Abstract
Radiology: Cardiothoracic Imaging publishes novel research and technical developments in cardiac, thoracic, and vascular imaging. The journal published many innovative studies during 2023 and achieved an impact factor for the first time since its inaugural issue in 2019, with an impact factor of 7.0. The current review article, led by the Radiology: Cardiothoracic Imaging trainee editorial board, highlights the most impactful articles published in the journal between November 2022 and October 2023. The review encompasses various aspects of coronary CT, photon-counting detector CT, PET/MRI, cardiac MRI, congenital heart disease, vascular imaging, thoracic imaging, artificial intelligence, and health services research. Key highlights include the potential for photon-counting detector CT to reduce contrast media volumes, utility of combined PET/MRI in the evaluation of cardiac sarcoidosis, the prognostic value of left atrial late gadolinium enhancement at MRI in predicting incident atrial fibrillation, the utility of an artificial intelligence tool to optimize detection of incidental pulmonary embolism, and standardization of medical terminology for cardiac CT. Ongoing research and future directions include evaluation of novel PET tracers for assessment of myocardial fibrosis, deployment of AI tools in clinical cardiovascular imaging workflows, and growing awareness of the need to improve environmental sustainability in imaging.
Keywords: Coronary CT, Photon-counting Detector CT, PET/MRI, Cardiac MRI, Congenital Heart Disease, Vascular Imaging, Thoracic Imaging, Artificial Intelligence, Health Services Research
© RSNA, 2024
Keywords: Coronary CT, Photon-counting Detector CT, PET/MRI, Cardiac MRI, Congenital Heart Disease, Vascular Imaging, Thoracic Imaging, Artificial Intelligence, Health Services Research
Summary
Cardiothoracic imaging continues to evolve with new cutting-edge research and innovative technical developments that can improve diagnosis and guide management decisions in cardiothoracic diseases.
Essentials
■ Photon-counting detector CT allowed a 25% reduction in contrast media volume as compared with energy-integrating detector CT, with similar image quality and contrast resolution.
■ Combined cardiac fluorine 18 fluorodeoxyglucose PET/MRI had a lower radiation dose and lower scan time compared with standard-of-care imaging in the evaluation of suspected cardiac sarcoidosis.
■ Left atrial late gadolinium enhancement at cardiac MRI predicted incident atrial fibrillation after adjusting for clinical risk factors and other cardiac MRI markers of left atrial remodeling.
■ Employing an artificial intelligence algorithm reduced the median detection and notification time for incidental pulmonary embolism at CT from several days to a few hours.
■ A new expert consensus document updated the standardized medical terminology for reporting in cardiac CT.
Introduction
Cardiothoracic imaging as a radiology subspecialty has benefited from new guidelines, innovative research, and technological advancements over the past year. Inspired by a review article highlighting key publications in 2022 (1), the current review aims to provide an overview of notable developments in cardiac, vascular, and thoracic imaging published in Radiology: Cardiothoracic Imaging between November 2022 and October 2023. This effort was led by the Radiology: Cardiothoracic Imaging trainee editorial board.
The article selection process was guided by a systematic approach. All manuscripts published within the eligibility date range were considered, except for those categorized under Images in Cardiothoracic Imaging, as those articles are considered for a separate trainee editorial board–led publication (2). All eligible articles were initially ranked within four separate categories based on data collected in November 2023: total number of downloads, number of downloads per month since online publication date, the Altmetric score, and total number of citations. The top 10% of articles ranked in each of the four categories were included in the review. Given that these metrics tend to favor articles published earlier in the time period, trainee editorial board members also considered timeliness, novelty, and reader interest during the review process. This approach aimed for a balanced blend of quantitative ranking and qualitative assessment. Articles were initially reviewed by trainee editorial board members (G.J.A. and D.M.), with additional editorial input from Radiology: Cardiothoracic Imaging editors.
Of the 56 total articles published between November 2022 and October 2023, 23 articles were selected for inclusion: 14 articles based on the top 10% of each of the four initial rank lists after removing duplicates ranked within the top 10% in multiple categories and nine additional articles based on timeliness, novelty, and reader interest. Included articles were grouped into nine categories: coronary CT, photon-counting detector CT (PCD CT), fluorodeoxyglucose (FDG) PET/MRI, cardiac MRI, congenital heart disease (CHD), vascular imaging, thoracic imaging, artificial intelligence (AI), and health services research. The writing group would like to acknowledge all authors of articles published in Radiology: Cardiothoracic Imaging. Each author has made a substantial contribution to the journal, its readers, and the field of cardiothoracic imaging.
Coronary CT
Coronary CT angiography (CCTA) plays a key role in the evaluation of coronary artery disease. In a post hoc analysis of a prospective study, Gulsin et al (3) explored how CT fractional flow reserve (CT-FFR) affects coronary artery disease management in 4290 participants, including 942 (22%) with diabetes mellitus (DM) and 3348 (78%) without DM. Participants with DM had higher rates of obstructive coronary artery disease, multivessel coronary artery disease, and vessels with a CT-FFR less than or equal to 0.8 compared with those without DM. Notably, treatment reclassification by CT-FFR was observed in two-thirds of participants regardless of DM status. Although those with DM had a higher 1-year rate of major adverse cardiovascular events (hazard ratio, 2.2; 95% CI: 1.2, 4.1; P = .01), no between-group differences were observed when stratified by stenosis severity (<50% or ≥50%) or CT-FFR positivity. This study provided further evidence of the value of CT-FFR in identifying coronary artery disease severity and guiding treatment strategies in patients with and without DM.
CCTA high-risk plaque characteristics are associated with higher risk of future acute coronary syndrome and lesion-specific ischemia (4). Tanisawa et al (5) investigated the association between CCTA low-attenuation plaque burden and plaque morphology determined with near-infrared spectroscopy intravascular US (NIRS-IVUS). In a retrospective analysis of 273 plaques in 141 patients, low-attenuation plaque burden increased with the number of high-risk features (P < .001) and had better discriminative ability for high-risk plaques than plaque attenuation by visual assessment (area under the receiver operating characteristic curve, 0.93 vs 0.89; P = .02) (Fig 1). The results of this study establish the value of quantification of low-attenuation plaques from CCTA compared with visual assessment, which could prove valuable in the future with the rise of PCD CT.
