Abstract
The tumor microenvironment is a complex network of cells, extracellular matrix, and signaling molecules that plays a critical role in tumor progression and metastasis. Lymphatic and blood vessels are major routes for solid tumor metastasis and essential parts of tumor drainage conduits. However, recent studies have shown that lymphatic endothelial cells (LECs) and blood endothelial cells (BECs) also play multifaceted roles in the tumor microenvironment beyond their structural functions, particularly in hepatocellular carcinoma (HCC). This comprehensive review summarizes the diverse roles played by LECs and BECs in HCC, including their involvement in angiogenesis, immune modulation, lymphangiogenesis, and metastasis. By providing a detailed account of the complex interplay between LECs, BECs, and tumor cells, this review aims to shed light on future research directions regarding the immune regulatory function of LECs and potential therapeutic targets for HCC.
Keywords: Lymphatic endothelial cells, Blood endothelial cells, Hepatocellular carcinoma, Tumor microenvironment
Core Tip: Lymphatic and blood endothelial cells are important components of stromal cells in the tumor microenvironment. Besides their essential function in the formation of tumor draining blood and lymphatic vessels, they can activate various signaling pathways to promote tumor development and metastasis. This review discusses lymphangiogenesis and angiogenesis, and summarizes the current knowledge on common markers of lymphatic and blood endothelial cells and their roles in tumor metastasis, particularly in hepatocellular carcinoma. Based on the available evidence, researchers are attempting to discover new targeted therapies for the prevention of tumor progression.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a prevalent form of cancer worldwide, particularly in Asia where the majority of cases are reported. According to the World Cancer Report released in GLOBOCAN 2020, there were an estimated 905677 new cases of HCC globally, with 72.5% of those occurring in Asia[1]. Liver cancer accounts for a significant proportion (8.3%) of cancer-related deaths[2]. Metastasis, the spread of cancer cells to other parts of the body, is the primary cause of mortality in patients with solid tumors. While surgical resection and liver transplantation are common treatment options for HCC, several other approaches (e.g., transhepatic arterial chemoembolization, microwave ablation, targeted drugs, and immunotherapy) are also employed. However, the emergence of resistance to drugs, such as sorafenib and lenvatinib[3], has prompted an investigation into alternative treatment strategies. Lymphatic and blood vessels are the primary routes for metastasis. Healthy tissues and solid tumors consist of two distinct regions, namely the parenchyma and the stromal region[4]. The term tumor microenvironment (TME) refers to the area where tumor cells reside, including the stromal region. It is a complex milieu comprising non-malignant cells, such as lymphatic endothelial cells (LECs), blood endothelial cells (BECs; also termed vascular endothelial cells), mesenchymal cells, pericytes, immune cells, as well as the extracellular matrix (ECM) and inflammatory mediators they secrete.
Pan-cancer analysis has revealed that the regulation of the TME significantly impacts tumor invasion. Studies have extensively investigated the effects of immune cells and inflammatory mediators secreted by stromal cells on the TME. For example, it has been demonstrated that CD8+ T cells and natural killer T cells cooperatively promote liver damage and carcinogenesis through interaction with hepatocytes in a non-alcoholic steatohepatitis-mouse model[5]. In addition, in human glioblastoma multiforme, macrophage-associated phosphoglycerate kinase 1 (PGK1) phosphorylation promotes aerobic glycolysis and tumorigenesis. CD8+ cytotoxic T cells kill tumor cells by granule exocytosis and Fas ligand-mediated (FasL-mediated) apoptosis. They induce cytotoxicity by secreting interferon-γ (IFN-γ) and tumor necrosis factor α (TNFα). Research using mouse melanoma models has shown that promoting fatty acid catabolism improves the ability of CD8+ tumor-infiltrating lymphocytes to delay tumor progression. In lung adenocarcinoma, hypoxia upregulates C-C motif chemokine ligand 28 (CCL28) to recruit regulatory T (Treg) cells, which are involved in the immune escape of tumor cells. However, Treg cells suppress effector T cells, including cytotoxic T cells[6-10]. Mesenchymal stem cells secrete hepatocyte growth factor (HGF), indoleamine 2,3-dioxygenase (IDO), nitric oxide (NO), prostaglandin E2 (PGE2), and transforming growth factor β (TGFβ), which inhibit cytotoxic activity and differentiation of T helper 1 cells. Interleukin-10 (IL-10) and PGE2 secreted by mesenchymal stem cells in the TME impair dendritic cell maturation, thereby reducing T cell activation[4]. In HCC, the action of IL-4, IL-13, and IL-10, and activation of toll-like receptors diminish antigen-presenting activity[11]. TGFβ and thymic stromal lymphopoietin (TSLP) inhibit T cells and promote T cell skewing towards a T helper 2 phenotype, respectively. Cancer-associated fibroblasts (CAFs) also secrete inflammatory cytokines, including CXC-chemokine ligand 8 (CXCL8), IL-4, and IL-6, further suppressing T cell activity. Of note, several chemokines secreted by CAFs in the TME inhibit immune cells: CXCL12 repels T cells; CXCL13 recruits B cells; and CCL2, CCL3, CCL4, and CCL5 recruit myeloid cells, including macrophages and myeloid-derived suppressor cells, and ECM[12]. ECM secreted by stromal cells in the TME also significantly influences anti-tumor immune responses. The role of LECs and BECs (representative stromal cells in the TME) in inhibiting tumor-associated lymphangiogenesis and neoangiogenesis has not been fully elucidated. In HCC, colorectal carcinoma, and breast invasive carcinoma, it has been shown that immune invasion is highly correlated with the expression of LECs and BECs. Previous studies have revealed associations between the presence of LECs and BECs in the TME and immune invasion in colorectal and breast cancer[13]. However, research studies on the role of LECs and BECs in HCC remain limited. Previous investigations have demonstrated interactions between LECs, BECs, and liver injuries. Chronic inflammation in the liver can induce the proliferation of LECs by promoting the production of chemoattractant cytokines. An increased number of LECs has been positively correlated with disease severity. The quantity of LECs is increasing during idiopathic portal hypertension, hepatitis C virus-associated cirrhosis, and primary biliary cirrhosis. Seemingly, changes in LECs reflect the type of peripheral inflammation[14]. The levels of bacterial products, such as lipopolysaccharide (LPS), are increased in cirrhosis; these products activate nuclear factor-κB (NF-κB) in LECs. Consequently, they upregulate the expression of prospero homeobox 1 (PROX1) and vascular endothelial growth factor receptor 3 (VEGFR-3). TGFβ1 is released in the TME of HCC to increase the expression of CD105 in BECs, thus enhancing the invasion and metastasis of liver cancer cells by inducing neoangiogenesis. This comprehensive review aims to provide valuable insights into the characteristics, effects, and intricate interactions of LECs and BECs in the TME. The article specifically focuses on their roles in tumor development, metastasis, and potential therapeutic interventions in HCC.
The levels of bacterial products, such as lipopolysaccharide (LPS), are increased in cirrhosis; these products activate NF-κB in LECs. Consequently, they upregulate the expression of prospero homeobox 1 (PROX1) and vascular endothelial growth factor receptor 3 (VEGFR-3). TGFβ1 is released in the TME of HCC to increase the expression of CD105 in BECs, thus enhancing the invasion and metastasis of liver cancer cells by inducing neoangiogenesis. This comprehensive review aims to provide valuable insights into the characteristics, effects, and intricate interactions of LECs and BECs in the TME. The article specifically focuses on their roles in tumor development, metastasis, and potential therapeutic interventions in HCC.
