Abstract
Bacterial strains CVO and FWKO B were isolated from produced brine at the Coleville oil field in Saskatchewan, Canada. Both strains are obligate chemolithotrophs, with hydrogen, formate, and sulfide serving as the only known energy sources for FWKO B, whereas sulfide and elemental sulfur are the only known electron donors for CVO. Neither strain uses thiosulfate as an energy source. Both strains are microaerophiles (1% O2). In addition, CVO grows by denitrification of nitrate or nitrite whereas FWKO B reduces nitrate only to nitrite. Elemental sulfur is the sole product of sulfide oxidation by FWKO B, while CVO produces either elemental sulfur or sulfate, depending on the initial concentration of sulfide. Both strains are capable of growth under strictly autotrophic conditions, but CVO uses acetate as well as CO2 as its sole carbon source. Neither strain reduces sulfate; however, FWKO B reduces sulfur and displays chemolithoautotrophic growth in the presence of elemental sulfur, hydrogen, and CO2. Both strains grow at temperatures between 5 and 40°C. CVO is capable of growth at NaCl concentrations as high as 7%. The present 16s rRNA analysis suggests that both strains are members of the epsilon subdivision of the division Proteobacteria, with CVO most closely related to Thiomicrospira denitrifcans and FWKO B most closely related to members of the genus Arcobacter. The isolation of these two novel chemolithotrophic sulfur bacteria from oil field brine suggests the presence of a subterranean sulfur cycle driven entirely by hydrogen, carbon dioxide, and nitrate.
During commercial production of petroleum, water is often coproduced with the oil. This produced water may be formation water (i.e., stratal) or any mixture of formation water, ground water, and surface water. Ground water or surface water is often injected into petroleum reservoirs to maintain reservoir pressure and improve the recovery of additional oil (12). Produced water, often referred to as “brine” in this paper, usually contains high concentrations of inorganic dissolved solids, such as sodium, chloride, magnesium, calcium, bicarbonate, and sulfate. However, dissolved solids in oil field brines can become more dilute following many years of injection with less-saline ground water or surface water. Many oil field brines also contain sulfides (H2S and HS−) as a result of the activity of sulfate-reducing bacteria (SRB) or other sulfidogenic bacteria. Sulfides are undesirable during oil production operations because of their toxicity, corrosiveness, and ability to form insoluble metal sulfides that can lead to a loss of reservoir permeability (40). Typically, control of SRB involves the use of oxidizing and nonoxidizing biocides, such as chlorine, bromine, aldehydes, amines, and quaternary phosphonium salts (28, 45). However, these chemicals can also be toxic, expensive, and ineffective (28, 62).
An alternative method for the mitigation and control of sulfides in oil field brines involve the addition of nitrate. Nitrates stimulate the activity of indigenous nitrate-reducing, sulfide-oxidizing bacteria (NR-SOB) to lower sulfide concentrations as well as stimulating heterotrophic denitrifiers that can outcompete SRB for energy sources (e.g., organic acids). The use of nitrate to control H2S odors in sewer and other wastewater streams has been known for many years and continues to be of commercial interest (1, 5, 8, 16, 22, 47). More recently, Jenneman et al. (32) and Jack et al. (27) demonstrated that addition of nitrate to sulfide-laden oil field brines could also be used to remove sulfides in these waters. McInerney et al. (41) added ammonium nitrate to an injector at the Southeast Vasser Vertz Sand Unit in Oklahoma and reported a 40 to 60% reduction in sulfide at three adjacent producing wells that they attributed to the activity of indigenous nitrate reducing bacteria (NRB). Reinsel et al. (48) observed lower concentrations of sulfides in the effluents of sandstone columns inoculated with produced water containing indigenous bacteria from two North Sea oil fields following injection with low concentrations of nitrate or nitrite (0.57 to 0.71 mM). However, they attributed the lower sulfide concentrations to nitrite inhibition of the indigenous SRB and not to oxidation of sulfides by indigenous NR-SOB. Sandbeck and Hitzman (50) claimed that nitrate controls sulfide in oil field brines by allowing the indigenous NRB to outcompete SRB for common electron donors (e.g., organic acids), thereby allowing NRB to exclude SRB (i.e., “biocompetitive exclusion”). Furthermore, they suggested that nitrate or nitrite in the presence of low concentrations of molybdate, an SRB inhibitor, is biocidal to SRB (24).
To further demonstrate the effectiveness of nitrate in mitigating sulfides in oil field brines, a recent field test was performed at the Coleville (CV) oil field in Saskatchewan, Canada (33). Ammonium nitrate (5 mM) and sodium phosphate (0.1 mM) were added continuously to injected brine for 50 days, resulting in complete removal of sulfide at one of two injectors used in the study. Reductions of 50 to 60% in sulfide levels at two adjacent producing wells were reported (33). Using nitrate-amended enrichments of CV brine, the products of this biological sulfide oxidation were determined to be elemental sulfur and dinitrogen gas (31). Telang et al. (61), using reverse sample genome probing, demonstrated that sulfide reductions in the oil field brine were accompanied by significant increases in a novel NR-SOB, referred to as strain CVO, while SRB concentrations remained unchanged or decreased slightly. Another novel NR-SOB, strain FWKO B, was also isolated and purified from CV-produced water but was not a dominant member of nitrate-amended CV brine enrichments. A preliminary report on the isolation and identification of strains CVO and FWKO B has been published (30). Voordouw et al. (70) provisionally identified CVO as a Campylobacter sp. and determined its presence in CV brine as well as several other western-Canadian oil field brines using reverse sample genome probing and hybridization of PCR-amplified 16S rRNA genes with a specific oligonucleotide probe. This led to the conclusion that NR-SOB may play key roles in the cycling of sulfur in these oil field brines. The purpose of this paper is to further describe and compare the properties of these two novel chemolithotrophic NR-SOB isolated from oil field brine.
MATERIALS AND METHODS
Source and collection of inoculum.
Produced water from the CV oil field near Kindersley, Saskatchewan, Canada, was used for enrichments and bacterial isolations. The water was collected near the bottom of the free-water-knockout (FWKO) tank which is used for primary separation of water, oil, and gas from producing wells. Approximately 4,800 m3 of water per day is produced, separated, and reinjected into 120 injection wells. Water is reinjected into the Bakken sandstone reservoir at a depth of 823 m with a bottomhole temperature of 29°C. The major constituents of the produced water include (all wt/vol) sodium, 0.29%; chloride, 0.41%; bicarbonate, 0.19%; and sulfate, 0.026%. The pH of the produced water at atmospheric pressure was 7.0 to 8.5. Total dissolved sulfide concentrations were between 100 and 200 mg/liter. The produced oil is a viscous asphaltic crude oil with a density of 0.984 g/cm3 at 15°C.
Produced water was collected in 1-liter sterile glass bottles that had been preincubated in an anaerobic chamber containing 10% H2, 5% CO2, and 85% N2 (Coy Laboratory Products, Inc., Ann Arbor, Mich.). Following collection, samples were shipped to the laboratory within 24 h and stored in the anaerobic chamber at room temperature. The time between sample collection and use varied between 3 days and 3 weeks.
Growth media.
CV synthetic brine (CSB) medium, a modified version of DTA medium (see below) used for growth of Beggiatoa, contained (all g/liter) NaCl, 7.0; MgSO4 · H2O, 0.68; CaCl2 · H2O, 0.24; NH4Cl, 0.02; KH2PO4, 0.027; NaC2H3O2 · 3H2O, 0.68; KNO3, 1.0; NaHCO3, 1.90; resazurin, 0.0001; and ND trace metals, 50 ml/liter (43). Following adjustment of the medium pH to between 7.0 and 7.5 and autoclaving, cooling, and equilibration of the medium with chamber gas overnight, sulfide (0.5 to 1.0 mM) was added aseptically from a sterile stock solution of 1.0 M Na2S · 9H2O and the medium was dispensed into sterile serum bottles or Balch tubes (18 by 150 mm; Bellco Glass, Inc., Vineland, N.J.) preincubated in the chamber overnight. The bottles were sealed with butyl rubber stoppers and aluminum crimp caps.
DTA brine medium was a semisynthetic CV brine that contained (all g/liter) (NH4)2SO4, 0.13; KNO3, 1.0; KH2PO4, 0.03; NaC2H3O2 · 3H2O, 0.78; CaCl2, 0.1; resazurin, 0.01; and 50 ml of ND trace metals (43)/liter added to CV oil field brine collected from the FWKO tank. This medium was sterilized by filtering it through a 0.22-μm-pore-size filter inside the anaerobic chamber or was dispensed into oxygen-free screw-cap glass bottles inside the anaerobic chamber and then sterilized by autoclaving (15 min at 121°C).
Enrichment and isolation.
Enrichments were prepared by adding 50 ml of produced water to sterile serum bottles preincubated for at least 24 h in the anaerobic chamber. Potassium nitrate (10 mM) and sodium phosphate, monobasic (0.1 mM), were then added from sterile stock solutions. The bottles were sealed and incubated at 30°C. Strain CVO was isolated by plating enrichments onto S-8 medium plates used for cultivation of Thiobacillus denitrificans (26). Isolated colonies from these plates were placed into DTA brine medium. Several rounds of endpoint dilution were used to further purify strain CVO. Strain FWKO B was isolated by plating enrichments in DTA brine medium on DTA brine medium plates. Three rounds of dilution to extinction in DTA brine medium were then used to further purify strain FWKO B. Extinction dilution was used to further purify CVO and FWKO B due to their poor growth on agar-based media used for primary isolation. CVO and FWKO B have been deposited in the Agricultural Research Service culture collection under accession numbers NRRL B-21473 and NRRL B-21472, respectively.