Figure 1:
Images show an example of low-attenuation plaques (LAPs) with multiple near-infrared spectroscopy intravascular US–derived high-risk features. (A) On curved planar reformatted coronary CT angiograms, the left circumflex coronary artery is subtotally occluded with a large mixed plaque. Image on the right with color-coded overlays shows the automated segmentation of the LAP (orange region). (B) Enlarged three-dimensional view shows large LAP clusters. (C) Near-infrared spectroscopy chemogram shows the plaque as lipid-rich, with a high maximum lipid core burden index at 4-mm segment (maxLCBI4 mm) value of 880. (D) Grayscale intravascular US cross-sectional view demonstrates extensive echo attenuation (arrows) and an echolucent zone (arrowhead). (Reprinted, with permission, from reference 5.)
PCD CT Imaging
PCD CT has shown improved contrast and spatial resolution compared with energy-integrating detector CT while providing spectral information with nearly every acquisition mode (6–8). However, to date, there are limited data to indicate whether these technical advantages translate into practical clinical benefits. In a prospective study of 100 participants by Higashigaito et al (9), PCD CT allowed a 25% reduction in contrast media volume as compared with energy-integrating detector CT, with similar image quality and contrast resolution (Fig 2). This method of reduction in contrast media volume could potentially lower costs and mitigate the risk of contrast-induced acute kidney injury in at-risk patients without compromising diagnostic confidence.
Figure 2:
Comparison of image quality between EID CT with standard contrast media protocol and PCD CT with low-volume contrast media protocol using a matched radiation dose. Transverse and three-dimensional cinematic rendered images from thoracoabdominal CTA in a 71-year-old woman in group 2 are shown. Group 2 was used to find whether a 25% reduction in contrast media dose at the optimal kiloelectron voltage led to noninferior image quality versus EID CT. (A–C) Images from third-generation EID CT with automated tube voltage selection of 90 kVp. BMI, effective diameter, CTDIvol, and SSDE were 23.7 kg/m2, 278 mm, 3.98 mGy, and 5.25 mGy, respectively; 70 mL of contrast media was used. (D–F) Images from PCD CT with reduced contrast media volume of 52.5 mL and VMI at 50 keV. Time interval between scans was 6 months. Mean BMI, effective diameter, CTDIvol, and SSDE at the time of the second scan were 24.2 kg/m2, 282 mm, 3.99 mGy, and 5.27 mGy, respectively. Mean contrast-to-noise ratio for EID CT and PCD CT were 17.2 and 17.9, respectively. BMI = body mass index, CTA = CT angiography, CTDIvol = volumetric CT dose index, EID = energy-integrating detector, PCD = photon-counting detector, SSDE = size-specific dose estimate, VMI = virtual monoenergetic images. (Reprinted, with permission, from reference 9.)
In another study, technical advantages of PCD CT were leveraged to reconstruct virtual noncontrast images from late enhancement PCD CT and were compared with true noncontrast images. Mergen et al (10) used PCD CT to derive virtual noncontrast images from late enhancement images obtained 5 minutes following contrast media administration in 90 patients undergoing transcatheter aortic valve replacement and compared calcium scores of multiple structures between virtual noncontrast and true noncontrast images. The study showed excellent agreement between calcification categories on virtual noncontrast images at 80 keV for aortic valve calcium (κ = 0.974) and on virtual noncontrast images at 70 keV for coronary artery calcium (κ = 0.967), highlighting the potential for cost- and radiation-dose savings with PCD CT.
With the growing use of transcatheter aortic valve replacement and the critical role of CT imaging in preprocedural planning, additional studies have evaluated PCD CT in this patient population. In a case series of five patients, van der Bie and Sharma et al (11) presented potential advantages of PCD CT in patients before and following transcatheter aortic valve replacement. The case series focused on the ability to obtain ultra-high-resolution images at 0.2-mm section thickness, which is not attainable with conventional energy-integrating detector CT. In these cases (Fig 3), there was improved quantification of calcium, visualization of complex valve-in-valve transcatheter aortic valve replacement, and assessment of in-stent stenosis and periprosthetic valve leakage. There was also substantial reduction of streak artifacts previously hindering femoral artery access route visualization.
Figure 3:
(A) Transcatheter heart valves scanned ex vivo in ultra-high-resolution (UHR) mode with photon-counting detector CT (PCD CT). Four different ex vivo transcatheter heart valves were scanned in UHR mode with 140 kV and reconstructed with a Qr89/Qr76 kernel at quantum iterative reconstruction strength 4. CT images are displayed as three-dimensional volume-rendered images. (B) UHR PCD CT image shows valve-in-valve transcatheter aortic valve replacement with hypoattenuated leaflet thickening in a 72-year-old male patient scanned with contrast medium, and the surgical valve and transcatheter heart valve frames are sharply depicted in the sagittal plane (left). Three-dimensional volume-rendered image (right). (C) Contrast-enhanced PCD CT images in an 85-year-old female patient following transcatheter aortic valve replacement with a stent in the left main coronary artery. UHR image shows incomplete stent expansion (arrow) (left). Images on the right show axial reconstructions perpendicular to the stent axis. (D) PCD CT scan for pre–transcatheter aortic valve replacement planning in an 81-year-old female patient (with contrast material). Axial reconstructions at the level of the femoral arteries (arrows) without (left) and with (right) iterative metal artifact reduction (IMAR) reconstruction. QIR = quantitative iterative reconstruction. (Reprinted, with permission, from reference 11.)
FDG PET/MRI
Hybrid cardiac imaging using combined PET/MRI provides complementary information, including morphologic, functional, and physiologic cardiac assessment. Initial cardiac PET/MRI applications focused on cardiac sarcoidosis (12). However, there are limited data on comparative effectiveness with standard imaging techniques.
In a prospective study of 40 participants with suspected cardiac sarcoidosis, Marschner et al (13) found that combined cardiac fluorine 18 (18F)–FDG PET/MRI had a 52% lower radiation dose and 43% shorter imaging duration compared with standard-of-care imaging with separate cardiac MRI, 18F-FDG PET/CT, and technetium 99m sestamibi SPECT perfusion imaging. Combined PET/MRI had the highest area under the receiver operating characteristic curve for diagnosis of cardiac sarcoidosis (0.84) (Fig 4). These results suggest that combined PET/MRI could lead to shorter overall wait times and potentially earlier diagnosis of cardiac sarcoidosis.
