Abstract
Confocal immunofluorescence microscopy was used to demonstrate that the Autographa californica nucleopolyhedrovirus (AcMNPV) chitinase was localized within the endoplasmic reticulum (ER) of virus-infected insect cells. This was consistent with removal of the signal peptide from the chitinase and an ER localization motif (KDEL) at the carboxyl end of the protein. Chitinase release from cells, a prerequisite for liquefaction of virus-infected insect larvae, appears to be aided by synthesis of the p10 protein. Deletion of p10 from the AcMNPV genome delayed the appearance of chitinase activity in the medium of virus-infected cells by 24 h and also delayed liquefaction of virus-infected Trichoplusia ni larvae by the same period.
The DNA genome of Autographa californica nucleopolyhedrovirus (AcMNPV) encodes a chitinase protein similar to chitinase A of Serratia marcescens (57% identity, 65% similarity [2, 6]). In virus-infected Spodoptera frugiperda cells, the AcMNPV chitinase gene (chiA) is expressed as a late protein (58 kDa) with endo- and exochitinase activities (6). The AcMNPV chiA gene can be deleted without affecting virus replication in cell culture or insects, but liquefaction or melting of larvae is abolished (7). A similar effect is observed when the cathepsin gene is deleted from the AcMNPV genome (18). We proposed previously that cathepsin removes the protein from chitin in the insect cuticle, thereby facilitating the digestion of exposed chitin by chitinase (7). This mechanism, however, is at variance with a number of observations. At least 90% of the chitinase activity induced in AcMNPV-infected S. frugiperda cells in culture remains intracellular (6). Further, immunofluorescence staining with a polyclonal chitinase-specific antiserum located the protein to the cytoplasm of virus-infected cells. This was inconsistent with the presence of a eukaryotic signal peptide at the N terminus of the chitinase protein (6), which should have served to attach it to the endoplasmic reticulum (ER), facilitating entry to the secretory pathway. Clearly, unless AcMNPV chitinase is released from cells in an inactive form, there must be a block in the secretion of this protein. In this study, we have determined the precise location of the chitinase within virus-infected cells.
Localization of AcMNPV chitinase in insect cells.
Confocal laser scanning microscopy (CLSM) was used to detect chitinase in AcMNPV-infected cells. One million S. frugiperda (Sf9 [19]) cells were seeded in 35-mm dishes which contained 13-mm sterile glass coverslips. The ce ells were incubated for 1 h at ambient temperature with AcMNPV C6 (17) (10 PFU/cell) or were mock infected with medium. The cells were then incubated at 28°C for 48 h. The coverslips were removed from the dishes, and immunofluorescence staining was performed as described previously (9). The antibodies employed were polyclonal serum raised against AcMNPV chitinase (6) and fluorescein isothiocyanate (FITC)-conjugated anti-guinea pig goat immunoglobulin G (IgG). The preparations were examined under a Zeiss LSM410 confocal laser scanning microscope with a 455-nm argon laser and appropriate filter sets (see Fig. 1).
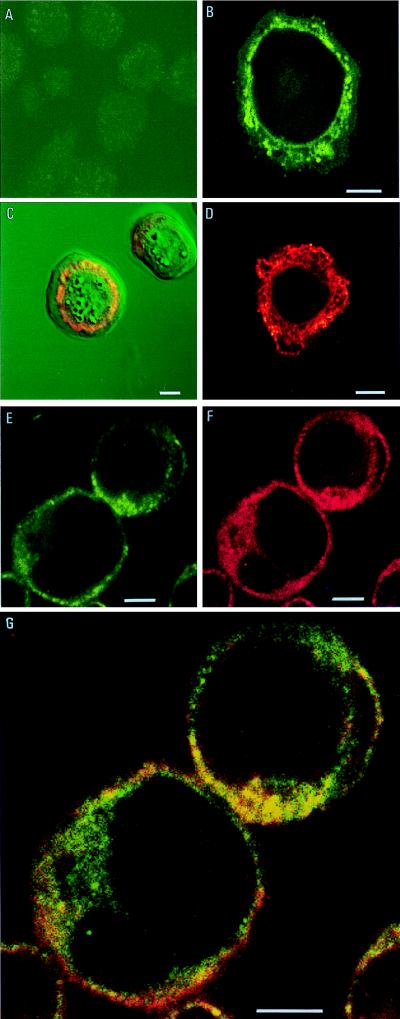
FIG. 1.