Growth and maintenance.
Routine growth and maintenance of both isolates were in CSB medium. Stock cultures of CVO and FWKO B were stored as lyophiles at −40°C. Working cultures from the lyophiles were grown in CSB medium soft agar stabs (0.4% agar). From the stabs, the isolates were cultured into serum bottles containing CSB medium.
Growth was detected from an increase in optical density (at 600 nm) or an increase in acridine orange direct counts (AODC). EpiCount membrane filters and stain (Nuclepore Corp., Pleasanton, Calif.) were used, and the EpiCount procedure was followed except that the membranes were rinsed with 3.0 ml of filtered (0.22-μm pore size) distilled water prior to being stained. An increase in optical density of at least 0.1 absorbance unit or at least a 10-fold increase in AODC was considered an indicator of significant cell growth. In addition, growth on elemental sulfur as an electron donor or acceptor was detected as an increase in sulfate or sulfide concentration, respectively, relative to uninoculated controls.
Growth of type cultures.
The following strains of sulfide-oxidizing bacteria were purchased from the American Type Culture Collection (ATCC): Thiobacillus denitrificans ATCC 23642, Thiomicrospira denitrificans ATCC 33889, Sulfurospirillum deleyianum ATCC 51133, Campylobacter sp. strain DSM 806, and Arcobacter nitrofigilis ATCC 33309. All type strains were grown in media as recommended by the ATCC (20).
Microscopic characterization.
All cells used in microscopic characterization were grown in CSB medium. The morphologies and dimensions of isolates were determined from photomicrographs using scanning electron microscopy (SEM), phase microscopy, or epifluorescence microscopy. The widths and lengths given represent the averages of measurements of several cells. Motility and the presence of spores were determined using phase microscopy of wet mounts. Gram staining was performed by the Hucker method (14).
For SEM, 1 ml of either CVO or FWKO B cells grown in CSB medium for about 24 h was centrifuged at 10,000 × g for 15 min. The pellet was resuspended in 1 ml of 0.1 M sodium phosphate (pH 7.0) containing 2.5% glutaraldehyde. After 30 min, the cells were washed three times with 0.1 M phosphate buffer and then fixed in 0.1% (wt/vol) osmium tetroxide. Following three more washes in 0.1 M phosphate buffer, a small portion of the cell suspension was removed and washed three times with distilled water. This suspension was pipetted onto a glass slide. Twenty minutes later, the slide was immersed in a liquid nitrogen-cooled isopentane bath. The frozen slides were dried overnight in a lyophilizer, sputter coated with Au-Pd, and observed with a scanning electron microscope.
Enzyme tests.
Cells of both strains were grown on CSB agar plates containing 1.0 mM sulfide. Colonies were used to determine oxidase activity with oxidase differentiation disks (Difco Laboratories, Detroit, Mich.) and catalase activity by the Tween 80-hydrogen peroxide assay (10).
Anaerobic substrate tests.
Sterilized, anaerobically equilibrated CSB medium was used for all substrate utilization tests. A 2.0 to 2.5% (vol/vol) inoculum of a 24- to 48-h-old culture of strain CVO or FWKO B grown in CSB medium with 0.5 mM sulfide was used. Balch tubes and bottles (50 ml) were sealed and incubated in the dark. Tests with elemental sulfur as an electron donor were performed by adding a slurry of elemental sulfur prepared according to the method of Widdel and Pfennig (72) for growth of Desulfuromonas. Ascorbic acid (0.1 g/liter) and thioglycollic acid (0.1 g/liter) were added as reducing agents in tests where sulfide was not the electron donor. Following inoculation, the headspaces in some tubes were exchanged and repressurized (172 kPa) with deoxygenated gas using a gassing manifold (4). All tests were run in triplicate, and growth was determined after at least 5 days of incubation.
(i) Nitrogen reduction.
Concentrated stock solutions of the following electron donors were prepared and added to CSB medium, minus sulfide and acetate, at a final concentration of 5 mM: acetate, succinate, formate, lactate, pyruvate, malate, fumarate, and propionate.
Tests with hydrogen as the electron donor were performed in CSB medium minus sulfide and acetate. The headspace was evacuated and replaced with H2-CO2 (90%:10%).
Tests with sulfide (0.5 mM) as the electron donor were performed in CSB medium equilibrated with chamber gas at atmospheric pressure. Tests with sodium thiosulfate (10 g/liter) or elemental sulfur (0.03 g/liter) as an electron donor were performed in CSB medium without sulfide using a headspace with N2-CO2 (90%:10%). The alternate electron acceptors, sodium nitrite (3.0 mM) and nitrous oxide (2%), were substituted for the nitrate in CSB medium in some tests. Nitrous oxide (Matheson, East Rutherford, N.J.) was added with a gas-tight syringe to the headspaces of bottles containing N2-CO2 (90%:10%).
(ii) Sulfur reduction.
Tests with hydrogen as the electron donor were performed in CSB medium minus nitrate, sulfide, and acetate, with elemental sulfur added as the electron acceptor and a headspace consisting of H2-CO2 (90%:10%).
(iii) Fermentation.
The following substrates were added to CSB medium minus nitrate, acetate, and sulfide (all 5 mM): acetate, pyruvate, succinate, fumarate, malate, aspartate, lactate, and glucose.
Aerobic substrate tests.
Aerobic growth in CSB medium minus acetate and nitrate was tested for the following organic substrates (all 5 mM): acetate, succinate, lactate, fumarate, malate, formate, pyruvate, glucose, and glycerol. Aerobic growth on thiosulfate (5 mM) and elemental sulfur was tested in CSB medium without nitrate, bicarbonate, and sulfide in 250-ml flasks containing 50 ml of medium with shaking on an incubator-shaker at 250 rpm.
Microaerophilic (1% O2) growth with sulfide (1.0 mM) as an electron donor was tested in CSB medium without nitrate, whereas S0 and H2 as the electron donors were tested in CSB medium without acetate and sulfide. The oxygen was added as 0.5 ml of sterile air (21% O2) to the headspace of each bottle containing 10 ml of N2-CO2 (90%:10%). A control for chemical oxidation of sulfide was run in sterile CSB medium without nitrate containing 1% oxygen in the headspace.
Carbon sources.
The ability of CVO and FWKO B to use CO2 as the sole source of carbon while growing on sulfide-nitrate was tested in CSB medium, with or without acetate. The headspace consisted of 90% N2 and 10% CO2. The utilization of acetate (10 mM) as the sole carbon source was tested in CSB medium containing 5 mM KH2PO4, minus sodium bicarbonate, and in the presence of an N2 headspace. Disappearance of sulfide and an increase in AODC were used to follow growth.
Growth parameters.
CSB medium containing 2 to 3 mM sulfide was used to study the effects of temperature and salinity, and CSB medium with 1.0 mM sulfide was used to study the effect of pH. The buffers (0.1 mM) used in pH experiments were citrate-phosphate (pHs 5.5 and 6.0), phosphate (pH 7.0), and Tris-hydrochloride (pH 8.5) (11). Growth was defined as the complete oxidation of sulfide within 7 days. All tests were run in triplicate. Controls without added cells were run to determine abiotic oxidation of sulfides.
Growth curves for both CVO and FWKO B were determined for cells growing in DTA brine medium. The medium was filter sterilized and dispensed into sterile 50-ml serum bottles. The bottles were inoculated with CVO and FWKO B previously grown in the same medium, and incubated. Specific growth rates were calculated for data (log AODC versus time) collected during early exponential growth. The data represent averages of three replicates.
Transformations of nitrate-nitrogen and sulfide-sulfur. (i) Effect of sulfide concentrations.
Strains CVO and FWKO B were grown in CSB medium with 0.5, 1.0, 2.0, or 3.0 mM sulfide. After 48 h of incubation, samples of culture media were analyzed for sulfide, soluble sulfur compounds (SSC), sulfate, sulfite, thiosulfate, nitrate, and nitrite.
(ii) 15N isotope studies.
For growth of CVO and FWKO B, CV field brine was amended with 5 mM K15NO3 and 0.1 mM NaH2PO4. For the growth of FWKO B, (NH4)2SO4 (130 mg/liter), CaCl2 (100 mg/liter), and 50 ml of ND trace metals (43) were also added. The medium was then filter sterilized (0.22-μm pore size) and dispensed as 100-ml aliquots into sterile 120-ml serum bottles. Following inoculation and sealing, the cultures were incubated overnight and analyzed for nitrate, nitrite, sulfate, sulfite, thiosulfate, sulfur, ammonia, N2O, and N2. The presence of 15N2O and 15N2 was determined by gas chromatography-mass spectrometry analysis of headspace gas.
Analytical procedures.
