Abstract
Background:
Adolescent solid organ transplant recipients (aSOTRs) who received three doses of the COVID-19 mRNA vaccine experience high seroconversion rates and antibody persistence for up to 3 months. Long-term antibody durability beyond this timeframe following three doses of the SARS-CoV-2 mRNA vaccine remains unknown. We describe antibody responses 6 months following the third vaccine dose (D3) of the BNT162b2 mRNA vaccination among aSOTRs.
Methods:
Participants in a multi-center, observational cohort who received the third dose of the vaccine were analyzed for antibodies to the SARS-CoV-2 spike protein receptor-binding domain (Roche Elecsys anti-SARS-CoV-2-S positive: ≥0.8, maximum: >2500U/mL). Samples were collected at 1-, 3-, and 6-months post-D3. Participants were surveyed at each timepoint and at 12-months post-D3.
Results:
All 34 participants had positive anti-RBD antibody titers 6 months post-D3. Variations in titers occurred between 3 and 6 months post-D3, with 8/28 (29%) having decreased antibody levels at 6 months compared to 3 months and 2/28 (7%) reporting increased titers at 6 months. The remaining 18/28 (64%) had unchanged antibody titers compared to 3-month post-D3 levels. A total of 4/34 (12%) reported breakthrough infection within 6 months and 3/32 (9%) reported infection after 6–12 months following the third dose of the SARS-CoV-2 mRNA vaccine.
Conclusions:
The results suggest that antibody durability persists up to 6 months following three doses of the SARS-CoV-2 mRNA in aSOTRs. Demography and transplant characteristics did not differ for those who experienced antibody weaning. Breakthrough infections did occur, reflecting immune-evasive nature of novel variants such as Omicron.
Keywords: adolescent solid organ transplant recipients, antibody, COVID-19, durability, mRNA vaccination, pediatric, SARS-CoV-2
1 |. INTRODUCTION
Immunocompetent adolescents maintain a distinctive immune profile in terms of their response to both SARS-CoV-2 vaccination and infection that differs from adults and younger children.1,2 Adolescents, particularly males, maintain a heightened risk of COVID-19 vaccine-induced myocarditis, a complication seldom seen in other age groups.3 These distinctive immunologic features underscore the importance of fully exploring the safety and immunogenicity of the SARS-CoV-2 vaccine among understudied adolescent populations, particularly the immunocompromised.
Adolescent solid organ transplant recipients (aSOTRs) who receive three doses of the SARS-CoV-2 mRNA vaccine experience high anti-spike antibody seroconversion rates and persistence for up to 3 months.4 Long-term durability remains unknown, though booster vaccination is recommended if ≥2 months have elapsed since completion of a primary series.5 We describe antibody responses 6 months after the third vaccine dose (D3) of the BNT162b2 mRNA vaccine among aSOTRs and the association of antibody titers with breakthrough infections.
2 |. METHODS
aSOTRs (12–18 years) in a multicenter, observational study who received three doses of SARS-CoV-2 mRNA vaccines submitted blood samples at 1, 3, 6 months following vaccination. Plasma was tested for anti-receptor binding domain IgG using the Roche Elecsys anti-SARS-CoV-2-S assay (positive: ≥0.8, maximum: >2500 U/mL, a putative threshold consistent with Omicron BA.1 neutralizing antibody).6 Select participants underwent additional titer dilution to a maximum of >25 000 U/mL due to a change in lab protocol.7,8 Characteristics between participants who did versus did not show detectable antibody waning below threshold (<2500 U/mL) at 6 months were compared using Fisher’s exact, Wilcoxon signed-rank, and McNemar’s tests as appropriate. Breakthrough infection was defined as participant-reported positive antigen or molecular test at any time following receipt of D3, ascertained through serial post vaccine surveys as well as via unsolicited virtual reporting. All analyses were performed using Stata 15.1 (StataCorp). This study was approved by the Johns Hopkins Medicine Institutional Review Board (IRB00248540).
3 |. RESULTS
Of the 70 active aSOTRs recruited to the original cohort, samples were available for 34 participants 6 months post-D3. Median (IQR) age was 16 (13.8–17); 47% were male and 66% were white. Patients were median 12 (IQR 8–14) years from transplant with heart transplant being most common (47%), followed by liver (34%), and kidney (15%). All 34 of these participants had available 1-month titers while 28 of the 34 had available 3-month titers.