TUMOR LYMPHANGIOGENESIS AND ANGIOGENESIS
Lymphangiogenesis
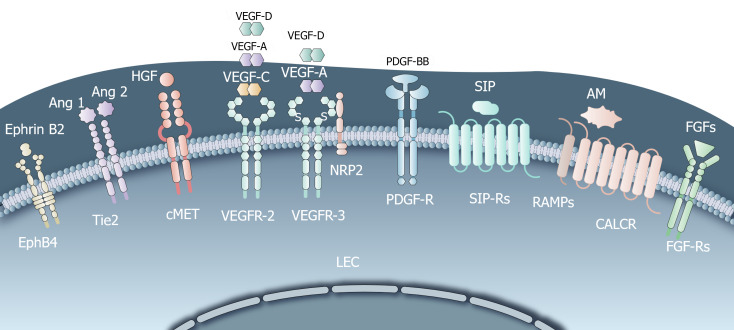
Lymphangiogenesis refers to the formation of new lymphatic vessels, and is closely associated with tumor metastasis[15,16]. In the TME, various lymphangiogenic growth factors contribute to the proliferation and morphological changes of LECs, thereby facilitating lymphangiogenesis (Figure 1).
Figure 1.
Common lymphangiogenesis-mediating receptors. Vascular endothelial growth factor receptor C (VEGF-C), fibroblast growth factors, hepatocyte growth factor (HGF), angiopoietins, adrenomedullin promote the survival, growth, and migratory ability of lymphatic endothelial cells (LECs); Neuropilin 2 forms a complex with VEGFR-3 upon binding with VEGF-C/D, enhancing lymphangiogenesis; Sphingosine-1-phosphate induces migration, sprouting, capillary-like tube formation of LECs; HGF, platelet-derived growth factor are directly involved in lymphangiogenesis. HGF indirectly promotes VEGF-C/D expression, contributing to lymphangiogenesis. LEC: Lymphatic endothelial cell; VEGF: Vascular endothelial growth factor receptor; HGF: Hepatocyte growth factor; S1P: Sphingosine-1-phosphate; AM: Adrenomedullin; FGFs: Fibroblast growth factors; PDGF: Platelet-derived growth factor.
Vascular endothelial growth factor-C: Vascular endothelial growth factor-C (VEGF-C) is one of the most potent stimulating factors for LEC growth. Studies have shown that VEGF-C is correlated with lymphangiogenesis, lymph node metastasis, and worse prognosis in patients with tumors. In murine models of human cancer, experiments involving supplementation with or blocking of VEGF-C have demonstrated its great importance[17,18]. VEGF-C binds to the receptor tyrosine kinase VEGFR-3, along with the less potent ligand VEGF-D[19,20]. This binding induces a series of downstream signaling events, including the activation of protein kinase C-dependent (PKC-dependent) pathways such as p42/p44 mitogen-activated protein kinase (MAPK) and AKT phosphorylation[19]. These signaling pathways promote the survival, growth, and migratory ability of LECs. Blocking VEGFR-3 effectively inhibits VEGF-C-induced lymphangiogenesis and tumor progression, thereby highlighting the central role of the VEGF-C/D-VEGFR-3 axis in LEC growth[21].
Neuropilin 2: Neuropilin 2 is another lymphangiogenic growth factor, a type 1 transmembrane glycoprotein highly expressed by LECs. It forms a complex with VEGFR-3 upon binding with VEGF-C/D, leading to the activation of VEGFR-3 and subsequent enhancement of lymphangiogenesis[22-24].
Fibroblast growth factor: Fibroblast growth factor receptor-3 (FGFR-3) has been identified as a novel PROX1 target gene. PROX1 induces the expression of the IIIc isoform, which is also the major isoform of FGFR-3 expressed in LECs. FGF-1 and FGF-2 promote the proliferation, migration, and survival of cultured LECs without involvement of blood endothelial cell growth factor receptor-3[25-27]. In mouse corneal tissue which lacks vascular and lymphatic vessels, FGF-2 directly acts on LECs to promote proliferation and migration via activation of the FGFR-1–mediated signaling pathway[26].
Sphingosine-1-phosphate: Sphingosine-1-phosphate (S1P) acts as a lymphangiogenic mediator in LECs. It induces migration, sprouting, capillary-like tube formation, and intracellular calcium mobilization in cultured human LECs in vitro and in a Matrigel plug assay in vivo[28]. In a murine model of breast cancer metastasis[29], S1P, suppressed by SK1-I, the specific sphingosine kinase 1 (a critical role in producing S1P and mediating tumor-induced lymphangiogenesis) inhibitor, reduced metastases to lymph nodes and lungs, and decreased overall tumor burden.
HGF: HGF plays a dual role in lymphangiogenesis. On one hand, HGF overexpression in transgenic mice or its intradermal delivery induces lymphatic vessel hyperplasia, indicating its direct involvement in lymphangiogenesis. On the other hand, experiments using prostate and breast tumor mouse models revealed that HGF can also promote the expression of VEGF-C/D, indirectly contributing to lymphangiogenesis[30,31]. Moreover, in oral squamous cell carcinoma, HGF significantly enhanced the proliferation, migration, invasion and tube formation of LECs; this process could be inhibited by downregulating the expression of c-Met, the receptor of HGF[32].
Platelet-derived growth factor: The platelet-derived growth factor (PDGF) family induces lymphatic vessel expansion independently of the VEGF-C/D/VEGFR-3 pathway. PDGF-BB, a member of this family, acts as a direct lymphangiogenic factor. Overexpression of PDGF-BB in a syngeneic fibrosarcoma tumor mouse model promoted tumor lymphangiogenesis and lymphatic metastasis, which could be reduced by blocking PDGF receptors (PDGFR). In vitro, PDGF-BB stimulated MAPK activity and the motility of isolated LECs[33].
Angiopoietins: Angiopoietins (ANGPTs) (Ang1, Ang2, and Ang3/Ang4) and their receptors Tie1 and Tie2 are involved in blood vessel maturation and patterning. However, they also play a role in lymphangiogenesis. Overexpression of ANGPTs promotes lymphangiogenesis in adult tissue in vivo, as observed in experimental pancreatic cancer models[34,35]. Holopainen et al[36] demonstrated that Ang2 blockade attenuated tumor lymphangiogenesis, dissemination of tumor cells via the lymphatic vessels, lung metastasis, and colonization of the lungs by tumor cells.
Adrenomedullin: High levels of adrenomedullin (AM) have been reported in several types of tumors in humans[37,38]. In a mouse model of lung carcinoma, AM overexpression has been correlated with increased tumor- and lymph node-associated lymphangiogenesis, as well as distant organ metastasis[39]. Berenguer-Daizé et al[40] and Fritz-Six et al[41] and found that histologic examination of anti-AM antibody-treated tumors showed evidence of disruption of tumor vascularity, with depletion of vascular, LECs, and pericytes, and increased LEC apoptosis. Another important finding was that anti-AM antibody potently blocks tumor-associated lymphangiogenesis, but does not affect established vasculature and lymphatic vessels in normal adult mice.
Angiogenesis
In 1971, Folkman[42] hypothesized that angiogenesis is essential for the development and growth of solid tumors beyond a size of 2-3 mm3. Subsequent evidence supported the notion that solid tumors rely on angiogenesis for sustained growth[43]. Angiogenesis involves the formation of new blood vessels from existing vasculature in disease. This process differs from vasculogenesis, the de novo formation of new blood vessels from endothelial progenitors[44]. Numerous studies have shown that metabolic stress, such as hypoxia, low pH, or hypoglycemia, as well as the immune and inflammatory response, can stimulate tumor angiogenesis[44,45].