Sulfide was detected either colorimetrically by a modification of the method of Fogo and Popowski (18) or using an Aquaquant hydrogen sulfide test kit (EM Sciences, Gibbstown, N.J.). Nitrate, nitrite, sulfate, thiosulfate, and sulfite were separated by ion chromatography and detected amperometrically. Ammonia was detected using an ion-specific electrode. Total SSC (which includes sulfate, sulfide, and thiosulfate), elemental sulfur, and calcite were qualitatively identified in culture broth by filtering solids onto a 0.22-μm-pore-size membrane filter, washing them with deionized water, drying them at room temperature, and analyzing them by X-ray diffraction spectroscopy. Elemental sulfur was analyzed by the method of Chan and Suzuki (9) using sulfur extraction in petroleum ether and ferric thiocyanate color formation for detection.
Molecular biology reagents.
Deoxyoligonucleotides were obtained from University Core DNA Services, The University of Calgary. PCR reagents (including Taq polymerase) and [α-35S]dATP were from Amersham-Pharmacia. Campylobacter sp. strain DSM 806 genomic DNA was kindly provided by W. Schumacher, Swiss Federal Institute for Environmental Science and Technology, Kastanienbaum, Switzerland.
Construction of a CVO-specific radiolabeled oligonucleotide probe (CLI) and hybridization test.
Whole cells (1 ml at 5 × 107/ml) were applied to a nylon membrane by slot blotting. The cells were then lysed, and the membrane was treated according to the method of Braun-Howland et al. (7). The membrane was then probed with the deoxyoligonucleotide CLI (5′-ATATGCTACCGTCATT) end labeled with [α-32P]ATP or with the general eubacterial probe EUB. Hybridization conditions have been previously described (70).
16S rRNA gene sequence analysis and phylogeny.
Nearly complete 16S rRNA gene sequences (positions 49 to 1313; Escherichia coli numbering) were obtained for CVO, FWKO B, and Campylobacter sp. strain DSM 806, using both manual sequencing with a Promega fmol cycle-sequencing kit and automated sequencing with an ABI PRISM dye terminator cycle-sequencing ready-reaction kit (Big Dyes). Automated sequencing was done on a model 377XL PRISM Sequencer (Applied Biosystems Inc.) at University Core DNA Services. The 1.4-kb PCR fragments used as templates for sequencing were amplified from genomic DNA with primers f8 (44) and r1406 (23) as explained elsewhere (61). The primers used for sequencing were f8, r1406, EUB 338 (3), p75 [ACCGCGGC(G/T)GCTGGC], p76 (complement of p75), and p78 [GTGAAAT(T/C)CGTAGA(G/T) ATC]. Sequences were aligned with the Staden package (56). The consensus sequence derived from this alignment was compared with all sequences in GenBank with a Blast search (2). Nineteen sequences that showed high homology with CVO, FWKO B, or DSM 806 were retrieved from GenBank. A multiple sequence alignment and corresponding dendrogram of these sequences and those of CVO, FWKO B, DSM 806, and E. coli was generated with the PileUp program of the Wisconsin Package version 9.1 (Genetics Computer Group [GCG], Madison, Wis.). The file of aligned sequences was also used to generate a phylogenetic tree with the phylogenetic analysis using parsimony (PAUP) software package (60) with 100 bootstrap replicates and using the E. coli sequence as an outgroup.
Nucleotide sequence accession numbers.
The sequences for CVO, FWKO B, and Campylobacter sp. strain DSM 806 have been deposited in GenBank under accession numbers U465062, AF144693, and AF144694, respectively.
RESULTS
Enrichment and isolation.
Addition of nitrate and phosphate to produced water from the CV oil field resulted in the anaerobic biological oxidation of sulfide (3 to 4 mM) and the production of elemental sulfur and dinitrogen gas according to the following stoichiometry (31): 5HS− + 2NO3− + 7H+ → 5S0 + N2 + 6H2O.
Microscopy of bacteria in the brine revealed the presence of nearly 107 cells/ml. SRB, denitrifying bacteria, fermentative bacteria, and spore-forming bacteria have all been previously detected in this brine (reference 70 and unpublished results). Lactate-oxidizing SRB compose nearly 1% of the total population, while NR-SOB have been detected at concentrations of 104 to 106/ml (20, 33). The NR-SOB, strains CVO and FWKO B, were isolated from enrichments of CV brine containing nitrate and phosphate as colonies on agar plates containing thiosulfate (26) and sulfide as energy sources, respectively. Subsequent purification was by dilution to extinction in DTA brine medium.
Characterization of isolates.
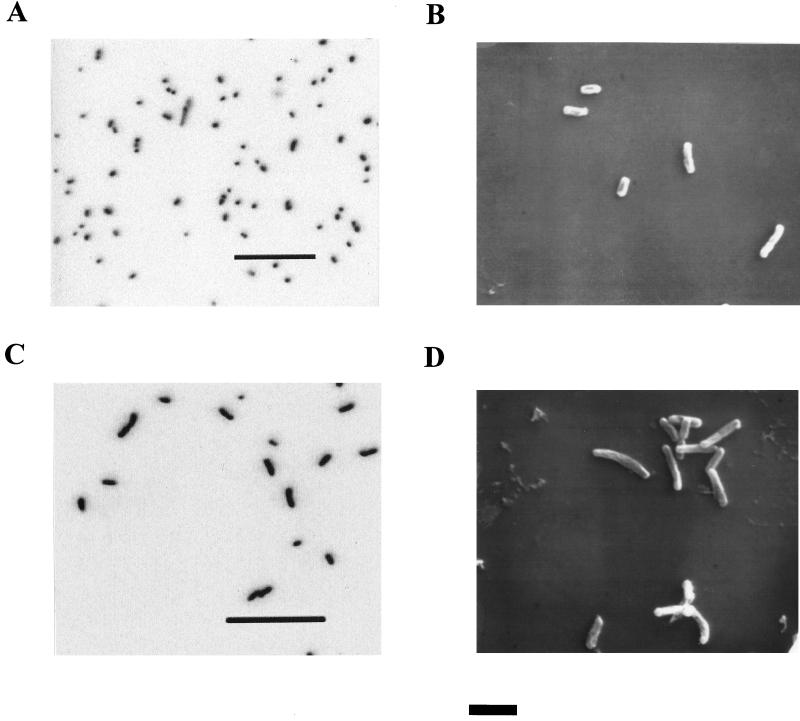
CVO (Fig. 1A and B) and FWKO B (Fig. 1C and D) are slightly curved rods with a diameter of 0.4 μm and lengths of 0.6 to 0.8 and 1.0 to 2.0 μm, respectively. Both are gram-negative non-spore formers. FWKO B is very motile, while CVO is nonmotile or very weakly motile. Both strains are catalase negative, while only CVO is positive for oxidase activity (Table 1).
FIG. 1.
(A and C) Epifluorescence photomicrographs of strains CVO (A) and FWKO B (C) grown with sulfide and nitrate (bars = 20 μm). (B and D) SEM photomicrographs of strains CVO (B) and FWKO B (D) grown on sulfide and nitrate (bar = 2 μm).
TABLE 1.
Major characteristics of strains CVO and FWKO B isolated from oil field brine
| Parameter | Valuea
|
|
|---|---|---|
| CVO | FWKO B | |
| Morphology | Slightly curved rods | Slightly curved rods |
| Width (μm) | 0.4 | 0.4 |
| Length (μm) | 0.6–0.8 | 1.0–2.0 |
| Spores | − | − |
| Motility | −b | + |
| Gram stain | − | − |
| Blood agar | − | − |
| Oxidase test | + | − |
| H2O2 decomposition | − | − |
| Aerobic growth | ||
| Acetate, succinate, lactate, fumarate, malate, formate, pyruvate, glucose, glycerol | − | − |
| S2O32− | − | − |
| S0 | − | NDd |
| Microaerophilic growth (1% O2) | ||
| HS− | + | + |
| S0 | + | ND |
| H2 | ND | + |
| Anaerobic growth on NO3− by oxidation of: | ||
| HS− | + | + |
| S2O32− | − | − |
| S0 | + | − |
| H2 | − | + |
| Formate | − | + |
| Acetate, pyruvate, succinate, fumarate, lactate, propionate, glucose | − | − |
| Anaerobic growth on NO223 by oxidation of: | ||
| HS− | + | − |
| S0 | + | − |
| H2 | ND | − |
| Anaerobic growth on N2O (2%) by oxidation of HS− | +c | − |
| Anaerobic growth on H2 by reduction of S0 | − | + |
| Anaerobic growth on acetate by reduction of S0 | ND | − |
| Growth by fermentation of pyruvate, succinate, lactate, fumarate, malate, aspartate | − | − |
+, positive; −, negative.
Nonmotile or only weakly motile.
Only a fivefold increase in cell numbers was observed after 5 days of incubation.
ND, not determined.
Growth rates.