All participants had positive antibody titers 6 months post-D3 (Figure 1) with a median level of >2500 U/mL (IQR: 1927, >2500). A total of 8/28 (29%) of aSOTRs showed detectable waning between 3 and 6 months, none of whom reported infection. Notably, two participants underwent additional sample dilution to maximum of >25 000 U/mL, with one participant remaining above threshold at 3 and 6 months and the other waning to 24 096 U/mL by 6 months. Demography and transplant characteristics did not statistically differ between those who did versus did not show titer waning to <2500 U/mL by 6 months (Table 1), though there was numerically more frequent use of 2-drug immunosuppression regimens in those with waning. Breakthrough infection was reported by 4/34 (13%) participants between 3-and 6-months post D3. This included three participants with antibody titers >2500 U/mL preceding infection, and one participant who weas seronegative at 3 months (preceding infection). Of the 34 participants with anti-RBD titers 6 months post-D3, 32/34 (94%) responded to 12-month survey regarding breakthrough infection, for which 3/32 (9%) reported infection between 6 and 12 months post-D3. Two of these participants who developed breakthrough infection had antibody titers >2500 U/mL at 1-, 3-, and 6-months post D3, while one participant had antibody titers 3.2, 22.4, and 29 U/mL, respectively. Mild upper respiratory symptoms were reported by 2/34 (6%) participants, while 2/34 (6%) reported constitutional symptoms of fever, sore throat, headache, and/or fatigue (one of whom was the participant seronegative at 3 months). 2/34 (6%) participants received monoclonal antibody treatment while no participants reported hospitalization during the study period.
FIGURE 1.
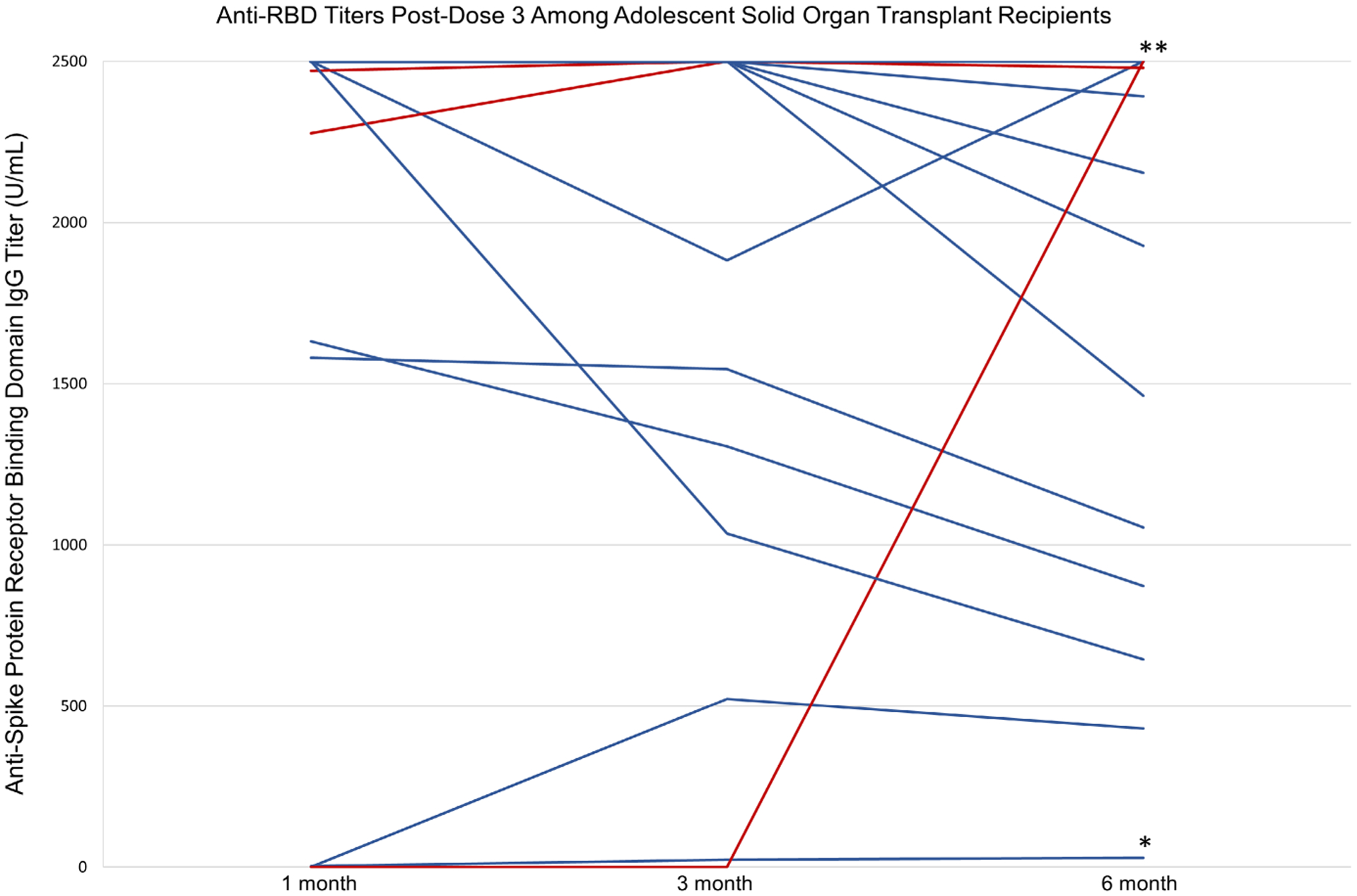
One-month post-dose 3, 3 months post-dose 3-, and 6-months post-dose 3 antibody titers among adolescent SOTRs. All participants had positive antibody titers 6 months post-D3 (n = 34). Those in red had self-reported infection between 3 and 6 months (n = 4), while those in blue with asterisks had self-reported infection between 6 and 12 months (n = 3). Of those with self-reported infection between 6 and 12 months, two participants (denoted with **) had anti-RBD titers >2500 U/mL, while the third (denoted with *) had persistently positive low-level antibody titers throughout the study period of 3.2, 22.4, and 29 U/mL, respectively (lowest blue line).