Immunofluorescent staining of S. frugiperda cells. Sf9 cells were infected with AcMNPV (10 PFU/cell) or were mock infected with medium. After 48 h, the cells were immunostained as described in the text and examined with a confocal laser scanning microscope. (A) Mock-infected cells stained sequentially with guinea pig chitinase-specific antiserum (1:10,000) and FITC conjugated with anti-guinea pig IgG polyclonal antiserum (1:40). (B) AcMNPV-infected insect cells stained as described for panel A. (C) Transmitted light and immunofluorescence image of an AcMNPV-infected insect cell immunostained as described for panel A. The pattern of immunostaining is depicted in red. (D) Mock-infected insect cell stained with mouse anti-HDEL monoclonal antibody (1:10) and FITC-conjugated goat anti-mouse IgG (1:40). (E to G) Colocalization of chitinase and ER proteins detected by double labelling of AcMNPV-infected Sf9 cells. Antichitinase antiserum and anti-HDEL monoclonal antibody were used as primary antibodies, followed by detection with, respectively, the following FITC-conjugated or Texas red-conjugated secondary antibodies: chitinase (E), HDEL (F), and dual staining (G). Bar = 5 μm.
Uninfected Sf9 cells (Fig. 1A) showed no fluorescence when stained with the chitinase-specific antibody. The image presented has been digitally enhanced to reveal the outline of the cells. In an AcMNPV-infected cell, the chitinase was located as a reticulate staining pattern which radiated out from the perinuclear region through the cytoplasm (Fig. 1B). The nuclear membrane was also stained, but no fluorescence was observed at the plasma membrane. This distribution of chitinase appeared to follow an ER staining pattern. Characteristically, in virus-infected cells, the ER becomes dilated due to the cytological effects of infection and extends from the nucleus as an irregular reticulate structure. Figure 1C is a transmitted light view of an AcMNPV-infected Sf9 cell with a superimposed image of the immunostained chitinase. The polyhedra can be seen within the nucleus of the cell. Surrounding the nucleus and extending from the nuclear membrane is the chitinase, which, in this image, is visualized in red to complement the green background.
Unambiguous evidence for the ER localization of the virus chitinase was provided by double labelling AcMNPV-infected cells with the chitinase-specific antibody and a mouse monoclonal antibody to an HDEL ER retention motif (12). The HDEL and KDEL motifs, located at the carboxyl ends of proteins, are widely used as markers for the ER in many cell types (14). HDEL is believed to be the principal ER retention sequence in insect cells (9), so the ER can be visualized by using an HDEL-specific antibody. Figure 1D shows uninfected Sf9 cells which have been immunostained with the HDEL-specific antibody and antimouse antibody conjugated with Texas red. The native ER proteins were stained to permit visualization of the characteristic reticulate network staining of this organelle. Figure 1E to G shows AcMNPV-infected Sf9 cells which have been incubated with both chitinase- and HDEL-specific primary antibodies prior to dual staining with secondary antibodies conjugated to FITC or Texas red, respectively. Figure 1E shows the immunofluorescence-labelled chitinase within the AcMNPV-infected Sf9 cells. The location of the labelled chitinase in this cell is in agreement with the previous result (Fig. 1B). Figure 1F is a view of the same cells, this time showing the immunolabelled HDEL to highlight the ER. Figure 1G shows the colocalization of the chitinase- and HDEL-specific antibodies with their red and green fluorescent secondary antibody conjugates depicted in yellow where colocalized. This confirmed that the chitinase was located in the ER of the AcMNPV-infected insect cells. Similar results have been obtained with S. frugiperda Sf21 cells (data not shown). Insect cells infected with an AcMNPV recombinant (AcchiA−) lacking the complete chiA gene (7) failed to produce a chitinase-specific signal in immunofluorescence studies (data not shown).
