Abstract
Objective:
To determine the effect of elective single ET (eSET) on live birth and multiple birth rates by a cycle-level and clinic-level analysis.
Design:
Retrospective cohort study.
Setting:
Not applicable.
Patient(s):
Patient ages <35 and 35–37 years old.
Intervention(s):
None.
Main Outcome Measure(s):
Clinics were divided into groups based on eSET rate for each age group and aggregate rates of live birth per ET and multiple birth per delivery were calculated. A cycle-level analysis comparing eSET and double ET (DET) live birth and multiple birth rates was also performed, stratified based on total number (2, 3, or 4+) of embryos available, embryo stage, and patient age.
Result(s):
There was a linear decrease in multiple birth rate with increasing eSET rate and no significant difference in clinic-level live birth rates for each age group. Cycle-level analysis found slightly higher live birth rates with double ET, but this was mainly observed in women aged 35–37 years or with four or more embryos available for transfer, and confirmed the marked reduction in multiple births with eSET.
Conclusion(s):
Our study showed a marked and linear reduction in multiple birth rates, and important, little to no effect on clinic-level live birth rates with increasing rates of eSET supporting the growing evidence that eSET is effective in decreasing the high multiple birth rates associated with IVF and suggests that eSET should be used more frequently than is currently practiced.
Keywords: Elective single embryo transfer, live birth rate, multiple birth rate, IVF
In vitro fertilization is the single most effective treatment for infertility, and it is used with increasing frequency worldwide. In 2013, IVF treatments in the United States helped to conceive >60,000 babies, approximately 1.6% of all infants born, which is consistent with many developed countries around the worl (1). Unlike many other developed countries, however, pregnancies resulting from IVF treatments in the United States are complicated by a very high rate of multiple gestations (41.1% of all IVF deliveries in 2013) (1), directly attributable to the common practice of transferring multiple embryos to the uterus to enhance pregnancy rates (PRs). During the past decade, reductions in the average number of transferred embryos have resulted in a marked decrease in high order multiple gestations (triplets and more) in the United States, but twinning rates have remained high due to the continued practice of transferring at least two embryos in most IVF cycles (2).
Twin pregnancies are associated with a number of short-term and long-term adverse health consequences, primarily related to the sevenfold increase in the rate of premature delivery compared to singletons (3, 4). In addition, twin gestations are costly to the healthcare system, largely due to expenses related to hospitalization and medical care of the premature infants (5). Because of these concerns, there is growing interest in reducing the incidence of twins after IVF treatments. One solution is to perform elective single ET (eSET), a practice that markedly reduces twinning rates after IVF (6–8). Some countries have adopted eSET policies, generally through legislation that requires eSET if monetary coverage for IVF procedures is provided by the government health system (9). In other countries, physicians have voluntarily embraced eSET as the standard practice for IVF, but this is often in the context of national healthcare coverage of IVF treatments (10). Compared with physicians in these countries, physicians in the United States have been slow to adopt eSET for a number of reasons; chief among them is the concern that PRs will decrease (9). With eSET, embryos not transferred to the uterus can be cryopreserved and transferred in another cycle with similar rates of pregnancy (11). However, the time and additional expenses incurred by the patient for additional cycles place a premium on high PRs in the initial cycle.
Although there is great variation in the rate of eSET among individual clinics in the United States, eSET is performed rarely nationwide, accounting for only 6% of all fresh transfers in 2010 (12). The most recent national data demonstrate somewhat higher rates of eSET, although still well below rates in many other countries (1). In the absence of a national mandate or policy, clinics voluntarily choose to emphasize eSET with their patients and do so to different degrees evidenced by differences in clinical opinion and the highly variable rates of eSET among United States IVF clinics (13). The purpose of our study was to assess eSET rates in IVF clinics throughout the United States, and to examine the relationship between eSET rates and clinic-level IVF outcomes, including live birth rates and multiple birth rates. Our hypothesis was that clinics performing higher rates of eSET would have reduced rates of multiple births, yet maintaining high PRs, as that has been the experience at our own clinic (14). To characterize the effect of eSET on individual patient outcomes, we also performed a cycle-level analysis comparing live birth and multiple birth rates for cycles using eSET versus those using double ET (DET).
MATERIALS AND METHODS
Primary IVF clinic data was collected by the Centers for Disease Control and Prevention National Assisted Reproductive Technology (ART) Surveillance System, a federally mandated reporting system that collects information regarding ART cycles (primarily IVF) performed in the United States. We analyzed the most recently available Centers for Disease Control and Prevention national data for cycles initiated during 2013, with study approval from the Institutional Review Board of the Centers for Disease Control and Prevention.
All IVF clinics reporting to the National ART Surveillance System in 2013 were included regardless of clinic size. This included 94% of all IVF clinics in the United States. The primary variable studied was the rate of eSET performed at a given clinic for all fresh autologous cycles in patient ages <35 and 35–37 years old performed in 2013. For this report we chose to focus on fresh ETs and, to avoid introducing biases from different treatments, excluded cryopreserved or “frozen” ETs and cycles using preimplantation genetic screening (PGS) or preimplantation genetic diagnosis. The primary outcomes of interest were the live birth rate per ET and the multiple birth rate per delivery. The eSET was defined as a cycle in which one embryo was transferred and at least one additional embryo was cryopreserved. This distinguishes fresh cycles in which SET was truly elective from fresh cycles in which only one embryo was available for transfer. The eSET rate was calculated as the total number of cycles that qualified as an eSET divided by the total number of ET cycles in a clinic for patients in each of the specified age groups. Live birth rate was defined as percentage of live births of at least one child (>20 weeks gestational age) divided by the total number of embryo transfer cycles in a clinic. Multiple birth rate was defined as the percentage of multiple births (twins and high order multiples) per live birth conceived by IVF in a clinic.