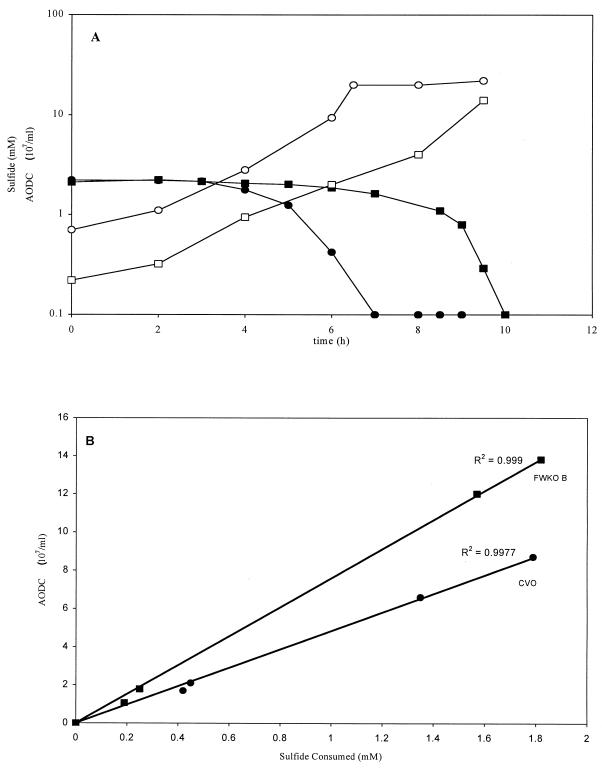
Consumption of sulfide (2 mM) in DTA brine medium was roughly proportional to growth for CVO and FWKO B (Fig. 2). Both strains reached a maximum cell density of over 108/ml at specific growth rates of 0.53 and 0.46 h−1, respectively.
FIG. 2.
(A) Growth curves and sulfide consumption rates, respectively, for CVO (○ and ●) and FWKO B (■ and □) growing at 30°C in DTA brine medium containing 2 mM sulfide and 10 mM KNO3. Cell numbers are expressed as AODC. (B) Cell density versus sulfide consumption for CVO and FWKO B plotted from data in panel A. Linear trend lines and R2 values were determined with Microsoft Excel 97 by setting the y intercept to 0.
Anaerobic growth on nitrate, nitrite, and nitrous oxide.
CVO grew anaerobically with sulfide (0.5 to 3.0 mM) as an electron donor and either nitrate, nitrite, or nitrous oxide (2%) as an electron acceptor. In contrast, FWKO B only grew with nitrate as an electron acceptor (Table 1). Growth on sulfide (0.5 mM) and nitrous oxide (2%) by strain CVO was slow, as only a fourfold increase in cell numbers and a 59% decrease in sulfide were observed, on average, after 5 days of incubation. At this time, the addition of more nitrous oxide (2%) did not result in any further sulfide oxidation or growth (results not shown). Sterile controls containing sulfide and nitrous oxide indicated no significant chemical oxidation of sulfide during this time. Both strains are capable of strictly autotrophic growth on sulfide and nitrate, whereas CVO presumably utilized acetate as a carbon source in the absence of added bicarbonate or CO2 (Table 2). FWKO B displayed hydrogenotrophic growth in the presence of nitrate. Growth of CVO on elemental sulfur in the presence of nitrate or nitrite was accompanied by sulfate production and clumping of sulfur crystals. Neither strain displayed an organotrophic mode of growth in the presence of nitrate and various organic substrates tested (Table 1). In addition, neither CVO nor FWKO B grew in thiosulfate-based media designed for the growth of Thiobacillus denitrificans (26) and Thiomicrospira denitrificans (66).
TABLE 2.
Growth of strains CVO and FWKO B on organic or inorganic carbon sources in the presence of various electron donors and acceptors
| Strain/donor/acceptor | Growth on carbon sourcea
|
||
|---|---|---|---|
| CO2-acetate | CO2 | Acetate | |
| CVO/HS−/NO3− | + | + | ± |
| CVO/S0/NO3− | + | ND | ND |
| FWKO B/HS−/NO3− | + | + | − |
| FWKO B/H2/S0 | + | + | ND |
ND, not determined; +, growth; −, no growth; ±, as much as 0.4 mM bicarbonate was transferred with the inoculum (2%) such that at least some of the growth yield (107 cells/ml) might have been due to assimilated HCO3−.
Anaerobic growth on sulfate and sulfur.
FWKO B grew by reduction of elemental sulfur to sulfide using hydrogen as an electron donor and CO2 as its sole carbon source (Tables 1 and 2). Attempts to growth CVO and FWKO B in a medium designed for growth of lactate-oxidizing SRB were unsuccessful (results not shown).
Fermentation.
Fermentative growth was not observed for strain CVO or FWKO B when tested on a variety of carbon substrates (Table 1), and neither CVO nor FWKO B grew in organic-rich, complex growth media designed for anaerobic growth of heterotrophic microorganisms.
Aerobic growth.
Neither strain was capable of growth on sulfur, thiosulfate, or various carbon sources under fully aerobic (21% O2) conditions (Table 1). However, autotrophic growth was observed for both strains under microaerophilic conditions (1% O2), with CVO using either sulfide or elemental sulfur and FWKO B using hydrogen as an electron donor (Table 1).
Temperature, salts, and pH ranges.
Growth of CVO occurred over a temperature range of 5°C (i.e., the lowest tested) to 35°C, a salinity range up to 7% NaCl, and a pH range from 5.5 to 8.5 (i.e., the highest tested). FWKO B grew over a temperature range of 10°C to 40°C, a salinity range of up to 3% NaCl, and a pH range from 6.0 to 8.5 (Table 3).
TABLE 3.
Effects of temperature, salinity, and pH on growth of strains CVO and FWKO B
| Growth conditionsa | Growthb
|
|
|---|---|---|
| CVO | FWKO B | |
| 5°C | + | − |
| 10°C | + | + |
| 35°C | + | + |
| 40°C | − | + |
| 45°C | − | − |
| 0% NaCl | + | + |
| 1.0% NaCl | + | + |
| 3.0% NaCl | + | + |
| 5.0% NaCl | + | − |
| 7.0% NaCl | + | − |
| 10.0% NaCl | − | ND |
| pH 5.5 | + | − |
| pH 6.0 | + | ±c |
| pH 7.0 | + | + |
| pH 8.5 | + | + |
Growth in CSB medium with 2 to 3 mM sulfide for temperature tests and 1 mM sulfide for salinity and pH tests. All tests were run in triplicate except where noted in the text.
ND, not determined; +, growth; −, no growth.
Complete oxidation of sulfide was noted in only one of two replicates.
Sulfur and nitrogen transformation.
Complete oxidation of 0.5 to 2.0 mM sulfide and 0.5 to 3.0 mM sulfide by CVO and FWKO B, respectively, occurred within 48 h under anaerobic conditions (Table 4). At the lower sulfide concentrations tested, CVO transformed 80 to 100% of the sulfide oxidized to SSC (Table 4), primarily in the form of sulfate, whereas at the higher sulfide concentrations tested, less than 15% of the sulfide oxidized was converted to SSC, suggesting that the majority of sulfide had been oxidized to elemental sulfur. However, only 29% of the sulfide-S, on average, was accounted for as elemental sulfur, while 61% of the total sulfur (Table 4, ΔS) remained unaccounted for. In contrast, oxidation of sulfide by FWKO B resulted in no increase in SSC regardless of the initial sulfide concentration. Instead, elemental sulfur accounted for 40 to 60% of the oxidized sulfide, on average, leaving the balance of the insoluble sulfur unaccounted for (Table 4, ΔS). These sulfur values are likely underestimates of the true values, since extraction of elemental sulfur from biological cultures via solvent extraction can be problematic due to the propensity of sulfur to form aggregates with the biomass and adhere to surfaces. However, the fact that SSC did not increase further suggests that elemental sulfur was the sole end product.
TABLE 4.
Transformation of nitrate-N (10 mM) and sulfide-S (0.5 to 3.0 mM) during sulfide oxidation by strains CVO and FWKO B
| Initial HS− (mM) | ΔHS− (mM) | ΔSSCa (mM) | ΔSO42− (mM) | S0 (mM) | ΔSd (mM) | ΔNO3− (mM) | ΔNO2− (mM) | ΔNe (mM) |
|---|---|---|---|---|---|---|---|---|
| Strain CVO | ||||||||
| 0.5 | −0.5 ± 0.0b | 0.5 ± 0.00 | 0.3 ± 0.12 | 0.04 ± 0.04 | −0.16 | −2.2 ± 0.50 | 1.5 ± 0.10 | −0.7 |
| 1.0 | −1.0 ± 0.0 | 0.8 ± 0.01 | 0.8 ± 0.10 | 0.02 ± 0.01 | −0.18 | −3.3 ± 0.38 | 2.2 ± 0.29 | −1.1 |
| 2.0 | −2.0 ± 0.0 | 0.3 ± 0.24 | 0.2 ± 0.18 | 0.58 ± 0.18 | −1.22 | −1.7 ± 0.40 | 0.66 ± 0.20 | −1.0 |
| 3.0 | −2.9 ± 0.16 | 0.0c | 0.2c | ND | ND | −2.8c | 1.1c | −1.7 |
| Strain FWKO B | ||||||||
| 0.5 | −0.5 ± 0.0b | −0.4 ± 0.12 | −0.2 ± 0.17 | 0.26 ± 0.04 | −0.24 | −0.9 ± 0.32 | 0.88 ± 0.03 | 0 |
| 1.0 | −1.0 ± 0.0 | −0.3 ± 0.0 | 0.0 ± 0.0 | 0.57 ± 0.08 | −0.43 | −1.2 ± 0.10 | 1.4 ± 0.15 | 0 |
| 2.0 | −2.0 ± 0.0 | −0.4 ± 0.12 | −0.2 ± 0.12 | 1.15 ± 0.09 | −0.85 | −2.3 ± 0.31 | 2.3 ± 0.06 | 0 |
| 3.0 | −3.0 ± 0.0 | −0.3 ± 0.25 | 0.0 ± 0.06 | 1.37 ± 0.04 | −1.63 | −3.2 ± 0.06 | 3.0 ± 0.08 | 0 |
Soluble sulfur as measrued by X-ray fluroescence spectroscopy.