Figure 4:
Images in a 52-year-old female participant with cardiac sarcoidosis. Standard-of-care imaging (top row) demonstrates a perfusion defect on SPECT images (top left) and corresponding fluorodeoxyglucose (FDG)–uptake on a fluorine 18 (18F)–FDG PET/CT image (top right) at the interventricular septum (green arrows). On combined 18F-FDG PET/MR images (bottom row), there is nodular late gadolinium enhancement (LGE) at the interventricular septum (orange arrows) with corresponding FDG uptake (blue arrows). (Reprinted, with permission, from reference 13.)
In another prospective study, Marschner et al (14) used combined cardiac FDG PET/MRI to evaluate potential cardiac sequelae of COVID-19 vaccination in relation to patient symptoms in 54 participants who received at least one dose of a COVID-19 vaccine. At 2-month follow-up, FDG PET/MRI showed evidence of myocardial inflammation in two of 17 participants diagnosed with acute myocarditis early after COVID-19 vaccination (Fig 5) but not in participants without acute myocarditis, regardless of the presence of symptoms. Lack of imaging, electrocardiography, and blood biomarker abnormalities in symptomatic participants without myocarditis suggests that cardiac symptoms alone are a poor indicator of myocardial inflammation or injury. These findings suggest that long-term cardiac investigations may not be needed for patients without acute myocarditis early after COVID-19 vaccination.
Figure 5:
Images from combined cardiac fluorine 18 (18F)–fluorodeoxyglucose (FDG) PET/MRI in a symptomatic female participant between 41 and 50 years of age 2 months after a diagnosis of myocarditis following COVID-19 vaccination. Short-axis midventricular native (A) T1 and (B) T2 maps demonstrate high T1 and T2 values in the subepicardial inferior and inferolateral walls (white and green arrows, respectively). (C) Short-axis late gadolinium enhancement (LGE) image demonstrates corresponding subepicardial LGE (red arrows). (D) Fused LGE and FDG PET image demonstrates corresponding focal FDG uptake (blue arrows), in keeping with myocardial inflammation. (Reprinted, with permission, from reference 14.)
Cardiac MRI
Cardiac MRI provides essential information, including evaluation of left atrial (LA) fibrosis and volumetry, in the risk assessment of atrial fibrillation. In a post hoc analysis of the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, Zghaib et al (15) found that LA late gadolinium enhancement was observed at baseline in 61% (1035 of 1697) of participants without atrial fibrillation. During the 4 years of follow-up, LA late gadolinium enhancement independently predicted incident atrial fibrillation after adjusting for clinical risk factors and other cardiac MRI markers of LA remodeling, including LA volume, function, and strain. These findings are particularly relevant due to the distorted LA geometry in patients with atrial fibrillation.
Maroun et al (16) proposed that asymmetric wall remodeling in atrial fibrillation poses a challenge for accurately assessing LA volume. In a retrospective cardiac MRI study of 64 patients with atrial fibrillation, LA volume assessment using biplanar volume on standard two- and four-chamber cine images underestimated LA volume by 24 mL and 31 mL, respectively, compared with three-dimensional volumetric methods such as late gadolinium–enhanced MRI and MR angiography. This difference was more pronounced in patients with larger atria, suggesting that LA enlargement does not conform to the ellipsoid shape assumed by the biplanar volume assessment method. These findings are particularly important as LA volumes are independent risk factors for adverse outcomes in patients with atrial fibrillation.
In a retrospective analysis comparing 14 patients with Wilson disease with 14 controls, Deng et al (17) reported that patients with Wilson disease had higher native myocardial T1 and T2 mapping values and higher extracellular volume despite no clinical cardiac symptoms or late gadolinium enhancement at MRI (Fig 6). These results suggest that early myocardial changes occur in Wilson disease, even in the absence of cardiac symptoms and ventricular dysfunction.
Figure 6:
Cardiac short-axis late gadolinium enhancement (LGE) image and native T1, extracellular volume (ECV), and T2 maps in a 25-year-old woman with Wilson disease with neurologic symptoms (top row) and a healthy control (26-year-old woman) (bottom row). Both the patient and control had negative LGE. Global native T1 times (1101 msec vs 1019 msec), ECV values (30% vs 26%), and global native T2 times (56 msec vs 49 msec) were higher in the patient. (Reprinted, with permission, from reference 17.)
Congenital Heart Disease Imaging
Evaluating cardiac anatomy at fetal cardiac MRI is challenging due to the small size of the fetal heart, high heart rates, and spontaneous fetal motion in addition to maternal breathing and voluntary movement. A direct electrocardiography-gating method using Doppler US has been recently applied to fetal cardiac MRI. In a prospective study, Vollbrecht et al (18) used Doppler US–gated fetal cardiac cine MRI in 23 female participants with fetuses suspected to have CHD and compared it with fetal echocardiography, with postnatal multimodality imaging serving as the reference standard. Diagnostic performance for CHD was similar between MRI and echocardiography (sensitivity, 91.8% vs 93.6%; specificity, 99.9% vs 99.9%, respectively; P > .05). Notably, in one case, the correct diagnosis was made using only fetal cardiac MRI (Fig 7). These results suggest that fetal MRI may be able to detect both cardiac and noncardiac findings not diagnosed at echocardiography which may be important for prognosis in CHD and will likely be an important contribution to establishing the clinical utility of fetal cardiac MRI.
Figure 7:
Cardiac MR images in a 30-year-old woman at a gestational age of 34 weeks 5 days with complex congenital heart disease of the fetus (fetus 8). Venoatrial connections and ventricular inversion could not be identified with echocardiography, leading to the incorrect diagnosis of a dextro-transposition of the great arteries with regular orifices of the systemic veins on the right side and of the pulmonary veins on the left side. (A–C) Axial and (D) coronal balanced steady-state free precession cine images demonstrate congenitally corrected transposition of the great arteries in complete situs inversus with isolated levocardia. (A) The pulmonary veins join the right-sided left atrium (LA), and the systemic veins are connected to the left-sided right atrium (RA). The LA (which lies anteriorly because of the levocardia) is connected to the right ventricle (RV), and the RA (which lies posteriorly because of the levocardia) is connected to the left ventricle (LV) (atrioventricular discordance). Note that in complete situs inversus with isolated levocardia, the LA and the LV would have both been positioned anteriorly, which is not the case here because of the congenitally corrected transposition of the great arteries (LV lies posteriorly). (B) The aorta arises from the RV, and the main pulmonary artery (MPA) arises from the LV (ventriculoarterial discordance). (C) The right-sided aortic arch and (D) the left-sided hepatic vein confluence indicate situs inversus. LSVC = left superior vena cava, PV = pulmonary valve. (Reprinted, with permission, from reference 18.)