Clinic eSET rates were classified into the following categories for patient ages <35 years: <10%, 10%–19%, 20%–29%, 30%–39%, 40%–49%, and ≥50%. For patients in the 35- to 37-year age group, clinics were classified into the following categories: <10%, 10%–19%, 20%–29% and ≥30%. Clinics were combined into a ≥30% group for the 35- to 37-year-old age group due to the small number of clinics performing high rates of eSET in this age group. Similarly, we could not study eSET in patients aged >37 years due to the relatively small number of clinics performing high rates of eSET in this age category. We compared the average number of cycles performed at the clinics, age of patients, number of prior ART cycles, parity, racial/ethnic distribution, eSET rate, number of embryos transferred, embryo stage at transfer, proportion of intracytoplasmic sperm injection (ICSI) cycles, and implantation rate across the clinic eSET categories using generalized linear models. Models were also constructed to estimate adjusted means for clinic-level live birth rates and multiple birth rates according to clinic eSET rates. Clinic-level confounding variables assessed included clinic size (number of cycles per year), average proportion of cycles where ICSI was used, frequency of blastocyst (days 5–6) and cleavage stage (days 2–3) ET, as well as average age of patient treated, number of prior ART cycles, parity, and the racial/ethnic distribution of a clinic’s patient population. Significant confounders (P<.05) were retained in the final models. The clinics were categorized by the eSET rate performed for the given age group, so they were not necessarily in the same eSET category for the <35 and 35- to 37-year-old age groups if they performed different rates of eSET for each group.
We then did a cycle-level analysis of all fresh, autologous ETs (excluding PGS/preimplantation genetic diagnosis cycles) to compare outcomes of eSET versus DET as previously defined using cycles performed in 2013 and reported to the National ART Surveillance System. We constructed study and comparison groups based on the total number of embryos available for transfer, defined as the number of embryos transferred plus the number of embryos cryopreserved for a patient (2, 3, or ≥4 embryos). This resulted in the following groups: eSET with one embryo frozen compared to DET with zero embryos frozen, eSET with two embryos frozen compared to DET with one embryo frozen, and eSET with three or more embryos frozen compared to DET with two or more embryos frozen. The rates of live birth and multiple live birth were stratified by age (<35 years, 35–37 years). Robust Poisson regression models with generalized estimating equations to account for clustering by clinic were used to estimate unadjusted and adjusted risk ratios for the association between eSET and the outcomes, stratified by day of transfer (days 2–3 vs days 5–6). The adjusted models included infertility diagnosis, parity, number of prior ART cycles, number of oocytes retrieved, use of assisted hatching, use of ICSI for fertilization, and the interaction of eSET and embryo stage.
All analyses were conducted using SAS version 9.2. Two-tailed P values <.05 were considered statistically significant.
RESULTS
In 2013, for patient aged <35 and 35–37 years, most clinics performed 0–9% of eSET (Table 1). When comparing clinics among eSET categories, some significant trends were noted. Higher eSET clinics performed a higher rate of blastocyst ETs (days 5–6 of culture) and a lower rate of cleavage-stage ETs (days 2–3 of culture) and had a higher average embryo implantation rate. There was no significant difference in average patient age, number of prior IVF cycles, or race/ethnicity; however, there was a large percentage of missing race data in the dataset.
TABLE 1.
United States fertility clinics categorized by elective single ET (eSET) rate in 2013 for fresh IVF cycles for patients aged < 35 y (A) and 35–37 y (B).
| Clinic eSET rate | P valuee comparing means between eSET categories | |||||||
|---|---|---|---|---|---|---|---|---|
| A. Patients aged < 35 y | All clinics (n = 464) | 0–9% (n = 219) | 10%–19% (n = 96) | 20%–29% (n = 55) | 30%–39% (n = 41) | 40%–49% (n = 17) | ≥50% (n = 36) | |