TABLE 1.
Demographic and transplant characteristics among adolescent solid organ transplant recipients with antibody waning versus without antibody waning between 3-and 6-months post third mRNA vaccine doses.
| n (%)a | Waned (n = 8) | Did not waneb (n = 20) | Total (n = 28) | p value |
|---|---|---|---|---|
| Age group, years | ||||
| 12–14 | 3 (38) | 8 (40) | 11 (39) | .42 |
| 15–16 | 2 (25) | 9 (45) | 11 (39) | |
| 17+ | 3 (38) | 3 (15) | 6 (21) | |
| Sex, male | 1 (13) | 10 (50) | 11 (39) | |
| Race, White | 5 (63) | 12c (60) | 17c (61) | .19 |
| Hispanic or Latino, yes | 0 (0) | 1d (5) | 1d (4) | >.99 |
| Transplant characteristics | >.99 | |||
| Organ | ||||
| Liver | 2 (22) | 9 (45) | 11 (39) | .75 |
| Kidney | 2 (22) | 3 (15) | 5 (18) | |
| Heart | 4 (50) | 8 (40) | 12 (43) | |
| Time since transplant, years | ||||
| <6 | 2 (22) | 1 (5) | 3 (11) | .09 |
| 6–12 | 2 (22) | 12 (60) | 14 (50) | |
| ≥13 | 4 (50) | 7 (35) | 11 (39) | |
| Reported prior infection | 1 (13) | 1 (5) | 2 (7) | |
| Number of immunosuppressive agents | .51 | |||
| 1 | 4 (50) | 15 (75) | 18 (68) | |
| 2 | 4 (50) | 5 (25) | 9 (32) | .38 |
| Immunosuppressive agents used | ||||
| Tacrolimus | 1 (13) | 11 (55) | 12 (43) | |
| Antimetabolite | 4 (50) | 6 (30) | 10 (36) | .13 |
| Sirolimus | 2 (22) | 3 (15) | 5 (18) | |
| Corticosteroids | 3 (36) | 1 (5) | 4 (14) | |
| Cyclosporine | 1 (13) | 3 (15) | 4 (14) | |
Participants who reported a SARS-CoV-2 infection during follow-up period were excluded.
Those who remained >2500 between 3 and 6 months were considered as not waned.
Race missing (n = 2).
Ethnicity missing (n = 1).
4 |. DISCUSSION
In this observational cohort of aSOTRs, antibody titers were positive in all participants at 6 months following D3, including 21/34 (62%) who had titer above 2500 U/mL suggestive of sustained, robust anti-RBD response. Clear waning below threshold was observed in 8/28 (29%) of participants between 3-and 6-months post D3, yet this was not statistically associated with particular transplant demography. Late breakthrough infections were relatively uncommon and not evidently associated with preceding antibody titer, though one seronegative participant at 3 months was among those experiencing breakthrough. Breakthrough symptoms were mild to moderate, with half receiving monoclonal antibody, and there were no reported hospitalizations.
