My parent gives comfort and understanding when I am upset), rated on a scale from 1 (almost never) to 5 (very often). For 95% of participants, this parent was their mother. There were no significant differences in adolescent--reported warmth as a function of the sex of the parent. We used a z-score transformation for analysis to facilitate interpretation of results.
Anxiety. At T1-T3, participants completed the Physical Symptoms and Social Anxiety subscales of the Multidimensional Anxiety Scale for Children (MASC; March et al., 1997). Consistent with previous work in this sample (Gotlib et al., 2023), we focused on these subscales as they assess anxiety symptoms most relevant to the age range of our sample. Participants assessed how often statements (e.g., My heart races or skips beats, I worry about other people laughing at me) are true on a scale from 0 (never true) to 3 (often true). We summed the raw scores of the two scales and applied z-score transformation across timepoints for use in analysis.
Adaptability. At T4 participants completed the Connor-Davidson Resilience Scale (CD-RISC-10; Connor and Davidson, 2003), a 10-item measure that assesses the degree to which participants are able to adapt and cope with difficult circumstances (e.g., I tend to bounce back after illness or hardship). Participants respond to items on a scale from 0 (not true at all) to 4 (true nearly all the time). We computed a total score for use in analyses.
Psychopathology Symptoms. At T4 adolescents completed the Youth Self Report (YSR) Scale (Achenbach and Rescorla, 2001) to report on their emotional and behavioral symptoms. The YSR has eight syndrome scales, of which the anxious/depressed, somatic complaints, and withdrawn scales comprise an internalizing symptoms score, and the aggressive behavior and rule-breaking scales comprise an externalizing symptoms score. We used both internalizing and externalizing symptoms scores. 16% of participants completed this assessment after the onset of the COVID-19 pandemic in March, 2020, and therefore completed the YSR remotely. Participants who completed the YSR during the pandemic had slightly (and nonsignificantly) higher levels of internalizing and externalizing symptoms than did participants who completed the YSR before the pandemic (internalizing symptoms: p=.302; externalizing symptoms: p=.120).
Neighborhood Disadvantage. Participants reported their address at T1, which we used to link each participant to a census tract. Of the 165 participants who reported an address at T1 and T3, 80.4% were still living in the same zip code. The California State Office of Environmental Health Hazard Assessment aggregates data on a number of indicators of socioeconomic, health, and pollutants and computes a score for each census tract in the state, called the California Communities Environmental Health Screening Tool, or “CalEnviroScreen” (CalEnviroScreen 3.0, 2017, Faust et al., 2017). Socioeconomic indicators of each census tract include educational attainment, housing burden, linguistic isolation, poverty, and unemployment. Health indicators include low birth weight infants and rates of asthma- and cardiovascular-related visits to hospital emergency departments. Pollutant indicators include ozone concentrations, particulate matter <2.5μm (PM2.5), diesel PM, drinking water contaminants, pesticide use, toxic releases, traffic density, cleanup sites, groundwater threats, hazardous waste, impaired water bodies, and solid waste sites. We used the CalEnviroScreen 3.0 which aggregates data from 2005 to 2016. Detailed information on each indicator and the computation of the CalEnviroScreen score is reported in the Supplement. To improve normality, we applied a square root transformation to CalEnviroScreen score. Hereafter we refer to this score as neighborhood disadvantage, with higher scores reflecting greater disadvantage.
Family Income-to-Needs Ratio. Participants and their guardians provided information about their demographic characteristics at baseline (T1). This included data on household size and household income on a ten-point scale: <$5000 (0.9%); $5001–$10,000 (2.7%); $10,001–$15,000 (0.9%); $15,001–$25,000 (2.7%); $25,001–$35,000 (1.8%); $35,001–$50,000 (2.7%); $50,001–$75,000 (12.7%); $75,000 –$100,000 (10.0%); $100,001–$150,000 (20.9%); >$150,000 (39.1%). An additional 5.5% did not report household income. These data were used to calculate an income-to-needs ratio; the midpoint of the endorsed household income was divided by the US Department of Housing and Urban Development’s low-income limit for Santa Clara County for the number of residents in the household (i.e., 80% of the median income; King et al., 2020).
MRI Scanning Acquisition. All scanning took place at the Center for Cognitive and Neurobiological Imaging located in the Department of Psychology at Stanford University. All participants at T1 and T2, and 86 participants at T3 were scanned on the 3 Tesla Discovery MR750 (GE Medical Systems, Milwaukee, WI; 32-channel Nova Medical headcoil); 22 participants at T3 were scanned on the 3 Tesla SIGNA Ultra High Performance (32-channel Nova Medical headcoil), which replaced the MR750 in December, 2019, prior to the COVID-19 pandemic. A high-resolution T1-weighted anatomical scan was acquired using an SPGR sequence in the anterior to posterior phase encoding direction (TR/TE/TI = 8.2/3.2/600 ms; flip angle = 12°; 156 axial slices; 1.0 mm isotropic voxels). All participants completed a 60-direction diffusion-weighted scan using an EPI sequence (TR/TE = 8500/93.5 ms; 64 axial slices; 2 mm isotropic voxels; 60 b = 2000 diffusion-weighted directions, and 6 b = 0 acquisitions).
Diffusion MRI Preprocessing and Deterministic Tractography of the Uncinate Fasciculus. Diffusion MRI data processing and tractography have been previously described in this sample (Ho et al., 2017, Ho et al., 2021), and details are available in the supplemental material. Briefly, we minimized the effects of motion on the data by first excluding directions (i.e., volumes) where relative motion in the translational directions or the rotational directions exceeded 5 mm or 1.5°, respectively. All participants included in the present study did not have more than 12 such outlier volumes (20% of volumes). As described in Yeatman et al. (2012) streamlines in the left and right UF, separately, were automatically generated using a two planar waypoint region of interest (ROI) approach and then seeding these two ROIs in accordance with probabilistic fiber groupings. Candidate fibers were then assessed based on similarity to the standard probability map (Mori et al., 2002, Wakana et al., 2007). Outlier fibers were defined as exceeding 4 standard deviations from the spatial core of the tract; these fibers were trimmed until no outliers remained in the final fiber group, with all resulting tracts visually inspected by TCH. Any tracts that were resolved inadequately were omitted from analyses (Ho et al., 2017, Ho et al., 2021). As in previous work from our group (Ho et al., 2021, Kircanski et al., 2019, Uy et al., 2023), mean FA was averaged along 10 evenly spaced nodes for each tract and used subsequent statistical analyses (see below). We computed a standardized residual of FA regressed on tract length and four motion parameters and averaged across the left and right hemisphere for analyses.
1.3. Statistical analysis
Identify UF and Anxiety Clusters. We conducted all analyses in R Studio v. 2022.2.0.443 (R core team, 2022). We first computed correlations among study variables (see Table 2). We then used the package kml3D, for k-means clustering for joint longitudinal data (Genolini et al., 2015), to cluster groups of participants with similar trajectories of anxiety symptoms and UF FA from T1 through T3. We used the package default options for k-means initialization (k-means++), distance (Euclidean with Gower adjustment), number of clusters tested (2−6), and data scaling. We set the number of times the algorithm was run to 100 and allowed for one missing timepoint of data. Missing data were imputed using the package default “Copy Mean” method, which uses linear interpolation and introduces variation to keep the trajectory the same shape as that of the population (Genolini et al., 2015). The kml3D package computes nonparametric (i.e., Calinski-Harabatz, Ray-Turi) and parametric (i.e., AIC, BIC) fit indices for each clustering solution; each index is computed by this package such that higher values represent a solution that is a better fit to the data. We chose the optimal number of clusters based on the solution for which the majority of the indices were maximized.