| No. of fresh cycles performed per clinica | 86 ± 119 | 60 ± 60 | 107 ±123 | 76 ± 73 | 126 ± 131 | 277 ± 375 | 71 ± 81 | < .0001 |
| Age of patients treated (y)a | 30.8 ± 0.7 | 30.7 ± 0.8 | 30.8 ± 0.6 | 31.0 ± 0.7 | 30.9 ± 0.5 | 31.1 ± 0.5 | 31.0 ± 0.8 | .05 |
| No. of prior ART cyclesa | 0.5 ± 0.4 | 0.5 ± 0.3 | 0.6 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.4 | 0.6 ± 0.3 | 0.6 ± 0.7 | .76 |
| Paritya | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | .002 |
| Racial distribution of clinic patientsa | ||||||||
| % non-Hispanic white | 53.0 ± 33.2 | 57.0 ± 32.6 | 51.0 ± 33.6 | 47.2 ± 33.9 | 50.0 ± 34.4 | 45.3 ± 30.3 | 49.3 ± 34.0 | .23 |
| % non-Hispanic black | 4.0 ± 6.2 | 4.3 ± 6.7 | 4.0 ± 5.7 | 4.3 ± 7.7 | 3.4 ± 4.1 | 4.7 ± 5.2 | 2.4 ± 4.0 | .61 |
| % Asian | 8.8 ± 13.4 | 7.4 ± 11.9 | 9.8 ± 14.9 | 7.3 ± 10.0 | 9.7 ± 15.5 | 10.0 ± 10.9 | 14.5 ± 18.8 | .07 |
| % Hispanic | 7.8 ± 15.1 | 10.2 ± 18.8 | 5.7 ± 9.8 | 5.4 ± 8.4 | 6.5 ± 11.1 | 3.9 ± 3.9 | 6.0 ± 14.5 | .05 |
| % other | 0.1 ± 0.8 | 0.1 ± 0.6 | 0.1 ± 0.4 | 0.1 ± 0.6 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.4 ± 2.1 | .34 |
| % unknown | 26.3 ± 37.9 | 20.9 ± 34.9 | 29.4 ± 39.5 | 35.7 ± 40.0 | 30.4 ± 41.4 | 35.9 ± 41.5 | 27.3 ± 39.4 | .07 |
| % eSETa,b | 17.0 ± 19.7 | 2.7 ± 3.3 | 14.0 ± 2.9 | 24.0 ± 2.9 | 34.6 ± 3.1 | 44.6 ± 2.8 | 67.8 ± 16.9 | < .0001 |
| No. of embryos transferreda,b | 1.9 ± 0.3 | 2.1 ± 0.2 | 1.9 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 | 1.3 ± 0.2 | < .0001 |
| % day 3 embryo transfersa,b | 34.4 ± 29.5 | 40.9 ± 32.7 | 32.6 ± 27.3 | 29.7 ± 25.7 | 28.6 ± 21.2 | 27.8 ± 21.5 | 16.0 ± 18.7 | < .0001 |
| % day 5 blastocyst transfersa,b | 61.9 ± 29.9 | 53.7 ± 32.6 | 64.9 ± 27.7 | 68.4 ± 25.3 | 68.1 ± 21.4 | 71.1 ± 21.8 | 83.0 ± 18.9 | < .0001 |
| % ICSI cyclesa,c | 79.2 ± 21.4 | 81.9 ± 20.9 | 80.2 ± 19.8 | 76.4 ± 20.8 | 73.0 ± 23.1 | 69.1 ± 20.8 | 77.2 ± 25.0 | .03 |
| Implantation ratea,d | 38.5 ± 14.4 | 35.2 ± 14.7 | 40.0 ± 11.4 | 41.2 ± 10.9 | 43.4 ± 13.4 | 39.4 ± 9.5 | 44.4 ± 21.5 | < .0001 |
| B. Patients aged 35–37 y | All clinics (n = 450) | 0–9% (n = 331) | 10%–19% (n = 61) | 20%–29% (n = 29) | ≥30% (n = 29) | |||
| No. of fresh cycles performed per clinica | 44 ± 68.3 | 34.8 ± 47.9 | 64.0 ± 72.1 | 73.7 ± 89.9 | 77.4 ± 156.9 | < .0001 | ||
| Age of patients treated (y)a | 35.9 ± 0.3 | 35.9 ± 0.3 | 36.0 ± 0.1 | 36.0 ± 0.2 | 35.9 ± 0.3 | .96 | ||
| No. of prior ART cyclesa | 0.8 ± 0.5 | 0.8 ± 0.5 | 0.9 ± 0.4 | 0.9 ± 0.5 | 0.8 ± 0.4 | .57 | ||
| Paritya | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.5 ± 0.2 | 0.3 ± 0.2 | .02 | ||
| Racial distribution of clinic patientsa | ||||||||
| % non-Hispanic white | 49.4 ± 33.5 | 51.1 ± 34.0 | 41.8 ± 31.9 | 48.6 ± 33.3 | 47.5 ± 30.7 | .25 | ||
| % non-Hispanic black | 4.8 ± 7.5 | 5.2 ± 8.1 | 4.7 ± 5.4 | 3.3 ± 5.8 | 2.7 ± 3.6 | .23 | ||
| % Asian | 10.6 ± 16.8 | 9.4 ± 16.1 | 11.9 ± 13.9 | 12.0 ± 18.4 | 20.0 ± 24.6 | .01 | ||
| % Hispanic | 8.4 ± 16.9 | 9.0 ± 18.5 | 8.6 ± 13.5 | 5.1 ± 8.6 | 4.3 ± 5.7 | .35 | ||
| % other | 0.2 ± 1.7 | 0.3 ± 2.0 | 0.1 ± 0.5 | 0.2 ± 0.6 | 0.1 ± 0.7 | .91 | ||
| % unknown | 26.4 ± 38.1 | 25.0 ± 37.5 | 33.0 ± 41.5 | 30.8 ± 39.6 | 25.3 ± 36.6 | .44 | ||
| % eSETa,b | 7.8 ± 13.0 | 2.0 ± 3.0 | 14.1 ± 2.9 | 23.2 ± 2.7 | 45.4 ± 19.6 | < .0001 | ||
| No. of embryos transferreda,b | 2.1 ± 0.4 | 2.2 ± 0.4 | 1.9 ± 0.1 | 1.8 ± 0.2 | 1.5 ± 0.2 | < .0001 | ||
| % day 3 embryo transfersa,b | 44.7 ± 31.3 | 47.6 ± 32.0 | 41.8 ± 29.3 | 38.6 ± 42.3 | 23.9 ± 22.3 | .0005 | ||
| % day 5 blastocyst transfersa,b | 52.4 ± 31.3 | 49.2 ± 31.9 | 56.3 ± 28.7 | 57.8 ± 26.1 | 75.4 ± 22.5 | < .0001 | ||
| % ICSI cyclesa,c | 79.7 ± 22.9 | 80.8 ± 23.6 | 75.4 ± 19.0 | 72.3 ± 22.3 | 82.3 ± 20.7 | .09 | ||
| Implantation rated | 28.0 ± 13.8 | 26.9 ± 13.8 | 27.5 ± 8.9 | 28.3 ± 10.1 | 41.3 ± 18.2 | < .0001 | ||
Note: ART = assisted reproductive technology; ICSI = intracytoplasmic sperm injection; PGD/PGS = preimplantation genetic diagnosis/preimplantation genetic screening.
Data presented as means ± SD.
Among fresh, autologous cycles where a transfer was attempted, PGD/PGS cycles were excluded.