Samples after 48 h of incubation at 30°C; average ± standard deviation; n = 3.
n = 1.
Total sulfur unaccounted for as either SO42− or S0.
Total nitrogen unaccounted for in the form of either N2O or N2; calculated as ΔNO3− + ΔNO2−.
At the lower sulfide concentrations tested, strain CVO converted over 60% of the nitrate reduced to nitrite, while less than 50% of the nitrate consumed was reduced to nitrite at the higher sulfide concentrations tested (2.0 and 3.0 mM). The nitrate-N unaccounted for is presumed to be in the form of nitrous oxide or dinitrogen gas. When strain CVO was added to CSB medium containing [15N]nitrate (5 mM) and 3.0 mM sulfide, the production of 15N2O was detected but not that of 15N2 (results not shown). Furthermore, there was no increase in ammonium. Growth of CVO did not occur, however, in CSB medium (0.5 mM sulfide) without nitrate in the presence of a headspace containing nitrous oxide (2%), nitrogen, and CO2, suggesting that CVO is capable of denitrification. In contrast, nitrate reduction by FWKO B at all sulfide concentrations tested resulted in complete conversion to nitrite.
16S RNA oligonucleotide probe.
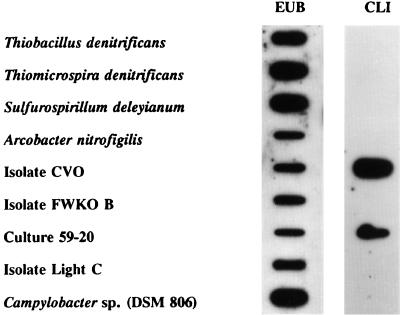
A 16-base oligonucleotide probe (CLI) targeting a unique region of 16S rRNA from strain CVO did not display a strong hybridization signal with nucleic acids (primarily 16S RNA) from other sulfide-oxidizing bacteria (Fig. 3). However, a weaker hybridization signal, not visible in Fig. 3, was observed with Thiomicrospira denitrificans, while lengthening the probe to 21 bases apparently decreased the specificity so that both Thiomicrospira denitrificans and CVO displayed signals of equal intensity (results not shown). No hybridization was observed between the CLI probe and nucleic acids from strain FWKO B (Fig. 3). However, the CLI probe did strongly hybridize with nucleic acids from strain CVO, as expected, as well as nucleic acids from an enrichment of NR-SOB from produced brine collected at producing well 59-20. No hybridization was evident with nucleic acids from isolate Light C, which is an unidentified photosynthetic NR-SOB (possibly Chromatium sp.) isolated from CV-produced brine. As a positive control, it was shown that nucleic acids from all cell types hybridized with the general eubacterial probe EUB 338 (3).
FIG. 3.
Hybridization of either labeled eubacterial (EUB 338) or labeled CVO-specific (CLI) oligonucleotide probe to nucleic acids from whole cells of bacteria known to be closely related to CVO (Fig. 4; isolate Light C is described in the text) or to nucleic acids from cells from an enrichment of sulfide-laden produced brine collected at CV Well 59-20 amended with nitrate and phosphate (culture 59-20).
Phylogenetic analysis.
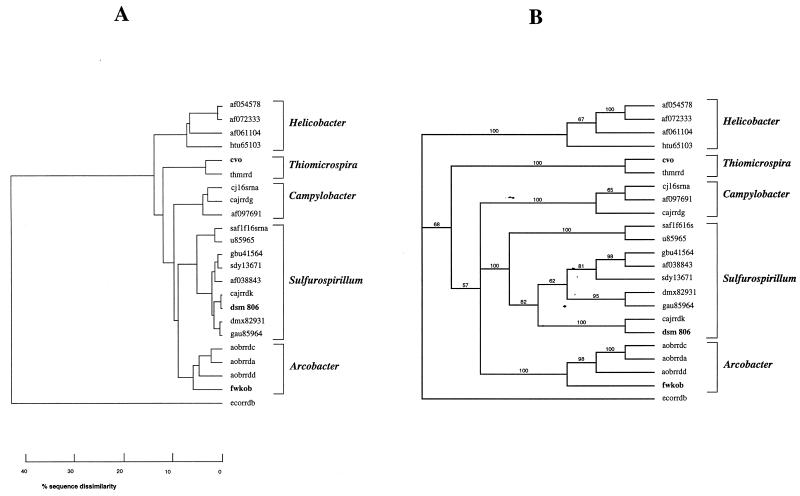
The top 100 hits of Blast searches with 16S rRNA gene sequences (positions 49 to 1313; E. coli numbering) of CVO, FWKO B, and Campylobacter sp. strain DSM 806 were all within the epsilon subdivision of the division Proteobacteria. Although these included matches with uncultured hydrothermal vent eubacteria and unidentified epsilon proteobacteria, only sequences of cultured and physiologically characterized representatives from this division were retrieved from GenBank. This included the best-matching sequences and sequences of representatives of genera within the epsilon subdivision. Pairwise sequence similarities calculated with the GCG program GAP ranged from 88 to 99%. The PileUp dendrogram derived from all pairwise similarity coefficients is shown in Fig. 4A. The 16S rRNA sequence of CVO was most similar to that of Thiomicrospira denitrificans (96.1% similarity), while the sequence of FWKO B was most closely related to that of Arcobacter spp. (92% similarity) and Sulfurospirillum spp. (90% similarity). The sequence obtained for Campylobacter sp. strain DSM 806 was nearly identical to that of Campylobacter sp. strain CCUG 1392 CLO. Both of these environmental Campylobacter spp. are most closely related to members of the newly defined genus Sulfurospirillum. The phylogenetic tree obtained with PAUP (Fig. 4B) closely matches that shown in Fig. 4A. Most branches are supported by high bootstrap values.
FIG. 4.
Phylogenetic trees of 16S rRNA sequences of strains CVO and FWKO B. (A) Tree based on pairwise homologies obtained with the GCG program PileUp. The horizontal distances reflect the pairwise sequence similarities as indicated on the scale. (B) Tree obtained by PAUP analysis with the sequence for E. coli as the outgroup and using 100 bootstrap replicates. The observed frequencies of the groups are indicated at the nodes. The horizontal distances are arbitrary. In addition to the sequences for CVO and FWKO B, the following sequences are represented in the tree: af054578, Helicobacter sp. strain UNSW1.7sp; af072333, Helicobacter sp. strain hamster B; af061104, Helicobacter typhlonicus; htu65103, Helicobacter trogontum; thmrrd, Thiomicrospira denitrificans; cj16srna, Campylobacter jejuni; cajrrdg, Campylobacter upsaliensis; af097691, Campylobacter hyointestinalis; saf1f16srna, Sulfurospirillum arcachonense; u85965, Sulfurospirillum sp. strain SM-5; gbu41564, S. barnesii; sdy13671, S. deleyianum; af038843, S. barnesii; cajrrdk, Campylobacter sp. strain CCUG 1392 CLO; dsm806, Campylobacter sp. strain DSM806 (determined in this study); dmx82931, D. multivorans; gau85964, S. arsenophilum; aobrrdc, A. butzleri; aobrrda, A. cryoaerophilus; aobrrdd, A. nitrofigilis; ecorrdb, E. coli. The strains sequenced in this study are in boldface.
DISCUSSION
Phylogeny.
Earlier analysis of a shorter 16S rRNA sequence for CVO indicated its classification as having the closest homology with Campylobacter sp. strain CCUG 1392 CLO (70). Since then, the genus Sulfurospirillum has been defined (17, 52) and a detailed analysis of the phylogeny of the genus Thiomicrospira has appeared (42). The epsilon subdivision of the division Proteobacteria currently comprises the Campylobacter group (genera Arcobacter and Campylobacter), the Helicobacter group (genera Flexispira, Gastrospirillum, Helicobacter, Thiovulum, and Wolinella), the genus Sulfurospirillum, and unclassified epsilon Proteobacteria which include Thiomicrospira denitrificans (http://www.ncbi.nlm.nih.gov/Taxonomy/tax/html). The genus Sulfurospirillum includes species previously indicated to be members of the genus Geospirillum (Sulfurospirillum barnesii, Sulfurospirillum arsenophilum, and Sulfurospirillum sp. strain SM-5 [59]) as well as Dehalospirillum multivorans (51), Campylobacter sp. strain CCUG 1392 CLO, and Campylobacter sp. strain DSM 806. All of these have high (97 to 99%) 16S rRNA sequence similarity to S. deleyianum (17), the type strain of the genus, as shown in Fig. 4A and have comparable morphological and physiological properties (52). Reclassification of Campylobacter spp. strains CCUG 1392 CLO and DSM 806 as Sulfurospirillum spp. makes the Campylobacter clade physiologically more homogeneous, i.e., strictly free-living organisms are now excluded and the remaining Campylobacter spp. all serve as human or animal pathogens. Strain CVO is a close relative of neither the Sulfurospirillum spp. nor the newly defined clade of Campylobacter spp. (Fig. 4); instead, it most closely resembles Thiomicrospira denitrificans. The genus Thiomicrospira has members in both the gamma (Thiomicrospira crunogena, Thiomicrospira pelophila, and Thiomicrospira sp. strain MA2-6) and epsilon (Thiomicrospira denitrificans only) subdivisions of proteobacteria (42). Strain CVO is thus the second well-characterized Thiomicrospira sp. in the epsilon subdivision. We propose to rename the organism Thiomicrospira sp. strain CVO from Campylobacter sp. strain CVO, the name proposed earlier (70).