Taken together, these results suggest long-term humoral immunogenicity of three-dose mRNA vaccine regimens in many aSOTRs, while outlining previously unreported late kinetics of vaccine response. It is notable that infections in the Omicron era did occur despite detection of higher-level antibody, reflecting the immune-evasive nature of novel variants of concern which can raise breakthrough risk amid expected antibody waning.9 Regardless, it is reassuring that COVID-19 outcomes were mild, which reflects vaccine immunoprotection as well as advances in therapeutic management.
Limitations of the study include reliance on both self-reported infection occurrence and outcomes, the antibody titer ceiling which limited detection of waning, and lack of neutralizing antibody measurement. Given the study design, asymptomatic SARS-CoV2 infection could not be captured and may have led to boosting of antibody responses between completion of dose 3 and the 6-month serology time point. Further studies evaluating long-term breakthrough rates and neutralizing responses to booster vaccination may help further inform correlates of protection and timing and role for booster vaccination in aSOTRs.
ACKNOWLEDGMENTS
This research was made possible with the generous support of the Ben-Dov family. This work was supported by grant number K24AI144954 (Segev) and K23AI157893 (Werbel) from the National Institute of Allergy and Infectious Diseases, and grant K08H2026510-01A1 from the Agency for Healthcare Research and Quality (Feldman). The authors acknowledge database management and data acquisition support from Benjamin L Salazar (Johns Hopkins University). JM is funded by a National Institutes of Health grant (T32-AI015071).
CONFLICT OF INTEREST STATEMENT
Lara Danziger-Isakov MD MPH has the following financial disclosures: Consulting member for Takeda and Merck. Contracted clinical research agreements paid to her institution by Ansun Bio-Pharma, Astellas, Merck, Pfizer, Takeda, and Viracor. Noelle Ebel MD has the following financial disclosures: consulting for Mirum. Evelyn Hsu MD has the following financial disclosures: contracted clinical research agreements paid to her institution by Gilead, Mirum, and Albireo. Emily R Perito MD has the following financial disclosures: contracted clinical research agreements paid to her institution by Gilead and Albireo. Dorry L. Segev MD PhD has the following financial disclosures: consulting and/or speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. William Werbel MD PhD has the following financial disclosures: consulting and/or speaking honoraria from AstraZeneca, GlobalData, the CDC/IDSA COVID-19 Real Time Learning Network, and advisory board fees from Novavax. Douglas Mogul MD MPH PhD has the following financial disclosures: consulting for Mirum. The remaining authors of this manuscript have no financial disclosures or conflicts of interest to disclose.
Abbreviations:
- aSOTRs
adolescent solid organ transplant recipients
- mRNA
messenger ribonucleic acid
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Lai YD, Chen YY, Sun JP, et al. Immune profiles of a COVID-19 adolescent with mild symptoms and anti-viral antibody deficiency. Fundam Res. 2021;1(2):117–123. doi: 10.1016/j.fmre.2021.02.004 [DOI] [Google Scholar]
- 2.Rotulo GA, Palma P. Understanding COVID-19 in children: immune determinants and post-infection conditions. Pediatr Res. 2023;94(2):434–442. doi: 10.1038/s41390-023-02549-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin CX, Auerbach SR, Charnaya O, et al. Antibody response to three SARS-CoV-2 mRNA vaccines in adolescent solid organ transplant recipients. Am J Transplant. 2022;22(10):2481–2483. doi: 10.1111/ajt.17085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Interim COVID-19 immunization schedule for persons 6 months of age and older. Accessed July 12, 2023. https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
- 6.Werbel WA, Segev DL. SARS-CoV-2 antibody testing for transplant recipients: a tool to personalize protection versus COVID-19. Am J Transplant. 2022;22(5):1316–1320. doi: 10.1111/ajt.16993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59(2):e02257–20. doi: 10.1128/JCM.02257-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roche Diagnostics. Elecsys® Anti-SARS-CoV-2 S. Accessed January 1, 2023. https://diagnostics.roche.com/us/en/products/params/elecsys-anti-sars-cov-2-s.html#productSpecs
- 9.Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608:603–608. doi: 10.1038/s41586-022-05053-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