Regarding postnatal CHD imaging, one of the biggest challenges for cardiac MRI is the need for repeated breath holds and long scan times. Thus, a free-breathing, electrocardiography-gated, fast cardiac MRI sequence would be ideal. Fotaki et al (19) proposed a three-dimensional, free-breathing, noncontrast, whole-heart Magnetization Transfer Contrast Bright-and-black blOOd phase-SensiTive (MTC-BOOST) sequence for use in imaging adults with CHD (20). In this prospective study, 120 adults with CHD underwent cardiac MRI with both the standard institutional protocol T2-prepared balanced steady-state free precession and the research MTC-BOOST sequence (Fig 8). The investigators found that the MTC-BOOST sequence reduced acquisition time (9 minutes vs 14 minutes; P < .001) and improved image quality.
Figure 8:
Comparison of MTC-BOOST and native T2prep-bSSFP cardiac MRI. (A) Multiplanar reformatted images in a 34-year-old man diagnosed with tetralogy of Fallot after repair with transannular patch. Severe pulmonary artery regurgitation caused signal voids in the right ventricle, right ventricular outflow tract, and main pulmonary artery because of flow artifact (yellow arrow) in the clinical native sequence. Off-resonance artifact is demonstrated in the left atrium (blue arrow). Artifacts are minimized with the proposed MTC-BOOST sequence (red arrows). (B) Multiplanar reformatted images in an 18-year-old man with hypoplastic left heart syndrome after total cavopulmonary connection completion with a fenestrated lateral tunnel Fontan pathway. Signal voids are observed in the lateral tunnel and right atrium because of stagnant flow (purple arrows) in the native T2prep-bSSFP clinical data set, which necessitate further imaging for the exclusion of obstruction. Residual respiratory artifact (red arrowhead) is also present. The MTC-BOOST sequence demonstrates the vascular lumen without substantial artifact and excludes obstruction (red arrows). (C) Multiplanar reformatted images in a 23-year-old man with tetralogy of Fallot after repair with transannular patch, followed by pulmonary valve replacement with homograft due to severe regurgitation. Off-resonance artifacts in the pulmonary veins in the native T2-prep bSSFP sequence (black arrows) impede the sequential segmental anatomic description. Pulmonary venous return can be established in the MTC-BOOST data set (red arrows). (D) Multiplanar reformatted images in a 23-year-old woman with a small perimembranous ventricular septal defect that has not been repaired, causing mild aortic regurgitation. Flow-related artifact in the left ventricle (blue arrow) observed in the clinical native data set is suppressed in the MTC-BOOST data set. The left anterior descending coronary artery is sharply delineated with the research sequence (white arrow), owing to the improved fat suppression. Ao = aorta, LA = left atrium, LAD = left anterior descending artery, LV = left ventricle, MPA = main pulmonary artery, MTC-BOOST = Magnetization Transfer Contrast Bright-and-black blOOd phase SensiTive, RPA = right pulmonary artery, RV = right ventricle, RVOT = RV outflow tract, SVC = superior vena cava, T2prep-bSSFP = T2-prepared balanced steady-state free precession. (Reprinted, with permission, from reference 19.)
Vascular Imaging
Limited intimal tear is an underrecognized imaging finding in patients with acute aortic syndrome characterized by partial-thickness tears of the intima and media without significant hematoma or dissection flap (21). Madani et al (22) described CT angiography morphologic patterns and clinical features of 42 patients with acute limited intimal tears (Fig 9). Linear tears were the most common, affecting mostly the ascending aorta. Chest pain was the most frequent symptom. Limited intimal tear prognosis was similar to other acute aortic syndromes. Despite its clinical significance, awareness of limited intimal tears among clinicians remains low. High-quality CT angiography is important to distinguishing the subtle CT features associated with limited intimal tears, and endoluminal aortic views derived from volume rendering reconstructions may be useful to delineate the pattern of limited intimal tears.
Figure 9:
Blood-pool inversion volume-rendered endoluminal CT images and axial contrast-enhanced CT angiographic images of the thoracic aorta showing different types of limited intimal tears (LITs; arrowheads). First row: linear-shaped LIT. Second row: T-shaped LIT. Third and fourth rows: T-shaped LIT. Fifth row: stellate-shaped LIT. (Reprinted, with permission, from reference 22.)
Uncomplicated type B aortic dissection is of growing interest due to high survival rates, necessitating long-term imaging surveillance and specialized treatment. Prior studies have evaluated false lumen chronic degeneration using patient-specific imaging features (23). In an article outlining study design and rationale, Mastrodicasa et al (24) focused on imaging features to predict late adverse events in patients with uncomplicated type B aortic dissection. The authors described the methodological approach of the Registry of Aortic Diseases to Model Adverse Events and Progression (ROADMAP) study, a retrospective and longitudinal analysis of patients with uncomplicated type B aortic dissection that aims to validate a previously developed risk prediction model. This multicenter study includes 10 institutions and will combine clinical and morphologic data from baseline and serial imaging to identify patients at high risk for late adverse events.
Regarding acute aortic syndromes, Bhat et al (25) conducted a study in New Zealand that revealed health care inequities among Māori and Pacific Islander ethnic groups. They found that Māori and Pacific Islanders had a higher risk for acute aortic syndrome but underwent CT angiography at disproportionately lower rates compared with other ethnic groups, highlighting an important health equity issue and racial disparity in imaging access.
Thoracic Imaging
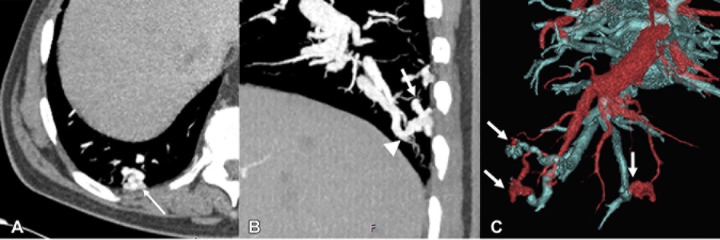
In a review article, Ota et al (26) illustrated multimodality imaging features of vascular malformations and tumors (Fig 10). The authors emphasize the replacement of old terms such as lymphangioma, cystic hygroma, and cavernous hemangioma with newer terminology, including terms such as lymphatic or venous malformation. The authors described the different subcategories of vascular malformations, such as simple, combined, malformations of major named vessels, and malformations associated with other anomalies. They also describe the spectrum, spanning from benign to malignant vascular tumors and tumorlike lesions, and review the clinical relevance and role that imaging plays in management.
