Abstract
The gp41 subunit of the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein plays a major role in the membrane fusion step of viral infection. The ectodomain of gp41 contains a six-helix structural domain that likely represents the core of the fusion-active conformation of the molecule. A monoclonal antibody (MAb), designated NC-1, was generated and cloned from a mouse immunized with the model polypeptide N36(L6)C34, which folds into a stable six-helix bundle. NC-1 binds specifically to both the α-helical core domain and the oligomeric forms of gp41. This conformation-dependent reactivity is dramatically reduced by point mutations within the N-terminal coiled-coil region of gp41 which impede formation of the gp41 core. NC-1 binds to the surfaces of HIV-1-infected cells only in the presence of soluble CD4. These results indicate that NC-1 is capable of reacting with fusion-active gp41 in a conformation-specific manner and can be used as a valuable biological reagent for studying the receptor-induced conformational changes in gp41 required for membrane fusion and HIV-1 infection.
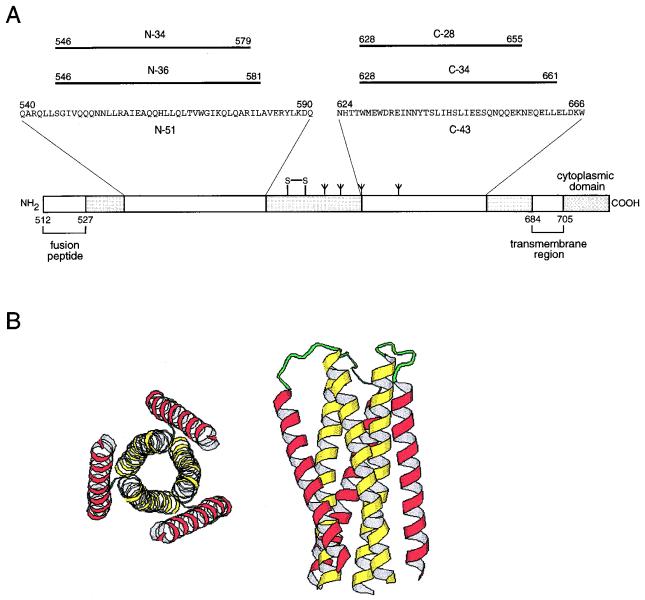
The envelope glycoprotein of human immunodeficiency virus type 1 (HIV-1) is synthesized as a precursor, gp160, that is proteolytically processed to generate two noncovalently associated subunits, gp120 and gp41 (1, 32). The surface glycoprotein, gp120, recognizes the target cell by binding to both CD4 and a coreceptor (reviewed in reference 23). The transmembrane glycoprotein, gp41, then promotes the fusion of viral and cellular membranes (22). The ectodomain (i.e., extracellular region) of gp41 contains a glycine-rich, N-terminal sequence, referred to as the fusion peptide, that is essential for membrane fusion (Fig. 1A). As in several other viral membrane fusion proteins, the fusion peptide region of gp41 is followed by two 4-3 hydrophobic (heptad) repeat regions predicted to form coiled-coils (5, 9, 14). The N-terminal heptad repeat region is located adjacent to the fusion peptide, while the C-terminal heptad repeat region precedes the transmembrane segment (Fig. 1A).
FIG. 1.
A six-helix core structure within the gp41 ectodomain composed of two interacting peptides. (A) Schematic representation of gp41. Its important functional features are shown. N and C peptides identified by protein dissection are indicated. The disulfide bond and four potential N glycosylation sites are depicted. The residues are numbered according to their positions in gp160. (B) Ribbon diagram of the N34(L6)C28 subdomain. The graphics representations are based on the crystal structure of the N34(L6)C28 trimer (31). The N-terminal helices are depicted in yellow and the C-terminal helices are in purple. The N-34 and C-28 termini are joined by the six-residue linker Ser-Gly-Gly-Arg-Gly-Gly. The left panel shows an end-on view of N34(L6)C28 looking down the three-fold axis of the trimer. The right panel shows a side view of the N34(L6)C28 trimer.