Table 2.
Multinomial logistic regression predicting cluster membership.
|
Model 1 |
Model 2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Cluster | B | SE | OR | 95% CI | p | B | SE | OR | 95% CI | p |
| Sex | Low-Risk | -0.967 | 0.688 | 0.380 | 0.099, 1.464 | .160 | -0.928 | 0.706 | 0.395 | 0.095, 1.578 | .189 |
| High-Risk | -0.142 | 0.693 | 0.868 | 0.223, 3.371 | .837 | -0.032 | 0.728 | 0.969 | 0.233, 4.032 | .965 | |
| Age at Baseline | Low-Risk | 0.018 | 0.335 | 1.018 | 0.528, 1.962 | .958 | -0.055 | 0.359 | 0.947 | 0.469, 1.912 | .879 |
| High-Risk | -0.364 | 0.351 | 0.695 | 0.349, 1.384 | .301 | -0.513 | 0.371 | 0.599 | 0.289, 1.239 | .167 | |
| Race | Low-Risk | -0.896 | 0.617 | 0.408 | 0.122, 1.368 | .147 | -0.974 | 0.627 | 0.378 | 0.111, 1.289 | .120 |
| High-Risk | 0.024 | 0.611 | 1.024 | 0.309, 3.391 | .969 | -0.115 | 0.635 | 0.896 | 0.257, 3.120 | .856 | |
| Income-to-Needs Ratio | Low-Risk | -0.438 | 0.624 | 0.645 | 0.190, 2.194 | .483 | -0.518 | 0.647 | 0.596 | 0.168, 2.117 | .423 |
| High-Risk | -0.658 | 0.602 | 0.518 | 0.159, 1.685 | .274 | -0.803 | 0.642 | 0.448 | 0.127, 1.578 | .211 | |
| Maternal Warmth | Low-Risk | -0.517 | 0.338 | 0.596 | 0.308, 1.156 | .126 | 0.788 | 1.459 | 2.200 | 0.126, 38.416 | .589 |
| High-Risk | -0.702 | 0.331 | 0.496 | 0.259, 0.948 | .034 | 2.044 | 1.410 | 7.725 | 0.487, 122.50 | .147 | |
| Neighborhood Disadvantage | Low-Risk | -1.038 | 0.353 | 0.354 | 0.177, 0.707 | .003 | -1.052 | 0.365 | 0.349 | 0.171, 0.714 | .004 |
| High-Risk | -0.827 | 0.321 | 0.437 | 0.233, 0.821 | .010 | -0.996 | 0.360 | 0.370 | 0.183, 0.748 | .006 | |
| Warmth* Neighborhood |
Low-Risk | -0.349 | 0.410 | 0.705 | 0.316, 1.575 | .394 | |||||
| High-Risk | -0.772 | 0.393 | 0.462 | 0.214, 0.999 | .0496 | ||||||
Note: “Resilient” cluster (i.e., low uncinate fasciculus fractional anisotropy and low anxiety symptoms) is set as reference for comparisons. “Low-Risk” cluster is high uncinate fasciculus fractional anisotropy and low anxiety symptoms. “High-Risk” cluster is low uncinate fasciculus fractional anisotropy and high anxiety symptoms.
Test Variables Associated with Cluster Membership. To determine whether cluster membership predicted psychological functioning at T4, we conducted a multivariate analysis of covariance (MANCOVA) with cluster predicting internalizing and externalizing symptoms and adaptability, adjusted for age at T4, participant sex, income-to-needs ratio, and Bonferonni correction for comparisons among the three clusters of participants (i.e., multiplied p-values by 3). Next, treating cluster membership as a known outcome, we conducted multinomial logistic regression using the nnet package (Venables and Ripley, 2002) and odds.ratio function from the questionr package (Barnier et al., 2022) to identify significant predictors of cluster membership. Due to small cell sizes in racial groups other than White, to test differences related to race we created a dummy variable coding non-White races as 1 and White race as 0. Because White and non-White participants differed in their CalEnviroScreen scores (t(177.75)=-4.08, p<.001), we included race as a covariate in analyses predicting cluster membership. We entered child sex, age at T1, race, income-to-needs ratio, transformed neighborhood disadvantage and, maternal warmth as predictors of cluster membership in Model 1, and added an interaction term between neighborhood disadvantage and maternal warmth in Model 2. To test whether the inclusion of race resulted in overfitting the model, we additionally conducted the same analyses without race. The interaction was probed and plotted using the alleffects function from the effects package (Fox and Hong, 2010, Fox and Weisberg, 2019).
Identify the Neighborhood Predictors. We conducted 100 multinomial LASSO regressions for feature selection of individual indicators in the CalEnviroScreen using the glmnet package (Friedman et al., 2010). With each run of the regression, the participants included in the train (60%) and test (40%) set were randomized. All 21 neighborhood disadvantage indicators were included as predictors of cluster membership. We report the number of times a nonzero coefficient was estimated for each indicator, as well as the minimum, maximum, and average coefficients for each predictor. We then conducted an ANOVA to evaluate group differences in these indicators.
Sensitivity Analysis. We entered anxiety symptoms and the forceps major of the corpus callosum (CC) FA into the longitudinal k-means clustering analysis to determine the sensitivity of findings to the UF. After conducting k-means clustering of anxiety symptoms and the CC, we entered child sex and age, race, family income-to-needs ratio, maternal warmth, and neighborhood disadvantage into a multinomial regression predicting cluster membership. We then used cluster membership to predict T4 psychopathology symptoms and adaptability.
2. Results
2.1. Participant characteristics
Demographic and other participants characteristics are presented in Table 1. We had an attrition rate of 29.1% between T1 and T2; importantly, however, youth who did not continue to participate at T2, T3, or T4 did not differ significantly from youth who did participate with respect to sex, race, income-to-needs ratio, maternal warmth, or neighborhood disadvantage (ps=.08–.739).
2.2. Correlations
Correlations among the measures in this study are presented in Supplemental Table 1. Income-to-needs ratio was negatively correlated with neighborhood disadvantage (p<.001) and anxiety symptoms at T3 (p=.034). Maternal warmth was negatively associated with anxiety symptoms at T2 (p=.017). Neighborhood disadvantage was negatively associated with UF FA at T3 (p=.032). UF FA at T1 was positively associated with externalizing symptoms at T4 (p=.023). Contrary to our predictions, UF FA was not significantly correlated with anxiety symptoms at any time point.