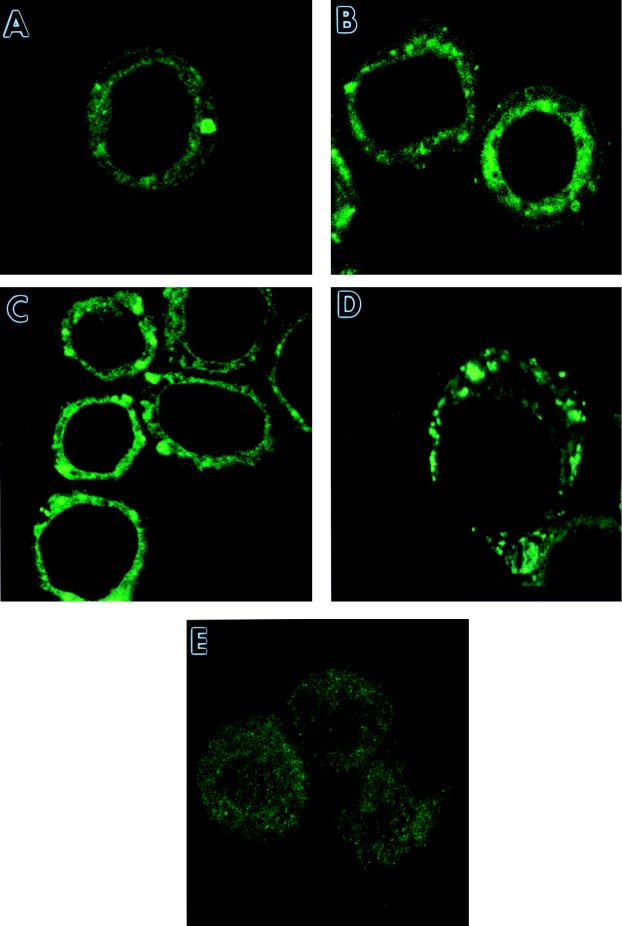
To confirm that the ER localization of chitinase was not confined to very late time points, AcMNPV-infected cells were examined at 12, 24, and 48 h postinfection (p.i.) by CLSM (Fig. 2). Faint ER staining with a chitinase-specific antibody was observed at 12 h p.i. (Fig. 2A), with stronger signals at 24 and 48 h p.i. (Fig. 2B and C, respectively). A strong ER staining pattern with the same antibody was also seen at 24 h p.i. in AcMNPV-infected Trichoplusia ni (Tn368) cells (Fig. 2D). This indicated that the localization of chitinase in the ER was not cell-type specific.
FIG. 2.
Temporal production of chitinase in AcMNPV-infected insect cells. Sf9 cells were infected with virus; stained with a primary guinea pig chitinase-specific antiserum; FITC conjugated with anti-guinea pig IgG at 12 (A), 24 (B), and 48 (C) h p.i.; and examined by CSLM as described in the text. T. ni (Tn368) cells were similarly infected and stained at 48 h p.i. (D). Mock-infected Sf9 cells were stained with the same antibodies (E).
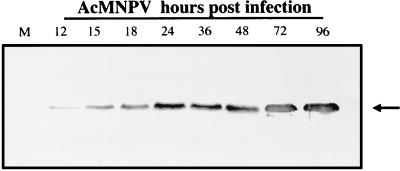
The ER localization of chitinase did not appear to be associated with proteolytic cleavage of the protein, since a 58-kDa product was evident in virus-infected cells between 12 and 96 h p.i. (Fig. 3). Enzyme assays using a range of substrates (6) showed that the intracellular chitinase was active in each of the samples harvested over the time course (data not shown).
