Abstract
Objective:
To assess endometriosis-associated infertility trends among assisted reproductive technology (ART) cycles, and to compare cancellation and hyperstimulation risks and pregnancy and live birth rates among women using ART for endometriosis-associated vs. male factor infertility.
Design:
Descriptive and multivariable analyses of Centers for Disease Control and Prevention (CDC) National ART Surveillance System data.
Setting:
Fertility centers.
Patient(s):
All reported fresh autologous ART cycles in the United States between 2000 and 2011 (n = 1,589,079).
Intervention(s):
None.
Main Outcome Measure(s):
Oocyte yield, hyperstimulation, cancellation, implantation, pregnancy, live birth.
Result(s):
The absolute number of ART cycles with an endometriosis diagnosis fell in recent years, from 16,751 (2000) to 15,311 (2011); the percentage fell over time, from 17.0% (2000) to 9.6% (2011) of all cycles. Compared with male factor (n = 375,557), endometriosis-associated cycles (n = 112,475) yielded fewer oocytes (50.5% vs. 42.5% of cycles with only 0–10 oocytes retrieved), lower risk of hyperstimulation (1.1% vs. 1.3%, adjusted risk ratio [aRR] 0.82, 95% confidence interval [CI] 0.74–0.91), and an increased risk of cancellation (12.9% vs. 10.1%, aRR 1.30, 95% CI 1.25–1.35). Endometriosis was associated with a statistically decreased but likely clinically insignificant difference in the following outcomes: chance of pregnancy per transfer (43.7% vs. 44.8%, aRR 0.96, 95% CI 0.95–0.98) among couples who did not also have tubal factor infertility and live birth per transfer (37.2% vs. 37.6%, aRR 0.96, 95% CI 0.94–0.98).
Conclusion(s):
The percentage of endometriosis-associated ART cycles has decreased over time. As compared with male factor infertility, endometriosis is associated with increased cancellation and decreased hyperstimulation risks. Despite decreased oocyte yield and higher medication dose, the difference in pregnancy and live birth rates may be of limited clinical significance, suggesting comparable pregnancy outcomes per transfer.
Keywords: Endometriosis, outcomes, ART, cancellation, hyperstimulation
Endometriosis, the presence of ectopic endometrial tissue, affects 25%–50% of infertile women, many of whom use assisted reproductive technology (ART) to conceive (1, 2). Endometriosis can cause adhesive disease that alters pelvic anatomy and may yield an inflammatory altered immune environment that has the potential to impact oocyte quality, embryogenesis, and implantation (3–5). The mechanism by which endometriosis contributes to infertility and its impact on fertility treatment success remain controversial (1, 6–12). Several studies, including systematic reviews and meta-analyses, have yielded conflicting results regarding the impact of endometriosis on ovarian reserve and IVF outcomes; several suggest comparable ART outcomes between women with and without endometriosis (8, 9, 13, 14), whereas others suggest that the presence of endometriosis negatively affects ART success (6, 8, 9, 12, 13). The National ART Surveillance System (NASS) allows us to study outcomes among a very large cohort of women using ART with endometriosis-associated infertility and to obtain additional diagnosis-specific prognostic information that may improve counseling of patients with endometriosis who plan to undergo ART.
The goal of this study was to use national data on women treated with ART to examine endometriosis-associated infertility incidence in this population and outcome trends, and to compare cancellation and hyperstimulation risks and pregnancy and live birth rates among those with endometriosis with those with male factor infertility.
MATERIALS AND METHODS
We assessed endometriosis trends among all ART cycles, including fresh, frozen, and banking cycles, performed in the United States between 2000 and 2011 (n = 1,589,079) using NASS, a nationally mandated reporting system that includes approximately 97% of all ART cycles performed in the United States (Fertility Clinic Success Rate and Certification Act of 1992 [15, 16], Public Law No. 102–493, October 24, 1992). The NASS data, which are ART cycle-based, include patient demographics, obstetric and medical history, infertility diagnoses, detailed parameters of each ART treatment cycle and, if applicable, the resultant pregnancy outcome.
Regression analysis was used to assess linear and quadratic trends over time in absolute number and percentage of ART cycles including any diagnosis of endometriosis and cancellation, pregnancy, and live birth rates among endometriosis-associated cycles for which a transfer was performed.