Figure 10:
Images in a 76-year-old-male patient with hereditary hemorrhagic telangiectasia and multiple pulmonary arteriovenous malformations. (A) Axial contrast-enhanced CT scan shows a tangle of vessels (arrow). (B) Sagittal contrast-enhanced chest CT scan shows a tangle of vessels with a feeding artery (arrow) and a draining vein (arrowhead). (C) Three-dimensional volume-rendered reconstruction demonstrates multiple pulmonary arteriovenous malformations (arrows), with arteries depicted in red and veins in blue. (Reprinted, with permission, from reference 26.)
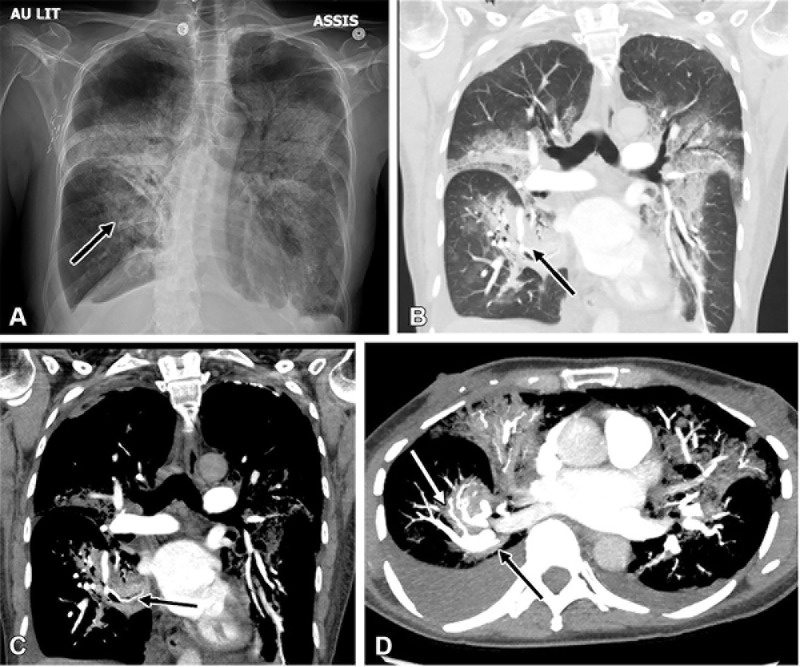
When assessing the vascular anatomy of the lung, especially in the immediate postoperative lung, a rare but clinically significant complication that may be encountered is lung torsion. Lung torsion may affect an entire lung or one of the lobes. Kanza et al (27) presented an extremely rare case of spontaneous segmental lung torsion and described its imaging findings (Fig 11). Spontaneous torsion can occur secondary to predisposing factors such as pneumonitis due to chemotherapy or radiation therapy. Unresolving consolidations with swirling vessels should raise concern for this rare surgical emergency.
Figure 11:
(A) Chest radiograph and (B–D) corresponding reconstructed CT pulmonary angiography images in coronal (B, lung window; C, mediastinal window) and axial (D, maximum intensity projection) views 1 week following admission in a 60-year-old female patient demonstrate deterioration with torsion of the medial basal segment (black arrows) of the right lower lobe. Note the presence of the antler sign (white arrow). (Reprinted, with permission, from reference 27.)
AI Clinical Applications
There is accelerating interest in clinical applications of AI in thoracic and cardiovascular imaging (28). Topff et al (29) evaluated the diagnostic efficacy of an AI software in detecting incidental pulmonary embolism at contrast-enhanced chest CT performed for oncologic indications (Fig 12). They also assessed the software’s ability to reduce the time to diagnosis by prioritizing positive studies from the radiologist reading worklist. Overall, 11 736 CT scans in 6447 oncology patients were evaluated, with high diagnostic accuracy achieved using the AI tool (sensitivity, 91.6%; specificity, 99.7%; negative predictive value, 99.9%). During prospective implementation, AI-based worklist prioritization reduced the median detection and notification time for incidental pulmonary embolism to 87 minutes (vs routine workflow of 7714 minutes). AI tools for worklist prioritization could be particularly useful in the current busy clinical environment with increasing CT volumes and associated delays in reporting (30).
Figure 12:
True-positive detection of incidental pulmonary embolism (PE) by the artificial intelligence (AI) software. (A, B) Images in a 68-year-old woman who underwent routine CT with intravenous contrast agent for outpatient follow-up of melanoma. (A) Axial CT image shows a large filling defect straddling the bifurcation of the pulmonary trunk (arrow) and extending into both pulmonary arteries, compatible with an incidental saddle PE. (B) Corresponding AI heatmap highlights the detected abnormality (red), thereby prioritizing the case in the radiologists’ worklist. (C, D) Images in a 58-year-old woman with a history of rectal cancer undergoing outpatient follow-up. (C) Axial restaging CT image with intravenous contrast agent shows a small incidental subsegmental PE in the right lower lung lobe (arrow). (D) Corresponding AI heatmap enables the radiologist to localize the finding (red). (Reprinted, with permission, from reference 29.)
The accuracy of CCTA can be limited by severe calcifications or coronary artery stents. Takafuji et al (31) retrospectively compared a super-resolution deep learning reconstruction with a conventional deep learning reconstruction algorithm in 58 patients who underwent CCTA, finding that super-resolution deep learning reconstruction improved vessel sharpness and significantly reduced image noise. In addition, in-stent lumen visualization was improved, as stent struts appeared thinner, resulting in improved detection of significant stenosis. These results highlight the potential role of AI in scenarios with severe calcifications or coronary stents where luminal visualization might prove difficult.
Quantitative assessment of regional myocardial wall motion is key to diagnosing and managing patients with ischemic heart disease. In a retrospective study, Masutani et al (32) developed a deep learning synthetic strain algorithm to improve the detection of wall motion abnormalities on routine short-axis cine steady-state free precession images (Fig 13). The study results demonstrated high diagnostic performance (area under the receiver operating characteristic curve, 0.90; accuracy, 86%) in identifying wall motion abnormalities for 223 cardiac MRI scans.