FIG. 3.
Immunoblot analysis of chitinase in AcMNPV-infected cells. Sf9 cells in suspension culture were mock infected (M) or inoculated with AcMNPV (10 PFU/cell). Virus-infected cells were harvested between 12 and 96 h p.i., and the pellets from 1-ml culture volumes were fractionated in a 12% polyacrylamide gel before immunoblotting with antichitinase antiserum (1/10,000) and alkaline phosphatase-conjugated goat anti-guinea pig IgG polyclonal antiserum (1/1,000). The position of the 58-kDa chitinase band is indicated with an arrow.
Immunogold labelling of chitinase in AcMNPV-infected cells.
Immunogold labelling and electron microscopy were used to confirm the localization of chitinase in AcMNPV-infected cells. The Sf9 cells were infected with either AcMNPV or AcchiA− or were mock infected with culture medium. The cells were harvested at 48 h p.i. and fixed, and ultrathin sections were cut as described previously (3, 20). The sections were incubated successively with antichitinase antiserum (1:8,000) for 16 h at 4°C and then with anti-guinea pig antiserum (1:20) conjugated to 10-nm colloidal gold for 1 h at ambient temperature. After immunolabelling, sections were poststained with 2% aqueous uranyl-acetate (5 min) and lead citrate (10 min) before examination with a JEOL 1200EXII transmission electron microscope.
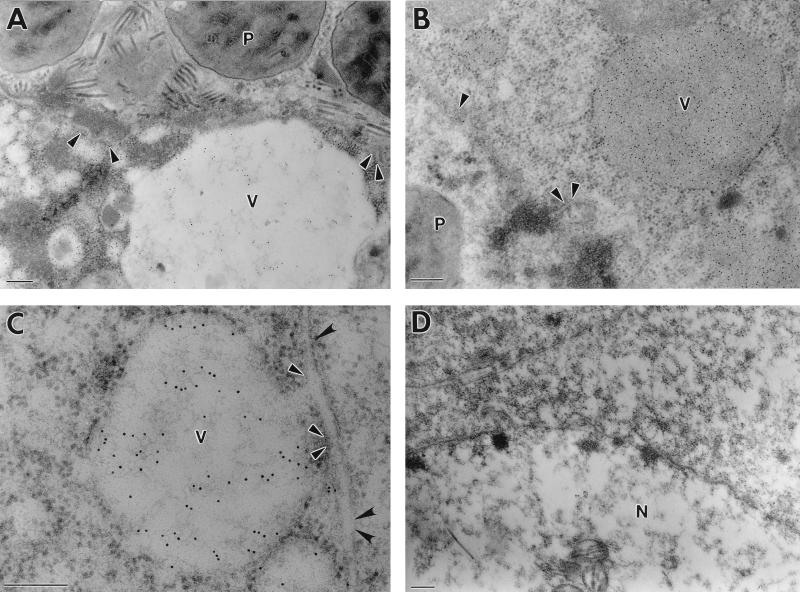
In cells infected with wild-type AcMNPV, polyhedra and nonoccluded virus particles were observed within the nuclei (Fig. 4A). Gold particles were observed around the nuclear membrane and also within vacuole-like structures which were apparent throughout the cytoplasm (Fig. 4A and B). In some vesicles, the concentration of gold particles was very high (Fig. 4B). The origin of the vacuoles was uncertain, but they were observed only in AcMNPV-infected cells. They most probably correspond to areas of degenerate or vacuolated ER. In Fig. 4C, note the outer nuclear membrane, which appears to be continuous with the membrane encompassing the vacuole containing gold particles. Virtually no gold particles were detected in the region of the plasma membrane in AcMNPV-infected cells (data not shown). In cells infected with AcchiA−, no immunogold labelling was detected in either the nucleus or cytoplasm, and the perinuclear region was completely devoid of gold particles (Fig. 4D). The vacuole-like structures observed in AcMNPV-infected cells were absent. There was no labelling in mock-infected cells or in AcMNPV-infected cells in which the primary antibody treatment was omitted (data not shown).