2.3. K-means clustering
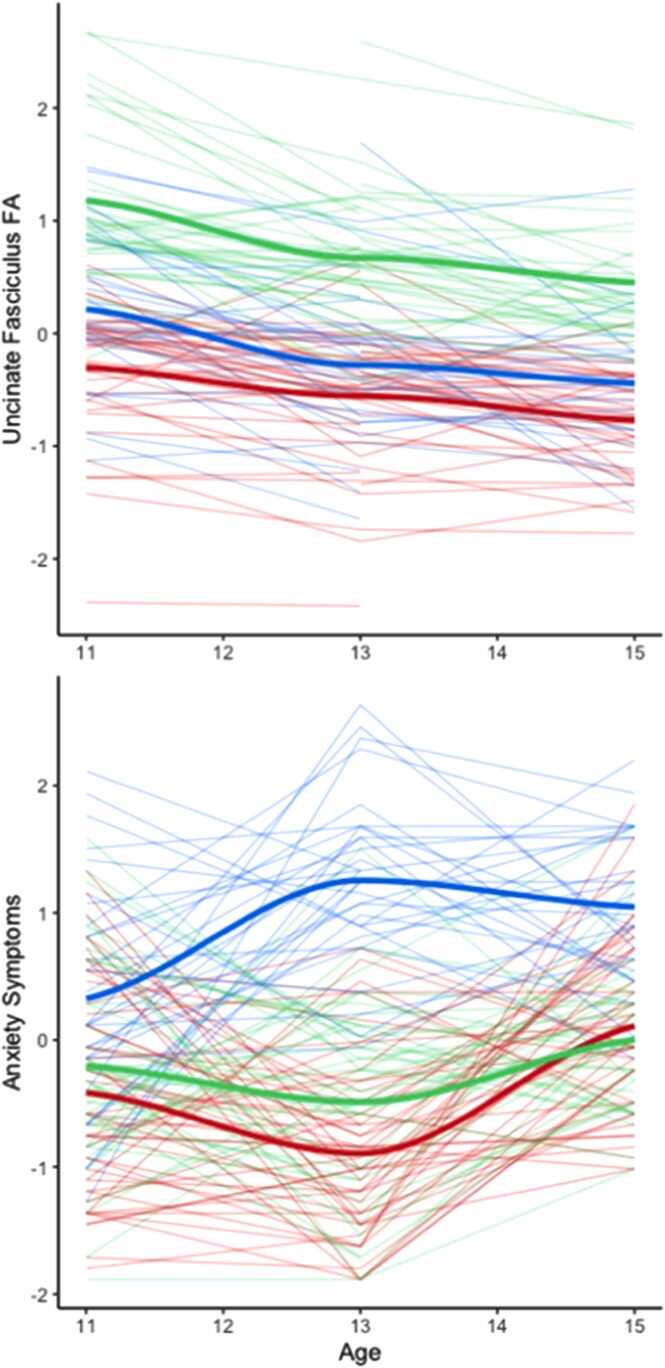
We entered UF FA and anxiety symptoms at T1-T3 into a longitudinal k-means clustering analysis. We hypothesized that we would find two clusters, one with high UF FA and low anxiety symptoms, and one with low UF FA and high anxiety symptoms. This analysis indicated that a 3-cluster solution was the best fit to the data (see Supplemental Table 2 for full results). The 3-cluster solution yielded the following clusters of participants: A) low UF FA and low anxiety symptoms; B) high UF FA and low anxiety symptoms; and C) low UF FA and high anxiety symptoms (see Fig. 1 for visualization of trajectories).
Fig. 1.
Longitudinal clustering of the uncinate fasciculus fractional anisotropy (FA) and anxiety symptoms. The three lines represent the cluster solutions, red=resilient; green=low-risk; blue=high-risk.
2.4. Cluster membership and psychopathology
To examine the association between cluster membership and subsequent symptoms of psychopathology, we conducted an ANCOVA predicting T4 internalizing and externalizing symptoms and adaptability. This analysis yielded a significant effect of cluster membership on internalizing symptoms (p=.017, partial η2=.117) and adaptability (p=.041, partial η2=.092), and a marginally significant effect of cluster membership on externalizing symptoms (p=.057, partial η2=.083), all adjusted for sex, age at T4, income-to-needs ratio, and multiple comparisons. The cluster of participants with low UF FA and low anxiety symptoms had lower levels of internalizing symptoms (p=.014, mean difference=-7.46, 95% CI=-13.73, −1.20) and higher adaptability scores (p=.035, mean difference=5.21, 95% CI=0.27, 10.15) than did participants in the low UF FA and high anxiety symptoms cluster (see Supplemental Fig. 1). There were no significant differences between the cluster of participants with low UF FA and low anxiety symptoms and the cluster of participants with high UF FA and low anxiety symptoms with respect to internalizing symptoms (p=1.00, mean difference=-1.68, 95% CI=-7.77, 4.40), externalizing symptoms (p=1.00, mean difference=-0.20, 95% CI=-5.13, 4.73), or adaptability (p=1.00, mean difference=1.86, 95% CI=-2.94, 6.65). Therefore, we refer to the cluster with low UF FA and low anxiety symptoms “resilient,” the cluster with low UF FA and high anxiety symptoms “high-risk,” and the cluster with high UF FA and low anxiety symptoms “low-risk.”
2.5. Predicting cluster membership
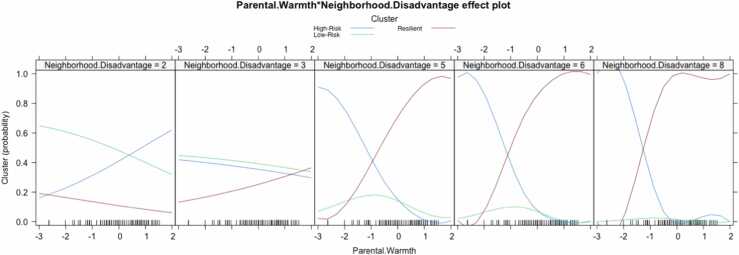
We used multinomial logistic regression to identify variables that are associated with cluster membership. We examined sex, age at baseline, income-to-needs ratio, maternal warmth, neighborhood disadvantage, and the interaction of maternal warmth and neighborhood disadvantage as predictors of cluster membership in Model 1 (see Table 2 for full results). With the resilient cluster as the reference group, we found that participants in both the low- and the high-risk clusters had less neighborhood disadvantage than did participants in the resilient cluster, indicating that resilient participants lived in more disadvantaged neighborhoods. Further, participants in the resilient cluster reported higher maternal warmth than did the participants in the high-risk cluster. In Model 2, the interaction between neighborhood disadvantage and maternal warmth was significant, such that participants living in more disadvantaged communities were more likely to be in the high-risk cluster if they reported low maternal warmth (B = −0.893, SE =.334, p =.009; see Fig. 2) and more likely to be in the resilient than in the high-risk cluster if they reported high maternal warmth (B = −0.793, SE =.334, p =.018). With the low-risk cluster as the reference group, there were no significant differences between the low- and high-risk cluster of participants (see Supplemental Table 3 for full results). There were no significant differences in cluster membership by sex, age at baseline, race, or family income-to-needs ratio. To assess whether the model was overfitted, we conducted the same analysis without race included as a covariate; this resulted in the interaction between maternal warmth and neighborhood disadvantage being only marginally significant (see Supplemental Table 4).
Fig. 2.
Plotting the probability of belonging to the resilient, low-risk, and high-risk clusters as a function of square root transformed neighborhood disadvantage score and maternal warmth.