Limited proteolysis of a recombinant fragment corresponding to the gp41 ectodomain generated a trimeric, α-helical complex composed of two peptides, designated N-51 and C-43, that are derived from the N- and C-terminal heptad repeat regions, respectively (18). By further protein dissection, a subdomain within gp41 composed of the N-36 and C-34 peptides was identified (19). A thermostable analog of this subdomain was constructed by a single-chain polypeptide, N34(L6)C28, consisting of N-34 and C-28 connected by a six-residue hydrophilic linker (Fig. 1A) (20). Biophysical studies suggest that these α-helical complexes fold into six-helix bundles (18). X-ray crystallographic analysis confirmed the proposed model (Fig. 1B) (6, 31, 34). Three N-terminal helices form an interior, parallel, coiled-coil trimer, while three C-terminal helices pack in the reverse direction into three hydrophobic grooves on the surface of this coiled-coil trimer.
Synthetic peptides corresponding to the N- and C-terminal coiled-coil sequences of gp41 (designated the N and C peptides, respectively) have potent antiviral activity (16, 35, 36). Previous studies suggested that these peptides inhibit membrane fusion, in a dominant-negative manner, by binding to viral gp41 (7, 13, 18, 36). Moreover, single-point mutations within the N-terminal heptad repeat region of gp41 abolish the fusion activity of gp41 (3, 8, 10). Taken together, these results suggest that formation of a coiled-coil structure in gp41, as in the influenza virus hemagglutinin (2, 4), is a critical step during virus entry.
Binding of gp120 to both CD4 and a coreceptor (e.g., CCR5 or CXCR4) results in extensive conformational changes in gp41 needed for initiating fusion (22, 23). These conformational changes are thought to be involved in the transition from a native (nonfusogenic) to a fusion-active (fusogenic) state. The six-helix core structure of gp41 resembles the proposed fusion-active conformation of hemagglutinin and the transmembrane subunit of Moloney leukemia virus (2, 4, 6, 12, 31, 34) and thus likely adopts the conformation of fusion-active gp41 (18). We show here that a conformation-specific monoclonal antibody (MAb), designated NC-1, specifically recognizes the fusogenic core structure of gp41. This MAb should facilitate the analysis of the CD4-induced conformational change in gp120 and gp41 and the identification of the effectors of this receptor-mediated activation of HIV-1 fusion.
Generation of MAbs directed against the six-helix core of gp41.
To generate mouse MAbs against the highly conserved core structure of gp41, three BALB/c mice were primarily immunized intraperitoneally with 100 μg of recombinant N36(L6)C34 polypeptide formulated with Freund’s complete adjuvant. N36(L6)C34 is a stable subdomain consisting of two peptides, N-36 and C-34, connected by a six-residue hydrophilic linker. The structure and characterization of the model polypeptide were described before (18–20). The secondary immunizations were carried out intraperitoneally at 3-week intervals with the same amount of antigen combined with Freund’s incomplete adjuvant. Murine sera were assayed 10 days later for reactivities specific to the N36(L6)C34 antigen by enzyme-linked immunosorbent assay (ELISA) as described below. One mouse having a strong serum antibody response to the antigen received a final intravenous booster via the tail. Four days later, the mouse was bled and sacrificed by cervical dislocation. The splenocytes from this mouse were fused with SP2/0 myeloma cells and cultured in hypoxanthine-aminopterin-thymidine medium in a 96-well plate. After incubation for 10 days, the culture supernatants were collected and screened by ELISA for antibodies to N36(L6)C34. After the first screening, 24 positive wells were selected for further cloning. Finally, one clone of hybridoma cells, designated NC-1, that continuously secreted antibody at high concentrations was established. Immunoglobulin G (IgG) was purified from the ascites fluid obtained from mice injected with NC-1 hybridoma cells and was used for the immunological studies. The isotype of this MAb is IgG2a.