FIG. 4.
Immunogold staining of virus-infected insect cells with chitinase-specific antiserum. Sf9 cells were infected (10 PFU/cell) with AcMNPV (A to C) or AcchiA− (D). Scale bars = 200 nm. (A) Polyhedra (P) can be seen within the nuclear membrane (arrowheads). Gold particles are visible around the nuclear membrane and within a vacuole-like structure (V) in the cytoplasm. (B) Dense gold staining was observed in the cytoplasmic vacuole and also in a perinuclear distribution following the nuclear membrane (arrowheads). (C) The vacuoles frequently appeared to be continuous with the outer nuclear membrane (black arrowheads), with the inner nuclear membrane remaining intact (white-bordered arrowheads). (D) The nucleus (N) is bounded by a normal membrane lacking attached vacuolar structures.
N-terminal protein sequence of the AcMNPV chitinase.
The ER location of chitinase suggested cleavage of the putative signal peptide. Whole-cell extracts were prepared from AcMNPV-infected Tn368 cells; the chitinase was isolated by high-performance liquid chromatography, and its identity was confirmed by Western blot analysis (data not shown). The N-terminal amino acid sequence of the protein was determined in triplicate. The data showed that the signal peptide was cleaved from the rest of the protein after the alanine residue (Fig. 5). The signal peptide sequence for AcMNPV has little similarity with the prokaryotic signal peptide for S. marcescens chitinase A (Fig. 5). Figure 5 also shows putative signal peptides for four other baculovirus chitinases: Bombyx mori NPV (BmNPV) (10), Orgyia pseudosugata MNPV (OpMNPV) (1), Heliothis zea SNPV (HzSNPV) (8), and Choristoneura fumiferana MNPV (CfMNPV). The signal peptides for BmNPV, OpMNPV, and CfMNPV are similar to the AcMNPV sequence, but the HzSNPV sequence is very different. It is probable that these sequences would also be cleaved from the nascent protein in virus-infected cells.
FIG. 5.
Alignment of the signal peptides and carboxyl termini of the predicted amino acid sequences of the chitinases from S. marcescens (16), AcMNPV (6), OpMNPV (1), BmNPV (10), CfMNPV (U72030), and HzSNPV (8). The prokaryotic signal peptide for S. marcescens comprises the first 23 residues. The eukaryotic signal peptide of AcMNPV chitinase is underlined, and the cleavage site of the signal peptidase is indicated by the vertical arrow. The corresponding sequences in the BmNPV, OpMNPV, CfMNPV, and HzSNPV chitinases are shown. The KDEL (RDEL for BmNPV and HNEL for HzSNPV) putative ER retention signals are underlined and shown in boldface type. The length of each protein is indicated at the end of each line.
Identification of ER retention signals in baculovirus chitinases.
The association of the AcMNPV chitinase with the ER of virus-infected cells and cleavage of the signal peptide suggested that secretion of chitinase was inhibited. This indicated that a specific signal may be preventing transport of the chitinase out of the ER. Analysis of the amino acid sequence of the AcMNPV chitinase revealed a KDEL motif at the C terminus of the protein (Fig. 5). This tetrapeptide sequence motif is a known ER retention-retrieval signal (11).
The OpMNPV and CfMNPV chitinase proteins also possess KDEL sequences at their C termini, but the BmNPV chitinase has an RDEL sequence motif (Fig. 5). This is consistent with published observations that the tetrapeptide sequence is not strictly observed and that certain changes to the motif still enable retention of proteins in the ER (15). Therefore, it appears that the KDEL motif (or a closely related sequence) is present in several baculovirus chitinases. The exception to this observation is the chitinase of HzSNPV, which has HNEL at the C terminus. This partial conservation may be sufficient to serve the same role as the KDEL motif. The HzSNPV chitinase also has a 13-residue extension at this end of the protein, relative to the AcMNPV chitinase. The significance of this is unknown. S. marcescens chitinase A does not possess a KDEL motif at its C terminus (Fig. 5).