Among these factors, hypoxia is a primary driver of tumor angiogenesis, leading to increased expression of VEGF and other angiogenesis stimulators from hypoxic cells[46]. Hypoxic tumor cells can activate the angiogenesis pathway by regulating pro-angiogenic genes through the hypoxia-inducible factor (HIF) pathway. Hypoxia occurs when there is insufficient oxygen reaching the tissue, often resulting from a mismatch between the demand for tumor growth and the supply of oxygen and nutrients[47,48]. Distinguished from angiogenesis under normal physiological conditions, tumor blood vessels exhibit immaturity and impaired functionality, including excessive permeability, poor perfusion, and increased hypoxia[49]. These effects are attributed to the secretion of abnormal levels of growth factors by tumor and stromal cells, among which VEGF plays a key role.
Markers of LECs and BECs
LECs and BECs exhibit specificity and sensitivity in expressing positive markers, while maintaining resistance to biological and chemical agents during histological processing. We did not search for markers that meet the above criteria; in actual practice, we often labeled specific cells with two or more markers. Common markers have been listed in Table 1.
Table 1.
Common Markers of Lymphatic endothelial cells and Blood endothelial cells
|
Marker
|
Definition
|
Expressed on cells
|
Addition
|
Types of cancer
|
Ref.
|
| PROX-1 | An evolutionarily conserved class of atypical homeodomain proteins | LECs | Located in the nucleus and promoting lymphangiogenesis | Breast cancer; Melanoma; Glioblastoma | [50-56] |
| PDPN | A transmembrane mucin type O-glycoprotein | LECs | Functioning in the downstream of PROX-1 | Angiosarcomas;Melanoma; Colorectal carcinoma; Breast cancer; Osteosarcoma | [57-60] |
| LYVE-1 | One of the hyaluronan-binding glyco-protein receptors | LECs | Acting with VEGFR and PDGFR in the LECs | Oral oncogenesis; Lung cancer | [62-66] |
| VEGFR-3 | An receptor tyrosine kinase | LECs/BECs | Functioning by activating RAS/RAF-1/MEK/ERK signaling pathway | Gastric cancer; Intrahepatic cholangiocarcinoma; Colorectal carcinoma | [67,68] |
| CD31 | One of the immunoglobulin superfamily | LECs/BECs | Promoting tumor angiogenesis by regulating TME indirectly | Breast cancer; Melanoma; Gastric cancer | [71,73-75] |
| CD105 | Homodimeric transmembrane glycoprotein, a coreceptor for ligands of the TGF-β family | BECs | Also called Endoglin | Esophageal squamous cell carcinoma; Colorectal carcinoma | [76-79] |
BECs: Blood endothelial cells; LECs: Lymphatic endothelial cells; LYVE-1: Lymphatic vessel endothelial hyaluronan receptor 1; PDGFR: Platelet-derived growth factor receptor; PDPN: Podoplanin; PROX-1: Prospero homeobox 1; VEGFR: Vascular endothelial growth factor receptor; TME: Tumor microenvironment.
PROX1: The transcription factor PROX1 is regarded as a constitutive marker of LECs due to its pivotal role in lymphangiogenesis[50,51]. It is consistently located in the nuclei of all LECs, regardless of their physiological or pathological state[52]. Recent studies showed that PROX1 could inhibit the proliferation of HCC cells, and reduced PROX1 expression was associated with poor prognosis of HCC[53]. Research has demonstrated that PROX1 can enhance tumor lymphangiogenesis in both breast cancer and melanoma[54,55]. This finding highlights the significance of PROX1 in promoting the formation of new lymphatic vessels within tumors, thereby facilitating the spread of cancer cells through the lymphatic system. In glioblastoma, overexpression of PROX1 enhanced the growth and proliferation of primary and implant focal tumor cells, and this invasive growth potential is regulated by activation of the NF-κB signaling pathway[56].
Podoplanin: The transmembrane glycoprotein podoplanin (PDPN) was initially identified on podocytes; it is also expressed on LECs, but not on BECs[57]. The anti-D2-40 antibody is a commonly used commercial antibody that specifically targets a fixation-resistant epitope of PDPN[58]. This antibody is widely utilized to detect and study PDPN expression in various research and diagnostic applications. Notably, both PROX1 and PDPN are mucin-type transmembrane proteins expressed in LECs. It has been hypothesized that PDPN functions downstream of PROX1[59]. Furthermore, it appears that PDPN expression is regulated by PROX1 in LECs at the transcriptional level[60]. Hence, both PROX1 and PDPN are excellent markers for the identification of LECs[58-61].
Lymphatic vessel endothelial hyaluronan receptor 1: The integral membrane glycoprotein lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) acts as a homologue of the CD44 hyaluronan receptor. Although it is not a signaling receptor, it is involved in cell interactions. It has been shown that the expression of LYVE-1 in tumors promotes lymphangiogenesis and facilitates the transfer of tumor cells to lymph nodes[62]. Studies have suggested that LYVE-1 induces signals indirectly through the tyrosine kinase Src and crosstalk with growth factor receptor tyrosine kinase-linked receptors. Furthermore, co-immune precipitation studies have indicated that LYVE-1 physically associates with VEGFR and PDGFR in the LEC plasma membrane[61]. While LYVE-1 is predominantly expressed in mature LECs, it is absent in some LECs; notably, it is also expressed in certain BECs. Consequently, the use of LYVE-1 alone as a marker for LECs can be challenging[63-66].
VEGFR-3: The receptor tyrosine kinase VEGFR-3, also termed fms-related receptor tyrosine kinase 4 (FLT4), plays a crucial role in both tumor angiogenesis and lymphangiogenesis. Binding of VEGF-C/D to VEGFR-3 triggers dimerization and transphosphorylation of the receptor, thereby activating the RAS/RAF-1/MEK/ERK signaling pathway and ultimately promoting lymphangiogenesis[67,68]. Sorafenib[69] and lenvatinib[70], which are widely used VEGFR-3 inhibitors, have demonstrated effectiveness as monotherapies. Recently, Paillasse et al[68] investigated EVT801, a novel selective VEGFR-3 inhibitor that specifically targets VEGFR-3-positive tumors and tumors with a VEGFR-3-positive TME. The results showed that EVT801 was effective in these settings without causing side effects, such as hypertension. The efficacy of EVT801 was correlated with the expression levels of VEGFR-3. HCC is primarily driven by angiogenesis, which is influenced by both tumor cells and the microenvironment. As a result, anti-vascular therapies have become increasingly popular for the treatment of HCC. For example, sorafenib is a VEGFR inhibitor that blocks the VEGF pathway to inhibit tumor angiogenesis. However, the effectiveness of sorafenib is limited; thus, this agent can only be used to treat advanced HCC. Therefore, it is likely that other unknown angiogenic mechanisms are involved in this process.
CD31: CD31, commonly termed platelet and endothelial cell adhesion molecule 1 (PECAM-1), is a widely used pan marker for endothelial cells. It has been utilized in various studies to isolate BECs[71]. CD31 belongs to the immunoglobulin (Ig) superfamily and is expressed on platelets, leukocytes, and endothelial cells. It is highly expressed at intercellular junctions on endothelial cells[72]. DeLisser et al[73] found that CD31 acts as a mediator of the late progression of metastatic tumors, driving advanced metastatic progression. Their experiments suggested that CD31-null mice had reduced tumor cell proliferation in non-vascularized, pre-angiogenic lesions[74]. These results indicated that CD31 may function as a modulator of the TME, rather than through direct stimulation of angiogenesis. However, CD31 is not a specific marker for BECs in the TME, as it is also expressed on the surface of normal cells, such as hematopoietic and immune cells (e.g., platelets, neutrophils, monocytes, megakaryocytes, natural killer cells, and some T cells)[75]. Therefore, researchers have identified other markers to more precisely distinguish BECs.
CD105: The transmembrane glycoprotein CD105, alternatively referred to as endoglin (ENG), is expressed on the surface of endothelial cells. It is a component of the TGFβ receptor complex. CD105 is involved in modulating TGFβ signaling to promote endothelial cell proliferation[76]. Sakurai et al[77] reported that CD105 is related to malignant tumor properties and prognosis in esophageal squamous cell carcinoma, and may be useful as a marker of angiogenesis. Additionally, increased CD105 expression has been observed in aggressive and metastatic colorectal cancer[78,79].
