Abstract
Background
Metabolic bariatric surgery the reduces risk of new-onset type 2 diabetes in individuals with obesity, but it is unclear whether the benefit varies by sex, age, or socioeconomic status. The aim was to assess the risk of new-onset type 2 diabetes after metabolic bariatric surgery in these subgroups.
Methods
The Finnish Public Sector study, a follow-up study with matched controls nested in a large employee cohort, included patients without type 2 diabetes and with a diagnosis of obesity or self-reported BMI of at least 35 kg/m2. For each patient who had laparoscopic metabolic bariatric surgery (2008–2016), two propensity-score matched controls were selected. New-onset type 2 diabetes was ascertained from linked records from national health registries.
Results
The study included a total of 917 patients and 1811 matched controls with obesity. New-onset type 2 diabetes was diagnosed in 15 of the patients who had metabolic bariatric surgery (4.1 per 1000 person-years) and 164 controls (20.2 per 1000 person-years). The corresponding rate ratio (RR) was 0.20 (95% c.i. 0.12 to 0.35) and the rate difference (RD) was −16.1 (−19.8 to −12.3) per 1000 person-years. The risk reduction was more marked in individuals of low socioeconomic status (RR 0.10 (0.04 to 0.26) and RD −20.6 (−25.6 to −15.5) per 1000 person-years) than in those with higher socioeconomic status (RR 0.35 (0.18 to 0.66) and RD −11.5 (−16.9 to −6.0) per 1000 person-years) (Pinteraction = 0.017). No differences were observed between sexes or age groups.
Conclusion
Metabolic bariatric surgery was associated with a reduced risk of new-onset type 2 diabetes in men and women and in all age groups. The greatest benefit was observed in individuals of low socioeconomic status.
The impact of metabolic bariatric surgery on the risk of new-onset type 2 diabetes was investigated in individuals with obesity. The findings indicate that metabolic bariatric surgery significantly reduces the risk of type 2 diabetes across sexes and age groups, with the most substantial benefit observed in individuals of low socioeconomic status.
Introduction
The age-standardized burden of chronic diseases has declined worldwide over the past 30 years, but the burden of metabolic disorders has not decreased1. The prevalence of obesity has nearly tripled since 1975 and, by year 2030, it is estimated that 20% of world’s population will have obesity (body BMI 30 kg/m2 or higher)2–4. Simultaneously, the global burden of diabetes has nearly doubled since 19901. Obesity is one of the leading causes of preventable premature death and the leading cause of type 2 diabetes (T2DM), a major risk factor for cardiovascular diseases5. Despite the fact that hyperglycaemia and obesity now rank as the third and fifth leading risk factors for the global burden of disease5, prevention and access to effective medical and surgical treatments for metabolic syndrome are insufficient6.
Metabolic bariatric surgery (MBS) has been established as the most effective treatment for severe obesity, often resulting in sustainable weight loss and remission of obesity-related co-morbidities7–9. Compared with non-surgical and medical treatments, bariatric surgery is superior in achieving glycaemic control and remission of T2DM in people with obesity10,11. Bariatric surgery is also associated with a reduced risk of cardiovascular diseases, cardiovascular mortality, and all-cause mortality2,12–14. International guidelines for MBS initially dating from 199115 were updated in October 202216 to expand this effective and safe surgical treatment to patients with a BMI of at least 35 kg/m2 regardless of metabolic disorders, lowering the threshold from the previous BMI value of 40 kg/m2. In 2018, a consensus statement17 by the American Diabetes Association and the European Association for the Study of Diabetes recommended metabolic surgery for patients with a BMI of between 30 and 34.9 kg/m2 with poorly controlled T2DM despite optimal medical therapy.
Although bariatric surgery is highly effective in treating patients with obesity and T2DM, its effect on preventing new-onset T2DM in patients with obesity and no T2DM remains unclear. The Swedish Obesity Study landmark trial18 suggested that bariatric surgery is significantly more efficient in the prevention of T2DM in patients with obesity than standard conservative weight-management treatment. Other studies assessing the preventive effect of MBS on new-onset T2DM are observational and mostly based on clinical cohorts. In seven follow-up studies19–25 of population-based cohorts with highly variable results, the relative risk for new-onset T2DM after bariatric surgery compared with controls ranged from 0.14 to 0.77. However, these studies were limited by insufficient control for major confounding factors, most importantly socioeconomic status (SES)2. SES may modify the effects of bariatric surgery for at least two reasons. First, the likelihood of receiving bariatric surgery may be affected by SES, the access being worse among those with socioeconomic disadvantage26. Second, some socioeconomic pattern factors, such as non-adherence to postoperative medical procedures, may be associated with weight loss outcomes after bariatric surgery27. There are no studies assessing either the association between bariatric surgery and risk of new-onset T2DM by SES or in comparison to individuals with different classes of obesity2,16,28.
In this cohort study, the risk of new-onset T2DM was compared between patients who underwent bariatric surgery and propensity score-matched controls, including subgroup analyses by sociodemographic characteristics and obesity class.
Methods
Study population
The study population of patients who underwent bariatric surgery and matched controls was obtained from the Finnish Public Sector (FPS) study, an ongoing dynamic cohort study with repeated questionnaire follow-up every 2–4 years and linkage to electronic health records. The FPS was established in 1997–1998 and comprises employees with a job contract for a minimum of 6 months in the municipal services of 6 largest cities and 5 smaller towns, and 21 public hospitals in Finland29. A total of 477 509 individuals participating in FPS had data on socioeconomic factors and were successfully linked to national health records until 31 December 2016. The ethics committee of the Hospital District of Helsinki and Uusimaa approved the FPS study (registration number HUS/1210/2016).