Release of chitinase from virus-infected cells.
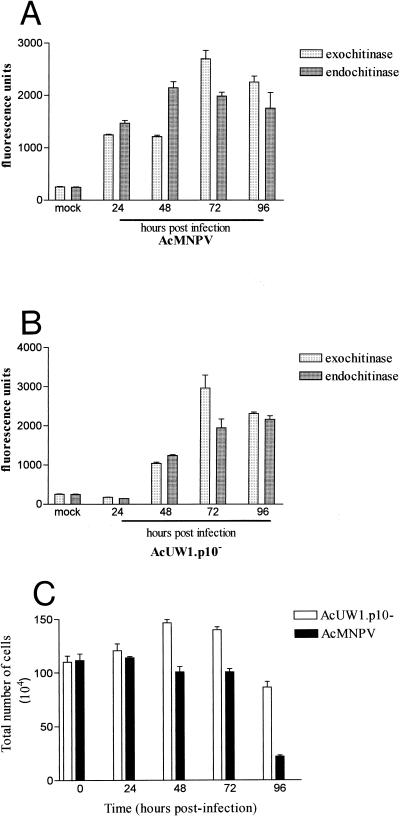
Our model for AcMNPV-induced liquefaction of virus-infected insect larvae requires chitinase to be released from cells to attack the cuticular chitin (7). It was reported that Sf21 cells infected with AcMNPV mutants lacking an intact p10 gene did not progress to cell lysis (23). We examined the release of active chitinase from Sf9 cells in a suspension culture infected with AcMNPV (10 PFU/cell) or a mutant (AcUW1.p10−) lacking an intact p10 gene (22).
Figure 6 shows that at 24 h p.i., there was approximately sixfold more chitinase in the medium supporting the growth of AcMNPV-infected cells than in that of AcUW1.p10−-infected cells. Only background levels of chitinase activity were recorded in the latter samples. Between 48 and 96 h p.i., however, similar levels of exo- and endochitinase were in the media of AcMNPV- and AcUW1.p10−-infected cell cultures. The viabilities of both virus-infected cell cultures declined from nearly 100% at 24 h p.i. to 0% at 96 h p.i. However, differences between the AcMNPV- and AcUW1.p10−-infected cell cultures were observed when the total numbers of cells at each time point were assessed. For AcMNPV, an initial concentration of 1.1 × 106 cells/ml remained static at 24 h p.i., declined slightly by 72 h p.i. (106 cells/ml), and then was reduced to about 2 × 105 cells/ml by 96 h p.i. In contrast, the concentration of cells in cultures infected with AcUW1.p10− slightly increased to 1.5 × 106 cells/ml by 48 h p.i. and declined only to 8 × 105 cells/ml at 96 h p.i.
FIG. 6.
Chitinase activities in the media of virus-infected cells and total cell numbers. Suspension cultures of Sf9 cells (106 cells/ml) were mock infected or inoculated with AcMNPV (A) or AcUW1.p10− (B) (10 PFU/cell). Medium from each culture was harvested at the times indicated and assessed for exo- and endochitinase activities (6). The cell concentration at each time point was also determined (C).
Infection of second-instar T. ni larvae with AcMNPV polyhedra resulted in host liquefaction at 5 days p.i. This process, however, was delayed by 1 day in insects infected with AcUW1.p10−.