ICSI, among fresh, autologous cycles that were not canceled before retrieval.
Calculated as the number of embryos implanted divided by the total number of embryos transferred, excluding PGD/PGS cycles; if number of fetal heartbeats and number of live and stillborn infants was missing, then implantation rate was considered missing.
P values adjusted for multiple comparisons using Holm-Bonferroni method.
When evaluating all fresh cycles for patient aged <35 years old, there was no significant difference in clinic-level live birth rates between eSET categories (P=.10; Fig. 1). The only significant confounding variable adjusted for in this model was blastocyst transfer rate. The multiple birth rate per delivery decreased in a linear fashion with increasing eSET from 37.5% in the 0–9% group to 15.2% in the ≥50% group after adjusting for blastocyst transfer rate and parity (P<.001; Fig. 1).
FIGURE 1.
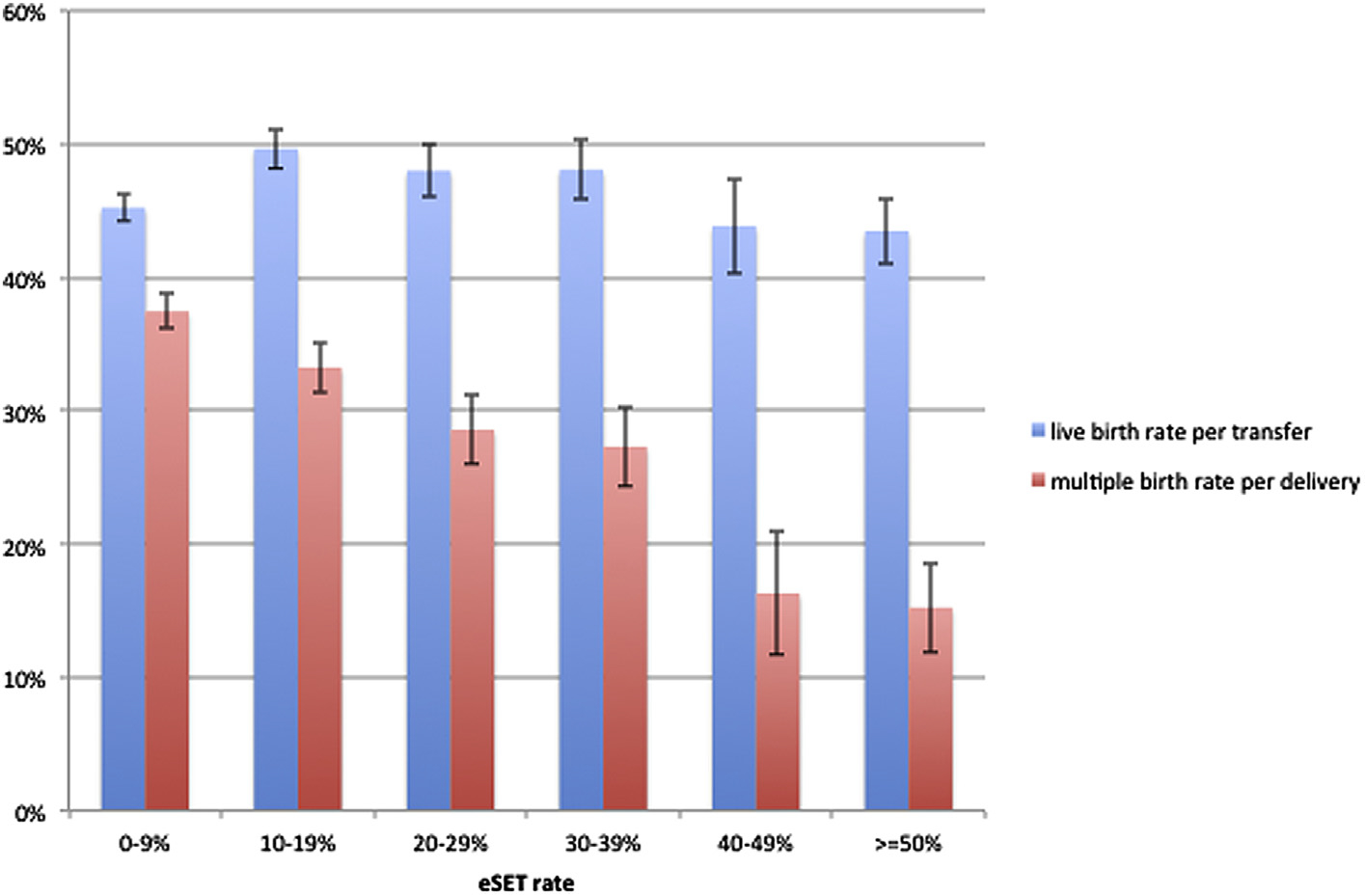
Live birth rate and multiple birth rate by clinic elective single ET (eSET) rate for fresh IVF cycles in patients <35 years old, US fertility clinics, 2013. Estimated means with standard error bars for live birth rates and multiple birth rates after adjusting for significant confounding variables. Live birth rates for patient aged <35 years adjusted for blastocyst transfer rate. Multiple birth rates for patient aged <35 years adjusted for blastocyst transfer rate and parity.
In fresh cycles for patient aged 35–37 years old, the eSET rate had no effect on clinic-level live birth rate after adjustment for blastocyst transfer rate, ICSI rate, and patient race (P=.14; Fig. 2) and the multiple birth rate per delivery decreased in a linear fashion with increasing eSET from 29.8% in the 0–9% group to 18.5% in the ≥30% group (P<.001; Fig. 2). No confounding variables were found for the multiple birth rate for age 35–37 years.
FIGURE 2.