Patients who had bariatric surgery and eligible comparison group
Individuals who underwent primary laparoscopic Roux-en-Y gastric bypass (LRYGB) or laparoscopic sleeve gastrectomy (LSG) between 2008 and 2016 were included. All study patients were recorded in the National Care Register for Health Care, maintained by the National Institute for Health and Welfare. The validity for bariatric surgery codes is high in this registry. It is mandatory practice for both public and private hospitals to record inpatient data including diagnoses, procedure codes, and dates of discharge of every patient in the National Care Register. The codes used to identify patients undergoing MBS are unique to the primary MBS and are used similarly in all hospitals performing MBS in Finland. A pool of individuals with obesity and no history of bariatric surgery were selected as controls. First, all cohort members were identified who had been hospitalized with the diagnoses for obesity (ICD-10 code E66) for reasons other than bariatric surgery, and those with a BMI of at least 35 kg/m2 based on self-reported body height and weight from one or more of the three surveys conducted between 2008 and 2014. Excluded were all participants from the bariatric and eligible comparison groups with prevalent diabetes (ICD-10 diagnoses E10–E14) at baseline.
Ascertainment of new-onset type 2 diabetes
The participants were linked to nationwide health and population registries using the unique personal identification numbers in Finland. T2DM was identified with ICD-10 code E11 in the hospital discharge registry and with the eligibility for special reimbursement in the Drug Prescription Register, as in the authors’ previous studies. The register maintained by the Social Insurance Institution listed all individuals fulfilling the diagnostic criteria for T2DM with physician-documented evidence (fasting plasma glucose over 7.0 mmol/l, or a non-fasting plasma glucose above 11.1 mmol/l and symptoms of diabetes)29–31. Follow-up started at the date of bariatric surgery for cases and the date of the recording of obesity (from hospital records or survey response) for controls and lasted until 31 December 2016.
Co-variables
Baseline co-variables were obtained via record linkage by the identification number of the participants to national registries. These included sociodemographic factors, area characteristics, and chronic medical conditions. Age, sex, and occupational titles were derived from employers’ records and educational attainment from Statistics Finland. SES was defined by occupational position, educational attainment, and level of neighbourhood disadvantage; detailed definitions have been published previously29. Based on records of granted work disability pensions and statutory pensions obtained from the Finnish Centre of Pensions, the participants were classified as not retired, retired owing to work disability, or retired based on age. Information on chronic medical conditions was obtained from national health registries. Healthcare provider-related differences were indicated by place of residence (city, town or rural) and hospital district (5 districts). Details of measurement of the co-variables are presented in the supplementary material, and the distribution of co-variables in the bariatric group and controls in Table S1.
Propensity score matching
For all patients in the bariatric surgery and non-surgery comparison groups, a logistic regression model was constructed with bariatric surgery as the outcome, and sex, age group, SES, retirement, diagnosed medical conditions, place of residence, and hospital district defined before the index date as co-variables. The model also included the interactions of the co-variables with sex, age group, and SES. For sensitivity analyses, four alternative control groups were defined: clinical controls only; non-clinical survey controls with BMI 30–34.9 kg/m2; those with BMI 35–39.9 kg/m2; and those with BMI 40 kg/m2 or higher.
Statistical analysis
Each bariatric surgery case was matched 1 : 2 with non-surgery controls with the same propensity score. The balance achieved by matching was studied using the χ2 test for each baseline variable to determine any imbalances.
To examine the risk of new-onset T2DM among bariatric surgery cases and their matched controls, follow-up started at the index date: hospitalization for bariatric surgery, and hospitalization with an ICD-10 code of obesity but no bariatric surgery, or the date of survey response with a BMI 35 kg/m2 or more for those not admitted to hospital with an ICD-10 code of obesity. Follow-up continued until disease onset, death, or end of follow-up (31 December 2016), whichever came first. To depict the association between bariatric surgery and new-onset T2DM across the follow-up, cumulative hazard curves for the bariatric surgery cases and their matched controls were prepared. Poisson regression models were used to estimate the rate of new-onset T2DM per 1000 person-years with 95% confidence intervals in the bariatric surgery and control groups, and the corresponding rate ratio (RR) and rate difference (RD) with 95% confidence intervals. To examine whether the associations varied between demographic subgroups, contrasts in the Poisson regression models were used. These included the demographic factor, the treatment group, and their interaction term to estimate the rates, RRs, and RDs for men and women, for those aged less than 50 years and 50 years or more, and for those of low SES or not.
In sensitivity analyses, first, cumulative hazard curves were constructed, and the rates, RRs, and RDs estimated as in the main analyses comparing the risk of patients undergoing bariatric surgery with four alternative propensity score-matched control groups (clinical controls only and non-clinical survey controls with BMI 40 or more, 35–39.9, or 30–34.9 kg/m2), matched 1 : 1. Second, the main analyses were replicated using a minimum 1-year lag between the index date and the date of the occurrence of T2DM to take into account the possibility that non-diagnosed prevalent T2DM would be more likely among the non-surgery controls than the bariatric surgery group. Third, the associations between bariatric surgery and T2DM onset were examined by type of operation (LRYGB or LSG). All analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA).