From the dendrograms in Fig. 4, it appears that FWKO B is most closely affiliated with members of the genus Arcobacter, which includes both free-living (A. nitrofigilis) and pathogenic (e.g., Arcobacter butzleri and Arcobacter cryaerophilus) species. FWKO B forms a deep branch in the Arcobacter clade and has some distinct physiological properties. However, the branching is not deeper than that in the genus Helicobacter (Fig. 4A), and the designation Arcobacter sp. strain FWKO B is, therefore, appropriate.
Phenotypic characteristics of CVO.
Thiomicrospira denitrificans and Thiomicrospira sp. strain CVO are obligate chemolithoautotrophs. Like Thiomicrospira sp. strain CVO, Thiomicrospira denitrificans oxidizes sulfide with nitrate as the electron acceptor. Thiomicrospira denitrificans, and probably also Thiomicrospira sp. strain CVO (see below), denitrifies nitrate to nitrogen. Thiomicrospira denitrificans oxidizes sulfide to sulfate (65), whereas Thiomicrospira sp. strain CVO forms sulfur and sulfate, depending on the sulfide-to-nitrate ratio. However, Thiomicrospira denitrificans also used thiosulfate as an electron donor, an activity that is not shared by Thiomicrospira sp. strain CVO (Table 1). Thiomicrospira denitrificans also has a distinct morphology, forming long spirals of 4 to 5 μm (36), whereas strain CVO forms only very small curved rods less than 1 μm in length (Fig. 1). The CVO-specific oligonucleotide probe CL1 reacted only weakly with nucleic acids from Thiomicrospira denitrificans due to the presence of two mismatches.
In contrast to this strict chemolithoautotrophic growth of strain CVO (Table 1), sulfurospirillae can grow as chemoorganoheterotrophs using organic acids or amino acids as carbon and energy sources. Growth by reduction of fumarate to succinate is a characteristic of S. deleyianum and strain DSM 806, supporting their close affiliation (Fig. 4), but not of strain CVO. S. deleyianum but not Thiomicrospira sp. strain CVO can use elemental sulfur as an electron acceptor. The similarities are that all are curved or vibroid, grow only microaerophilically or anaerobically, are oxidase positive, and do not ferment glucose or reduce sulfate.
S. deleyianum dissimilates nitrate to ammonium (52), whereas CVO reduces nitrate to nitrous oxide and presumably dinitrogen: the growth of CVO in the presence of sulfide (0.5 mM) and nitrous oxide (2%) suggests that nitrogen is produced. The accumulation of nitrous oxide in the presence of 3.0 mM sulfide and nitrate indicates inhibition of nitrous oxide reductase by sulfide. As little as 0.3 mM H2S resulted in almost total inhibition of nitrous oxide reduction by denitrifying bacteria (54). Gram-negative aerobic and microaerobic denitrifiers reported to produce nitrous oxide as an end product in the absence of sulfide have also been described (74). A strictly anaerobic, thermophilic, chemolithoautotrophic archaeum, Ferroglobus placidus, dissimilates nitrate to nitrous oxide while oxidizing either sulfide, H2, or ferrous iron (71). The colorless sulfur-oxidizing bacteria of the genera Thiobacillus, Thermothrix, Thiosphaera, and Thiomicrospira are also capable of denitrification, but unlike CVO, they also oxidize thiosulfate as an alternate energy source.
Phenotypic characteristics of Arcobacter sp. strain FWKO B.
Arcobacter spp., like FWKO B, are gram-negative, motile, curved rods. They do not ferment glucose, and they grow at temperatures between 15 and 40°C, reduce nitrate, and grow under microaerophilic conditions (38, 39, 64, 68). However, unlike other Arcobacter spp., FWKO B is catalase and oxidase negative. In addition, Arcobacter spp. are chemoorganotrophs unable to grow lithotrophically, unlike FWKO B, which is a chemolithotroph capable of oxidizing sulfide and hydrogen but incapable of using any of the organic compounds tested, except formate, as an energy source (Table 1). Also, FWKO B grows autotrophically and could not use acetate as a carbon source in the absence of CO2, while Arcobacter spp. use amino acids and other organics as carbon sources (68). Furthermore, FWKO B is a hydrogenotrophic sulfur reducer, whereas hydrogen is reported to only stimulate growth of Arcobacter spp. (67). Widdel and Pfennig (72) indicated that growth by hydrogenotrophic, sulfur-reducing eubacteria typically requires 1 to 2 mM acetate in addition to CO2. Although several thermophilic chemolithoautotrophic sulfur reducers from the domain Archaea (25, 53) and several from the domain Bacteria (21) have been reported, FWKO B represents the only mesophilic chemolithoautotrophic sulfur reducer in the domain Bacteria.
Although both A. nitrofigilis and FWKO B reduce nitrate only to nitrite, FWKO B grows (Fig. 2) but A. nitrofigilis does not, due to the toxicity of the nitrite formed (38). Certain members of the neutrophilic, chemolithotrophic sulfur bacteria also produce nitrite and typically require the presence of a nitrite-reducing bacterium for growth in the presence of nitrate (49).
Growth and sulfur production by CVO and FWKO B.
Growth was proportional to sulfide oxidation for both CVO and FWKO B, indicating that both are capable of electron transport from sulfide to nitrate (Fig. 2B). The doubling times of 1.3 and 1.5 h for CVO and FWKO B, respectively, are significantly lower than the minimum doubling time of 2.85 h reported for the aerobic chemolithoautotrophic sulfide oxidizers Thiobacillus neapolitanus and Thiobacillus sp. strain O (29).
Both CVO and FWKO B oxidized sulfide to elemental sulfur, but only CVO was capable of complete oxidation to sulfate, which was dependent on the nitrate-to-sulfide ratio (Table 4). A shift from sulfate to sulfur production was also observed for two aerobic sulfide-oxidizing Thiobacillus spp. at increasing sulfide concentrations (29, 67, 69). Sulfur production by aerobic, sulfide-oxidizing thiobacilli was determined to be maximal at an oxygen/sulfide uptake ratio near the expected theoretical value of 0.56 (69). Sulfur production by CVO occurred at a nitrate/sulfide ratio of 0.52 (Table 4), which is also close to its expected theoretical ratio when nitrous oxide is the end product: HS− + 0.5 NO3− + 1.5 H+ → S0 + 0.25 N2O + 1.25 H2O. Therefore, CVO responds similarly to the aerobic thiobacilli by producing elemental sulfur when nitrate is limiting.
S. deleyianum oxidizes sulfide to elemental sulfur while reducing nitrate to ammonium (15). Its ability to shift electrons from production of sulfur to production of sulfate cannot be evaluated, since the tests were conducted in the presence of high sulfide (4 to 5 mM) and limiting nitrate (1.2 and 5 mM) concentrations. Elemental sulfur was also the end product of fumarate-dependent sulfide oxidation by Wolinella succinogenes and S. deleyianum (37, 73).
Ecological significance.
Colorless sulfur bacteria are widespread in nature and have been isolated from sulfur deposits, hot sulfur springs, soils, freshwater, and seawater (35). Bharathi et al. (6) isolated over 100 strains of anaerobic, colorless NR-SOB from seawater and a sulfide-laden creek. However, few reports have been made of the isolation of NR-SOB from petroleum reservoir brines, even though their activity has been observed (28, 41, 55, 61, 70). Dannenberg et al. (13) reported that the SRB Desulfobulbus propionicus also displays NR-SOB activity, but its presence in oil field brines has not been reported.
Sulfide-oxidizing microaerophiles such as CVO might utilize limiting amounts of oxygen or nitrate diffusing from surface water or shallow groundwater to oxidize sulfide produced by indigenous SRB in petroleum reservoirs (63, 70). Cycling of sulfur in oil reservoirs, such as CV, likely occurs between sulfide and elemental sulfur in view of the high concentrations of sulfide (>3 mM), which favor the formation of elemental sulfur (Table 4) (30).
Since CVO is the dominant NR-SOB in CV brine amended with nitrate (61, 63), the role of FWKO B may be that of a sulfur reducer and not an NR-SOB. Pfennig and Biebl (46) reported the cycling of sulfide and elemental sulfur by a phototrophic green sulfur bacterium and the acetate-oxidizing sulfur reducer Desulfuromonas acetoxidans. Likewise, efficient cycling of sulfide and elemental sulfur by D. acetoxidans and CVO has been demonstrated (63).