Generally, proteins that are located in the organelles of the secretory pathway encode signals for their retention at the correct location. Proteins resident in the lumen of the ER usually carry a signal motif (KDEL or a closely related sequence) at their carboxy terminus. The sequence KDEL is predominantly found in mammalian cells (11), HDEL is found in the yeast Saccharomyces cerevisiae (13), and (H/K/R)DEL is found in plants (21). The precise sequence of the motif varies, although generally conservative changes are seen (R for K or D for E) (15). The demonstration of similar protein retention signals in widely divergent species suggests that it is a universal feature of eukaryotes (15). The signal serves to retain proteins in the ER lumen of mammalian (11), yeast (4), and plant (5) cells. Proteins escaping the ER interact with a KDEL receptor in the cis-Golgi and are returned via a retrograde vesicle-mediated pathway to the ER. If the sequence is deleted, or if it is extended by the addition of other amino acids, the protein is secreted from the cell instead of remaining in the lumen of the ER. Conversely, if the tetrapeptide sequence is added to the C terminus of non-ER resident proteins such as lysozyme, a known secretory protein, the enzyme is retained in the ER lumen instead of being secreted (11).
This study has described the identification of a KDEL motif at the C terminus of the chitinase protein of AcMNPV. This sequence motif is not observed in the S. marcescens chitinase, but has been identified as KDEL in the chitinase proteins of OpMNPV and CfMNPV and as RDEL in BmNPV. Our data do not prove that the KDEL motif in chitinase is solely responsible for retaining the protein in the ER, and we will have to perform site-directed mutagenesis on the chitinase gene to alter this sequence and determine the effect on protein location.
The retention of chitinase within virus-infected cells is surprising given that in the insect larva, our model for the mechanism of liquefaction requires that the enzyme should be extracellular to attack cuticular chitin (7). We have not examined virus-infected cells from larvae to determine if the chitinase is also retained within the ER. It may be advantageous for the virus to delay liquefaction of the host until the maximum yield of polyhedra has been attained. If chitinase were secreted from virus-infected cells as soon as it was synthesized (7 to 10 h p.i. [6]), the insect might disintegrate too rapidly and reduce polyhedron production. The eventual release of chitinase probably occurs after cell lysis, which, at least in part, is mediated by production of the p10 protein. Cells infected with a virus unable to synthesize p10 released active chitinase 24 h later than did AcMNPV-infected controls and remained intact for longer. The same p10 deletion mutant virus also induced liquefaction 1 day later in insects. Although we need to examine cell lysis in virus-infected insects, these preliminary observations suggest that chitinase release is also associated with p10 production in vivo. The fact that insects infected with a p10 deletion mutant virus eventually liquefy suggests that this protein is not the only factor with a role in the process. Attempts to measure chitinase activity in extracts from virus-infected insects have been made difficult by the high levels of host chitinases present in these samples and in uninfected controls.
Acknowledgments
We thank Barry Martin for expert assistance with electron microscopy.
This study was supported by an NERC grant awarded to R.D.P. and L.A.K.
REFERENCES
- 1.Ahrens C H, Russell R L Q, Funk C J, Evans J T, Harwood S H, Rohrmann G F. The sequence of the Orgyia pseudotsugata multinucleocapsid nuclear polyhedrosis virus genome. Virology. 1997;229:381–399. doi: 10.1006/viro.1997.8448. [DOI] [PubMed] [Google Scholar]
- 2.Ayres M D, Howard S C, Kuzio J, Lopez-Ferber M, Possee R D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- 3.Cole L, Dewey F M, Hawes C R. Infection mechanisms of Botrytis species: pre-penetration and pre-infection processes of dry and wet conidia. Mycol Res. 1996;100:277–286. [Google Scholar]
- 4.Dean N, Pelham H R B. Recycling of proteins from the Golgi compartment to the ER in yeast. J Cell Biol. 1990;111:369–377. doi: 10.1083/jcb.111.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denecke J, De Rycke R, Botterman J. Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 1992;11:2345–2355. doi: 10.1002/j.1460-2075.1992.tb05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawtin R E, Arnold K, Ayres M D, Zanotto P M D A, Howard S C, Gooday G W, Chappell L H, Kitts P A, King L A, Possee R D. Identification and preliminary characterisation of a chitinase gene in the Autographa californica nuclear polyhedrosis virus genome. Virology. 1995;212:673–685. doi: 10.1006/viro.1995.1525. [DOI] [PubMed] [Google Scholar]
- 7.Hawtin R E, Zarkowska T, Arnold K, Thomas C J, Gooday G W, King L A, Possee R D. Liquefaction of Autographa californica nucleopolyhedrovirus-infected insects is dependent on the integrity of virus-encoded chitinase and cathepsin genes. Virology. 1997;238:243–253. doi: 10.1006/viro.1997.8816. [DOI] [PubMed] [Google Scholar]
- 8.Hoa Le T, Wu T, Robertson A, Bulach D, Cowan P, Goodge K, Tribe D. Genetically variable triplet repeats in a RING-finger of Helicoverpa species baculovirus. Virus Res. 1997;49:67–77. doi: 10.1016/s0168-1702(97)01454-8. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald H, Henderson J, Napier R M, Venis M A, Hawes C, Lazarus C M. Authentic processing and targeting of active maize auxin-binding protein in the baculovirus expression system. Plant Physiol. 1994;105:1049–1057. doi: 10.1104/pp.105.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda, S. 1994. GenBank accession no. L33180.