The ELISA was carried out as previously described (25). Briefly, a peptide or protein antigen dissolved in 0.1 M Tris (pH 8.8) was used to coat wells of a 96-well polystyrene plate (Immulon II; Dynatech Laboratories, Inc., Chantilly, Va.) and post-coat blocked with a blocking buffer (phosphate-buffered saline plus 5% horse serum). Mouse sera and culture supernatants containing antibodies or purified IgG were added to the wells at various concentrations. Then, biotin-labeled goat anti-mouse IgG (Boehringer Mannheim, Indianapolis, Ind.), streptavidin-labeled horseradish peroxidase (Zymed, South San Francisco, Calif.), and the substrate 3,3′,5,5′-tetramethylbenzidine (Sigma Chemical Co., St. Louis, Mo.) were added sequentially. The optical density at 450 nm (OD450) was read in an ELISA reader (Dynatech Laboratories, Inc.). Each sample was tested in triplicate.
Specific recognition of conformational epitopes on the six-helix core of gp41 by NC-1.
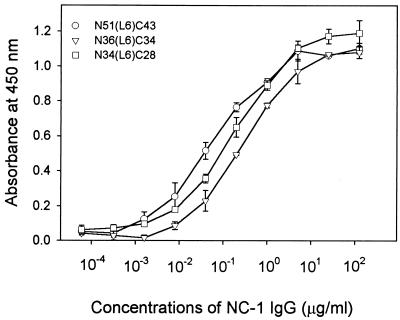
Previous studies have shown that the model polypeptide N51(L6)C43 represents the larger domain of gp41 (18, 19), while the N34(L6)C28 polypeptide folds into a minimal six-helix core (20). To examine whether the NC-1 MAb is capable of binding to the gp41 core, we examined its reactivity to the N36(L6)C34 immunogen and the N51(L6)C43 and N34(L6)C28 polypeptides by ELISA. As shown in Fig. 2, the antibody-binding properties of these polypeptides are similar, with dilution endpoints of 2.0 ng of IgG/ml for N51(L6)C43, 6.1 ng of IgG/ml for N36(L6)C34, and 2.3 ng of IgG/ml for N34(L6)C28.
FIG. 2.
Binding of NC-1 to the model polypeptides containing N and C peptides. Each of the N51(L6)C43, N36(L6)C34, and N34(L6)C28 polypeptides (∼1 μM) were used to coat wells of a microplate. The binding of the purified NC-1 IgG at the indicated concentrations to each coated polypeptide was tested by ELISA.
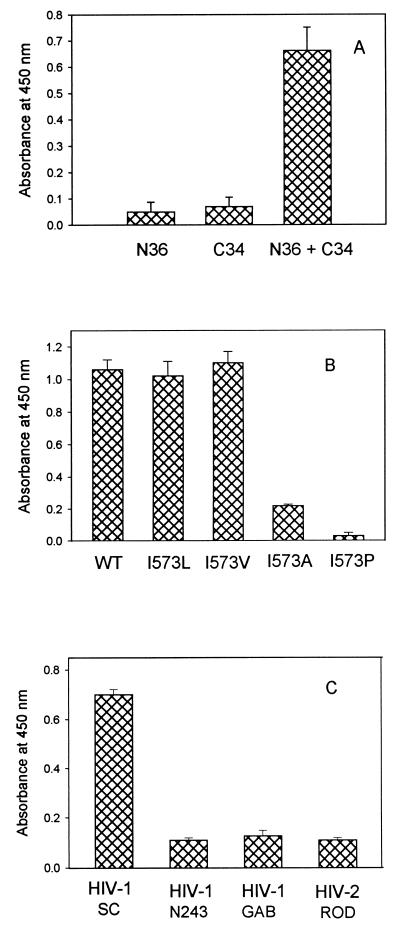
Protein dissection studies demonstrated that, in isolation, the N-36 peptide is predominantly aggregated, while the C-34 peptide is unfolded; upon mixing, these peptides form a stable trimer of heterodimers (Fig. 1B) (18, 19). To test whether the NC-1 MAb recognizes conformational and/or sequential epitopes, 96-well plates were coated with the isolated N-36 and C-34 peptides and an equimolar mixture of N-36 and C-34. As measured by ELISA, NC-1 exhibits a strong reactivity to the N-36–C and 34 complex (Fig. 3A). In contrast, the isolated N-36 and C-34 peptides fail to bind the antibody (Fig. 3A). These results indicate that the NC-1 MAb recognizes conformational epitopes on the gp41 core.
FIG. 3.