The ability of FWKO B and CVO to grow lithoautotrophically with sulfide and nitrate suggests the potential for a subterranean sulfur cycle driven solely by the inorganic nutrients hydrogen, nitrate, and CO2. We recently demonstrated that cycling between sulfide and elemental sulfur occurs in a CV brine enrichment by alternating the addition of nitrate and hydrogen gas to the enrichment (results not shown). Hydrogen gas in this shallow reservoir could be generated from fermentation or by geochemical mechanisms (34, 57, 58). Bicarbonate ions are plentiful in this sandstone reservoir brine (approximately 23 mM), while nitrates, used as fertilizer in this agricultural area, could enter the reservoir by diffusion from nitrate-contaminated surface waters or via injection of nitrate-contaminated groundwater used for improving oil recovery. Alternatively, the presence of these lithoautotrophic NR-SOB in oil field waters provides opportunities for the application of biologically mediated removal of sulfides in oil field waters through the simple addition of inexpensive nutrients, such as nitrate and phosphate (28, 33, 41).
ACKNOWLEDGMENTS
This work was supported by Phillips Petroleum Company and was performed as an in-kind contribution to the U.S. DOE-funded project Bioreactor Design and Demonstration for Microbial Oxidation of Sulfides under contract DOE AC-10-15 and under contract FWP 4340-42, which is being conducted under a Cooperative Research and Development Agreement (99-CR-01) with The Idaho National Engineering and Environmental Laboratory, Idaho Falls, Idaho. Research in G.V.'s laboratory was supported by a Strategic Grant from the Natural Science and Engineering Research Council of Canada (NSERC).
We thank Miriam Wright, Glenna Thompson, Robert H. Webb, and Michael Moradi-Araghi for their technical assistance. We also thank Michael Madigan for his helpful comments.
REFERENCES
- 1.Allen L A. The effect of nitro-compounds and some other substances on production of hydrogen sulfide by sulphate-reducing bacteria in sewage. Proc Soc Appl Bacteriol. 1949;2:26–38. [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Amann R I, Stromley J, Devereux R, Key R, Stahl D A. Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl Environ Microbiol. 1992;58:614–623. doi: 10.1128/aem.58.2.614-623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balch W E, Fox G E, Magrum L J, Woese C R, Wolfe R S. Methanogens: reevaluation of a unique group. Microbiol Rev. 1979;43:260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentzen G, Smith A T, Bennett D, Webster N J, Reinholt F, Sletholt E, Hobson J. Controlled dosing of nitrate for prevention of H2S in a sewer network and the effects on the subsequent treatment processes. Water Sci Technol. 1995;31:293–302. [Google Scholar]
- 6.Bharathi P A L, Nair S, Chandramohan D. Anaerobic sulfide-oxidation in marine colorless sulfur-oxidizing bacteria. Mar Biotechnol. 1997;5:172–177. [Google Scholar]
- 7.Braun-Howland E B, Vescio P A, Nierzwicki-Bauer S A. Use of a simplified cell blot technique and 16S RNA directed probes for identification of common environmental isolates. Appl Environ Microbiol. 1993;59:3219–3224. doi: 10.1128/aem.59.10.3219-3224.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter W T. Sodium nitrate used to control nuisance. Water Works Sewage. 1932;79:75–176. [Google Scholar]
- 9.Chan C W, Suzuki I. Quantitative extraction and determination of elemental sulfur and stoichiometric oxidation of sulfide to elemental sulfur by Thiobacillus thiooxidans. Can J Microbiol. 1993;39:1166–1168. [Google Scholar]
- 10.Collins C H, Lyne P M. Microbiological methods. London, United Kingdom: Butterworth & Co. Ltd.; 1976. [Google Scholar]
- 11.Costilow R N. Biophysical factors in growth. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. pp. 66–78. [Google Scholar]
- 12.Craig F F., Jr . Reservoir engineering aspects of waterflooding. Monograph series. Dallas, Tex: Society of Petroleum Engineers; 1971. [Google Scholar]
- 13.Dannenberg S, Kroder M, Dilling W, Cypionka H. Oxidation of H2, organic compounds and inorganic sulfur compounds coupled to reduction of O2 or nitrate by sulfate-reducing bacteria. Arch Microbiol. 1992;158:93–99. [Google Scholar]
- 14.Doetsch R N. Determinative methods of light microscopy. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. pp. 21–33. [Google Scholar]
- 15.Eisenmann E, Beurle J, Sulger K, Kronek P M H, Schumacher W. Lithotrophic growth of Sulfurospirillum deleyianum with sulfide as electron donor coupled to respiratory reduction of nitrate to ammonia. Arch Microbiol. 1995;164:180–185. [Google Scholar]
- 16.Fales A L. Treatment of industrial wastes from paper mills and tannery on Neponsit River. J Ind Eng Chem. 1929;21:216. [Google Scholar]
- 17.Finster K, Liesack W, Tindall B J. Sulfurospirillum arcachonense sp. nov., a new microaerophilic sulfur-reducing bacterium. Int J Syst Bacteriol. 1997;47:1212–1217. doi: 10.1099/00207713-47-4-1212. [DOI] [PubMed] [Google Scholar]
- 18.Fogo J K, Popowski M. Spectrophotometric determination of hydrogen sulfide. Anal Biochem. 1949;21:732–734. [Google Scholar]
- 19.Gevertz D, Jenneman G E, Zimmerman S, Stevens J. Microbial oxidation of soluble sulfides in produced water from the Bakken sands. In: Byrant R, Sublette K L, editors. Proceedings of the 5th International Conference on Microbial Enhanced Oil Recovery and Related Biotechnology for Solving Environmental Problems. Springfield, Va: National Technical Information Services; 1995. pp. 295–310. [Google Scholar]
- 20.Gherna R, Pienta P, Cote R, editors. ATCC catalogue of bacteria & bacteriophages. Rockville, Md: American Type Culture Collection; 1992. [Google Scholar]
- 21.Hedderich R, Klimmek O, Kroger A, Dirmeier R, Keller M, Stetter K O. Anaerobic respiration with elemental sulfur and with disulfides. FEMS Microbiol Rev. 1999;22:353–381. [Google Scholar]
- 22.Heukelekian H. Effect of the addition of sodium nitrate to sewage on hydrogen sulfide production and B.O.D. reduction. Sewage Works J. 1943;15:255–261. [Google Scholar]
- 23.Hicks R E, Amann R I, Stahl D A. Dual staining of natural bacterioplankton with 4′, 6-diamidino-2-phenylindole and fluorescent oligonucleotide probes targeting kingdom-level 16S rRNA sequences. Appl Environ Microbiol. 1992;58:2158–2163. doi: 10.1128/aem.58.7.2158-2163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hitzman D O, Sperl G T, Sandbeck K A. U.S. patent 5,405,593. April 1995.. [Google Scholar]
- 25.Huber R, Stetter K O. The order Thermoproteales. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. I. New York, N.Y: Springer-Verlag; 1991. pp. 677–683. [Google Scholar]
- 26.Hutchinson M, Johnstone K I, White D. The taxonomy of anaerobic Thiobacilli. J Gen Microbiol. 1967;47:17–23. doi: 10.1099/00221287-47-1-17. [DOI] [PubMed] [Google Scholar]
- 27.Jack T R, Lee E, Mueller J. Anaerobic gas production: controlling factors. In: Zajic J E, Donaldson E C, editors. Microbes and oil recovery. Proceedings of the International Conference on Microbial Enhancement of Oil Recovery. El Paso, Tex: Petroleum Bioresources; 1985. pp. 167–180. [Google Scholar]
- 28.Jack T R, Westlake D W S. Control in industrial settings. In: Barton L L, editor. Sulfate-reducing bacteria. New York, N.Y: Plenum Press; 1995. pp. 265–292. [Google Scholar]
- 29.Janssen A J H, Sleyster R, van der Kaa C, Jochemsen A, Bontsema J, Lettinga G. Biological sulphide oxidation in a fed-batch reactor. Biotechnol Bioeng. 1995;47:327–333. doi: 10.1002/bit.260470307. [DOI] [PubMed] [Google Scholar]
- 30.Jenneman, G. E., and D. Gevertz. Identification, characterization, and application of sulfide-oxidizing bacteria in oil fields. In Proceedings of the 8th International Meeting on Microbial Ecology, Halifax, Nova Scotia, in press.