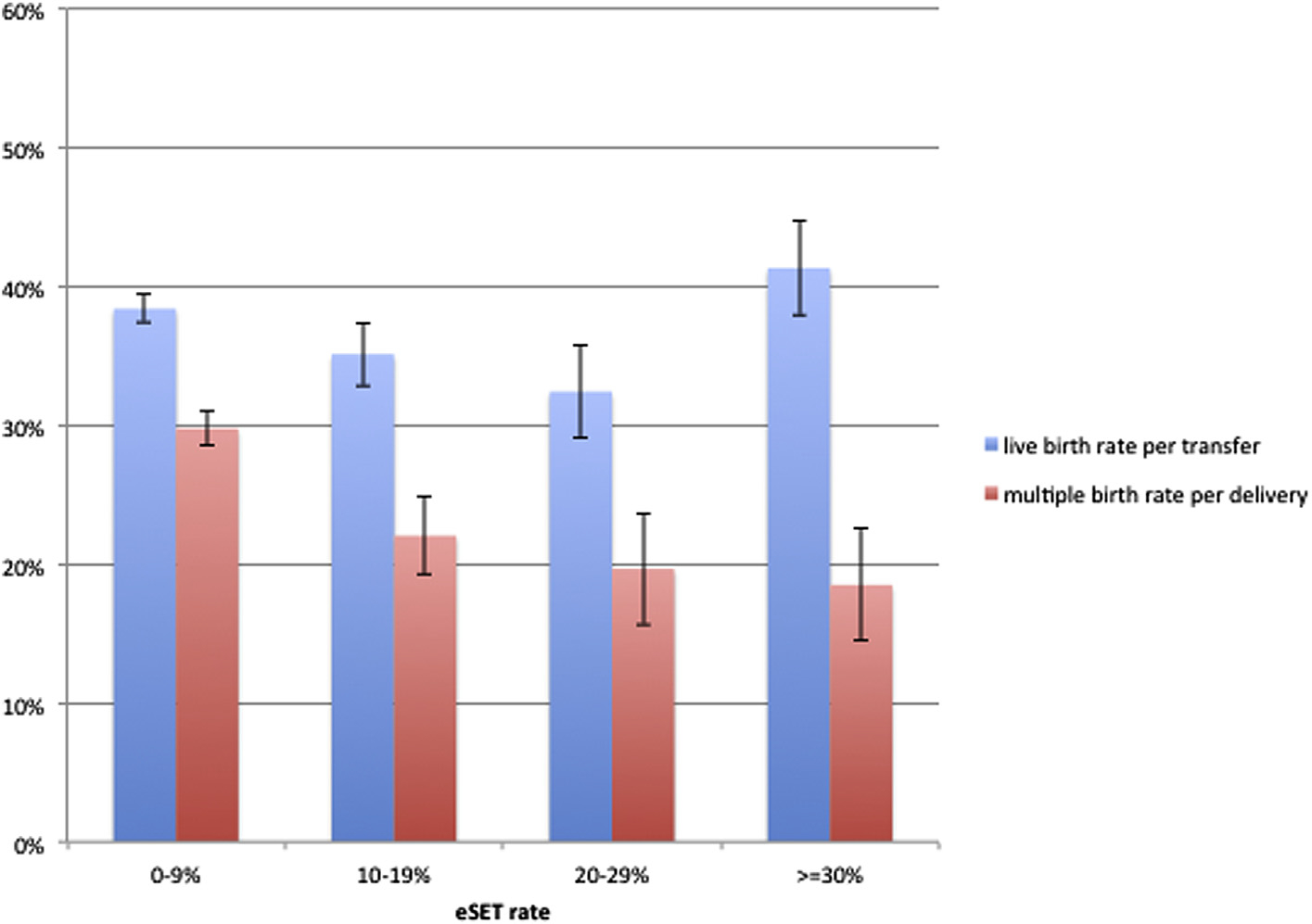
Live birth rate and multiple birth rate by clinic elective single ET (eSET) rate for fresh IVF cycles in patients 35–37 years old, US fertility clinics, 2013. Estimated means with standard error bars for live birth rates and multiple birth rates after adjusting for significant confounding variables. Live birth rates for patient aged 35–37 years, adjusted for blastocyst transfer rate, intracytoplasmic sperm injection (ICSI) rate, and patient race. No significant confounding variables were found for multiple birth rates for ages 35–37 years.
The cycle-level analysis included 6,167 eSET cycles and 20,968 DET cycles for patient aged <35 years, and 1,541 eSET cycles and 9,436 DET cycles for patient aged 35–37 years (Table 2). Because the live birth outcomes differed, we separated this analysis by cleavage-stage ETs and blastocyst transfers. Looking first at blastocyst transfers, the unadjusted absolute live birth rates for eSET and DET cycles are very similar. We found that the total number of embryos available for transfer (embryos transferred plus those cryopreserved) was a significant confounding factor with higher delivery rates in patients with more total embryos. We therefore performed our analysis stratified by the total number of embryos available. For women <35 years, there was no significant difference in live birth rates when two or three total embryos were available after adjusting for significant confounding variables. The DET was associated with a higher live birth rate in women aged <35 years when four or more total embryos were available. For women aged 35–37 years, DET was associated with a higher live birth rate when three or more total embryos were available.
TABLE 2.