Reactivity of NC-1 with the gp41 core domains. (A) Binding of NC-1 to complexes formed by N and C peptides. Wells of polystyrene plate were coated with the isolated N-36 and C-34 peptides and a complex containing the peptides at equimolar concentrations (2 μM) and then were reacted with the purified NC-1 IgG (10 μg/ml). The following controls were included: wells coated with unrelated peptides derived from the HIV-1IIIB V3 loop (OD450 [mean ± standard deviation] = 0.052 ± 0.018) and from the immunodominant region of gp41 (residues 572 to 598) (OD450 = 0.061 ± 0.040) and wells with the N-36 and C-34 complex reacted with normal mouse IgG (10 μg/ml) (OD450 = 0.132 ± 0.033). (B) NC-1 reactivity is abolished by point mutations that disrupt the six-helix core formations. The conserved Ile 573 in the minimal subdomain N34(L6)C28 was replaced by Leu, Val, Ala, and Pro. The binding of the purified NC-1 IgG to the wild type polypeptide was compared to binding to mutant polypeptides in an ELISA. (C) Reactivity of NC-1 to the core domains formed by N-36 and C peptides derived from the transmembrane glycoprotein sequences of different HIV strains.
Single-point mutations within the highly conserved N-terminal heptad repeat region abolish the ability of gp120 and gp41 to mediate membrane fusion (3, 8, 10). Studies of model peptides demonstrated that these mutations also can disrupt formation of the minimal N34(L6)C28 core subdomain (20). It was of interest to see whether NC-1 reactivity to the N34(L6)C28 subdomain was abolished by these fusion-defective mutations. Single-point mutations (I573L, I573V, I573A, and I573P) were introduced into pN34/C28-L6 by oligonucleotide-directed mutagenesis, and the recombinant proteins were expressed and purified as previously described (20). As shown in Fig. 3B, the mutant N34(L6)C28 peptides with conserved mutations (I573L and I573V) had binding activities for NC-1 similar to that of the wild-type molecule, but those with the fusion-defective mutations (I573P and I573A) did not bind to the NC-1 MAb. These results are strong evidence that NC-1 specifically recognizes the six-helix core structure of the gp41 molecule.
To determine whether MAb NC-1 has broad reactivity, we tested the binding of NC-1 to the complexes reconstituted with N-36 and C peptides derived from the transmembrane glycoprotein sequences of HIV-1SC (clade B), HIV-1N243 (clade E), HIV-1GAB (clade O), and HIV-2ROD. As shown in Fig. 3C, NC-1 strongly bound to the complex formed by N-36 and the C peptide from HIV-1SC, which belongs to the same clade as HIV-1IIIB, but not to those formed by N-36 and C peptides from other HIV-1 and HIV-2 strains. These results indicate that NC-1 recognizes the gp41 core domain derived from strains closely related to HIV-1IIIB. A recent study has demonstrated that N peptide from HIV-1 formed a heterotypic complex with C peptide from simian immunodeficiency virus (21). It is likely that the N peptide from HIV-1IIIB and C peptides from HIV-2 and other HIV-1 strains may also form six-helix core domains. Therefore, the inability of NC-1 to bind to these heterotypic complexes is probably due to the variation of the residues on the surface of the helical core domain that participate in the formation of discontinuous epitopes for MAb NC-1.
NC-1 reacts with the oligomeric forms of gp41.