The primary outcomes of interest were treatment complications (hyperstimulation, hospitalization, and cancellation) and pregnancy outcomes (pregnancy, live birth, and miscarriage). Cycles were considered to result in pregnancy if they had an outcome of clinical intrauterine gestation, defined as ultrasound confirmation of gestational sac(s) within the uterus, regardless of whether a heartbeat(s) was observed or fetal pole established. When ultrasound data were not available, confirmation was achieved through documented birth, spontaneous miscarriage, or induced abortion. A cycle was considered to result in live birth when at least one live-born infant was delivered at ≥20 weeks’ gestation and as a miscarriage if the pregnancy outcome occurred at <20 weeks’ gestation. Secondary characteristics of interest included total FSH medication dose and implantation rate, defined as the number of fetal heartbeats at 6-week ultrasound per number of embryos transferred.
Bivariate analyses were conducted to explore the relationship between infertility diagnosis and other maternal and cycle characteristics. Infertility diagnosis was classified as endometriosis or male factor, excluding those with both endometriosis and male factor infertility, but allowing for other concomitant diagnoses. Male factor infertility was chosen as the comparison group because it suggests a female without known infertility. We did not include women with unexplained infertility because women with either endometriosis or male factor infertility were, by definition, not “unexplained.” Chi-square tests were used to test for statistical significance between infertility type and categorical characteristics, whereas the Wilcoxon rank sum test was used for associations between infertility type and continuous characteristics.
Log binomial regression was used to generate unadjusted and adjusted risk ratios (RRs), 95% confidence intervals (CI), and P values to compare complication rates per cycle start and success rates per ET among fresh, nondonor ART cycles performed for couples with endometriosis-associated infertility (n = 112,475) as compared with male factor infertility (n = 375,557). Poisson regression was used to model the number of embryos implanted per number of embryos transferred, and linear regression was used to model FSH levels. Generalized estimating equations with an independent correlation matrix were used to account for clustering by clinic. Characteristics controlled for in the multivariable models varied by outcome, as determined by their statistical significance in bivariate analysis. For complication outcomes, the possible list of covariates included maternal age, obstetric history (number of prior pregnancies, spontaneous miscarriages, preterm births, and full-term births), number of prior ART cycles, concomitant infertility diagnoses, and year. For pregnancy outcomes, the possible list of covariates included the same variables previously listed as well as use of intracytoplasmic sperm injection (ICSI), use of assisted hatching, the embryo stage at transfer, the number of embryos transferred, the number of supernumerary embryos cryopreserved, and the number of oocytes retrieved. Race or ethnicity was included in the bivariate tables but not in the multivariable analysis because this factor was missing for approximately 40% of the observations. We explored the possibility of an interaction between type of infertility (endometriosis or male factor) and tubal factor because women with endometriosis-induced tubal factor may have a noninfectious cause as compared with those with tubal factor that resulted from history of prior pelvic infection. The interaction was significant when modeling live birth; therefore, RRs are reported by the presence or absence of tubal factor.
Statistical significance was determined using an α of 0.05. All analyses were conducted using SAS v. 9.3 (SAS Institute). This study was approved by the institutional review board of the Centers for Disease Control and Prevention.
RESULTS
The absolute number and percentage of ART cycles with an endometriosis diagnosis showed a significant quadratic trend (Fig. 1A). The number rose from 16,751 in 2000 to 17,902 in 2003 and then fell to 15,311 in 2011. The percentage of ART cycles with any diagnosis of endometriosis fell over time, from 17.0% in 2000 to 9.6% in 2011. A similar significant decreasing quadratic trend was also noted among endometriosis-associated first ART cycles. The average number of oocytes retrieved among endometriosis-associated cycles also had a significant quadratic trend, rising to a peak of 12.3 in 2006 (ranging from 11.9 to 12.3). The percentage of cancelled ART cycles among patients with endometriosis significantly decreased over time, from 14.5% in 2000 to 10.0% in 2011 (Fig. 1B), whereas the pregnancy rate and live birth rates per transfer showed a significant quadratic trend (Fig. 1C). The pregnancy rate rose from 40.0% (2000) to 48.3% (2008) and then fell to 45% (2011). The live birth rate rose from 33.6% (2000) to 40.0% (2008) and then fell to 37.3% (2011).
FIGURE 1.