ROLE OF LYMPHANGIOGENESIS AND ANGIOGENESIS IN CANCER PROGRESSION
Role of lymphangiogenesis and angiogenesis in tumor metastasis
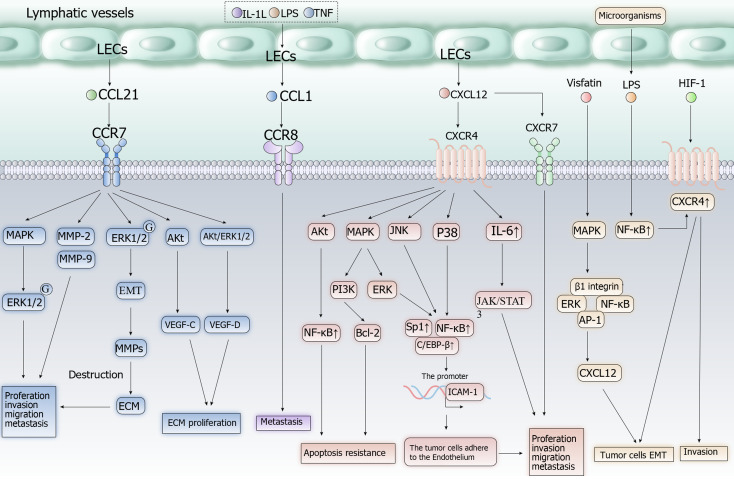
An increase in the number and density of LECs in and around tumor masses creates more opportunities for contact between these cells and tumor cells. Lymphatic vessels provide a relatively comfortable environment for tumor cells within the tumor mass, offering better survival conditions compared with the bloodstream due to lower hydrodynamic stress. This aids in their survival during lymphatic metastasis[80]. Moreover, an increased number of functional lymphatic vessels can lead to better drainage of lymphatic fluid and lower interstitial pressure. This, in turn, can result in increased blood perfusion and nutrient supply to tumor cells, promoting their growth and proliferation[21]. Migration of tumor cells along LECs and lymphatic vessels is a crucial step in lymphatic metastasis. LECs facilitate this process by secreting chemokines that attract tumor cells expressing the corresponding receptors, such as C-C motif chemokine receptor 7 (CCR7) and C-X-C motif chemokine receptor 4 (CXCR4)[81]. The chemokine gradient towards tumor-draining lymph nodes is generated by the flow of interstitial fluid, ensuring the unidirectional migration of tumor cells along lymphatic vessels and into tumor-draining lymph nodes[82]. Notably, LECs may utilize distinct mechanisms (Figure 2) to attract tumor cells along collecting lymphatic vessels or into the sinus system of the lymph node[83].
Figure 2.
Cell signaling pathways of CXCL12/CXCR4, CCL21/CCR7, CCL1/CCR8 axis in tumor cells. Proinflammatory mediators increase the production of CCL1 by lymphatic endothelial cells (LECs) and enhance the migration of tumor cells towards LECs. CCL1/CCR8 Axis is involved in recruiting Treg cells into the tumor microenvironment and converting CD4+ T cells into Treg cells. CXCR4 expression caused by products of the microbiota and chronic hypoxia stimulates tumor proliferation and migration of LECs into lymphatic vessels. Vascular endothelial growth factor receptor can enhances the effect of CXCL12/CXCR4 Axis. The CCL21/CCR7 axis induces epithelial-mesenchymal transition and promotes proliferation of tumor cells, LECs and extracellular matrix. Akt: Serine/threonine kinase; AP-1: Activated protein 1; Bcl-2: Anti-apoptotic gene; ECM: Extracellular matrix; EMT: Epithelial-mesenchymal transition; ERK: Extracellular signal-regulated kinase; HIF-1: Hypoxia-inducible factor 1; ICAM-1: Intercellular adhesion molecule 1; IL-1L: Interleukin 1L; IL-6: Interleukin 6; JAK/STAT: Janus kinase/signal transducer and activator of transcription; JNK: Jun N-terminal kinase; LPS: Lipopolysaccharide; MAPK: Mitogen-activated protein kinase; MMP: Matrix metalloproteinase; NF-κB: Nuclear factor kappa B; PI3K: Phosphatidylinositol-3-kinase; TNF: Tumor necrosis factor; VEGF: Vascular endothelial growth factor receptor.
CCL1/CCR8 axis: The CCL1/CCR8 axis plays a pivotal role in metastasis to lymph nodes. Proinflammatory mediators, including TNF, IL-1β, and LPS, increase the production of CCL1 by LECs and enhance the migration of tumor cells towards LECs. CCR8, the receptor for CCL1, is highly expressed in human malignant melanomas. Blocking CCR8 or CCL1 can inhibit the migration of tumor cells towards LECs. Additionally, this axis is involved in recruiting Treg cells into the tumor niche and converting CD4+ T cells into Treg cells[83,84].
CXCL12/CXCR4 or CXCR7 axis: CXCL12 is a key regulator of tumor progression with two receptors, namely CXCR4 and CXCR7. Overexpression of CXCL12 is associated with increased risk and poor prognosis in several common types of cancer, including HCC, colorectal carcinoma, and breast invasive carcinoma. Chronic hypoxia-induced increase in CXCR4 expression stimulates cancer cell proliferation and leads to the migration of LECs into lymphatic vessels, thus facilitating the invasion of cancer cells into adjacent tissues and organs. Epithelial–mesenchymal transition (EMT) is considered an important process in tumor metastasis. In CRC, LPS (normally produced by the microbiota) use NF-κB signaling, which can suppress apoptotic signaling, to induce CXCR4 expression in tumor cells. This process promotes EMT and metastasis. VEGF enhances the effect of CXCL12 on LECs by increasing CXCR4 expression on endothelial cells[85-91].
CCL21/CCR7 axis: CCL21 is mainly produced by LECs and interacts with CCR7 on immune cells, such as dendritic and T cells, playing a crucial role in their migration to lymph nodes. Tumor cells expressing CCR7 can also utilize this mechanism to enter lymphatic vessels for lymphatic metastasis. The CCL21/CCR7 axis induces EMT in tumor cells, upregulates the expression of matrix metalloproteinases (MMPs) by activating the ERK1/2 pathway, and promotes cancer cell proliferation. It can also promote LEC proliferation by activating the AKT pathway and the AKT/ERK1/2 pathway in tumor cells. Tumor cells expressing CCR7 can sense the gradient of CCL21 concentrations in lymphatic vessels, and migrate from low to high concentrations into those vessels[82,92].
The Ang/Tie axis is involved in regulating vascular development, vascular homeostasis, pathological inflammation, and angiogenic responses[93,94]. In addition to the above mentioned abnormal growth factors, the Ang/Tie axis also plays an important role in tumor angiogenesis. Tie is the receptor for Ang, including Tie1 and Tie2. Tie1 is an orphan receptor that does not bind to Ang; in contrast, Tie2 is expressed in BECs, pericytes, monocytes, and macrophages, and binds to members of the Ang family. The Ang family mainly includes Ang1, Ang2, Ang3, and Ang4. Although Ang1 and Ang2 bind to Tie2 with similar affinity, they exert different regulatory effects on BECs. Ang3 and Ang4 have been rarely studied thus far; hence, data on their characteristics and functions are inconclusive. Ang1, expressed in pericytes and smooth muscle cells, binds to Tie2 in a paracrine manner and phosphorylates Tie2 to maintain vascular stability and survival of BECs. Ang2 is expressed only in BECs; it acts on BECs in an autocrine manner, and is a partial competitive antagonist of Ang1 for Tie2. Qian et al[95] found that, by blocking the phosphorylation of Tie2, Ang1 affects downstream phosphatidylinositol-3-kinase (PI3K) and the growth factor receptor bound protein 2 (GRB2) signaling pathway. These effects inhibit the proliferation and migration of BECs, and result in an incomplete tumor vascular basement membrane and intercellular space. Consequently, circulating tumor cells pass through leaky tumor blood vessels and tumor external lumen, thereby facilitating tumor metastasis.