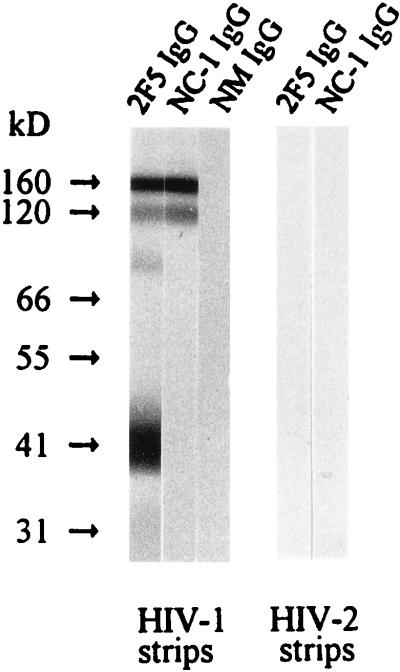
The gp120 and gp41 complex exists as either trimers or tetramers on the surfaces of virions and HIV-1-infected cells (11, 27, 29, 33). We examined the reactivity of the NC-1 MAb to viral gp41 by Western blot assay (25, 27). Strips with electrophoretically separated HIV-1IIIB and HIV-2GB122B1 proteins were obtained from Cambridge Biotech, Worcester, Mass. The attachment of the MAb NC-1 IgG was detected with biotinylated goat anti-mouse IgG antibody (Boehringer Mannheim) followed by streptavidin-conjugated horseradish peroxidase and the substrate from the Western blot kit. Human MAb 2F5, which recognizes an epitope encompassing residues 662 to 667 (ELDKWA) (24), was purchased from Polymun Scientific Immunbiologische Forschung GmbH, Vienna, Austria, and was used as a control. As shown in Fig. 4, NC-1 binds to two bands with molecular masses of about 120 and 160 kDa in the HIV-1 strip. Since NC-1 did not react with gp120 and gp160 (MicroGenesis, Meriden, Conn.) in a separate Western blot assay (data not shown), we assume that these two bands are probably gp41 trimers and tetramers. MAb 2F5 bound to bands in the HIV-1 strip with molecular masses of about 40, 80, 120, and 160 kDa, which correspond to gp41 monomers, dimers, trimers, and tetramers, respectively, consistent with previous observation (26). By contrast, neither MAb NC-1 nor 2F5 reacted with any protein in the HIV-2 strip (Fig. 4). These results indicate that the NC-1 MAb can recognize the discontinuous epitopes on the oligomers of HIV-1IIIB gp41 but cannot react with the transmembrane glycoprotein of an HIV-2 strain. We have not yet able to coprecipitate the transmembrane glycoproteins with MAb NC-1 in a radioimmunoprecipitation assay.
FIG. 4.
Detection of antibody binding to different forms of transmembrane proteins from HIV-1 and HIV-2. The binding of the purified NC-1 IgG (10 μg/ml) to the electrophoretically separated protein from HIV-1IIIB and HIV-2GB122B1 was tested by Western blotting. Normal mouse (NM) IgG and MAb 2F5 IgG were used as controls.
Binding of NC-1 to gp41 upon addition of sCD4.
Numerous studies have led to the proposal that the binding of gp120 to the CD4 receptor triggers a major conformational change in gp41 that induces fusion of viral membranes with target cell membranes (22, 23). Evidence for this conformational change includes soluble-CD4 (sCD4)-induced dissociation (shedding) of gp120 from the viral surface (15, 30) and an increased exposure of epitopes on gp41 (28). Several lines of evidence strongly suggest that the six-helix structure within the gp41 ectodomain represents the fusion-active conformation (6, 13, 18, 31, 34). We surmised that the NC-1 MAb would bind to gp41 only after its conformational change to the fusion-active state.
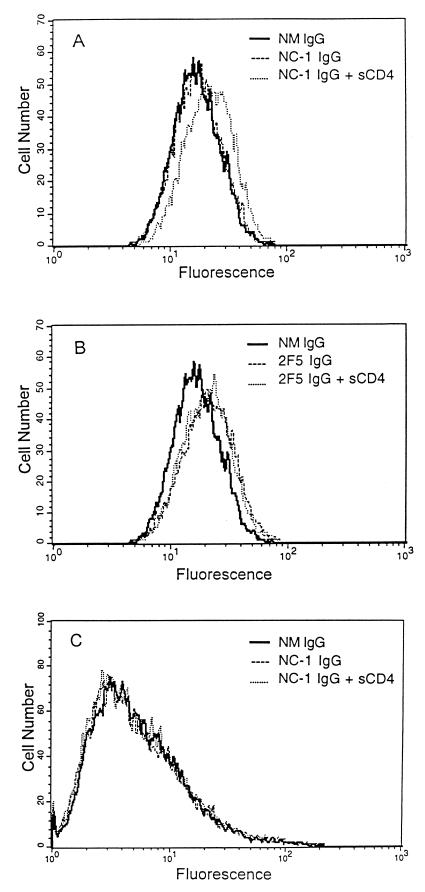
To test this hypothesis, we used flow cytometry to analyze the binding of the NC-1 MAb to HIV-infected cells. The NC-1 IgG was incubated with HIV-1IIIB-infected H9 cells and HIV-2ROD-infected U937 cells in the presence or absence of sCD4 (ImmunoDiagnostics, Bedford, Mass.) at 37°C for 30 min. MAb 2F5 IgG was used as a control. The cells were incubated with biotinylated goat anti-mouse IgG (or anti-human IgG for 2F5) and streptavidin-conjugated fluorescein isothiocyanate (Zymed) sequentially. After extensive washes, the cells were fixed with 1% formaldehyde and analyzed by flow cytometry. Remarkably, the binding of NC-1 to the surfaces of HIV-1-infected cells was detected only after addition of sCD4 (Fig. 5A). In contrast, the 2F5 MAb had similar reactivities with HIV-1-infected cells in the presence and absence of sCD4 (Fig. 5B). NC-1 did not bind to HIV-2-infected cells even in the presence of sCD4 (Fig. 5C). We conclude that the NC-1 MAb recognizes conformation-specific epitopes on fusion-active HIV-1 gp41.