2.6. LASSO regressions
Finally, we conducted a series of LASSO regressions to explore specific aspects of neighborhood SES associated with cluster membership. Supplemental Table 5 shows the variables selected as predictors, the frequency with which a nonzero coefficient was computed, and the smallest, largest, and average coefficient of each predictor in relation to cluster membership. Of the 100 regressions that we conducted, nonzero coefficients were estimated for pesticides, impaired bodies of water, unemployment, drinking water contaminants, groundwater threats, asthma rates, cardiovascular disease, poverty, PM2.5, and traffic density. We conducted supplementary ANOVAs (see Supplemental Fig. 1) on the features selected by the LASSO regressions, which showed that the resilient cluster of participants, compared to the high-risk cluster, lived in neighborhoods with higher rates of asthma (p=.010, η2=.070), more cardiovascular disease (p=0.003, η2=.092), more households living in poverty (p=.004, η2=.094), and greater traffic density (p=.042, η2=.053). Compared with participants in the low- and high-risk clusters, participants in the resilient cluster lived in neighborhoods in which more households lived in poverty (p=.029, η2=.094). Finally, the low-risk cluster of participants had greater traffic density than did participants in the high-risk cluster (p=.047, η2=.053). However, group differences in neighborhood pesticides, impaired water bodies, unemployment, drinking water contaminants, or PM2.5 were not statistically significant.
2.7. Sensitivity analysis
We entered CC FA and anxiety symptoms from T1-T3 into a longitudinal k-means clustering analysis. A two cluster solution was the best fit to the data; one cluster was characterized by participants with high CC FA and high anxiety symptoms, and the other cluster was characterized by participants with low CC FA and low anxiety symptoms (see Supplemental Fig. 2). We then predicted membership to these clusters from child sex, age at baseline, race, family income-to-needs ratio, maternal warmth, and neighborhood disadvantage in one model, and the same variables in addition to an interaction between maternal warmth and neighborhood disadvantage in a second model (see Supplemental Table 6 for full results). In model 1, participants who were not White were less likely to be in the low CC FA + low anxiety cluster than the high CC + high anxiety cluster (OR=-0.969, 95% CI=0.148, 0.977). Further, higher neighborhood disadvantage was associated with increased risk of being in the high CC + high anxiety cluster (OR=0.42, 95% CI=1.001, 2.306). In model 2, race remained a significant predictor of cluster membership, neighborhood disadvantage was no longer a significant predictor, and the interaction term between maternal warmth and neighborhood disadvantage was not significant.
We next predicted T4 internalizing symptoms, externalizing symptoms, and adaptability from cluster membership. This analysis yielded a significant effect of cluster membership on internalizing symptoms (p=.005, partial η2=.103) and externalizing (p=.001, partial η2=.133), and a marginally significant effect of cluster membership on adaptability (p=.064, partial η2=.047), all adjusted for sex, age at T4, income-to-needs ratio, and multiple comparisons. Participants in the high CC FA + high anxiety symptoms cluster had higher internalizing (p=.005, mean difference=5.73, 95% CI=1.75, 9.71) and externalizing symptoms (p=.001, mean difference=5.11, 95% CI=2.05, 8.18) than participants in the low CC FA + low anxiety symptoms cluster (see Supplemental Figure 3). Participants in the low CC FA + low anxiety symptoms cluster had marginally higher adaptability scores than participants in the hight CC FA + high anxiety symptoms cluster (p=.064, mean difference=-3.07, 95% CI=-6.32, 0.18).
3. Discussion
In this study we examined trajectories of UF FA and anxiety symptoms across 6 years in adolescents. Anxiety symptoms increased for some participants and remained relatively stable for others. UF FA, which was regressed on motion parameters and tract length, tended to decrease. This direction was unexpected, given the increase in UF FA typically observed through adulthood (Lebel et al., 2012). We found that found three data-driven clusters of adolescent anxiety symptoms and UF FA: a “low-risk” cluster of participants with low anxiety symptoms and high UF FA, a “high-risk” cluster of participants with high anxiety symptoms and low UF FA, and an unexpected “resilient” cluster of participants with low anxiety symptoms and UF FA. The latter participants had fewer internalizing symptoms and higher adaptability than did participants in the high-risk cluster. Importantly, participants in the resilient cluster reported more maternal warmth than did the high-risk cluster of participants and were more likely to live in disadvantaged communities than both the low- and the high-risk clusters of participants. Further, an interaction between neighborhood disadvantage and maternal warmth indicated that participants living in disadvantaged neighborhoods were more likely to be in the resilient cluster than in the high-risk cluster if they reported experiencing more maternal warmth. In contrast, participants living in disadvantaged neighborhoods with lower maternal warmth were more likely to be in the high-risk cluster than in the resilient cluster. However, this interaction was only marginally significant when participant race was not included in the model. These findings support the formulation that positive parenting behaviors can promote the ability to adapt to disadvantageous neighborhood environments.
We had hypothesized that we would identify the low- and high-risk clusters of participants based on prior work linking poor UF structure with higher anxiety symptoms (Lee and Lee, 2020). We did not, however, expect to also identify a cluster of participants with low levels of both FA and anxiety symptoms (i.e., resilient). We expected that the low-risk cluster of participants would report high maternal warmth and low neighborhood disadvantage relative to the high-risk cluster of participants. We found instead that the resilient cluster of participants tended to live in more disadvantaged neighborhoods relative to both the low- and high-risk participant clusters. We also found that the resilient cluster of participants, but not the low-risk participants, reported more maternal warmth than the high-risk cluster of participants. There were no significant differences between the low- and high-risk clusters of participants except in the density of community traffic in exploratory follow-up analyses of neighborhood disadvantage indicators, in which the low-risk cluster of participants had greater traffic density. Examining other variables, such as genetic risk or family history of mental illness, may have yielded significant differences between the low- and high-risk clusters of participants.
Our results expand on prior work indicating that both family and neighborhood environments are associated with neural and behavioral risk for psychopathology. In this context, our finding that maternal warmth buffers neighborhood disadvantage is supported by previous research (Mrug et al., 2022, Whittle et al., 2017). We have also extended this work by showing that neighborhood disadvantage in the context of high maternal warmth is associated with an adaptable, rather than with a high-risk, profile of trajectories of UF FA and anxiety symptoms. Researchers have identified positive parenting as a resilience factor for children exposed to adversity (Vazquez et al., 2023). In the current study, we found that for youth growing up in more disadvantaged neighborhoods, maternal warmth was implicated in a resilient profile characterized by lower symptoms of psychopathology and better adaptability despite reduced FA structural integrity. Our findings suggest that maternal warmth promotes adaptability and mitigates risk for mental health difficulties related to neighborhood disadvantage and low UF integrity. We did not expect participants with low UF FA to have high levels of adaptability given research linking this neural characteristic with psychopathology (e.g., Lee and Lee, 2020; Xu et al., 2023). It is noteworthy, however, that research findings examining the association between resilience and UF structural integrity have been equivocal (Costanzo et al., 2016, van der Werff et al., 2017). Moreover, resilience is not simply the absence of psychopathology, but rather, the ability to positively adapt and cope with negative circumstances (Fletcher and Sarkar, 2013). Therefore, our findings suggest that maternal warmth promotes the ability to adapt to disadvantageous neighborhood circumstances without developing mental health challenges.