Role of LECs and BECs in tumor metastasis
The process of tumor cell dissemination through either blood or lymphatic vessels is complex. Moreover, the pathways preferred by tumor cells remain partly understood. Several factors can influence this preference, including characteristics specific to the tumor cells themselves, the TME, and the newly formed vasculature[96]. In terms of the TME, factors that attract tumor cells into blood vessels or lymphatic vessels should be considered. These factors include inflammation, host hematopoietic precursors, and soluble factors (e.g., chemokines, growth factors, and soluble receptors). Studies have shown that gene expression profiles can differentiate between LECs and BECs, highlighting their distinct physiological functions and potential as metastatic pathways for tumor cells[97]. Besides their direct association with the development of cancer, BECs are one of the sources of CAFs[98]. The heterogeneous group of CAFs is the main inducer of migration and invasion of cancer cells. Nutrition and oxygen are provided to HCC by discontinuous BECs, thereby contributing to its growth and development. BECs are also involved in intravasation, allowing HCC cells to translocate into the blood vessel lumen[99].
Additionally, the specific coreceptors expressed by tumor cells play a role in determining whether they migrate through LECs or BECs, as these cells express different receptors and signaling molecules. Furthermore, the choice between lymphangiogenesis and angiogenesis may depend on the balance of different inducers in the local TME[100]. The selection of a specific route for dissemination may also depend on various factors, such as the structural and mechanical properties of blood vessels, the expression of adhesion molecules, the secretion of chemokines, and the activity of specific signaling pathways.
SPECIAL FOCUS ON LECS AND BECS IN THE TME OF HCC
The role of LECs and BECs in HCC has also been gradually explored. Preliminary results have implied a correlation between lymphangiogenesis and cancer prognosis. Apart from its well-established functions (i.e., processing of gut-derived nutrients, clearance of toxins, and bile production), the liver is also considered a lymphoid organ. For example, hepatic stellate cells and liver sinusoidal endothelial cells exhibit antigen-presenting and immunomodulatory functions to create a tolerant microenvironment. Therefore, LECs in the TME of HCC participate in the nearby arising immune responses[14,101].
Chronic inflammation in the liver can induce the production of chemoattractant cytokines and activate NF-κB in LECs, leading to upregulation of PROX1 and VEGFR-3 expression[102]. This, in turn, increases the sensitivity to VEGF-C/D, thus influencing lymphangiogenesis. It has been confirmed that high expression of VEGF-C in patients with HCC is associated with poor prognosis[103]. Lymphatic vessel endothelial hyaluronan receptor 1 (Lyve-1+) cells have been identified in the tumor-surrounding environment of human HCC samples. Liver tumors expressing VEGF-C/D are more likely to spread within the liver, resulting in poorer outcomes and reduced patient survival[104,105].
HCC is a solid tumor with a high degree of capillarization and arterialization[106,107]. Thus, angiogenesis also plays a critical role in its development and metastasis. HCC-released TGFβ1 promotes the expression of CD105 in BECs, acting as a promoter of tumor angiogenesis[108]. CD105, in turn, enhances the invasion and metastasis of liver cancer cells by increasing VEGF expression and inducing neoangiogenesis[109]. Researchers are actively searching for additional anti-angiogenic targets. For instance, the sphingosine-1-phosphate receptor 1 (S1PR1), which binds to bioactive molecule S1P involved in angiogenesis, may serve as an important target for suppressing angiogenesis in HCC. Inhibiting S1PR1 shows promise as an approach to anti-tumor therapy against HCC[110,111].
CONCLUSION
In this manuscript, we chiefly discussed the current knowledge regarding tumor lymphangiogenesis and angiogenesis. In addition, we summarized the markers of LECs and BECs, as well as their roles in tumor metastasis (especially in HCC).
LECs can play a significant role in the TME by producing growth factors to sustain tumor cells or present tumor antigens to the immune cells. Nevertheless, there is a need to reveal the cellular and molecular mechanisms involved in these processes. Furthermore, several distinct subpopulations of LECs have been identified, which may fulfill diverse types of functions. Further studies are required to investigate whether subpopulations of LECs could be involved in different aspects of anti-tumor immunity and related to the sequential steps of tumor metastasis. Further investigation is warranted to examine the possibility of preventing tumor progression by removing LECs that promote tumor cell metastasis and by preserving LECs that inhibit tumor cells. Thus far, the mechanisms underlying the inhibitory effect of BECs on anti-tumor immunity remain unclear. Blockage of this process can avoid tumor development by regulating self-immunity without the occurrence of intolerable complications. Hence, it is important to investigate the mechanisms involved in this process. Additionally, high-risk factors of HCC, such as chronic infection with hepatitis B virus or nonalcoholic steatohepatitis, are linked to different mechanisms. Therefore, the distinct functions of the TME components during the development of HCC warrant further research. It is currently established that BECs and LECs can play a markedly more complex role than merely offering nutrition and forming the conduits of tumor cell metastasis. Thus, additional research is required to decipher the mechanisms involved. Such work may result in the development of effective therapies targeting BECs and LECs.
Footnotes
Conflict-of-interest statement: The authors declared that there is no conflict of interests.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 10, 2023
First decision: December 15, 2023
Article in press: March 18, 2024
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gutiérrez-Cuevas J, Mexico S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
Contributor Information
Jing-Jing Li, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Jia-Xi Mao, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Han-Xiang Zhong, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Yuan-Yu Zhao, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Fei Teng, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Xin-Yi Lu, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Li-Ye Zhu, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Yang Gao, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Hong Fu, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China.
Wen-Yuan Guo, Department of Liver Surgery and Organ Transplantation, Shanghai Changzheng Hospital, Naval Medical University, Shanghai 200003, China. guowenyuan@smmu.edu.cn.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf .
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, Wang Q, Wang S, Rong D, Reiter FP, De Toni EN, Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. doi: 10.1038/s41392-020-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez-Cuevas J, Lucano-Landeros S, López-Cifuentes D, Santos A, Armendariz-Borunda J. Epidemiologic, Genetic, Pathogenic, Metabolic, Epigenetic Aspects Involved in NASH-HCC: Current Therapeutic Strategies. Cancers (Basel) 2022;15 doi: 10.3390/cancers15010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiry F, Torok M, Shakya R, Agrawal K, Deems A, Benner B, Hinton A, Shaffer J, Blaser BW, Noonan AM, Williams TM, Dillhoff M, Conwell DL, Hart PA, Cruz-Monserrate Z, Bai XF, Carson WE 3rd, Mace TA. CD200 promotes immunosuppression in the pancreatic tumor microenvironment. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono H, Ito D, Fujii R, Watanabe S, Sai A, Fukuoka S, Sugiyama E, Watanabe G, Owari T, Nishinakamura H, Sugiyama D, Maeda Y, Kawazoe A, Yukami H, Chida K, Ohara Y, Yoshida T, Shinno Y, Takeyasu Y, Shirasawa M, Nakama K, Aokage K, Suzuki J, Ishii G, Kuwata T, Sakamoto N, Kawazu M, Ueno T, Mori T, Yamazaki N, Tsuboi M, Yatabe Y, Kinoshita T, Doi T, Shitara K, Mano H, Nishikawa H. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40:201–218.e9. doi: 10.1016/j.ccell.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Wei C. Hypoxia Induces Overexpression of CCL28 to Recruit Treg Cells to Enhance Angiogenesis in Lung Adenocarcinoma. J Environ Pathol Toxicol Oncol. 2021;40:65–74. doi: 10.1615/JEnvironPatholToxicolOncol.2020035859. [DOI] [PubMed] [Google Scholar]
- 9.Feng Q, Lu H, Wu L. Identification of M2-like macrophage-related signature for predicting the prognosis, ecosystem and immunotherapy response in hepatocellular carcinoma. PLoS One. 2023;18:e0291645. doi: 10.1371/journal.pone.0291645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang G, Hudaihed A, Filisio F, Giles-Davis W, Xu X, Karakousis GC, Schuchter LM, Xu W, Amaravadi R, Xiao M, Sadek N, Krepler C, Herlyn M, Freeman GJ, Rabinowitz JD, Ertl HCJ. Enhancing CD8(+) T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell. 2017;32:377–391.e9. doi: 10.1016/j.ccell.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novikova MV, Khromova NV, Kopnin PB. Components of the Hepatocellular Carcinoma Microenvironment and Their Role in Tumor Progression. Biochemistry (Mosc) 2017;82:861–873. doi: 10.1134/S0006297917080016. [DOI] [PubMed] [Google Scholar]
- 12.Mhaidly R, Mechta-Grigoriou F. Fibroblast heterogeneity in tumor micro-environment: Role in immunosuppression and new therapies. Semin Immunol. 2020;48:101417. doi: 10.1016/j.smim.2020.101417. [DOI] [PubMed] [Google Scholar]
- 13.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 14.Lukacs-Kornek V. The Role of Lymphatic Endothelial Cells in Liver Injury and Tumor Development. Front Immunol. 2016;7:548. doi: 10.3389/fimmu.2016.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 16.Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009;13:1405–1416. doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 18.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 21.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 22.Kärpänen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006;20:1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivée B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188:115–130. doi: 10.1083/jcb.200903137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M, Hong YK. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao R, Ji H, Feng N, Zhang Y, Yang X, Andersson P, Sun Y, Tritsaris K, Hansen AJ, Dissing S, Cao Y. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A. 2012;109:15894–15899. doi: 10.1073/pnas.1208324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platonova N, Miquel G, Regenfuss B, Taouji S, Cursiefen C, Chevet E, Bikfalvi A. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood. 2013;121:1229–1237. doi: 10.1182/blood-2012-08-450502. [DOI] [PubMed] [Google Scholar]
- 28.Yoon CM, Hong BS, Moon HG, Lim S, Suh PG, Kim YK, Chae CB, Gho YS. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood. 2008;112:1129–1138. doi: 10.1182/blood-2007-11-125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, Takabe K. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang WG, Davies G, Martin TA, Parr C, Watkins G, Mansel RE, Mason MD. The potential lymphangiogenic effects of hepatocyte growth factor/scatter factor in vitro and in vivo. Int J Mol Med. 2005;16:723–728. [PubMed] [Google Scholar]
- 31.Cao R, Björndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 32.Gao P, Li C, Chang Z, Wang X, Xuan M. Carcinoma associated fibroblasts derived from oral squamous cell carcinoma promote lymphangiogenesis via c-Met/PI3K/AKT in vitro. Oncol Lett. 2018;15:331–337. doi: 10.3892/ol.2017.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Schulz P, Fischer C, Detjen KM, Rieke S, Hilfenhaus G, von Marschall Z, Böhmig M, Koch I, Kehrberger J, Hauff P, Thierauch KH, Alves F, Wiedenmann B, Scholz A. Angiopoietin-2 drives lymphatic metastasis of pancreatic cancer. FASEB J. 2011;25:3325–3335. doi: 10.1096/fj.11-182287. [DOI] [PubMed] [Google Scholar]
- 35.Fagiani E, Lorentz P, Kopfstein L, Christofori G. Angiopoietin-1 and -2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 2011;71:5717–5727. doi: 10.1158/0008-5472.CAN-10-4635. [DOI] [PubMed] [Google Scholar]
- 36.Holopainen T, Saharinen P, D'Amico G, Lampinen A, Eklund L, Sormunen R, Anisimov A, Zarkada G, Lohela M, Heloterä H, Tammela T, Benjamin LE, Ylä-Herttuala S, Leow CC, Koh GY, Alitalo K. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst. 2012;104:461–475. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay DL, Walker CS, Poyner DR. Adrenomedullin and calcitonin gene-related peptide receptors in endocrine-related cancers: opportunities and challenges. Endocr Relat Cancer. 2011;18:C1–14. doi: 10.1677/ERC-10-0244. [DOI] [PubMed] [Google Scholar]
- 38.Zudaire E, Martínez A, Cuttitta F. Adrenomedullin and cancer. Regul Pept. 2003;112:175–183. doi: 10.1016/s0167-0115(03)00037-5. [DOI] [PubMed] [Google Scholar]
- 39.Karpinich NO, Kechele DO, Espenschied ST, Willcockson HH, Fedoriw Y, Caron KM. Adrenomedullin gene dosage correlates with tumor and lymph node lymphangiogenesis. FASEB J. 2013;27:590–600. doi: 10.1096/fj.12-214080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berenguer-Daizé C, Boudouresque F, Bastide C, Tounsi A, Benyahia Z, Acunzo J, Dussault N, Delfino C, Baeza N, Daniel L, Cayol M, Rossi D, El Battari A, Bertin D, Mabrouk K, Martin PM, Ouafik L. Adrenomedullin blockade suppresses growth of human hormone-independent prostate tumor xenograft in mice. Clin Cancer Res. 2013;19:6138–6150. doi: 10.1158/1078-0432.CCR-13-0691. [DOI] [PubMed] [Google Scholar]
- 41.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 43.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 45.Carmeliet P. Developmental biology. Controlling the cellular brakes. Nature. 1999;401:657–658. doi: 10.1038/44304. [DOI] [PubMed] [Google Scholar]
- 46.Dor Y, Porat R, Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol. 2001;280:C1367–C1374. doi: 10.1152/ajpcell.2001.280.6.C1367. [DOI] [PubMed] [Google Scholar]
- 47.Fan TP, Jaggar R, Bicknell R. Controlling the vasculature: angiogenesis, anti-angiogenesis and vascular targeting of gene therapy. Trends Pharmacol Sci. 1995;16:57–66. doi: 10.1016/s0165-6147(00)88979-8. [DOI] [PubMed] [Google Scholar]