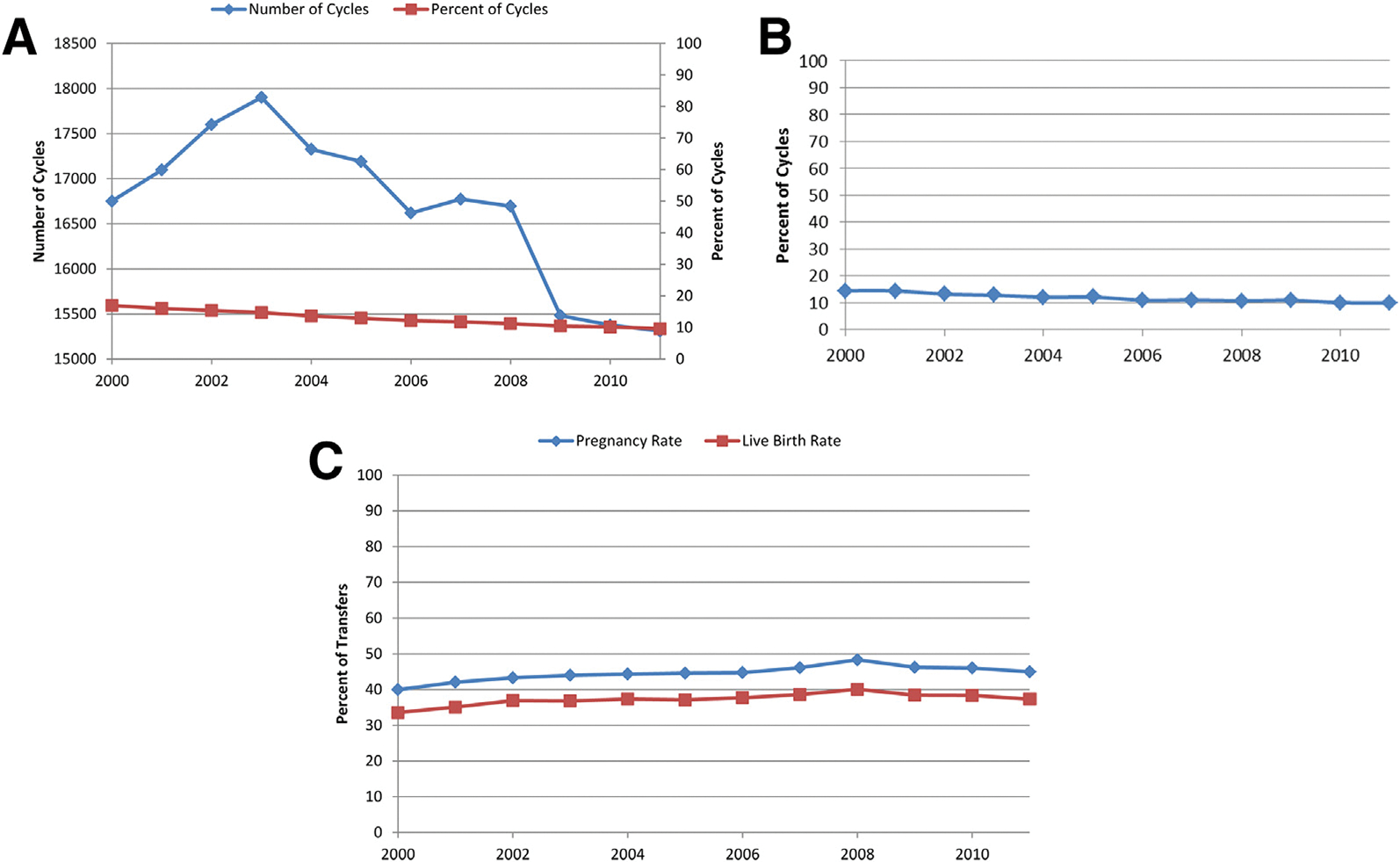
(A) Absolute number and percentage of all ART cycles including a diagnosis of endometriosis, 2000–2011. (B) Percentage of fresh ART cycles including any diagnosis of endometriosis that were cancelled, 2000–2011. (C) Pregnancy and live birth rates for all fresh, non-donor transfers with any diagnosis of endometriosis, 2000–2011.