Results
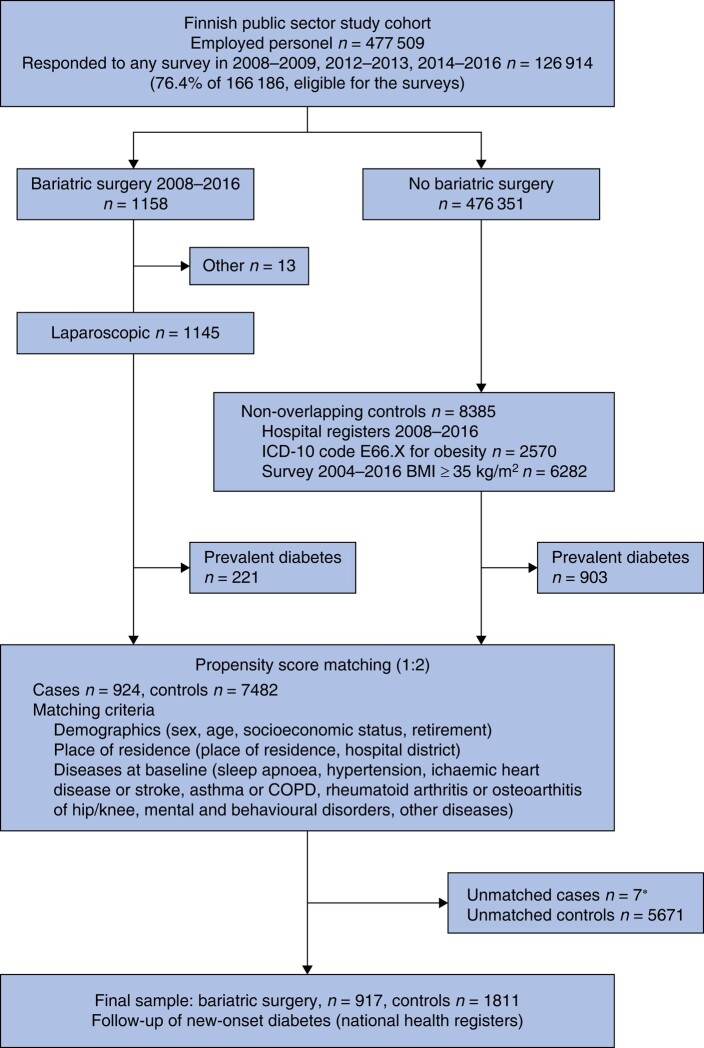
A total of 1158 patients underwent laparoscopic LRYGB or LSG. There were 8385 potential controls with no history of bariatric surgery who had either been hospitalized with the diagnosis of obesity or who had a self-reported BMI of at least 35 kg/m2 (Fig. 1). After excluding individuals with prevalent diabetes at baseline (221 in the bariatric surgery group and 903 controls, prevalence 19.3% and 10.8% respectively), each bariatric surgery case was matched with two non-surgery controls with the same propensity score, leaving only seven bariatric patients unmatched. None of these seven patients had new-onset T2DM during follow-up. The study group consisted of 917 patients who had MBS and 1811 matched controls (Table 1). There were no statistically significant differences between the two groups; there were over 90% women, mean age was 45 years, and half had a low SES.
Fig. 1.
Study flow chart
*No incident type 2 diabetes during follow-up. COPD, chronic obstructive pulmonary disease.
Table 1.
Baseline characteristics of propensity score-matched cohort of patients with bariatric surgery and controls
| Bariatric surgery (n = 917) |
Controls (n = 1811) | P§ | |
|---|---|---|---|
| Sex | 0.491 | ||
| Male | 81 (8.8) | 146 (8.1) | |
| Female | 836 (91.2) | 1665 (91.9) | |
| Age (years), mean(s.d.) | 44.8(9.6) | 44.9(9.4) | 0.757 |
| Socioeconomic status | 0.875 | ||
| Low | 465 (50.7) | 936 (51.7) | |
| Intermediate | 361 (39.4) | 695 (38.4) | |
| Higher-grade non-manual | 91 (9.9) | 180 (9.9) | |
| Retirement status | 0.212 | ||
| Not retired | 755 (82.3) | 1525 (84.2) | |
| Disability retirement | 157 (17.1) | 282 (15.6) | |
| Statutory retirement | 5 (0.6) | 4 (0.2) | |
| Diseases | |||
| Sleep apnoea | 139 (15.2) | 257 (14.2) | 0.498 |
| Hypertension | 116 (13.0) | 212 (11.7) | 0.474 |
| Cardiovascular diseases* | 20 (2.2) | 32 (1.8) | 0.455 |
| Asthma or COPDø | 131 (14.3) | 223 (12.3) | 0.148 |
| Musculoskeletal disorders† | 108 (12.0) | 203 (11.2) | 0.659 |
| Mental or behavioural disorders | 71 (7.7) | 138 (7.6) | 0.910 |
| Other‡ | 34 (3.7) | 51 (2.8) | 0.206 |
| Place of residence | 0.988 | ||
| City | 455 (49.6) | 901 (49.8) | |
| Town | 254 (27.7) | 504 (27.8) | |
| Rural | 208 (22.7) | 406 (22.4) | |
| Hospital district | 0.987 | ||
| Helsinki and Uusimaa | 347 (37.8) | 691 (38.2) | |
| South-West Finland | 146 (15.9) | 280 (15.5) | |
| Pirkanmaa | 151 (16.5) | 307 (16.9) | |
| Northern Ostrobothnia | 105 (11.5) | 198 (10.9) | |
| Other | 168 (18.3) | 335 (18.5) | |
| Propensity score, mean(s.d.) | 0.16(0.10) | 0.16(0.09) | 0.187¶ |
Values are n (%) unless indicated otherwise. *Ischaemic heart disease, heart failure, stroke. †Rheumatoid arhritis and related disorders, osteoarthritis of hip, osteoarthritis of knee. ‡Parkinson disease, multiple sclerosis, epilepsy, alcoholic liver disease, pancreatitis, renal failure. øCOPD, chronic obstructive pulmonary disease. §P-values derived from Chi2 test for categorial variables and T-test for continous variables.