- 48.Manuelli V, Pecorari C, Filomeni G, Zito E. Regulation of redox signaling in HIF-1-dependent tumor angiogenesis. FEBS J. 2022;289:5413–5425. doi: 10.1111/febs.16110. [DOI] [PubMed] [Google Scholar]
- 49.Torrence D, Antonescu CR. The genetics of vascular tumours: an update. Histopathology. 2022;80:19–32. doi: 10.1111/his.14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park KJ, Cho SB, Park YL, Kim N, Park SY, Myung DS, Lee WS, Kweon SS, Joo YE. Prospero homeobox 1 mediates the progression of gastric cancer by inducing tumor cell proliferation and lymphangiogenesis. Gastric Cancer. 2017;20:104–115. doi: 10.1007/s10120-015-0592-y. [DOI] [PubMed] [Google Scholar]
- 51.Rudzińska M, Grzanka M, Stachurska A, Mikula M, Paczkowska K, Stępień T, Paziewska A, Ostrowski J, Czarnocka B. Molecular Signature of Prospero Homeobox 1 (PROX1) in Follicular Thyroid Carcinoma Cells. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20092212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu D, Wang R, Wang Y, Wang L. Prospero homeobox 1 promotes proliferation, migration, and invasion of osteosarcoma cells and its clinical significance. Bioengineered. 2022;13:2259–2271. doi: 10.1080/21655979.2021.2024330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang TM, Hung WC. The homeobox transcription factor Prox1 inhibits proliferation of hepatocellular carcinoma cells by inducing p53-dependent senescence-like phenotype. Cancer Biol Ther. 2013;14:222–229. doi: 10.4161/cbt.23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gramolelli S, Cheng J, Martinez-Corral I, Vähä-Koskela M, Elbasani E, Kaivanto E, Rantanen V, Tuohinto K, Hautaniemi S, Bower M, Haglund C, Alitalo K, Mäkinen T, Petrova TV, Lehti K, Ojala PM. PROX1 is a transcriptional regulator of MMP14. Sci Rep. 2018;8:9531. doi: 10.1038/s41598-018-27739-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reger de Moura C, Landras A, Khayati F, Maskos U, Maouche K, Battistella M, Menashi S, Lebbé C, Mourah S. CD147 Promotes Tumor Lymphangiogenesis in Melanoma via PROX-1. Cancers (Basel) 2021;13 doi: 10.3390/cancers13194859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X, Wan X, Wei X. PROX1 promotes human glioblastoma cell proliferation and invasion via activation of the nuclear factor-κB signaling pathway. Mol Med Rep. 2017;15:963–968. doi: 10.3892/mmr.2016.6075. [DOI] [PubMed] [Google Scholar]
- 57.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonne SB, Herlihy AS, Hoei-Hansen CE, Nielsen JE, Almstrup K, Skakkebaek NE, Marks A, Leffers H, Rajpert-De Meyts E. Identity of M2A (D2-40) antigen and gp36 (Aggrus, T1A-2, podoplanin) in human developing testis, testicular carcinoma in situ and germ-cell tumours. Virchows Arch. 2006;449:200–206. doi: 10.1007/s00428-006-0223-4. [DOI] [PubMed] [Google Scholar]
- 59.Leong SP, Naxerova K, Keller L, Pantel K, Witte M. Molecular mechanisms of cancer metastasis via the lymphatic vs the blood vessels. Clin Exp Metastasis. 2022;39:159–179. doi: 10.1007/s10585-021-10120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Y, Wang WD, Yago T. Transcriptional regulation of podoplanin expression by Prox1 in lymphatic endothelial cells. Microvasc Res. 2014;94:96–102. doi: 10.1016/j.mvr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Takemoto A, Takagi S, Ukaji T, Gyobu N, Kakino M, Takami M, Kobayashi A, Lebel M, Kawaguchi T, Sugawara M, Tsuji-Takayama K, Ichihara K, Funauchi Y, Ae K, Matsumoto S, Sugiura Y, Takeuchi K, Noda T, Katayama R, Fujita N. Targeting Podoplanin for the Treatment of Osteosarcoma. Clin Cancer Res. 2022;28:2633–2645. doi: 10.1158/1078-0432.CCR-21-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nunomiya K, Shibata Y, Abe S, Inoue S, Igarashi A, Yamauchi K, Kimura T, Aida Y, Nemoto T, Sato M, Kishi H, Nakano H, Sato K, Kubota I. Relationship between Serum Level of Lymphatic Vessel Endothelial Hyaluronan Receptor-1 and Prognosis in Patients with Lung Cancer. J Cancer. 2014;5:242–247. doi: 10.7150/jca.8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karinen S, Juurikka K, Hujanen R, Wahbi W, Hadler-Olsen E, Svineng G, Eklund KK, Salo T, Åström P, Salem A. Tumour cells express functional lymphatic endothelium-specific hyaluronan receptor in vitro and in vivo: Lymphatic mimicry promotes oral oncogenesis? Oncogenesis. 2021;10:23. doi: 10.1038/s41389-021-00312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saha S, Fan F, Alderfer L, Graham F, Hall E, Hanjaya-Putra D. Synthetic hyaluronic acid coating preserves the phenotypes of lymphatic endothelial cells. Biomater Sci. 2023;11:7346–7357. doi: 10.1039/d3bm00873h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carvalho AM, Reis RL, Pashkuleva I. Hyaluronan Receptors as Mediators and Modulators of the Tumor Microenvironment. Adv Healthc Mater. 2023;12:e2202118. doi: 10.1002/adhm.202202118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson LA, Jackson DG. Hyaluronan and Its Receptors: Key Mediators of Immune Cell Entry and Trafficking in the Lymphatic System. Cells. 2021;10 doi: 10.3390/cells10082061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paillasse MR, Esquerré M, Bertrand FA, Poussereau-Pomié C, Pichery M, Visentin V, Gueguen-Dorbes G, Gaujarengues F, Barron P, Badet G, Briaux A, Ancey PB, Sibrac D, Erdociain E, Özcelik D, Meneyrol J, Martin V, Gomez-Brouchet A, Selves J, Rochaix P, Battistella M, Lebbé C, Delord JP, Dol-Gleizes F, Bono F, Blanc I, Alam A, Hunneyball I, Whittaker M, Fons P. Targeting Tumor Angiogenesis with the Selective VEGFR-3 Inhibitor EVT801 in Combination with Cancer Immunotherapy. Cancer Res Commun. 2022;2:1504–1519. doi: 10.1158/2767-9764.CRC-22-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 70.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 71.Hewett PW. Isolation and Culture of Human Endothelial Cells from Micro- and Macro-vessels. Methods Mol Biol. 2016;1430:61–76. doi: 10.1007/978-1-4939-3628-1_4. [DOI] [PubMed] [Google Scholar]
- 72.Lertkiatmongkol P, Liao D, Mei H, Hu Y, Newman PJ. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31) Curr Opin Hematol. 2016;23:253–259. doi: 10.1097/MOH.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeLisser H, Liu Y, Desprez PY, Thor A, Briasouli P, Handumrongkul C, Wilfong J, Yount G, Nosrati M, Fong S, Shtivelman E, Fehrenbach M, Cao G, Moore DH, Nayak S, Liggitt D, Kashani-Sabet M, Debs R. Vascular endothelial platelet endothelial cell adhesion molecule 1 (PECAM-1) regulates advanced metastatic progression. Proc Natl Acad Sci U S A. 2010;107:18616–18621. doi: 10.1073/pnas.1004654107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao G, Fehrenbach ML, Williams JT, Finklestein JM, Zhu JX, Delisser HM. Angiogenesis in platelet endothelial cell adhesion molecule-1-null mice. Am J Pathol. 2009;175:903–915. doi: 10.2353/ajpath.2009.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 76.Sier VQ, van der Vorst JR, Quax PHA, de Vries MR, Zonoobi E, Vahrmeijer AL, Dekkers IA, de Geus-Oei LF, Smits AM, Cai W, Sier CFM, Goumans MJTH, Hawinkels LJAC. Endoglin/CD105-Based Imaging of Cancer and Cardiovascular Diseases: A Systematic Review. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22094804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakurai T, Okumura H, Matsumoto M, Uchikado Y, Owaki T, Kita Y, Setoyama T, Omoto I, Kijima Y, Ishigami S, Natsugoe S. Endoglin (CD105) is a useful marker for evaluating microvessel density and predicting prognosis in esophageal squamous cell carcinoma. Anticancer Res. 2014;34:3431–3438. [PubMed] [Google Scholar]