Association between live birth rates and use of elective single ET (eSET) versus double ET (DET), stratified by embryo stage and by number of embryos cryopreserved.
| Days 2–3 transfers | eSET with 1 embryo frozen (n = 204) | DET with 0 embryos frozen (n = 7,142) | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient age (y) | Total transfers | No. of live births | Unadjusted live birth rate (%) | Total transfers | No. of live births | Unadjusted live birth rate (%) | RR (95% CI) | aRR (95% CI) |
| <35 | 142 | 50 | 35.2 | 4,621 | 2,946 | 36.3 | 0.97 (0.79–1.20) | 0.93 (0.77–1.13) |
| 35–37 | 62 | 11 | 17.7 | 2,521 | 751 | 29.8 | 0.60 (0.31–1.15) | 0.56 (0.29–1.10) |
| eSET with 2 embryos frozen (n = 125) | DET with 1 embryo frozen (n = 1,400) | |||||||
| <35 | 93 | 34 | 36.6 | 944 | 443 | 46.9 | 0.78 (0.55–1.10) | 0.76 (0.54–1.07) |
| 35–37 | 32 | 9 | 28.1 | 456 | 190 | 41.7 | 0.68 (0.36–1.28) | 0.67 (0.37–1.24) |
| eSET with 3 or more embryos frozen (n = 242) | DET with 2 or more embryos frozen (n = 3,100) | |||||||
| <35 | 181 | 65 | 35.9 | 2,258 | 1,106 | 49.0 | 0.73 (0.57–0.94) | 0.71 (0.56–0.90) |
| 35–37 | 61 | 14 | 23.0 | 842 | 363 | 43.1 | 0.53 (0.32–0.87) | 0.52 (0.32–0.86) |
| Days 5–6 transfers | eSET with 1 embryo frozen (n = 814) | DET with 0 embryos frozen (n = 6,278) | ||||||
| Patient age (y) | Total transfers | No. of live births | Unadjusted live birth rate (%) | Total transfers | No. of live births | Unadjusted live birth rate (%) | ||
| <35 | 619 | 285 | 46.0 | 4,241 | 1,987 | 46.9 | 0.98 (0.89–1.09) | 0.95 (0.86–1.05) |
| 35–37 | 195 | 71 | 36.4 | 2,037 | 818 | 40.2 | 0.91 (0.75–1.10) | 0.87 (0.72–1.06) |
| eSET with 2 embryos frozen (n = 1,135) | DET with 1 embryo frozen (n = 2,032) | |||||||
| <35 | 866 | 454 | 52.4 | 1,333 | 713 | 53.5 | 0.98 (0.90–1.06) | 0.95 (0.87–1.03) |
| 35–37 | 269 | 112 | 41.6 | 699 | 348 | 49.8 | 0.84 (0.70–1.00) | 0.82 (0.69–0.98) |
| eSET with 3 or more embryos frozen (n = 5,188) | DET with 2 or more embryos frozen (n = 10,452) | |||||||
| <35 | 4,266 | 2,273 | 53.3 | 7,571 | 4,487 | 59.3 | 0.90 (0.57–0.94) | 0.88 (0.85–0.92) |
| 35–37 | 922 | 433 | 47.0 | 2,881 | 1,554 | 53.9 | 0.87 (0.80–0.95) | 0.85 (0.78–0.93) |
Note: The adjusted models included infertility diagnosis, parity, number of prior ART cycles, number of oocytes retrieved, embryo stage, use of assisted hatching, use of intracytoplasmic sperm injection (ICSI) for fertilization, and the interaction of eSET and embryo stage. aRR = adjusted relative risk; ART = assisted reproductive technology; CI = confidence interval; RR = relative risk.
In the United States in 2013, eSET was performed in 4.7% of days 2–3 ETs and these cycles represented only 7.4% of all eSET cycles. Due to the relatively small numbers of eSET cycles among cleavage-stage ETs, estimates of delivery rates are not as robust as those for blastocyst transfers. Compared to blastocyst transfers, the unadjusted live birth rates are lower and there is a greater disparity in outcomes with eSET versus DET. Nevertheless, after adjusting for confounding variables, DET of cleavage stage embryos was associated with a statistically higher live birth rate only when four or more total embryos were available for transfer in <35 and 35–37 years age categories.
For multiple birth calculations, we combined all cleavage stage and blastocyst transfers as multiples birth rates did not differ by stage of ET. As expected, the multiple birth rates were lower for eSET compared with DET with a multiple birth rate of 1.7% (n = 54) for eSET and 39.4% (n = 4,152) for DET for patient aged <35 years and a multiple birth rate of 1.7% (n = 11) for eSET and 32.0% (n = 1,301) for DET for patient aged 35–37 years.
DISCUSSION
When eSET is used in the younger age groups of patients (<38 years) we found seemingly paradoxical results for live birth per transfer when evaluating this outcome on a cycle-level or a clinic-level basis. On one hand, at the cycle level, DET is associated with higher live birth rates than eSET, particularly in women aged 35–37 years and when more total embryos are available from the cycle. On the other hand, clinics are able to perform high rates of eSET with no apparent reduction in their clinic-level live birth rates. We believe this paradox may be explained by several findings in this study. At the cycle-level, the unadjusted live birth rates for eSET versus DET are very similar, particularly for blastocyst ETs. When adjusting for confounding variables, DET is associated with a higher delivery rate per transfer, but the difference is much less than would be expected from prior prospective randomized trials of SET versus DET, which have found an odds ratio of 0.5 for live birth per transfer after SET when considering only the fresh ET (7). One important difference between previous randomized trials and IVF performed at present is the increasing utilization of extended embryo culture and blastocyst transfer. Higher embryo implantation rates have been demonstrated for embryos cultured for 5–6 days to the blastocyst stage (15), which is likely because aneuploid embryos are more likely to arrest in development during extended culture, thus enriching the blastocyst-stage embryos with euploid embryos capable of producing a viable pregnancy (16, 17). We found that clinics performing a high rate of eSET had higher utilization of blastocyst ETs and higher embryo implantation rates, which likely accounts for their ability to maintain equal clinic-level live birth rates despite the slightly lower cycle-level live birth rates associated with eSET. Our findings suggest that many clinics can judiciously apply eSET with no negative effects on their clinic-level live birth rates. Our national database study supports previous individual clinic reports of increased rates of eSET with blastocyst embryos resulting in equal and competitive live birth rates relative to past experience and national averages (14, 18, 19).