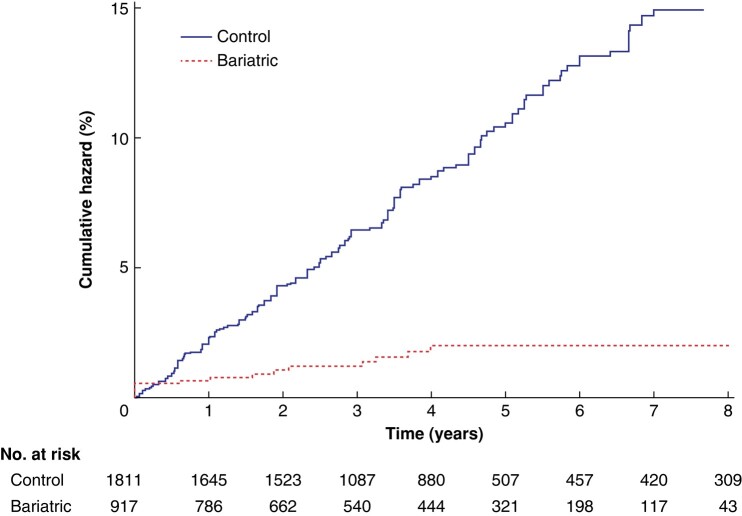
During a median follow-up of 3.8 (range 0–9.0) years for the MBS group and 3.8 (range 0–9.0) years for the control group, altogether 179 patients were diagnosed with new-onset T2DM. New-onset T2DM was diagnosed in 164 of 1811 controls (9.1%) and in 15 of 917 patients who had bariatric surgery (1.6%) (Fig. 2). The rate of T2DM was 20.2 (95% c.i. 17.3 to 23.5) per 1000 person-years in the control group and 4.1 (2.5 to 6.9) per 1000 person-years in the bariatric surgery group; the RR for the bariatric group compared with the controls was 0.20 (0.12 to 0.35) and the corresponding RD was −16.1 (−19.8 to −12.3) per 1000 person-years. All new-onset T2DM after MBS occurred during the first 4 years, except in 1 patient after 8 years. In the control group, new-onset T2DM accumulated linearly (Fig. 2).
Fig. 2.
Eight-year cumulative hazard of new-onset type 2 diabetes after metabolic bariatric surgery and in matched controls
Rate ratio 0.20 (95% c.i. 0.12 to 0.35), P < 0.001; rate difference –16.1 (–19.8 to –12.3) per 1000 person-years, P < 0.001.
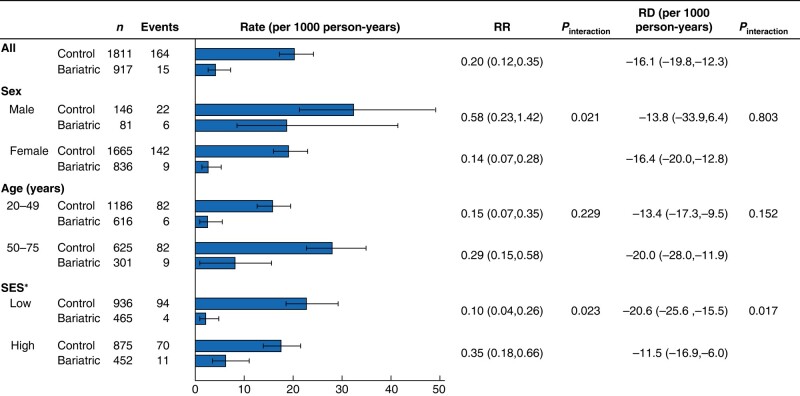
Figure 3 shows the results for subgroups of sex, age, and SES. The rate of new-onset T2DM was higher in men than in women in the bariatric surgery group and the controls, resulting in a lower relative risk among women. The RD was the same magnitude (−13.8 (−33.9 to 6.4) per 1000 person-years in men and −16.4 (−20.0 to −12.8) per 1000 person-years in women; Pinteraction = 0.803). No statistically significant difference in the relative or absolute risk for T2DM after bariatric surgery between age groups was observed (interaction P = 0.152).
Fig. 3.
Bariatric surgery and risk of new-onset type 2 diabetes compared with matched controls by patient subgroups
Values are n (%) unless otherwise indicated. Rates, rate ratios (RRs), and risk differences (RDs) are shown with 95% confidence intervals. Socioeconomic status (SES): low—basic eduction, manual occupation, or residence in disadvantaged neighbourhood; high— all other combinations.