- 31.Jenneman G E, Gevertz D, Wright M. Proceedings of the 3rd International Petroleum Environmental Conference, Albuquerque, N.M. [CD-ROM.] 1996. Sulfide bioscavenging of soured produced water by natural microbial populations; pp. 693–701. [Google Scholar]
- 32.Jenneman G E, McInerney M J, Knapp R M. Effect of nitrate on biogenic sulfide production. Appl Environ Microbiol. 1986;51:1205–1211. doi: 10.1128/aem.51.6.1205-1211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenneman G E, Moffitt P D, Bala G A, Webb R H. Sulfide removal in reservoir brine by indigenous bacteria. Soc Petroleum Eng Prod Facilities. 1999;14:219–225. [Google Scholar]
- 34.Krumholtz L R. Microbial ecosystems in the Earth's subsurface. ASM News. 1998;64:197–202. [Google Scholar]
- 35.Kuenen J G. Colourless sulfur bacteria and their role in the sulfur cycle. Plant Soil. 1975;43:49–76. [Google Scholar]
- 36.Kuenen J G, Robertson L A, Tuovinen O H. The genera Thiobacillus, Thiomicrospira, and Thiosphaera. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. III. New York, N.Y: Springer-Verlag; 1991. pp. 2638–2657. [Google Scholar]
- 37.Macy J M, Schroder I, Thauer R K, Kroger A. Growth of Wolinella succionogenes on H2S plus fumarate and on formate plus sulfur as energy sources. Arch Microbiol. 1986;144:147–150. [Google Scholar]
- 38.McClung C R, Patriquin D G. Isolation of a nitrogen-fixing Campylobacter species from the roots of Spartina alterniflora Loisel. Can J Microbiol. 1980;26:881–886. doi: 10.1139/m80-153. [DOI] [PubMed] [Google Scholar]
- 39.McClung C R, Patriquin D G, Davis R E. Campylobacter nitrofigilis sp. nov., a nitrogen-fixing bacterium associated with roots of Spartina alterniflora Loisel. Int J Syst Bacteriol. 1983;33:605–612. [Google Scholar]
- 40.McInerney M J, Sublette K L. Petroleum microbiology: biofouling, souring and improved oil recovery. In: Hurst C J, Knudsen G R, McInerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C.: American Society for Microbiology; 1997. pp. 600–607. [Google Scholar]
- 41.McInerney M J, Sublette K L, Bhuparthiaraju V K, Coates J D, Knapp R M. Causes and control of microbially induced souring. In: Premuzic E T, Woodhead A, editors. Developments in petroleum science. 39. Microbial enhancement of oil recovery—recent advances. Proceedings of the 1992 International Conference on Microbial Enhanced Oil Recovery. Amsterdam, The Netherlands: Elsevier; 1993. pp. 363–371. [Google Scholar]
- 42.Muyzer G, Teske A, Wirsen C O, Jannasch H W. Phylogenetic relationships of Thiomicrospira species and their identification in deep sea hydrothermal vent samples by denaturing gradient electrophoresis of 16S rRNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- 43.Nelson DC. The genus Beggiatoa. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. IV. New York, N.Y: Springer-Verlag; 1991. pp. 3171–3180. [Google Scholar]
- 44.Olsen G J, Lane D J, Giovannoni S J, Pace N R, Stahl D A. Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol. 1986;40:337–365. doi: 10.1146/annurev.mi.40.100186.002005. [DOI] [PubMed] [Google Scholar]
- 45.Okabe S, Jones W L, Lee W, Characklis W G. Anaerobic SRB biofilms in industrial water systems: a process analysis. In: Geesey G G, Lewandowski Z, Flemming H-C, editors. Biofouling and biocorrosion in industrial water systems. Boca Raton, Fla: Lewis Publishers; 1994. pp. 189–204. [Google Scholar]
- 46.Pfennig N, Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976;110:3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- 47.Poduska R A, Anderson B D. Successful storage lagoon odor control. J Water Pollut Control Fed. 1981;53:299–310. [Google Scholar]
- 48.Reinsel M A, Sears J T, Stewart P S, McInerney M J. Control of microbial souring by nitrate, nitrite or glutaraldehyde injection in a sandstone column. J Ind Microbiol. 1996;17:128–136. [Google Scholar]
- 49.Robertson L A, Kuenen J G. The colorless sulfur bacteria. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. I. New York, N.Y: Springer-Verlag; 1991. pp. 385–413. [Google Scholar]
- 50.Sandbeck K A, Hitzman D O. Biocompetitive exclusion technology: a field system to control reservoir souring and increase production. In: Byrant R, Sublette K L, editors. Proceedings of the 5th International Conference on Microbial Enhanced Oil Recovery and Related Biotechnology for Solving Environmental Problems. Springfield, Va: National Technical and Information Services; 1995. pp. 311–320. [Google Scholar]
- 51.Scholz-Muramatsu H, Neumann A, Messmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 52.Schumacher W, Kroneck P M H, Pfennig N. Comparative systematic study on “Spirillum” 5175, Campylobacter and Wolinella species. Arch Microbiol. 1992;158:287–293. [Google Scholar]
- 53.Segerer A H, Stetter K O. The order Sulfobales. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. I. New York, N.Y: Springer-Verlag; 1991. pp. 684–701. [Google Scholar]
- 54.Sørensen J, Tiedje J M, Firestone R B. Inhibition by sulfide of nitric and nitrous oxide by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol. 1980;39:105–110. doi: 10.1128/aem.39.1.105-108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sperl G T, Sperl P L, Hitzman D O. A use of natural microflora, electron acceptors and energy sources for enhanced oil recovery. In: Premuzic E T, Woodhead A, editors. Developments in petroleum science. 39. Microbial enhancement of oil recovery—recent advances. Proceedings of the 1992 International Conference on Microbial Enhanced Oil Recovery. Amsterdam, The Netherlands: Elsevier; 1993. pp. 17–25. [Google Scholar]
- 56.Staden R. Computer handling of DNA sequencing projects. In: Bishop M J, Rawlings C J, editors. Nucleic acid and protein sequence analysis. A practical approach. Oxford, United Kingdom: IRL Press; 1987. pp. 173–217. [Google Scholar]
- 57.Stevens T. Lithoautotrophy in the subsurface. FEMS Microbiol Rev. 1997;20:327–337. [Google Scholar]
- 58.Stevens T O, McKinley J P. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science. 1995;270:450–454. [Google Scholar]
- 59.Stolz J F, Ellis D J, Switzer Blum J, Ahmann D, Lovley D R, Oremland R S. Sulfurospirillum barnesii sp. nov. and Sulfurospirillum arsenophilum sp. nov., new members of the Sulfurospirillum clade of the epsilon Proteobacteria. Int J Syst Bacteriol. 1999;49:1177–1180. doi: 10.1099/00207713-49-3-1177. [DOI] [PubMed] [Google Scholar]
- 60.Swofford D L. Phylogenetic analysis using parsimony, version 3.1. Champaign, Ill: Illinois Natural History Survey; 1993. [Google Scholar]
- 61.Telang A J, Ebert S, Foght J M, Westlake D W S, Jenneman G E, Gevertz D, Voordouw G. Effect of nitrate injection on the microbial community in an oil field as monitored by reverse genome probing. Appl Environ Microbiol. 1997;63:1785–1793. doi: 10.1128/aem.63.5.1785-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Telang A J, Ebert S, Foght J M, Westlake D W S, Voordouw G. Effects of two diamine biocides on the microbial community from an oil field. Can J Microbiol. 1998;44:1060–1065. [Google Scholar]
- 63.Telang A J, Jenneman G E, Voordouw G. Sulfur cycling in mixed cultures of sulfide-oxidizing and sulfate- or sulfur-reducing oil field bacteria. Can J Microbiol. 1999;45:905–913. [Google Scholar]
- 64.Tenover F C, Fennell C L. The genera Campylobacter and Helicobacter. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. IV. New York, N.Y: Springer-Verlag; 1991. pp. 3488–3511. [Google Scholar]
- 65.Timmer-Ten Hoor A. A new type of thiosulphate oxidizing, nitrate reducing microorganism: Thiomicrospira denitrificans sp. nov. Neth J Sea Res. 1975;9:344–350. [Google Scholar]
- 66.Timmer-Ten Hoor A. Cell yield and bioenergetics of Thiomicrospira denitrificans compared with Thiobacillus denitrificans. Antonie Leeuwenhoek. 1981;47:231–243. doi: 10.1007/BF00403394. [DOI] [PubMed] [Google Scholar]
- 67.Van den Emde F P, van Gemerden H. Sulfide oxidation under oxygen limitation: a Thiobacillus thioparus isolated from a marine microbial mat. FEMS Microbiol Ecol. 1993;13:69–78. [Google Scholar]
- 68.Vandamme P, Falsen E, Rossau R, Hoste B, Segers P, Tytgat R, De Ley J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. syst. Bacteriol. 1991;41:88–103. doi: 10.1099/00207713-41-1-88. [DOI] [PubMed] [Google Scholar]
- 69.Visser J M, Robertson L A, Van Verseveld H W, Kuenen J G. Sulfur production by obligately chemolithoautotrophic Thiobacillus species. Appl Environ Microbiol. 1997;63:2300–2305. doi: 10.1128/aem.63.6.2300-2305.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voordouw G, Armstrong S M, Reimer M F, Fouts B, Telang A J, Shen Y, Gevertz D. Characterization of 16S rRNA genes from oil field microbial communities indicates the presence of a variety of sulfate-reducing, fermentative, and sulfide-oxidizing bacteria. Appl Environ Microbiol. 1996;62:1623–1629. doi: 10.1128/aem.62.5.1623-1629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vorholt J A, Hafenbradl D, Stetter K O, Thauer R K. Pathways of autotrophic CO2 fixation and of dissimilatory nitrate reduction to N2O in Ferroglobus placidus. Arch Microbiol. 1997;167:19–23. doi: 10.1007/s002030050411. [DOI] [PubMed] [Google Scholar]
- 72.Widdel F, Pfennig N. The genus Desulfuromonas and other Gram-negative sulfur-reducing eubacteria. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. IV. New York, N.Y: Springer-Verlag; 1991. pp. 3379–3389. [Google Scholar]
- 73.Wolfe R S, Pfennig N. Reduction of sulfur by Spirillum 5175 and syntrophism with Clorobium. Appl Environ Microbiol. 1977;33:427–433. doi: 10.1128/aem.33.2.427-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zumft W G. The denitrifying prokaryotes. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. I. New York, N.Y: Springer-Verlag; 1991. pp. 554–582. [Google Scholar]