In our cycle-level analysis, live birth rates with eSET and DET were influenced by the stage of embryos transferred and by the total number of embryos available in a cycle. A larger cohort of embryos progressing to advanced stages of development is a predictor of higher delivery rates as has been previously reported for human blastocyst culture (20). Although the adjusted live birth rates tended to favor DET, it was only in patients who received four or more embryos where DET was consistently and statistically associated with higher delivery rates than eSET for women less than age 35 years. However, DET is also associated with a 39.4% multiple birth rate (data not shown) in this good prognosis group of patients. Although we cannot determine the cumulative delivery rate in this analysis, we suggest it will be high for both groups of patients, particularly when accounting for the extra embryo cryopreserved in patients having eSET. This asks the question if the slightly higher PR with DET in the fresh cycle is worth the risk of a much higher multiple birth rate.
A very consistent finding at the cycle level and the clinic level was that as utilization of eSET increased, there was a marked linear reduction in multiple birth rates after IVF. Reducing the high multiple birth rate after IVF is a desirable outcome. Studies have shown that although some couples prefer multiple births and particularly twins as a means of faster family building, this preference can be diminished through education about the risks of multiple gestation (6, 21). Risks of twins and high order multiples include increased rates of preeclampsia, premature rupture of membranes, cesarean deliveries, and premature labor for the mother compared with singleton deliveries. For infants, these risks include prolonged hospital stays, increased neonatal intensive care unit (ICU) admissions, and higher rates of long-term morbidity and perinatal mortality compared with singletons (3–5). Twins have a sevenfold increase in delivery before 32 weeks, even when compared to two singleton deliveries after IVF (3).
Multiple gestations also pose a significant financial burden to healthcare delivery systems. A recent study (22) estimated the annual cost of iatrogenic multiple births at $6.3 billion in the United States. A comprehensive analysis (5) of all healthcare costs of the mother during her pregnancy and all infants from delivery up to 1 year of age concluded that twins were five times more expensive ($105,000) and triplets twenty times more expensive (~$400,000) than a singleton delivery ($21,500).
Our findings support increasing utilization of eSET as one solution to the medical and financial problem of multiple gestations after IVF. A Canadian study (23) estimated that implementation of an eSET policy in their country would prevent 30–40 perinatal deaths per year, 34–46 severe intracranial hemorrhages per year, and ≤42,000 neonatal ICU patient-days per year; numbers that would be multiplied by 14.6 if assumptions were extrapolated to United States IVF volumes. Recognizing these health improvements and savings, some countries with national healthcare services have required eSET for reimbursement of IVF cycles (9). In Australia, physicians have voluntarily embraced eSET as a standard practice with IVF, and eSET rates now exceed 70% of IVF cycles, resulting in multiple birth rates of <10% (10).
The fact that eSET lowers multiple birth rates is intuitive, but although well-known, the practice is still underused, due to concerns about possibly lowering clinic-level delivery rates per cycle, as reported nationally by the Centers for Disease Control and Prevention and Society of Assisted Reproductive Technology (SART) data, which is available to potential patients. However, our report demonstrates that clinics can perform high rates of eSET, yet maintaining competitive live birth rates per transfer. With a greater emphasis on the cumulative PR per patient in the 2014 national report (www.sart.org), this may be less of a concern.
We deliberately excluded cycles using PGS to select genetically normal embryos for transfer. Although this practice holds great promise, it may introduce increased cycle costs, a delay in ET while awaiting results, and the possibility for testing error. A recent randomized trial (24) found equivalent live birth rates between eSET and DET if PGS is used. Our findings suggest that PGS is not essential for clinics to successfully use eSET in a high percentage of IVF cycles.
Limitations of our study include the retrospective analysis of data, which is now several years old due to the constraints of national reporting of live birth outcomes. Second, data are collected from multiple clinics treating potentially different patient populations that cannot be completely accounted for in this analysis. Although we attempted to control for the most important variables affecting live birth rates, there may be others that cannot be assessed. These might include duration of infertility, number of previous treatment cycles, and individual laboratory practices and quality. We are unable to determine the “optimal” eSET rate in an individual IVF program, which would balance live birth rates with multiple birth rates as a complication of IVF. However, we can conclude that many IVF clinics are able to perform eSET at much higher rates than the national average with no apparent detrimental effect on their live birth rates. If it exists, the upper limit of eSET above which clinic-level live birth rates may decrease is not apparent from this study as live birth rates were unaffected by eSET rates >50% in women less than age 35 years. At the same time, the linear association between increasing eSET rates and decreased multiple birth rates is clear. From our analysis it would appear that quite high rates of eSET are required if one is to effectively address the complication of multiple births after IVF considering that clinics performing eSET in 40%–49% of cycles still had multiple birth rates in excess of 15%. With ongoing improvements in embryo cryopreservation and embryo genetic analysis and selection, IVF outcomes for patients having eSET will likely continue to improve, leading to greater acceptance and utilization of this practice (25, 26). In the meantime, the benefits of increasing use of eSET in United States IVF clinics are evident.
Footnotes
A.C.M. has nothing to disclose. S.L.B. has nothing to disclose. E.D. has nothing to disclose. E.M. has nothing to disclose. D.M.K. has nothing to disclose. B.J.V.V. has nothing to disclose.