The risk reduction was greater for low SES (RR 0.10 (0.04 to 0.26) and RD −20.6 (−25.6 to −15.5) per 1000 person-years) compared with higher status (RR 0.35 (0.18 to 0.66) and RD −11.5 (−16.9 to −6.0) per 1000 person-years; Pinteraction = 0.017). There were no statistically significant differences in baseline characteristics between patients who had MBS and controls in the low- and high-SES subgroups (Table S2). Comparing baseline characteristic between low- and high-SES controls, a higher proportion of those in the high-SES group were in employment and a higher proportion of those in the low-SES group had been granted disability retirement. There were no differences in baseline characteristics in patients who had LRYGB or LSG, such as age, co-morbidities, SES, retirement, or mean propensity score (Table S3).
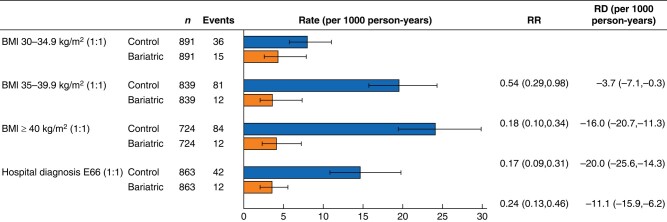
The results of the sensitivity analyses with alternative matched control groups were similar to those of the main analyses (Figs 4 and S1). Compared with matched hospital controls, the relative risk of new-onset T2DM in the bariatric surgery group was 0.24 (0.13 to 0.46) and the RD was −11.1 (−15.9 to −6.2) per 1000 person-years. Compared with matched survey controls with a BMI of at least 40 kg/m2 and those with a BMI between 35 and 35.9 kg/m2, the risk reduction was more pronounced than that for hospital controls. Compared with survey controls with a BMI between 30 and 34.9 kg/m2, the control group with the lowest diabetes risk, patients with bariatric surgery had a significantly reduced risk of new-onset T2DM (RR 0.54 (0.29 to 0.98) and RD −3.7 (−7.1 to −0.3) per 1000 person-years).
Fig. 4.
Bariatric surgery and risk of new-onset type 2 diabetes compared with alternative matched control groups
Values are n (%) unless otherwise indicated. Rates, rate ratios (RRs), and risk differences (RDs) are shown with 95% confidence intervals.
The sensitivity analysis with a 1-year lag before the start of the follow-up of new-onset T2DM also replicated the findings of the main analyses. Compared with matched controls, the RR among patients who had surgery was 0.16 (0.08 to 0.32) and the RD was −13.3 (−16.5 to −10.0) per 1000 person-years.
The sensitivity analysis examining the association between bariatric surgery and T2DM by the type of surgery found that both LSG (234 patients) and RYGBP (683) were equally effective in preventing T2DM. The RR and RD were 0.21 (0.08 to 0.57) and −15.9 (−21.1 to −10.7) per 1000 person-years, and 0.20 (0.11 to 0.37) and −16.1 (−20.0 to −12.2) per 1000 person-years respectively.
Discussion
This study identified a substantial risk reduction in developing new-onset T2DM associated with MBS compared with controls. There were no differences between men and women or age groups, but individuals of low SES benefited more from MBS than those of higher SES. The association with reduced risk of new-onset T2DM risk after MBS was substantial even in comparison to individuals with class I obesity.
These results are in agreement with those of a recent systematic review and meta-analysis2 that included six heterogeneous population cohort studies19–24 showing an association between bariatric surgery and reduced incidence of new-onset T2DM. Data on obesity in the control group were derived from hospital registers20, measured BMI21–24 or the information was missing19. The comparators in these studies were matched mainly by age, sex, and clinical characteristics21,23. One study19 also controlled for education and another24 for residential neighbourhood disadvantage. None of these studies included occupational status, which is another socioeconomic indicator known to be related to obesity and new-onset T2DM risk29.
The present study adds to understanding of the ability of bariatric surgery to lower the risk of new-onset T2DM, particularly in individuals of low SES. This finding is not explained by the differences in baseline characteristics between MBS and control groups in low- or high-SES subgroups, or differences between low- and high-SES controls. Plausible explanations are multifactorial as people with individual or neighbourhood-level socioeconomic disadvantage may have an accumulation of risk factors and thus may experience a greater preventive effect of bariatric surgery in relation to new-onset T2DM risk. Low SES is a risk factor for a spectrum of interconnected mental and physical illnesses and health conditions, including obesity, T2DM, and other cardiometabolic disorders29. In addition, low SES has been found to be associated with worse access to healthcare, including MBS26.
With obesity being a chronic disease, the risk of new-onset T2DM could be expected to increase with time and possible weight regain after MBS. However, most cases of T2DM in the present study occurred within the few first years after surgery. This finding is consistent with other studies32–34 on new-onset T2DM risk after MBS during longer follow-up.
This study benefits from information on sociodemographic characteristics, occurrence and timing of the bariatric surgery, and major chronic co-morbidities based on linkage to reliable national registers. The incidence of new-onset T2DM was ascertained using these national health registers with virtually no loss to follow-up. Additional strengths include a matched control group based on a well balanced propensity score taking into account a wide set of potential confounders, including demographics, socioeconomic factors, clinical characteristics, and living environment features. The present study was conducted in a Scandinavian welfare country offering universal health care for all citizens, which diminishes biases from selection for treatment by, for example, SES.