Figure 13:
Example case of a 66-year-old man with catheter angiography–proven left anterior descending coronary artery occlusion. The images show focal wall motion abnormality of the anteroseptal (blue arrow) and inferoseptal (purple arrow) walls with decreased peak radial strain and strain rate. Corresponding strain and strain rate curves show the severity of this abnormality relative to the other myocardial segments in the same section. Following intravenous contrast agent administration, the septal wall shows a matching perfusion defect and transmural delayed enhancement, indicating myocardial ischemia and infarction. LV = left ventricle, RV = right ventricle. (Reprinted, with permission, from reference 32.)
Health Services Research
The COVID-19 pandemic impacted health care services worldwide. Hirschfeld et al (33) evaluated diagnostic cardiovascular procedure volumes in the United States following the COVID-19 outbreak compared with worldwide trends. The authors found that diagnostic cardiovascular procedure volumes dropped considerably worldwide in April 2020 (−66% for the U.S. and −71% for non-U.S. facilities) compared with the prepandemic baseline and rebounded to prepandemic levels the following year in the United States and other high-income countries. However, 2021 diagnostic cardiovascular procedure volumes remained low in lower-income countries, highlighting a potential health equity disparity. Hirschfield et al also presented data to show that the recovery phase of the pandemic has shown increasing use of CCTA and cardiac MRI above prepandemic levels.
Barbosa et al (34) published results of a living systematic review and meta-analysis of randomized controlled trials comparing the effectiveness of CCTA and standard of care in the evaluation of acute chest pain. In their analysis of 22 randomized controlled trials (9379 total participants; 4956 assigned to CCTA arms and 4423 to standard-of-care arms), they found a 14% reduction in the length of stay and a 17% reduction in immediate costs for the CCTA arm compared with the standard of care arm. These results support the use of CCTA as a safe, rapid, and less expensive strategy to exclude acute coronary syndrome in low- to intermediate-risk patients presenting with acute chest pain.
Future Perspectives
Expert consensus documents are important to establish guidelines and synthesize information from multiple studies. Koweek et al (35) outline updated standardized medical terminology for cardiac CT in 2023 with the input and endorsement of multiple cardiac imaging societies. This document recommends appropriate terminology and incorporates terminologies related to newer technologies that have evolved since the initial version, including PCD CT (36).
The emergence of PCD CT has revolutionized cardiothoracic imaging practices, offering exciting possibilities for the future. This includes high-risk coronary plaque visualization, lower imaging costs through contrast material–saving capabilities, increased use of CT for myocardial tissue characterization, as well as simultaneous morphologic and functional pulmonary imaging (9,37–44).
Technical developments in cardiac MRI, including generative AI for fast cardiac MRI and AI approaches to automate cardiac MRI segmentation, hold the potential to improve diagnosis and risk stratification of cardiac disease (28,45,46). Regarding cardiac PET applications, there is heightened interest in novel tracer development and translation to cardiac applications, including the use of gallium 68–labeled fibroblast activation protein inhibitor as a potential early marker of myocardial fibrosis in patients with heart failure (47).
Given worsening human health effects related to climate change, there has been substantial interest in environmental sustainability in radiology, particularly in the past year (48). Key actions to decrease energy and associated greenhouse gas emissions related to producing and powering medical imaging equipment have been highlighted (49). The human health impacts of climate change will be particularly relevant to cardiothoracic imagers given the exacerbation of cardiovascular and respiratory disease and the potential for increased health care resource utilization related to extreme heat and other weather events (50).
Conclusion
Cardiothoracic imaging is evolving, with ongoing innovation driving increased utilization and expanded clinical applications. Novel techniques have the potential to improve diagnostic accuracy and patient outcomes for a wide range of cardiothoracic diseases.
G.J.A. and D.M. contributed equally to this work.
S. Alabed is supported by the National Institute for Health and Care Research Sheffield Biomedical Research Centre (NIHR203321).
Disclosures of conflicts of interest: G.J.A. Radiology: Cardiothoracic Imaging trainee editorial board member. D.M. Radiology: Cardiothoracic Imaging trainee deputy editor for Images in Cardiothoracic Imaging; consulting fees from Segmed; stock options in Segmed. S. Alabed Radiology: Cardiothoracic Imaging trainee deputy editor for Images in Cardiothoracic Imaging; previous grants from the British Heart Foundation, The Wellcome Trust, and the Royal College of Radiologists. S. Abohashem Radiology: Cardiothoracic Imaging trainee editorial board member. L.W. Radiology: Cardiothoracic Imaging trainee editorial member. R.R.G. Radiology: Cardiothoracic Imaging associate editor and trainee editorial board mentor. D.M.E.B. Radiology: Cardiothoracic Imaging associate editor and trainee editorial board mentor. S. Abbara Editor of Radiology: Cardiothoracic Imaging; textbook author royalties from Elsevier; member of the board of directors of the Society of Cardiovascular Computed Tomography; RSNA editor-in-chief stipend to employer. K.H. Associate editor for Radiology and Radiology: Cardiothoracic Imaging.