We also explored neighborhood pollutants, SES, and health indicators to examine whether specific aspects of neighborhood disadvantage were associated with risk. Prior work has largely evaluated neighborhood disadvantage in terms of relative SES, but SES is likely to be a proxy for pollution (e.g., air and water contaminants), instability (e.g., safety), lack of community resources (e.g., healthcare, education), and/or the cumulative effects of many different contributing factors. We found that measures of poorer physical health, poverty, and traffic density predicted cluster membership, with participants in the resilient cluster tending to live in neighborhoods with higher levels of these variables. Although we cannot be sure that participants were directly exposed to these factors (see discussion of study limitations, below), they were at least at higher risk for exposure. These factors have been identified in previous work as contributing to risk for health challenges (e.g., Kulla et al., 2023; Manczak et al., 2020; Miller, Buthmann, et al., 2022); few studies, however, have considered these factors simultaneously, in addition to parenting. Although we did not expect to find that participants in a cluster characterized by greater neighborhood disadvantage would be resilient to psychopathology symptoms, we also did not measure neighborhood aspects that can promote adaptability to negative evironments. For example, prior work has identified higher levels of resilience to adversity in individuals living in areas with more neighborhood social cohesion, vegetation, and walkability (Ma et al., 2023, Romero et al., 2020). Future work should consider the possibility that positive and negative neighborhood qualities can co-occur. We should also note that race did not predict cluster membership. Although census tract-level neighborhood disadvantage data do not always fully explain disparities in access to resources and exposure to harmful environments (Riley, 2018), in the present study race did not explain variance in cluster differences beyond the factors that comprised the CalEnviroScreen.
Contrary to our hypotheses, we found no significant differences between the low- and high-risk groups with respect to maternal warmth, neighborhood disadvantage, or any of the covariates we tested. This was surprising given previous findings of associations between parenting and both anxiety (Yap et al., 2014) and alterations in corticolimbic circuitry (Gard et al., 2022, Hanson et al., 2015). It is important to note, however, that research examining parenting and corticolimbic circuitry has focused on extreme maltreatment (Hanson et al., 2015), on brain circuit function rather than structure (Gard et al., 2022), or has been conducted with samples of adults (Farber et al., 2019). The association between neighborhood disadvantage and internalizing symptoms, including symptoms of anxiety, has been documented by several researchers (e.g., King et al., 2022; Rudolph et al., 2014); these associations, however, may be more nuanced than previously appreciated. For example, Alegría et al. (2014) found that association between neighborhood disadvantage and anxiety varied by racial group, with Latinos at increased risk for anxiety disorders and Asians at lower risk. Further, studies of the association between neighborhood disadvantage and UF structural integrity have focused on specific aspects of the community, such as community violence (Bell et al., 2021) or socioeconomic status (Kulla et al., 2023). Our measure of neighborhood disadvantage was a composite score of pollutants, SES, and health, and may have obscured associations between specific neighborhood characteristics and UF structure.
In sensitivity analyses, we found two clusters of CC FA and anxiety symptoms: one composed of participants with high FA and high anxiety symptoms, and one composed of participants with low CC FA and low anxiety symptoms. The latter cluster had higher neighborhood disadvantage and marginally higher adaptability, whereas the former had higher internalizing and externalizing symptoms at T4. Participants in both the low UF FA + low anxiety symptoms and the low CC FA + low anxiety symptoms clusters had higher levels of neighborhood disadvantage and higher adaptability; however, participants in the high CC FA + high anxiety symptoms cluster had high internalizing and externalizing symptoms, as did participants in the low UF FA + high anxiety symptoms cluster. Thus, neighborhood disadvantage may be associated with lower FA in multiple white matter tracts; future research is required to examine this formulation more explicitly and systematically. Notably, the white matter development of the CC follows a different trajectory than the UF, with research suggesting it peaks earlier in adolescence, whereas the UF peaks in adulthood (Lebel et al., 2012). The lack of association between maternal warmth and CC FA clusters suggests that parenting is associated more specifically with the UF, consistent with prior research (Eluvathingal et al., 2006). Further, the higher psychopathology symptoms found in participants in both the low UF FA + high anxiety and in the high CC FA + high anxiety clusters challenge the idea that higher FA values are generally associated with more positive outcomes. Certainly, however, these findings need to be replicated in an independent sample, and should only be interpreted here with full consideration of this study’s limitations.
This study has several limitations. First, we began assessing our participants when they were in late childhood/early adolescence and, therefore, cannot distinguish between exposure to parenting or neighborhood disadvantage in early vs. late childhood. In this context, Gard et al. (2021) found that whereas earlier exposure to disadvantage is more strongly associated with amygdala development, later context is related more strongly to prefrontal development. Second, we did not measure direct exposure to these neighborhood variables and cannot make causal statements about the contributions of the variables assessed in this study to UF integrity, anxiety, or adaptability; rather, we found associations involving risk of exposure to those factors. We should note that only 80% of participants were still living in the same zip code at T3 as they were at baseline. Further, not all neighborhood variables that were selected as important features by LASSO regressions yielded group differences that were significant at the p<.05 level; thus we urge caution in interpreting these exploratory findings. Third, our measure of neighborhood disadvantage, although more comprehensive than some operationalizations that only consider SES, did not account for community factors such as school quality, green spaces, libraries, safety, etc. Further, we assessed parenting behaviors only for the primary caregiver, which for 95% of participants was the mother; certainly, adolescents may also benefit or suffer from the parenting behaviors of secondary and tertiary caregivers. Future work should explore the contributions from fathers and other caregivers. Fourth, we relied on self-reports of anxiety symptoms, parenting perceptions, internalizing and externalizing symptoms, and adaptability, which may bias responses. Further, the sample is drawn from a relatively high SES region and our findings need to be replicated in a more socioeconomically diverse sample. As a related point, although fewer than half of the participants were White, we did not have enough participants of other races to test for differences among non-White races, which is an important direction for future research. Finally, we relied on only one metric of white matter tract structural organization (i.e., FA); scientists have called for the analysis of multiple measures before drawing strong conclusions about tract organization (Figley et al., 2022), and it is important that our findings be replicated and extended in future studies. Additionally, our measure of UF FA was regressed on motion parameters and tract length. This makes interpretation of the general decline in UF FA over time more difficult to interpret, and we caution readers not to generalize these findings to raw values of FA.
Despite these limitations, this study is important in identifying three clusters of adolescents with different profiles of anxiety symptoms and UF structural integrity. We also found positive parenting behaviors promoted adaptability in adolescents from disadvantaged neighborhoods whose UF integrity suggested that they were vulnerable to developing symptoms of psychopathology. This may indicate that parenting interventions and other resources to promote positive caregiving could be particularly effective in disadvantaged communities. A key strength of this study is that we used a data-driven clustering approach that led us to identify an unexpected “resilient” profile of low UF integrity and low anxiety symptoms; model-based approaches may not have detected this resilient group of participants. Further, in addition to examining multiple environmental contexts (i.e., parenting and neighborhood), both independently and in interaction, we explored multidimensional aspects of neighborhood disadvantage that were associated with cluster membership. Future work should examine additional neural metrics, such as structure-function coupling, incorporate both positive and negative qualities of neighborhoods, and explore the contributions of more than one caregiver, as well as peer relationships, on adolescent development.
Compliance with Ethical Standards
Informed consent was obtained from parents/guardians and informed assent was obtained from minors participating in research activities. All research activities were conducted in accordance with the Stanford University Institutional Review Board and the Declaration of Helsinki.
Funding
This research was supported by the National Institute of Mental Health (R37MH101495 to IHG) and the Brain and Behavior Research Foundation Young Investigator Grant to JLB.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the participating families and research staff for their time and effort.
Data Statement
Data have been uploaded to the NIMH Data Archive here: https://nda.nih.gov/edit_collection.html?id=2135.
Code used for analyses is available here: https://osf.io/2pz8v/.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2024.101368.