However, the study has limitations. Among the patients who had MBS, greater or lesser weight loss might have been associated with a preventive effect on new-onset T2DM. Hence, the lack of data on bodyweight is a major limitation. Conversely, more weight loss may be an additional preventive factor for new-onset T2DM. This is supported by the sensitivity analyses, which showed that the relative benefit among patients who had MBS was highest in the group with a BMI of at least 40 kg/m2 compared with the controls. In the main analysis, both survey-based BMI over 35 kg/m2 and the ICD-10 code E66 for obesity were used to identify potential control patients. However, the code E66 may not always be recorded for patients with obesity needing other hospital care other than MBS or treatment of obesity-related co-morbidities. Therefore, alternative matched control groups based only on an E66 diagnosis or survey-based BMI categories were used for the analyses, but the results remained unchanged. Seven patients who had MBS were excluded because no potential controls with the same propensity score were available. However, none of these individuals developed T2DM during follow-up. In the matched controls who had not been treated in hospital for obesity, BMI was determined using self-reported height and weight, which was open to reporting bias. However, the cumulative hazard curves for new-onset T2DM followed a dose–response pattern in these self-reported survey controls between class I to III obesity, and the results did not differ from those observed among the matched hospital controls. This suggests that using self-reported data did not create an important bias35. It is possible that the patients who had MBS were more likely to be diagnosed with T2DM during active multidisciplinary treatment compared with the controls; this represents a potential ascertainment bias which could have led to an underestimation of the benefits of MBS in reducing T2DM risk during the first years of follow-up. The results of the analyses suggest that this bias, if anything, is small. The result of the sensitivity analysis using a minimum 1-year lag between the index date and new-onset T2DM was similar to that of the main analysis. Thus, undetected T2DM in the control group seems not to be an important source of underestimation. There was limited information on health-related behaviours, such as smoking or alcohol intake. This could have influenced the seeking of treatment and adherence to dietary recommendations after operation, and affected the risk of new-onset T2DM. However, information on hospitalizations for mental and behavioural disorders, which correlate with the use of addictive substances29, was included in the propensity score. Finally, an ethnically homogeneous occupational cohort from a single welfare country was used, and so further research is needed to examine the effect of bariatric surgery in non-white ethnic groups and among population segments outside the workforce.
Research is also needed to assess the effect of the new antiobesity medications (AOMs) on the risk of new-onset T2DM. The management of severe obesity should follow the principle of treatment of any chronic disease treatment with combination therapies. With the better availability of potent AOMs, the practice of combination therapy will grow as MBS and AOMs work in synergy on both the treatment of severe obesity and in enabling increased access to effective obesity treatment.
In conclusion, MBS was associated with a significantly reduced risk of new-onset T2DM and this preventive effect was most pronounced in individuals of low SES. These findings underline the importance of improving treatment accessibility for patients with severe obesity.
Supplementary Material
Acknowledgements
P.S. and J.V. contributed equally to this work.
Contributor Information
Viiko Vahtera, Department of Surgery, Päijät-Häme Central Hospital, Lahti, Finland; Department of Surgery, University of Turku, Turku, Finland.
Jukka Pajarinen, Department of Plastic and Reconstructive Surgery, University of Helsinki and Helsinki University Central Hospital, Helsinki, Finland.
Mika Kivimäki, Finnish Institute of Occupational Health, Finland; UCL Brain Sciences, University College London, London, UK; Clinicum, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Jenni Ervasti, Finnish Institute of Occupational Health, Finland.
Jaana Pentti, Clinicum, Faculty of Medicine, University of Helsinki, Helsinki, Finland; Department of Public Health, University of Turku and Turku University Hospital, Turku, Finland.
Sari Stenholm, Department of Public Health, University of Turku and Turku University Hospital, Turku, Finland; Centre for Population Health Research, University of Turku and Turku University Hospital, Turku, Finland.
Jussi Vahtera, Department of Public Health, University of Turku and Turku University Hospital, Turku, Finland; Centre for Population Health Research, University of Turku and Turku University Hospital, Turku, Finland.
Paulina Salminen, Department of Surgery, University of Turku, Turku, Finland; Division of Digestive Surgery and Urology, Turku University Hospital, Turku, Finland.
Funding
V.V. was supported by ERVA research funding from the Hospital District of Southwest Finland (VTR 13136), the Finnish Society of Gastrointestinal Surgeons, the Finnish Association of Bariatric and Metabolic Surgery (LIME), and the Doctoral Programme in Clinical Research of University of Turku, Turku, Finland. M.K. was supported by the Wellcome Trust (221854/Z/20/Z), UK Medical Research Council (MRC S011676/1, R024227/1), the US National Institute on Aging (R01AG056477), the Academy of Finland (350426), and the Finnish Foundation for Cardiovascular Research (a86898). S.S. was supported by the Academy of Finland (332030). J.V. was supported by the Academy of Finland (321409 and 329240). P.S. was supported by the Sigrid Jusélius Foundation.
Author contributions
Viiko Vahtera (Writing—original draft), Jukka Pajarinen (Writing—review & editing), Mika Kivimäki (Data curation, Writing—review & editing), Jenni Ervasti (Writing—review & editing), Jaana Pentti (Data curation, Writing—review & editing), Sari Stenholm (Supervision, Writing—review & editing), Jussi Vahtera (Data curation, Methodology, Supervision, Writing—review & editing), and Paulina Salminen (Supervision, Writing—review & editing)
Disclosure
P.S. reports lecture fees from Novo Nordisk. The authors declare no other conflict of interest.