Abbreviations:
- AI
- artificial intelligence
- CCTA
- coronary CT angiography
- CHD
- congenital heart disease
- CT-FFR
- CT fractional flow reserve
- DM
- diabetes mellitus
- FDG
- fluorodeoxyglucose
- LA
- left atrial
- MTC-BOOST
- Magnetization Transfer Contrast Bright-and-black blOOd phase-SensiTive
- PCD CT
- photon-counting detector CT
References
- 1. Mastrodicasa D , Aquino GJ , Ordovas KG , et al . Radiology: Cardiothoracic Imaging Highlights 2022 . Radiol Cardiothorac Imaging 2023. ; 5 ( 3 ): e230042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mastrodicasa D , Gunasekaran S , Alabed S , Gulsin GS , Hanneman K . Top 2023 Images in Cardiothoracic Imaging . Radiol Cardiothorac Imaging 2023. ; 5 ( 6 ): e230259 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gulsin GS , Tzimas G , Holmes KR , et al . Impact of Coronary CT Angiography-derived Fractional Flow Reserve on Downstream Management and Clinical Outcomes in Individuals with and without Diabetes . Radiol Cardiothorac Imaging 2023. ; 5 ( 5 ): e220276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cury RC , Leipsic J , Abbara S , et al . CAD-RADS™ 2.0 - 2022 Coronary Artery Disease - Reporting and Data System An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America Society of Cardiovascular Imaging (NASCI) . Radiol Cardiothorac Imaging 2022. ; 4 ( 5 ): e220183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanisawa H , Matsumoto H , Cadet S , et al . Quantification of Low-Attenuation Plaque Burden from Coronary CT Angiography: A Head-to-Head Comparison with Near-Infrared Spectroscopy Intravascular US . Radiol Cardiothorac Imaging 2023. ; 5 ( 5 ): e230090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajendran K , Petersilka M , Henning A , et al . First Clinical Photon-counting Detector CT System: Technical Evaluation . Radiology 2022. ; 303 ( 1 ): 130 – 138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Si-Mohamed SA , Boccalini S , Lacombe H , et al . Coronary CT Angiography with Photon-counting CT: First-In-Human Results . Radiology 2022. ; 303 ( 2 ): 303 – 313 . [DOI] [PubMed] [Google Scholar]
- 8. Sandfort V , Persson M , Pourmorteza A , Noël PB , Fleischmann D , Willemink MJ . Spectral photon-counting CT in cardiovascular imaging . J Cardiovasc Comput Tomogr 2021. ; 15 ( 3 ): 218 – 225 . [DOI] [PubMed] [Google Scholar]
- 9. Higashigaito K , Mergen V , Eberhard M , et al . CT Angiography of the Aorta Using Photon-counting Detector CT with Reduced Contrast Media Volume . Radiol Cardiothorac Imaging 2023. ; 5 ( 1 ): e220140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mergen V , Ghouse S , Sartoretti T , et al . Cardiac Virtual Noncontrast Images for Calcium Quantification with Photon-counting Detector CT . Radiol Cardiothorac Imaging 2023. ; 5 ( 3 ): e220307 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Bie J , Sharma SP , van Straten M , et al . Photon-counting Detector CT in Patients Pre- and Post-Transcatheter Aortic Valve Replacement . Radiol Cardiothorac Imaging 2023. ; 5 ( 2 ): e220318 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanneman K , Kadoch M , Guo HH , et al . Initial Experience With Simultaneous 18F-FDG PET/MRI in the Evaluation of Cardiac Sarcoidosis and Myocarditis . Clin Nucl Med 2017. ; 42 ( 7 ): e328 – e334 . [DOI] [PubMed] [Google Scholar]
- 13. Marschner CA , Aloufi F , Aitken M , et al . Combined FDG PET/MRI versus Standard-of-Care Imaging in the Evaluation of Cardiac Sarcoidosis . Radiol Cardiothorac Imaging 2023. ; 5 ( 5 ): e220292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marschner CA , Thavendiranathan P , Gustafson D , et al . Myocardial Inflammation on FDG PET/MRI and Clinical Outcomes in Symptomatic and Asymptomatic Participants after COVID-19 Vaccination . Radiol Cardiothorac Imaging 2023. ; 5 ( 2 ): e220247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zghaib T , Quinaglia A C Silva T , Ambale-Venkatesh B , et al . (MESA) . Radiol Cardiothorac Imaging 2023. ; 5 ( 4 ): e220047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maroun A , Baraboo JJ , Gunasekaran S , et al . Comparison of Biplane Area-Length Method and 3D Volume Quantification by Using Cardiac MRI for Assessment of Left Atrial Volume in Atrial Fibrillation . Radiol Cardiothorac Imaging 2023. ; 5 ( 2 ): e220133 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng W , Zhang J , Zhao R , et al . T1 Mapping Values May Be Associated with Early Myocardial Involvement in Young Patients with Wilson Disease . Radiol Cardiothorac Imaging 2022. ; 4 ( 6 ): e220145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vollbrecht TM , Hart C , Zhang S , et al . Fetal Cardiac Cine MRI with Doppler US Gating in Complex Congenital Heart Disease . Radiol Cardiothorac Imaging 2023. ; 5 ( 1 ): e220129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fotaki A , Pushparajah K , Hajhosseiny R , et al . Free-breathing, Contrast Agent-free Whole-Heart MTC-BOOST Imaging: Single-Center Validation Study in Adult Congenital Heart Disease . Radiol Cardiothorac Imaging 2023. ; 5 ( 1 ): e220146 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ginami G , Neji R , Phinikaridou A , Whitaker J , Botnar RM , Prieto C . Simultaneous bright- and black-blood whole-heart MRI for noncontrast enhanced coronary lumen and thrombus visualization . Magn Reson Med 2018. ; 79 ( 3 ): 1460 – 1472 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chin AS , Willemink MJ , Kino A , et al . Acute Limited Intimal Tears of the Thoracic Aorta . J Am Coll Cardiol 2018. ; 71 ( 24 ): 2773 – 2785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Madani MH , Turner VL , Hallett RL , et al . Limited Aortic Intimal Tears: CT Imaging Features and Clinical Characteristics . Radiol Cardiothorac Imaging 2022. ; 4 ( 6 ): e220155 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuellar-Calabria H , Burcet G , Roque A , et al . Differences in the Area of Proximal and Distal Entry Tears at CT Angiography Predict Long-term Clinical Outcomes in Aortic Dissection . Radiol Cardiothorac Imaging 2021. ; 3 ( 6 ): e210029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mastrodicasa D , Willemink MJ , Turner VL , et al . Registry of Aortic Diseases to Model Adverse Events and Progression (ROADMAP) in Uncomplicated Type B Aortic Dissection: Study Design and Rationale . Radiol Cardiothorac Imaging 2022. ; 4 ( 6 ): e220039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhat S , Bir S , Schreve F , Bergin CJ , Jones PG , Waqanivavalagi SWFR . Ethnic Disparities in CT Aortography Use for Diagnosing Acute Aortic Syndrome . Radiol Cardiothorac Imaging 2022. ; 4 ( 6 ): e220018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ota Y , Lee E , Sella E , Agarwal P . Vascular Malformations and Tumors: A Review of Classification and Imaging Features for Cardiothoracic Radiologists . Radiol Cardiothorac Imaging 2023. ; 5 ( 4 ): e220328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanza RE , Heniche Y , Hubert J , Bérubé M . Segmental Lung Torsion . Radiol Cardiothorac Imaging 2023. ; 5 ( 3 ): e220258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanneman K , Playford D , Dey D , et al. ; American Heart Association Council on Cardiovascular Radiology and Intervention; and Council on Lifelong Congenital Heart Disease and Heart Health in the Young . Value Creation Through Artificial Intelligence and Cardiovascular Imaging: A Scientific Statement From the American Heart Association . Circulation 2024. ; 149 ( 6 ): e296 – e311 . [DOI] [PubMed] [Google Scholar]