Appendix A. Supplementary material
Supplementary material
Data availability
I have shared the link to the data and code at the Attach File step
References
- Achenbach T.M., Rescorla L.A. University of Vermont; Burling: 2001. Manual for the ASEBA school-age forms & profiles. [Google Scholar]
- Alegría M., Molina K.M., Chen C.-N. Neighborhood characteristics and differential risk for depressive and anxiety disorders across racial/ethnic groups in the United States. Depress Anxiety. 2014;31(1):27–37. doi: 10.1002/da.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnier, J., Briatte, F., & Larmarange, J. (2022). questionr: Functions to Make Surveys Processing Easier (R package version 0.7.7) [Computer software]. 〈https://CRAN.R-project.org/package=questionr〉.
- Beckwith T., Cecil K., Altaye M., Severs R., Wolfe C., Percy Z., Maloney T., Yolton K., LeMasters G., Brunst K., Ryan P. Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLOS ONE. 2020;15(1) doi: 10.1371/journal.pone.0228092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K.L., Purcell J.B., Harnett N.G., Goodman A.M., Mrug S., Schuster M.A., Elliott M.N., Emery S.T., Knight D.C. White matter microstructure in the young adult brain varies with neighborhood disadvantage in adolescence. Neuroscience. 2021;466:162–172. doi: 10.1016/j.neuroscience.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CalEnviroScreen 3.0. (2017). [dataset]. 〈https://oehha.ca.gov/calenviroscreen/report/calenviroscreen-30〉.
- Chen Y., Kubzansky L.D., VanderWeele T.J. Parental warmth and flourishing in mid-life. Soc. Sci. Med. 2019;220:65–72. doi: 10.1016/j.socscimed.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger R.D., Conger K.J., Martin M.J. Socioeconomic status, family processes,and individual development. J. Marriage Fam. 2010;72(3):685–704. doi: 10.1111/j.1741-3737.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K.M., Davidson J.R.T. Development of a new resilience scale: the connor-davidson resilience scale (CD-RISC) Depress Anxiety. 2003;18(2):76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- Costanzo M.E., Jovanovic T., Pham D., Leaman S., Highland K.B., Norrholm S.D., Roy M.J. White matter microstructure of the uncinate fasciculus is associated with subthreshold posttraumatic stress disorder symptoms and fear potentiated startle during early extinction in recently deployed Service Members. Neurosci. Lett. 2016;618:66–71. doi: 10.1016/j.neulet.2016.02.041. [DOI] [PubMed] [Google Scholar]
- Dadvand P., Pujol J., Macià D., Martinez-Vilavella G., Blanco L., Mortamais M., Alvarez-Pedrerol M., Fenoll R., Esnaola M., Dalmau-Bueno A., López-Vicente M., Basagaña X., Jerrett M., Nieuwenhuijsen M.J., Sunyer J. The association between lifelong greenspace exposure and 3-dimensional brain magnetic resonance imaging in barcelona schoolchildren. Environ. Health Perspect. 2018;126(2) doi: 10.1289/EHP1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko M.I., Ip K.I., Kelly D.P., Constante K., Goetschius L.G., Keating D.P. Ecological stress, amygdala reactivity, and internalizing symptoms in preadolescence: Is parenting a buffer? Cortex. 2021;140:128–144. doi: 10.1016/j.cortex.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff S.J.A., Elzinga B.M., Smit A.S., van der Wee N.J.A. Structural brain correlates of resilience to traumatic stress in Dutch police officers. Psychoneuroendocrinology. 2017;85:172–178. doi: 10.1016/j.psyneuen.2017.08.019. [DOI] [PubMed] [Google Scholar]
- Eluvathingal T.J., Chugani H.T., Behen M.E., Juhász C., Muzik O., Maqbool M., Chugani D.C., Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Farber M.J., Kim M.J., Knodt A.R., Hariri A.R. Maternal overprotection in childhood is associated with amygdala reactivity and structural connectivity in adulthood. Dev. Cogn. Neurosci. 2019;40 doi: 10.1016/j.dcn.2019.100711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust, J., August, L., Bangia, K., Galaviz, V., Leichty, J., Prasad, S., Schmitz, R., Slocombe, A., Welling, R., & Zeise, L. (2017). Update to the California Communities Environmental Health Screening Tool, CalEnviroScreen 3.0. 〈https://oehha.ca.gov/calenviroscreen/report/calenviroscreen-30〉.
- Figley C.R., Uddin M.N., Wong K., Kornelsen J., Puig J., Figley T.D. Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Front. Neurosci. 2022;15 doi: 10.3389/fnins.2021.799576. 〈https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2021.799576〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A.C., Buehler C., McCurdy A.L., Weymouth B.B. Skin conductance reactivity as a moderator of associations between youth perceptions of neighborhood stress and depressive symptoms. J. Early Adolesc. 2019;39(8):1154–1176. doi: 10.1177/0272431618812164. [DOI] [Google Scholar]
- Fletcher D., Sarkar M. Psychological resilience. Eur. Psychol. 2013;18(1):12–23. doi: 10.1027/1016-9040/a000124. [DOI] [Google Scholar]
- Flouri E., Midouhas E., Joshi H., Tzavidis N. Emotional and behavioural resilience to multiple risk exposure in early life: the role of parenting. Eur. Child Adolesc. Psychiatry. 2015;24(7):745–755. doi: 10.1007/s00787-014-0619-7. [DOI] [PubMed] [Google Scholar]
- Fox J., Hong J. Effect displays in r for multinomial and proportional-odds logit models: extensions to the effects package. J. Stat. Softw. 2010;32:1–24. doi: 10.18637/jss.v032.i01. [DOI] [Google Scholar]
- Fox J., Weisberg S. An R Companion to Applied Regression. 3rd ed. Sage; 2019. 〈https://socialsciences.mcmaster.ca/jfox/Books/Companion/index.html〉 [Google Scholar]
- Friedman, J., Hastie, T., Tibshirani, R., Narasimhan, B., Tay, K., Simon, N., & Qian, J. (2010). Glmnet: Lasso and elastic-net regularized generalized linear models [Computer software]. 〈http://CRAN〉. R-project. org/package= glmnet. R package version,
- Gard A.M., Maxwell A.M., Shaw D.S., Mitchell C., Brooks-Gunn J., McLanahan S.S., Forbes E.E., Monk C.S., Hyde L.W. Beyond family-level adversities: Exploring the developmental timing of neighborhood disadvantage effects on the brain. Dev. Sci. 2021;24(1) doi: 10.1111/desc.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A.M., Hein T.C., Mitchell C., Brooks-Gunn J., McLanahan S.S., Monk C.S., Hyde L.W. Prospective longitudinal associations between harsh parenting and corticolimbic function during adolescence. Dev. Psychopathol. 2022;34(3):981–996. doi: 10.1017/S0954579420001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Generaal E., Timmermans E.J., Dekkers J.E.C., Smit J.H., Penninx B.W.J.H. Not urbanization level but socioeconomic, physical and social neighbourhood characteristics are associated with presence and severity of depressive and anxiety disorders. Psychol. Med. 2019;49(1):149–161. doi: 10.1017/S0033291718000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genolini C., Alacoque X., Sentenac M., Arnaud C. kml and kml3d: R packages to cluster longitudinal data. J. Stat. Softw. 2015;65:1–34. doi: 10.18637/jss.v065.i04. [DOI] [Google Scholar]