Between 2000 and 2011, NASS included 488,032 fresh autologous IVF cycles for which either endometriosis or male factor was among the diagnoses for infertility, excluding those with both endometriosis-associated and male factor infertility (Table 1). A higher percentage of women in the endometriosis-associated group as compared with male factor reported race as non-Hispanic white, held a concomitant diagnosis of tubal factor infertility, and had to be cancelled before retrieval. A lower percentage of women in the endometriosis-associated group held a concomitant diagnosis of ovulatory dysfunction or diminished ovarian reserve, and reported a complication of ovarian hyperstimulation.
TABLE 1.
Cycle characteristic by infertility diagnosis, all fresh nondonor IVF cycles 2000–2011.
| Primary diagnosis | |||||
|---|---|---|---|---|---|
| Endometriosis | Male factor | ||||
| Characteristic | n | % | n | % | P value |
| Total | 112,475 | 375,557 | |||
| Age (y) | < .0001 | ||||
| <35 | 56,156 | 49.9 | 180,322 | 48.0 | |
| 35–37 | 27,330 | 24.3 | 83,319 | 22.2 | |
| 38–40 | 20,037 | 17.8 | 70,508 | 18.8 | |
| 41–42 | 6,441 | 5.7 | 27,600 | 7.4 | |
| 43–44 | 2,092 | 1.9 | 10,878 | 2.9 | |
| ≥45 | 419 | 0.4 | 2,930 | 0.8 | |
| Race/ethnicitya | < .0001 | ||||
| Non-Hispanic white | 54,288 | 80.4 | 171,507 | 76.0 | |
| Non-Hispanic black | 2,642 | 3.9 | 13,657 | 6.1 | |
| Asian/Pacific islander | 6,142 | 9.1 | 22,199 | 9.8 | |
| Hispanic | 4,414 | 6.5 | 17,880 | 7.9 | |
| Other | 84 | 0.1 | 391 | 0.2 | |
| Concomitant diagnosis | |||||
| Ovulatory dysfunction | 9,951 | 8.9 | 47,387 | 12.6 | < .0001 |
| Tubal factor | 21,198 | 18.9 | 37,391 | 10.0 | < .0001 |
| Diminished ovarian reserve | 14,863 | 13.2 | 61,618 | 16.4 | < .0001 |
| No. of prior ART cycles | .0077 | ||||
| 0 | 62,221 | 55.4 | 209,571 | 55.8 | |
| 1 | 23,356 | 20.8 | 76,625 | 20.4 | |
| 2+ | 26,820 | 23.9 | 89,091 | 23.7 | |
| No. of prior pregnancies | < .0001 | ||||
| 0 | 59,921 | 53.3 | 198,509 | 52.9 | |
| 1 | 30,747 | 27.4 | 98,867 | 26.4 | |
| 2+ | 21,701 | 19.3 | 77,575 | 20.7 | |
| No. of prior spontaneous abortions | < .0001 | ||||
| 0 | 80,761 | 72.0 | 281,045 | 75.2 | |
| 1 | 21,866 | 19.5 | 65,906 | 17.6 | |
| 2+ | 9,573 | 8.5 | 27,000 | 7.2 | |
| No. of prior preterm births | < .0001 | ||||
| 0 | 110,110 | 98.2 | 366,005 | 98.0 | |
| 1 | 1,873 | 1.7 | 6,608 | 1.8 | |
| 2+ | 159 | 0.1 | 737 | 0.2 | |
| No. of prior full-term births | < .0001 | ||||
| 0 | 88,712 | 79.0 | 284,526 | 76.0 | |
| 1 | 20,678 | 18.4 | 71,211 | 19.0 | |
| 2+ | 2,862 | 2.6 | 18,594 | 5.0 | |
| Mean maximum serum FSH (SD)a | 7.98 | 4.9 | 7.54 | 4.4 | < .0001 |
| Cycle history | < .0001 | ||||
| First IVF, no previous live birth | 52,453 | 46.8 | 171,171 | 45.7 | |
| First IVF, ≥1 previous live birth | 9,653 | 8.6 | 37,668 | 10.1 | |
| ≥1 previous IVF, no previous live birth | 34,627 | 30.9 | 107,960 | 28.9 | |
| ≥1 previous IVF, ≥1 previous live birth | 15,462 | 13.8 | 57,413 | 15.3 | |
| Stimulation protocol (only 2004+ data)a | < .0001 | ||||
| Agonist standard | 34,515 | 48.7 | 132,966 | 49.7 | |
| Agonist flare | 9,057 | 12.8 | 29,472 | 11.0 | |