- 29. Topff L , Ranschaert ER , Bartels-Rutten A , et al . Artificial Intelligence Tool for Detection and Worklist Prioritization Reduces Time to Diagnosis of Incidental Pulmonary Embolism at CT . Radiol Cardiothorac Imaging 2023. ; 5 ( 2 ): e220163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reeves RA , Halpern EJ , Rao VM . Cardiac Imaging Trends from 2010 to 2019 in the Medicare Population . Radiol Cardiothorac Imaging 2021. ; 3 ( 5 ): e210156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takafuji M , Kitagawa K , Mizutani S , et al . Super-Resolution Deep Learning Reconstruction for Improved Image Quality of Coronary CT Angiography . Radiol Cardiothorac Imaging 2023. ; 5 ( 4 ): e230085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masutani EM , Chandrupatla RS , Wang S , et al . Deep Learning Synthetic Strain: Quantitative Assessment of Regional Myocardial Wall Motion at MRI . Radiol Cardiothorac Imaging 2023. ; 5 ( 3 ): e220202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirschfeld CB , Dorbala S , Shaw LJ , et al. ; INCAPS COVID 2 Investigators Group . Cardiovascular Testing in the United States during the COVID-19 Pandemic: Volume Recovery and Worldwide Comparison . Radiol Cardiothorac Imaging 2023. ; 5 ( 5 ): e220288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbosa MF , Canan A , Xi Y , et al . Comparative Effectiveness of Coronary CT Angiography and Standard of Care for Evaluating Acute Chest Pain: A Living Systematic Review and Meta-Analysis . Radiol Cardiothorac Imaging 2023. ; 5 ( 4 ): e230022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koweek L , Achenbach S , Berman DS , et al . Standardized Medical Terminology for Cardiac Computed Tomography 2023 Update: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), American Association of Physicists in Medicine (AAPM), American College of Radiology (ACR), North American Society for Cardiovascular Imaging (NASCI), and Radiological Society of North America (RSNA) with endorsement by the Asian Society of Cardiovascular Imaging (ASCI), the European Association of Cardiovascular Imaging (EACI), and the European Society of Cardiovascular Radiology (ESCR) . Radiol Cardiothorac Imaging 2023. ; 5 ( 4 ): e230167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts J , Hanneman K . Standardized Medical Terminology for Cardiac CT: What’s in a Name? Radiol Cardiothorac Imaging 2023. ; 5 ( 4 ): e230213 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mergen V , Eberhard M , Manka R , Euler A , Alkadhi H . First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography . Front Cardiovasc Med 2022. ; 9 : 981012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Emrich T , O’Doherty J , Schoepf UJ , et al . Reduced Iodinated Contrast Media Administration in Coronary CT Angiography on a Clinical Photon-Counting Detector CT System: A Phantom Study Using a Dynamic Circulation Model . Invest Radiol 2023. ; 58 ( 2 ): 148 – 155 . [DOI] [PubMed] [Google Scholar]
- 39. Aquino GJ , O’Doherty J , Schoepf UJ , et al . Myocardial Characterization with Extracellular Volume Mapping with a First-Generation Photon-counting Detector CT with MRI Reference . Radiology 2023. ; 307 ( 2 ): e222030 . [DOI] [PubMed] [Google Scholar]
- 40. Weir-McCall JR , Alabed S . Myocardial Tissue Characterization With CT-Derived Extracellular Volume: Closing the Gap With CMR? JACC Cardiovasc Imaging 2023. ; 16 ( 10 ): 1318 – 1320 . [DOI] [PubMed] [Google Scholar]
- 41. Zsarnóczay E , Varga-Szemes A , Emrich T , et al . Characterizing the Heart and the Myocardium With Photon-Counting CT . Invest Radiol 2023. ; 58 ( 7 ): 505 – 514 . [DOI] [PubMed] [Google Scholar]
- 42. Si-Mohamed SA , Miailhes J , Rodesch PA , et al . Spectral Photon-Counting CT Technology in Chest Imaging . J Clin Med 2021. ; 10 ( 24 ): 5757 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alkadhi H , Runge V . The Future Arrived: Photon-Counting Detector CT . Invest Radiol 2023. ; 58 ( 7 ): 439 – 440 . [DOI] [PubMed] [Google Scholar]
- 44. Scharm SC , Schaefer-Prokop C , Winther HB , et al . Regional Pulmonary Morphology and Function: Photon-counting CT Assessment . Radiology 2023. ; 308 ( 1 ): e230318 . [DOI] [PubMed] [Google Scholar]
- 45. Alabed S , Alandejani F , Dwivedi K , et al . Validation of Artificial Intelligence Cardiac MRI Measurements: Relationship to Heart Catheterization and Mortality Prediction . Radiology 2022. ; 305 ( 1 ): 68 – 79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoon S , Nakamori S , Amyar A , et al . Accelerated Cardiac MRI Cine with Use of Resolution Enhancement Generative Adversarial Inline Neural Network . Radiology 2023. ; 307 ( 5 ): e222878 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song W , Zhang X , He S , et al . 68Ga-FAPI PET visualize heart failure: from mechanism to clinic . Eur J Nucl Med Mol Imaging 2023. ; 50 ( 2 ): 475 – 485 . [DOI] [PubMed] [Google Scholar]
- 48. Chaban YV , Vosshenrich J , McKee H , et al . Environmental Sustainability and MRI: Challenges, Opportunities, and a Call for Action . J Magn Reson Imaging 2023. . 10.1002/jmri.28994. Published online September 11, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown M , Schoen JH , Gross J , Omary RA , Hanneman K . Climate Change and Radiology: Impetus for Change and a Toolkit for Action . Radiology 2023. ; 307 ( 4 ): e230229 . [DOI] [PubMed] [Google Scholar]
- 50. Khraishah H , Alahmad B , Ostergard RL Jr , et al . Climate change and cardiovascular disease: implications for global health . Nat Rev Cardiol 2022. ; 19 ( 12 ): 798 – 812 . [DOI] [PubMed] [Google Scholar]