FIG. 5.
Binding of MAb NC-1 to transmembrane glycoproteins expressed on HIV-infected cells by flow cytometric analysis. HIV-1IIIB-infected cells were reacted with the MAbs NC-1 (A) and 2F5 (B) (5 μg of IgG/ml) in the presence or absence of sCD4 (10 μg/ml) and analyzed by flow cytometry. Binding of NC-1 to HIV-2ROD-infected cells was also tested (C). Normal mouse (NM) IgG was used as a control.
MAb NC-1 has no detectable inhibitory activity for HIV-1 infection.
To determine whether the NC-1 MAb has neutralizing activity, we examined its ability to block fusion between HIV-1IIIB-infected cells and uninfected MT-2 cells (16, 17). The MAb had no detectable inhibitory activity, even at a concentration of 200 μg/ml. In contrast, the 2F5 MAb inhibited HIV-1 infection at a 50% inhibitory concentration of about 12 μg/ml (data not shown). There are two possible explanations for the inability of the NC-1 MAb to block HIV-1 membrane fusion. Firstly, the conformational epitope(s) in the gp41 core recognized by the antibody may not be a virus-neutralizing epitope(s). Secondly, the NC-1 epitopes may be buried in the gp120 and gp41 complex and therefore not accessible to the antibody. Nevertheless, MAb NC-1 is directed against the conformational epitopes on the putative fusogenic core of gp41 and will facilitate the characterization of the receptor-induced conformational changes in gp41.
Acknowledgments
We thank A. Robert Neurath for critical reading of the manuscript. We are grateful to Thomas Moran and Helen Parks at the Hybridoma Core Facility of the Mount Sinai Medical Center for assistance in preparation of MAbs. We thank Edward Beharry for assistance in flow cytometric analysis, Divakaramenon Venugopal for synthesis of peptides, and Tellervo Huima-Byron for photography.
This work was supported by an NIH grant (AI42693) to S.J. and by the City of New York Council Speaker’s Fund for Biomedical Research to M.L.