Data availability
The pseudonymized questionnaire data used in the FPS study can be shared by request to the investigators after approval from the Finnish Institute of Occupational Health scientific committee. Linked electronic health records require separate permission from the National Institute of Health and Welfare and Statistics Finland.
References
- 1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR.. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and meta-analysis. PLoS Med 2020;17:e1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep Ket al. . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO. Global Health Observatory (GHO) Data: Overweight and Obesity. WHO, 2020 [Google Scholar]
- 5. GBD 2019 Risk Factor Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patnode CD, Evans CV, Senger CA, Redmond N, Lin JS.. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: updated evidence report and systematic review for the US preventive services task force. JAMA 2017;318:175–193 [DOI] [PubMed] [Google Scholar]
- 7. Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PRet al. . Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019;322:1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schauer PR, Mingrone G, Ikramuddin S, Wolfe B.. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016;39:902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandoval DA, Patti ME.. Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia. Nat Rev Endocrinol 2023;19:164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni Get al. . Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–973 [DOI] [PubMed] [Google Scholar]
- 11. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SAet al. . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE.. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care 2012;35:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS Jret al. . Association between bariatric surgery and long-term survival. JAMA 2015;313:62–70 [DOI] [PubMed] [Google Scholar]
- 14. Cohen R, Le Roux CW, Junqueira S, Ribeiro RA, Luque A.. Roux-en-Y gastric bypass in type 2 diabetes patients with mild obesity: a systematic review and meta-analysis. Obes Surg 2017;27:2733–2739 [DOI] [PubMed] [Google Scholar]
- 15. NIH conference . Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956–961 [PubMed] [Google Scholar]
- 16. Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RVet al. . 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis 2022;18:1345–1356 [DOI] [PubMed] [Google Scholar]
- 17. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone Get al. . Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson Bet al. . Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704 [DOI] [PubMed] [Google Scholar]
- 19. Backman O, Bruze G, Naslund I, Ottosson J, Marsk R, Neovius Met al. . Gastric bypass surgery reduces de novo cases of type 2 diabetes to population levels: a nationwide cohort study from Sweden. Ann Surg 2019;269:895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailly L, Schiavo L, Sebastianelli L, Fabre R, Morisot A, Pradier Cet al. . Preventive effect of bariatric surgery on type 2 diabetes onset in morbidly obese inpatients: a national French survey between 2008 and 2016 on 328 509 morbidly obese patients. Surg Obes Relat Dis 2019;15:478–487 [DOI] [PubMed] [Google Scholar]
- 21. Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L.. Bariatric surgery in the UK: a cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med 2015;12:e1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pontiroli AE, Zakaria AS, Fanchini M, Osio C, Tagliabue E, Micheletto Get al. . A 23-year study of mortality and development of co-morbidities in patients with obesity undergoing bariatric surgery (laparoscopic gastric banding) in comparison with medical treatment of obesity. Cardiovasc Diabetol 2018;17:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer Iet al. . Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA 2018;319:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thereaux J, Lesuffleur T, Czernichow S, Basdevant A, Msika S, Nocca Det al. . Association between bariatric surgery and rates of continuation, discontinuation, or initiation of antidiabetes treatment 6 years later. JAMA Surg 2018;153:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ebadinejad A, Barzin M, Abiri B, Mahdavi M, Khalaj A, Ebrahimi Det al. . The effect of bariatric surgery in comparison with the control group on the prevention of comorbidities in people with severe obesity: a prospective cohort study. BMC Surg 2022;22:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keating C, Backholer K, Moodie M, Stevenson C, Peeters A.. Differences in the rates of treatment of severe obesity using bariatric surgery across socioeconomic groups. JAMA Surg 2015;150:367–368 [DOI] [PubMed] [Google Scholar]
- 27. Stenberg E, Naslund I, Persson C, Szabo E, Sundbom M, Ottosson Jet al. . The association between socioeconomic factors and weight loss 5 years after gastric bypass surgery. Int J Obes (Lond) 2020;44:2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZet al. . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Obes Surg 2017;27:2–21 [DOI] [PubMed] [Google Scholar]
- 29. Kivimaki M, Batty GD, Pentti J, Shipley MJ, Sipila PN, Nyberg STet al. . Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 2020;5:e140–e149 [DOI] [PubMed] [Google Scholar]
- 30. Xu T, Clark AJ, Pentti J, Rugulies R, Lange T, Vahtera Jet al. . Characteristics of workplace psychosocial resources and risk of diabetes: a prospective cohort study. Diabetes Care 2022;45:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kivimaki M, Tabak AG, Lawlor DA, Batty GD, Singh-Manoux A, Jokela Met al. . Antidepressant use before and after the diagnosis of type 2 diabetes: a longitudinal modeling study. Diabetes Care 2010;33:1471–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nor Hanipah Z, Punchai S, Brethauer SA, Schauer PR, Aminian A.. Development of de novo diabetes in long-term follow-up after bariatric surgery. Obes Surg 2018;28:2247–2251 [DOI] [PubMed] [Google Scholar]
- 33. Booth H, Khan O, Prevost T, Reddy M, Dregan A, Charlton Jet al. . Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diabetes Endocrinol 2014;2:963–968 [DOI] [PubMed] [Google Scholar]
- 34. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel Het al. . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 35. Flegal KM, Kit BK, Orpana H, Graubard BI.. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The pseudonymized questionnaire data used in the FPS study can be shared by request to the investigators after approval from the Finnish Institute of Occupational Health scientific committee. Linked electronic health records require separate permission from the National Institute of Health and Welfare and Statistics Finland.