Discuss: You can discuss this article with its authors and with other ASRM members at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/10953-elective-single-embryo-transfer-in-women-less-than-age-38-years-reduces-multiple-birth-rates-but-not-live-birth-rates-in-united-states-fertility-clinics
REFERENCES
- 1.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted Reproductive Technology Surveillance—United States, 2013. MMWR Surveill Summ 2015;64:1–25. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni AD, Jamieson DJ, Jones HW Jr, Kissin DM, Gallo MF, Macaluso M, et al. Fertility treatments and multiple births in the United States. N Engl J Med 2013;369:2218–25. [DOI] [PubMed] [Google Scholar]
- 3.Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Neonatal and maternal outcomes comparing women undergoing two in vitro fertilization (IVF) singleton pregnancies and women undergoing one IVF twin pregnancy. Fertil Steril 2013;99:731–7. [DOI] [PubMed] [Google Scholar]
- 4.Pinborg A Morbidity in a Danish National cohort of 472 IVF/ICSI twins, 1132 non-IVF/ICSI twins and 634 IVF/ICSI singletons: health-related and social implications for the children and their families. Hum Reprod 2003;18:1234–43. [DOI] [PubMed] [Google Scholar]
- 5.Lemos EV, Zhang D, van Voorhis BJ, Hu XH. Healthcare expenses associated with multiple vs singleton pregnancies in the United States. Am J Obstet Gynecol 2013;209:586.e1–11. [DOI] [PubMed] [Google Scholar]
- 6.Ryan GL, Sparks AE, Sipe CS, Syrop CH, Dokras A, van Voorhis BJ. A mandatory single blastocyst transfer policy with educational campaign in a United States IVF program reduces multiple gestation rates without sacrificing pregnancy rates. Fertil Steril 2007;88:354–60. [DOI] [PubMed] [Google Scholar]
- 7.McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ 2010;341:c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinborg A IVF/ICSI twin pregnancies: risks and prevention. Hum Reprod Update 2005;11:575–93. [DOI] [PubMed] [Google Scholar]
- 9.Maheshwari A, Griffiths S, Bhattacharya S. Global variations in the uptake of single embryo transfer. Hum Reprod Update 2011;17:107–20. [DOI] [PubMed] [Google Scholar]
- 10.Chambers GM, Illingworth PJ, Sullivan EA. Assisted reproductive technology: public funding and the voluntary shift to single embryo transfer in Australia. Med J Aust 2011;195:594–8. [DOI] [PubMed] [Google Scholar]
- 11.Pandian Z, Marjoribanks J, Ozturk O, Serour G, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev 2013;7:cd003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg ML, Boulet S, Kissin D, Warner L, Jamieson DJ. Elective single embryo transfer trends and predictors of a good perinatal outcome—United States, 1999 to 2010. Fertil Steril 2013;99:1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleicher N, Barad D. The relative myth of elective single embryo transfer. Hum Reprod 2006;21:1337–44. [DOI] [PubMed] [Google Scholar]
- 14.Kresowik JD, Stegmann BJ, Sparks AE, Ryan GL, van Voorhis BJ. Five-years of a mandatory single-embryo transfer (mSET) policy dramatically reduces twinning rate without lowering pregnancy rates. Fertil Steril 2011;96:1367–9. [DOI] [PubMed] [Google Scholar]
- 15.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod 2008;23:91–9. [DOI] [PubMed] [Google Scholar]
- 16.Fragouli E, Lenzi M, Ross R, Katz-Jaffe M, Schoolcraft WB, Wells D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum Reprod 2008;23:2596–608. [DOI] [PubMed] [Google Scholar]
- 17.Kroener L, Ambartsumyan G, Briton-Jones C, Dumesic D, Surrey M, Munne S, et al. The effect of timing of embryonic progression on chromosomal abnormality. Fertil Steril 2012;98:876–80. [DOI] [PubMed] [Google Scholar]
- 18.Styer AK, Wright DL, Wolkovich AM, Veiga C, Toth TL. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil Steril 2008;89:1702–8. [DOI] [PubMed] [Google Scholar]
- 19.Criniti A, Thyer A, Chow G, Lin P, Klein N, Soules M. Elective single blastocyst transfer reduces twin rates without compromising pregnancy rates. Fertil Steril 2005;84:1613–9. [DOI] [PubMed] [Google Scholar]
- 20.Thomas MR, Sparks AE, Ryan GL, van Voorhis BJ. Clinical predictors of human blastocyst formation and pregnancy after extended embryo culture and transfer. Fertil Steril 2010;94:543–8. [DOI] [PubMed] [Google Scholar]
- 21.Hojgaard A, Ottosen LD, Kesmodel U, Ingerslev HJ. Patient attitudes towards twin pregnancies and single embryo transfer—a questionnaire study. Hum Reprod 2007;22:2673–8. [DOI] [PubMed] [Google Scholar]
- 22.Allen BD, Adashi EY, Jones HW. On the cost and prevention of iatrogenic multiple pregnancies. Reprod Biomed Online 2014;29:281–5. [DOI] [PubMed] [Google Scholar]
- 23.Janvier A, Spelke B, Barrington KJ. The epidemic of multiple gestations and neonatal intensive care unit use: the cost of irresponsibility. J Pediatr 2011;159:409–13. [DOI] [PubMed] [Google Scholar]
- 24.Forman EJ, Hong KH, Franasiak JM, Scott RT Jr. Obstetrical and neonatal outcomes from the BEST Trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol 2014;210:157.e1–6. [DOI] [PubMed] [Google Scholar]
- 25.Forman EJ, Tao X, Ferry KM, Taylor D, Treff NR, Scott RT Jr. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Hum Reprod 2012;27:1217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grifo JA, Hodes-Wertz B, Lee HL, Amperloquio E, Clarke-Williams M, Adler A. Single thawed euploid embryo transfer improves IVF pregnancy, miscarriage, and multiple gestation outcomes and has similar implantation rates as egg donation. J Assist Reprod Genet 2013;30:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