REFERENCES
- 1.Allan J S, Coligan J E, Barin F, McLane M F, Sodroski J G, Rosen C A, Haseltine W A, Lee T H, Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985;228:1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- 2.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 3.Cao J, Bergeron L, Helseth E, Thali M, Repke H, Sodroski J. Effects of amino acid changes in the extracellular domain of the human immunodeficiency virus type 1 gp41 envelope glycoprotein. J Virol. 1993;67:2747–2755. doi: 10.1128/jvi.67.5.2747-2755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr C M, Kim P S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- 5.Chamber P, Pringle C R, Easton A J. Heptad-repeat regions are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J Gen Virol. 1990;71:3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- 6.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-H, Matthews T J, McDanal C B, Bolognesi D P, Greenberg M L. A molecular clasp in the human immunodeficiency virus (HIV) type 1 TM protein determines the anti-HIV activity of gp41 derivatives: implication for viral fusion. J Virol. 1995;69:3771–3777. doi: 10.1128/jvi.69.6.3771-3777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S S-L, Lee C-N, Lee W-R, McIntosh K, Lee T-H. Mutational analysis of the leucine zipper-like motif of the human immunodeficiency virus type 1 envelope transmembrane glycoprotein. J Virol. 1993;67:3615–3619. doi: 10.1128/jvi.67.6.3615-3619.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delwart E L, Mosialos G, Gilmore T. Retroviral envelope glycoproteins contain a leucine zipper-like repeat. AIDS Res Hum Retroviruses. 1990;6:703–706. doi: 10.1089/aid.1990.6.703. [DOI] [PubMed] [Google Scholar]
- 10.Dubay J W, Roberts S J, Brody B, Hunter E. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J Virol. 1992;66:4748–4756. doi: 10.1128/jvi.66.8.4748-4756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fass D, Harrison S C, Kim P S. Retrovirus envelope domain at 1.7 angstrom resolution. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 13.Furuta R, Wild C T, Weng Y, Weiss C D. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 14.Gallaher W R, Ball J M, Garry R F, Griffin M C, Montelaro R C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989;5:431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- 15.Hart T K, Kirsh R, Ellens H, Sweet R W, Lambert D M, Petteway S R, Jr, Leary J, Bugelski P J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci USA. 1991;88:2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang S, Lin K, Strick N, Neurath A R. HIV-1 inhibition by a peptide. Nature. 1993;365:113. doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 17.Jiang S, Lin K. Effect of amino acid replacements, additions and deletions on the antiviral activity of a peptide derived from the HIV-1 gp41 sequences. Peptide Res. 1995;8:345–348. [PubMed] [Google Scholar]
- 18.Lu M, Blacklow S C, Kim P S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 19.Lu M, Kim P S. A trimeric structural subdomain of the HIV-1 transmembrane glycoprotein. J Biomol Struct Dyn. 1997;15:465–471. doi: 10.1080/07391102.1997.10508958. [DOI] [PubMed] [Google Scholar]
- 20.Lu, M., J. Hong, and S. Shen. Subdomain folding of the core structure from HIV-1 gp41: implications for viral membrane fusion. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 21.Malashkevich V, Chan D C, Chutkowski C T, Kim P S. Crystal structure of the simian immunodeficiency virus (SIV) gp41 core: conserved helical interactions underlie the broad inhibitory activity of gp41 peptides. Proc Natl Acad Sci USA. 1998;95:9134–9139. doi: 10.1073/pnas.95.16.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore J P, Jameson B A, Weiss R A, Sattentau Q J. The HIV-cell fusion reaction. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 233–289. [Google Scholar]
- 23.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 24.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neurath A R, Strick N, Jiang S. Synthetic peptides and anti-peptide antibodies as probes to study inter-domain interactions involved in virus assembly: the envelope of the human immunodeficiency virus (HIV-1) Virology. 1992;188:1–13. doi: 10.1016/0042-6822(92)90729-9. [DOI] [PubMed] [Google Scholar]
- 26.Neurath A R, Strick N, Lin K, Jiang S. Multifaceted consequences of anti-gp41 monoclonal antibody 2F5 binding to HIV-1 virions. AIDS Res Hum Retroviruses. 1995;11:687–696. doi: 10.1089/aid.1995.11.687. [DOI] [PubMed] [Google Scholar]
- 27.Pinter A, Honnen W J, Tilley S A, Bona C, Zaghouani H, Gorny M K, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sattentau Q J, Moore J P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schawaller M, Smith G E, Skehel J J, Wiley D C. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology. 1989;172:367–369. doi: 10.1016/0042-6822(89)90142-6. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci USA. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veronese F D, DeVico A L, Copeland T D, Oroszlan S, Gallo C, Sarngadharan M G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985;229:1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- 33.Weiss C D, Levy J A, White J M. Oligomeric organization of gp120 on infectious human immunodeficiency virus type 1 particles. J Virol. 1990;64:5674–5677. doi: 10.1128/jvi.64.11.5674-5677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 35.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wild C T, Shugars D C, Greenwell T K, McDanal C B, Matthews T J. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]