References
- 1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR.. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and meta-analysis. PLoS Med 2020;17:e1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep Ket al. . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO. Global Health Observatory (GHO) Data: Overweight and Obesity. WHO, 2020 [Google Scholar]
- 5. GBD 2019 Risk Factor Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patnode CD, Evans CV, Senger CA, Redmond N, Lin JS.. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: updated evidence report and systematic review for the US preventive services task force. JAMA 2017;318:175–193 [DOI] [PubMed] [Google Scholar]
- 7. Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PRet al. . Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019;322:1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schauer PR, Mingrone G, Ikramuddin S, Wolfe B.. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016;39:902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandoval DA, Patti ME.. Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia. Nat Rev Endocrinol 2023;19:164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni Get al. . Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–973 [DOI] [PubMed] [Google Scholar]
- 11. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SAet al. . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE.. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care 2012;35:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS Jret al. . Association between bariatric surgery and long-term survival. JAMA 2015;313:62–70 [DOI] [PubMed] [Google Scholar]
- 14. Cohen R, Le Roux CW, Junqueira S, Ribeiro RA, Luque A.. Roux-en-Y gastric bypass in type 2 diabetes patients with mild obesity: a systematic review and meta-analysis. Obes Surg 2017;27:2733–2739 [DOI] [PubMed] [Google Scholar]
- 15. NIH conference . Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956–961 [PubMed] [Google Scholar]
- 16. Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RVet al. . 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis 2022;18:1345–1356 [DOI] [PubMed] [Google Scholar]
- 17. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone Get al. . Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson Bet al. . Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704 [DOI] [PubMed] [Google Scholar]
- 19. Backman O, Bruze G, Naslund I, Ottosson J, Marsk R, Neovius Met al. . Gastric bypass surgery reduces de novo cases of type 2 diabetes to population levels: a nationwide cohort study from Sweden. Ann Surg 2019;269:895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailly L, Schiavo L, Sebastianelli L, Fabre R, Morisot A, Pradier Cet al. . Preventive effect of bariatric surgery on type 2 diabetes onset in morbidly obese inpatients: a national French survey between 2008 and 2016 on 328 509 morbidly obese patients. Surg Obes Relat Dis 2019;15:478–487 [DOI] [PubMed] [Google Scholar]
- 21. Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L.. Bariatric surgery in the UK: a cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med 2015;12:e1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pontiroli AE, Zakaria AS, Fanchini M, Osio C, Tagliabue E, Micheletto Get al. . A 23-year study of mortality and development of co-morbidities in patients with obesity undergoing bariatric surgery (laparoscopic gastric banding) in comparison with medical treatment of obesity. Cardiovasc Diabetol 2018;17:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer Iet al. . Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA 2018;319:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thereaux J, Lesuffleur T, Czernichow S, Basdevant A, Msika S, Nocca Det al. . Association between bariatric surgery and rates of continuation, discontinuation, or initiation of antidiabetes treatment 6 years later. JAMA Surg 2018;153:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ebadinejad A, Barzin M, Abiri B, Mahdavi M, Khalaj A, Ebrahimi Det al. . The effect of bariatric surgery in comparison with the control group on the prevention of comorbidities in people with severe obesity: a prospective cohort study. BMC Surg 2022;22:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keating C, Backholer K, Moodie M, Stevenson C, Peeters A.. Differences in the rates of treatment of severe obesity using bariatric surgery across socioeconomic groups. JAMA Surg 2015;150:367–368 [DOI] [PubMed] [Google Scholar]
- 27. Stenberg E, Naslund I, Persson C, Szabo E, Sundbom M, Ottosson Jet al. . The association between socioeconomic factors and weight loss 5 years after gastric bypass surgery. Int J Obes (Lond) 2020;44:2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZet al. . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Obes Surg 2017;27:2–21 [DOI] [PubMed] [Google Scholar]
- 29. Kivimaki M, Batty GD, Pentti J, Shipley MJ, Sipila PN, Nyberg STet al. . Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 2020;5:e140–e149 [DOI] [PubMed] [Google Scholar]
- 30. Xu T, Clark AJ, Pentti J, Rugulies R, Lange T, Vahtera Jet al. . Characteristics of workplace psychosocial resources and risk of diabetes: a prospective cohort study. Diabetes Care 2022;45:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kivimaki M, Tabak AG, Lawlor DA, Batty GD, Singh-Manoux A, Jokela Met al. . Antidepressant use before and after the diagnosis of type 2 diabetes: a longitudinal modeling study. Diabetes Care 2010;33:1471–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nor Hanipah Z, Punchai S, Brethauer SA, Schauer PR, Aminian A.. Development of de novo diabetes in long-term follow-up after bariatric surgery. Obes Surg 2018;28:2247–2251 [DOI] [PubMed] [Google Scholar]
- 33. Booth H, Khan O, Prevost T, Reddy M, Dregan A, Charlton Jet al. . Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diabetes Endocrinol 2014;2:963–968 [DOI] [PubMed] [Google Scholar]
- 34. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel Het al. . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 35. Flegal KM, Kit BK, Orpana H, Graubard BI.. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]