- 78.Zhu DJ, Chen XW, Zhang WJ, Wang JZ, Ouyang MZ, Zhong Q, Liu CC. Twist1 is a potential prognostic marker for colorectal cancer and associated with chemoresistance. Am J Cancer Res. 2015;5:2000–2011. [PMC free article] [PubMed] [Google Scholar]
- 79.Romani AA, Borghetti AF, Del Rio P, Sianesi M, Soliani P. The risk of developing metastatic disease in colorectal cancer is related to CD105-positive vessel count. J Surg Oncol. 2006;93:446–455. doi: 10.1002/jso.20456. [DOI] [PubMed] [Google Scholar]
- 80.Dieterich LC, Tacconi C, Ducoli L, Detmar M. Lymphatic vessels in cancer. Physiol Rev. 2022;102:1837–1879. doi: 10.1152/physrev.00039.2021. [DOI] [PubMed] [Google Scholar]
- 81.Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, Sahin A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 82.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 83.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, Gordon R, Nagi CS, Wang Y, Entenberg D, Condeelis J, Skobe M. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korbecki J, Grochans S, Gutowska I, Barczak K, Baranowska-Bosiacka I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21207619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, Mantovani A, Allavena P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–8427. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 86.Zeng H, Wei W, Xu X. Chemokine (C-X-C motif) receptor 4 RNA interference inhibits bone metastasis in breast cancer. Oncol Lett. 2014;8:77–81. doi: 10.3892/ol.2014.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishikawa T, Nakashiro K, Klosek SK, Goda H, Hara S, Uchida D, Hamakawa H. Hypoxia enhances CXCR4 expression by activating HIF-1 in oral squamous cell carcinoma. Oncol Rep. 2009;21:707–712. [PubMed] [Google Scholar]
- 88.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 89.Andre F, Xia W, Conforti R, Wei Y, Boulet T, Tomasic G, Spielmann M, Zoubir M, Berrada N, Arriagada R, Hortobagyi GN, Hung MC, Pusztai L, Delaloge S, Michiels S, Cristofanilli M. CXCR4 expression in early breast cancer and risk of distant recurrence. Oncologist. 2009;14:1182–1188. doi: 10.1634/theoncologist.2009-0161. [DOI] [PubMed] [Google Scholar]
- 90.Hung CS, Su HY, Liang HH, Lai CW, Chang YC, Ho YS, Wu CH, Ho JD, Wei PL, Chang YJ. High-level expression of CXCR4 in breast cancer is associated with early distant and bone metastases. Tumour Biol. 2014;35:1581–1588. doi: 10.1007/s13277-013-1218-9. [DOI] [PubMed] [Google Scholar]
- 91.Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, Vannier J, Soria J, Vasse M, Soria C. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99:587–594. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S, Wang H, Xu Z, Bai Y, Xu L. Lymphatic Metastasis of NSCLC Involves Chemotaxis Effects of Lymphatic Endothelial Cells through the CCR7-CCL21 Axis Modulated by TNF-α. Genes (Basel) 2020;11 doi: 10.3390/genes11111309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 94.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017;16:635–661. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 95.Chen Q, Zheng WW, Zou W, Yang CM, Zhang S, Wu YY, Lu Y, Wang AY. Research progress on the role of Ang/Tie axis in angiogenesis and metastasis. Acta Pharm Sin. 2020;55:2291–2297. [Google Scholar]
- 96.Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr) 2016;39:397–410. doi: 10.1007/s13402-016-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Langheinrich MC, Schellerer V, Perrakis A, Lohmüller C, Schildberg C, Naschberger E, Stürzl M, Hohenberger W, Croner RS. Molecular mechanisms of lymphatic metastasis in solid tumors of the gastrointestinal tract. Int J Clin Exp Pathol. 2012;5:614–623. [PMC free article] [PubMed] [Google Scholar]
- 98.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 99.Sobierajska K, Ciszewski WM, Sacewicz-Hofman I, Niewiarowska J. Endothelial Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1234:71–86. doi: 10.1007/978-3-030-37184-5_6. [DOI] [PubMed] [Google Scholar]
- 100.Van den Eynden GG, Van der Auwera I, Van Laere SJ, Trinh XB, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Comparison of molecular determinants of angiogenesis and lymphangiogenesis in lymph node metastases and in primary tumours of patients with breast cancer. J Pathol. 2007;213:56–64. doi: 10.1002/path.2211. [DOI] [PubMed] [Google Scholar]
- 101.Xu Y, Huang Y, Xu W, Zheng X, Yi X, Huang L, Wang Y, Wu K. Activated Hepatic Stellate Cells (HSCs) Exert Immunosuppressive Effects in Hepatocellular Carcinoma by Producing Complement C3. Onco Targets Ther. 2020;13:1497–1505. doi: 10.2147/OTT.S234920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, Pepper MS, Zawieja DC, Ran S. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. 2010;115:418–429. doi: 10.1182/blood-2008-12-196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adachi Y, Matsuki M, Watanabe H, Takase K, Kodama K, Matsui J, Funahashi Y, Nomoto K. Antitumor and Antiangiogenic Activities of Lenvatinib in Mouse Xenograft Models of Vascular Endothelial Growth Factor-Induced Hypervascular Human Hepatocellular Carcinoma. Cancer Invest. 2019;37:185–198. doi: 10.1080/07357907.2019.1601209. [DOI] [PubMed] [Google Scholar]
- 104.Kitagawa K, Nakajima G, Kuramochi H, Ariizumi SI, Yamamoto M. Lymphatic vessel endothelial hyaluronan receptor-1 is a novel prognostic indicator for human hepatocellular carcinoma. Mol Clin Oncol. 2013;1:1039–1048. doi: 10.3892/mco.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thelen A, Scholz A, Benckert C, von Marschall Z, Schröder M, Wiedenmann B, Neuhaus P, Rosewicz S, Jonas S. VEGF-D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int J Cancer. 2008;122:2471–2481. doi: 10.1002/ijc.23439. [DOI] [PubMed] [Google Scholar]
- 106.Taskaeva I, Bgatova N. Microvasculature in hepatocellular carcinoma: An ultrastructural study. Microvasc Res. 2021;133:104094. doi: 10.1016/j.mvr.2020.104094. [DOI] [PubMed] [Google Scholar]
- 107.Kmeid M, Park YN, Chung T, Lukose G, Sullivan L, Brar R, Lee H. PSMA Immunohistochemistry in Hepatic Neoplasms: A Promising Diagnostic Marker With Potential Theranostic Applications. Am J Surg Pathol. 2022;46:1688–1699. doi: 10.1097/PAS.0000000000001971. [DOI] [PubMed] [Google Scholar]
- 108.Benetti A, Berenzi A, Gambarotti M, Garrafa E, Gelati M, Dessy E, Portolani N, Piardi T, Giulini SM, Caruso A, Invernici G, Parati EA, Nicosia R, Alessandri G. Transforming growth factor-beta1 and CD105 promote the migration of hepatocellular carcinoma-derived endothelium. Cancer Res. 2008;68:8626–8634. doi: 10.1158/0008-5472.CAN-08-1218. [DOI] [PubMed] [Google Scholar]
- 109.Li Y, Zhai Z, Liu D, Zhong X, Meng X, Yang Q, Liu J, Li H. CD105 promotes hepatocarcinoma cell invasion and metastasis through VEGF. Tumour Biol. 2015;36:737–745. doi: 10.1007/s13277-014-2686-2. [DOI] [PubMed] [Google Scholar]
- 110.Balaji Ragunathrao VA, Anwar M, Akhter MZ, Chavez A, Mao Y, Natarajan V, Lakshmikanthan S, Chrzanowska-Wodnicka M, Dudek AZ, Claesson-Welsh L, Kitajewski JK, Wary KK, Malik AB, Mehta D. Sphingosine-1-Phosphate Receptor 1 Activity Promotes Tumor Growth by Amplifying VEGF-VEGFR2 Angiogenic Signaling. Cell Rep. 2019;29:3472–3487.e4. doi: 10.1016/j.celrep.2019.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu S, Ni C, Zhang D, Sun H, Dong X, Che N, Liang X, Chen C, Liu F, Bai J, Lin X, Zhao X, Sun B. S1PR1 regulates the switch of two angiogenic modes by VE-cadherin phosphorylation in breast cancer. Cell Death Dis. 2019;10:200. doi: 10.1038/s41419-019-1411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]