- Gotlib I.H., Miller J.G., Borchers L.R., Coury S.M., Costello L.A., Garcia J.M., Ho T.C. Effects of the COVID-19 pandemic on mental health and brain maturation in adolescents: implications for analyzing longitudinal data. Biol. Psychiatry Glob. Open Sci. 2023;3(4):912–918. doi: 10.1016/j.bpsgos.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Yang X., Yuan Z., Qiu J., Lu W. A comparison between diffusion tensor imaging and generalized q-sampling imaging in the age prediction of healthy adults via machine learning approaches. J. Neural Eng. 2022;19(1) doi: 10.1088/1741-2552/ac4bfe. [DOI] [PubMed] [Google Scholar]
- Hackman D.A., Cserbik D., Chen J.-C., Berhane K., Minaravesh B., McConnell R., Herting M.M. Association of local variation in neighborhood disadvantage in metropolitan areas with youth neurocognition and brain structure. JAMA Pediatr. 2021;175(8) doi: 10.1001/jamapediatrics.2021.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Knodt A.R., Brigidi B.D., Hariri A.R. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 2015;27(4pt2) doi: 10.1017/S0954579415000978. Article 4pt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., King L.S., Leong J.K., Colich N.L., Humphreys K.L., Ordaz S.J., Gotlib I.H. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc. Cogn. Affect. Neurosci. 2017;12(9):1460–1469. doi: 10.1093/scan/nsx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., Sisk L.M., Kulla A., Teresi G.I., Hansen M.M., Wu H., Gotlib I.H. Sex differences in myelin content of white matter tracts in adolescents with depression. Neuropsychopharmacology. 2021;46(13) doi: 10.1038/s41386-021-01078-3. Article 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., Teresi G.I., Segarra J.R., Ojha A., Walker J.C., Gu M., Spielman D.M., Sacchet M.D., Jiang F., Rosenberg-Hasson Y., Maecker H., Gotlib I.H. Higher levels of pro-inflammatory cytokines are associated with higher levels of glutamate in the anterior cingulate cortex in depressed adolescents. Front. Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.642976. 〈https://www.frontiersin.org/articles/10.3389/fpsyt.2021.642976〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins A.A., McTeague L.M., Davis M.M., Bustos N., Crum K.I., Polcyn R., Adams Z.W., Carpenter L.A., Hajcak G., Halliday C.A., Joseph J.E., Danielson C.K. Neighborhood disadvantage associated with blunted amygdala reactivity to predictable and unpredictable threat in a community sample of youth. Biol. Psychiatry Glob. Open Sci. 2022;2(3):242–252. doi: 10.1016/j.bpsgos.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Gard A.M., Tomlinson R.C., Burt S.A., Mitchell C., Monk C.S. An ecological approach to understanding the developing brain: examples linking poverty, parenting, neighborhoods, and the brain. Am. Psychol. 2020;75(9):1245–1259. doi: 10.1037/amp0000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleque A. Perceived parental warmth, and children’s psychological adjustment, and personality dispositions: a meta-analysis. J. Child Fam. Stud. 2013;22(2):297–306. doi: 10.1007/s10826-012-9579-z. [DOI] [Google Scholar]
- King C., Huang X., Dewan N.A. Continuity and change in neighborhood disadvantage and adolescent depression and anxiety. Health Place. 2022;73 doi: 10.1016/j.healthplace.2021.102724. [DOI] [PubMed] [Google Scholar]
- King L.S., Dennis E.L., Humphreys K.L., Thompson P.M., Gotlib I.H. Cross-sectional and longitudinal associations of family income-to-needs ratio with cortical and subcortical brain volume in adolescent boys and girls. Dev. Cogn. Neurosci. 2020;44 doi: 10.1016/j.dcn.2020.100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K., Sisk L.M., Ho T.C., Humphreys K.L., King L.S., Colich N.L., Ordaz S.J., Gotlib I.H. Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Dev. Psychopathol. 2019;31(3):1011–1022. doi: 10.1017/S0954579419000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulla A., Coury S., Garcia J.M., Teresi G.I., Sisk L.M., Hansen M., Miller J.G., Gotlib I.H., Ho T.C. Neighborhood socioeconomic disadvantage and white matter microstructure of the arcuate fasciculus and uncinate fasciculus in adolescents. Biol. Psychiatry Glob. Open Sci. 2023 doi: 10.1016/j.bpsgos.2023.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Lee S.H. In: Rethinking and Understanding Recent Discoveries (pp. Kim Y.-K., editor. Springer; 2020. White Matter-Based Structural Brain Network of Anxiety; pp. 61–70. (Anxiety Disorders). [DOI] [Google Scholar]
- Linke J.O. The uncinate fasciculus in anxiety disorders: a potential treatment target? Biol. Psychiatry. 2019;86(12):e47–e48. doi: 10.1016/j.biopsych.2019.09.020. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang M. Parental corporal punishment and child anxiety in China: the moderating role of HPA-axis Activity. J. Affect. Disord. 2020;273:500–507. doi: 10.1016/j.jad.2020.04.055. [DOI] [PubMed] [Google Scholar]
- Lubczyńska M.J., Muetzel R.L., El Marroun H., Basaga X., Strak M., Denault W., Jaddoe V.W.V., Hillegers M., Vernooij M.W., Hoek G., White T., Brunekreef B., Tiemeier H., Guxens M. Exposure to air pollution during pregnancy and childhood, and white matter microstructure in preadolescents. Environ. Health Perspect. 2020;128(2) doi: 10.1289/EHP4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Ye R., Ettema D., van Lierop D. Role of the neighborhood environment in psychological resilience. Landsc. Urban Plan. 2023;235 doi: 10.1016/j.landurbplan.2023.104761. [DOI] [Google Scholar]
- Manczak E.M., Miller J.G., Gotlib I.H. Water contaminant levels interact with parenting environment to predict development of depressive symptoms in adolescents. Dev. Sci. 2020;23(1) doi: 10.1111/desc.12838. [DOI] [PubMed] [Google Scholar]
- Manczak E.M., Miller J.G., Gotlib I.H. Census tract ambient ozone predicts trajectories of depressive symptoms in adolescents. Dev. Psychol. 2022;58(3):485–492. doi: 10.1037/dev0001310. [DOI] [PubMed] [Google Scholar]
- March J.S., Parker J.D.A., Sullivan K., Stallings P., Conners C.K. The multidimensional anxiety scale for children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Miller J.G., Ho T.C., Kirshenbaum J.S., Chahal R., Gifuni A.J., Gotlib I.H. Testing a developmental model of positive parenting, amygdala–subgenual anterior cingulate cortex connectivity, and depressive symptoms in adolescents before and during the COVID-19 pandemic. Biol. Psychiatry Glob. Open Sci. 2021;1(4):291–299. doi: 10.1016/j.bpsgos.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.G., López V., Buthmann J.L., Garcia J.M., Gotlib I.H. A social gradient of cortical thickness in adolescence: relationships with neighborhood socioeconomic disadvantage, family socioeconomic status, and depressive symptoms. Biol. Psychiatry Glob. Open Sci. 2022;2(3):253–262. doi: 10.1016/j.bpsgos.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.G., Buthmann J.L., Gotlib I.H. Hippocampal volume indexes neurobiological sensitivity to the effect of pollution burden on telomere length in adolescents. N. Dir. Child Adolesc. Dev. 2022 doi: 10.1002/cad.20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Elsevier; 2007. Introduction to Diffusion Tensor Imaging. [Google Scholar]