- 11.Munro S, Pelham H R B. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 12.Napier R M, Fowke L C, Hawes C R, Lewis M, Pelham H R B. Immunological evidence that plants use both HDEL and KDEL for targeting proteins to the endoplasmic reticulum. J Cell Sci. 1992;102:261–271. doi: 10.1242/jcs.102.2.261. [DOI] [PubMed] [Google Scholar]
- 13.Pelham H R B. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988;7:913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelham H R B. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- 15.Pelham H R B. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990;15:483–487. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- 16.Perrakis A, Tews I, Dauter Z, Oppenheimer A B, Chet I, Wilson K S, Vorgias C E. Crystal structure of a bacterial chitinase at 2.3Å resolution. Structure. 1994;2:1169–1180. doi: 10.1016/s0969-2126(94)00119-7. [DOI] [PubMed] [Google Scholar]
- 17.Possee R D. Cell-surface expression of influenza virus haemagglutinin in insect cells using a baculovirus vector. Virus Res. 1986;5:43–47. doi: 10.1016/0168-1702(86)90064-x. [DOI] [PubMed] [Google Scholar]
- 18.Slack J M, Kuzio J, Faulkner P. Characterisation of v-cath, a cathepsin L-like proteinase expressed by the baculovirus Autographa californica multiple nuclear polyhedrosis virus. J Gen Virol. 1995;76:1091–1098. doi: 10.1099/0022-1317-76-5-1091. [DOI] [PubMed] [Google Scholar]
- 19.Summers M D, Smith G E. A manual of methods for baculovirus vectors and insect cell culture procedures. Texas Agricultural Experiment Station bulletin no. 1555. College Station, Tex: Texas Agricultural Experiment Station; 1987. [Google Scholar]
- 20.Van den Bosch K. Immunogold labelling. In: Hall J L, Hawes C, editors. Electron microscopy of plant cells. London, United Kingdom: Academic Press; 1991. pp. 181–218. [Google Scholar]
- 21.Vitale A, Sturm A, Bollini R. Regulation of a plant glycoprotein in the Golgi complex: a comparative study using Xenopus oocytes. Planta. 1987;169:108–116. doi: 10.1007/BF01369781. [DOI] [PubMed] [Google Scholar]
- 22.Weyer U, Knight S, Possee R D. Analysis of very late gene expression by Autographa californica nuclear polyhedrosis virus and the further development of multiple expression vectors. J Gen Virol. 1990;71:1525–1534. doi: 10.1099/0022-1317-71-7-1525. [DOI] [PubMed] [Google Scholar]
- 23.Williams G V, Rohel D Z, Kuzio J, Faulkner P. A cytopathological investigation of Autographa californica nuclear polyhedrosis virus p10 gene function using insertion/deletion mutants. J Gen Virol. 1989;70:187–202. doi: 10.1099/0022-1317-70-1-187. [DOI] [PubMed] [Google Scholar]