- Mori S., Kaufmann W.E., Davatzikos C., Stieltjes B., Amodei L., Fredericksen K., Pearlson G.D., Melhem E.R., Solaiyappan M., Raymond G.V., Moser H.W., van Zijl P.C.M. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn. Reson. Med. 2002;47(2):215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Morris N.M., Udry J.R. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Mrug S., Barker-Kamps M., Orihuela C.A., Patki A., Tiwari H.K. Childhood neighborhood disadvantage, parenting, and adult health. Am. J. Prev. Med. 2022;63(1, Supplement 1):S28–S36. doi: 10.1016/j.amepre.2022.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., Heide R.J.V.D., Alm K.H., Vyas G. Development of the uncinate fasciculus: implications for theory and developmental disorders. Dev. Cogn. Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M. Associations of parenting dimensions and styles with externalizing problems of children and adolescents: an updated meta-analysis. Dev. Psychol. 2017;53(5):873. doi: 10.1037/dev0000295. [DOI] [PubMed] [Google Scholar]
- Pinquart M., Gerke D.-C. Associations of parenting styles with self-esteem in children and adolescents: a meta-analysis. J. Child Fam. Stud. 2019;28(8):2017–2035. doi: 10.1007/s10826-019-01417-5. [DOI] [Google Scholar]
- Rakesh D., Zalesky A., Whittle S. Similar but distinct – Effects of different socioeconomic indicators on resting state functional connectivity: findings from the Adolescent Brain Cognitive Development (ABCD) Study®. Dev. Cogn. Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D., Seguin C., Zalesky A., Cropley V., Whittle S. Associations between neighborhood disadvantage, resting-state functional connectivity, and behavior in the adolescent brain cognitive development study: the moderating role of positive family and school environments. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2021;6(9):877–886. doi: 10.1016/j.bpsc.2021.03.008. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Zalesky A., Whittle S. Assessment of parent income and education, neighborhood disadvantage, and child brain structure. JAMA Netw. Open. 2022;5(8) doi: 10.1001/jamanetworkopen.2022.26208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A., Rutherford G.W., James J., Razani N. Association of neighborhood parks with child health in the United States. Prev. Med. 2020;141 doi: 10.1016/j.ypmed.2020.106265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley A.R. Neighborhood disadvantage, residential segregation, and beyond—lessons for studying structural racism and health. J. Racial Ethn. Health Disparities. 2018;5(2):357–365. doi: 10.1007/s40615-017-0378-5. [DOI] [PubMed] [Google Scholar]
- Robinson C., Mendleco B., Olsen S.F., Hart C.H. In: Handbook of family measurement …. Perlmutter B.F., Touliatos J., Holden G.W., editors. SAGE Publications, Inc; 2001. The parenting styles and dimensions questionnaire (PSDQ) pp. 319–321.〈https://www.academia.edu/13707474/The_parenting_styles_and_dimensions_questionnaire_PSDQ_〉 [Google Scholar]
- Romero A.J., White R.M.B., Anguas M.M., Curlee A., Rodas J.M. Resilience of Mexican descent youth in a low-income neighborhood: examining family and neighborhood factors. J. Lat. Psychol. 2020;8(4):265–279. doi: 10.1037/lat0000149. [DOI] [Google Scholar]
- Roos L.E., Salisbury M., Penner-Goeke L., Cameron E.E., Protudjer J.L.P., Giuliano R., Afifi T.O., Reynolds K. Supporting families to protect child health: parenting quality and household needs during the COVID-19 pandemic. PLoS ONE. 2021;16(5) doi: 10.1371/journal.pone.0251720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K.E., Stuart E.A., Glass T.A., Merikangas K.R. Neighborhood disadvantage in context: The influence of urbanicity on the association between neighborhood disadvantage and adolescent emotional disorders. Soc. Psychiatry Psychiatr. Epidemiol. 2014;49(3):467–475. doi: 10.1007/s00127-013-0725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D., Khoddam H., Piero L.D., Stoycos S.A., Gimbel S.I., Margolin G., Kaplan J.T. Community violence exposure in early adolescence: longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Dev. Sci. 2018;21(6) doi: 10.1111/desc.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist J., Li X., Ohlsson H., Råstam M., Winkleby M., Sundquist K., Kendler K.S., Crump C. Familial and neighborhood effects on psychiatric disorders in childhood and adolescence. J. Psychiatr. Res. 2015;66–67:7–15. doi: 10.1016/j.jpsychires.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T., Millner A., Galvan A., Davidson M.C., Eigsti I.-M., Thomas K.M., Freed P.J., Booma E.S., Gunnar M.R., Altemus M., Aronson J., Casey B.J. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation: Previous institutionalization. Dev. Sci. 2010;13(1) doi: 10.1111/j.1467-7687.2009.00852.x. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy J.P., Ho T.C., Buthmann J.L., Coury S.M., Gotlib I.H. Early life stress, sleep disturbances, and depressive symptoms during adolescence: the role of the cingulum bundle. Dev. Cogn. Neurosci. 2023;63 doi: 10.1016/j.dcn.2023.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez A.Y., Shewark E.A., Hyde L.W., Klump K.L., Burt S.A. Parental nurturance moderates the etiology of youth resilience. Behav. Genet. 2023 doi: 10.1007/s10519-023-10150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D. Modern Applied Statistics with S. 4th ed. Springer; 2002. 〈https://www.stats.ox.ac.uk/pub/MASS4/〉 [Google Scholar]
- Versace A., Ladouceur C.D., Graur S., Acuff H.E., Bonar L.K., Monk K., McCaffrey A., Yendiki A., Leemans A., Travis M.J., Diwadkar V.A., Holland S.K., Sunshine J.L., Kowatch R.A., Horwitz S.M., Frazier T.W., Arnold L.E., Fristad M.A., Youngstrom E.A.…Phillips M.L. Diffusion imaging markers of bipolar versus general psychopathology risk in youth at-risk. Neuropsychopharmacology. 2018;43(11) doi: 10.1038/s41386-018-0083-z. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Hua K., Zhang J., Jiang H., Dubey P., Blitz A., van Zijl P., Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Yap M.B.H., Sheeber L., Dudgeon P., Yücel M., Pantelis C., Simmons J.G., Allen N.B. Hippocampal volume and sensitivity to maternal aggressive behavior: A prospective study of adolescent depressive symptoms. Dev. Psychopathol. 2011;23(1):115–129. doi: 10.1017/S0954579410000684. [DOI] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Dennison M., Schwartz O., Simmons J.G., Sheeber L., Allen N.B. Observed measures of negative parenting predict brain development during adolescence. PLOS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J.G., Dennison M., Schwartz O., Pantelis C., Sheeber L., Byrne M.L., Allen N.B. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry. 2017;74(8):824–832. doi: 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E.P., Nguyen L., Leibenluft E., Stange J.P., Linke J.O. A meta-analysis on the uncinate fasciculus in depression. Psychol. Med. 2023;53(7):2721–2731. doi: 10.1017/S0033291723000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap M.B.H., Pilkington P.D., Ryan S.M., Jorm A.F. Parental factors associated with depression and anxiety in young people: a systematic review and meta-analysis. J. Affect. Disord. 2014;156:8–23. doi: 10.1016/j.jad.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract profiles of white matter properties: automating fiber-tract quantification. PLOS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
I have shared the link to the data and code at the Attach File step