| Antagonist | 21,788 | 30.7 | 84,912 | 31.7 | |
| Clomid ± FSH | 1,015 | 1.4 | 3,336 | 1.3 | |
| FSH only | 2,160 | 3.0 | 7,856 | 2.9 | |
| Unstimulated | 611 | 0.9 | 2,759 | 1.0 | |
| Other | 1,797 | 2.5 | 6,489 | 2.4 | |
| Mean total FSH dose (SD)a | 3,274.32 | 1,732.2 | 3,106.54 | 1,672.1 | < .0001 |
| Ovarian hyperstimulation | < .0001 | ||||
| Yes | 1,187 | 1.1 | 4,929 | 1.3 | |
| No | 111,287 | 98.9 | 370,625 | 98.7 | |
| Cycle cancellation total | < .0001 | ||||
| Before retrieval | 14,474 | 12.9 | 37,936 | 10.1 | |
| Between retrieval and transfer | 6,281 | 5.6 | 23,084 | 6.2 | |
| Not cancelled | 91,720 | 81.6 | 314,537 | 83.8 | |
| Hospitalization | 337 | 0.3 | 1,175 | 0.3 | .4832 |
All missing <1% except race/ethnicity (39.9%), maximum serum FSH (25.5%), and mean FSH dose (3.7%).
Between 2000 and 2011, NASS included 406,255 noncancelled fresh autologous IVF cycles resulting in transfer among women with endometriosis or male factor infertility (Table 2); 81,777 cycles were cancelled either before retrieval or before transfer. Statistically significant (P<.0001 for all comparisons) differences were detected between male factor- and endometriosis-associated cycles in the distribution of the following cycle characteristics: number of oocytes retrieved (endometriosis-associated with 50.5% vs. male factor with 42.5% of cycles with 0–10 oocytes retrieved), use of ICSI (endometriosis-associated 52.3% vs. male factor 92.3%), day of ET (endometriosis-associated 67.4% day 3 vs. male factor 63.4% day 3), number of embryos transferred (endometriosis-associated 48.3% transfer of three or more embryos vs. male factor 45.0%), and elective single embryo transfer (endometriosis-associated 1.9% vs. male factor 2.6%). For both groups, the majority of cycles (67.0% endometriosis and 65.6% male factor) had no embryos cryopreserved. Of the cycles resulting in pregnancy, the majority of women in both groups delivered a singleton live-born infant (55.5% endometriosis and 56.1% male factor); the incidence of twins was also comparable (25.8% endometriosis and 25.0% male factor).
TABLE 2.
Cycle characteristic by infertility diagnosis, noncancelled fresh nondonor IVF cycles resulting in transfer 2000–2011.
| Primary diagnosis | |||||
|---|---|---|---|---|---|
| Endometriosis | Male factor | ||||
| Characteristic | n | % | n | % | P value |
| No. of oocytes retrieved | < .0001 | ||||
| 0–10 | 46,274 | 50.5 | 133,674 | 42.5 | |
| 11–20 | 35,435 | 38.6 | 133,526 | 42.5 | |
| 21 + | 10,006 | 10.9 | 47,323 | 15.1 | |
| Use of ICSI | < .0001 | ||||
| Used ICSI | 47,922 | 52.3 | 289,920 | 92.3 | |
| Did not use ICSI | 43,720 | 47.7 | 24,366 | 7.8 | |
| Use of assisted hatching | < .0001 | ||||
| Used assisted hatching | 39,066 | 42.6 | 136,556 | 43.4 | |
| Did not use assisted hatching | 52,652 | 57.4 | 177,981 | 56.6 | |
| Embryo stage at transfer | < .0001 | ||||
| Day 3 | 61,581 | 67.4 | 198,848 | 63.4 | |
| Day 5 | 21,522 | 23.6 | 83,834 | 26.8 | |
| Other (1, 2, 4, 6) | 8,231 | 9.0 | 30,764 | 9.8 | |
| No. of embryos transferred | < .0001 | ||||
| 1 | 8,206 | 9.0 | 31,021 | 9.9 | |
| 2 | 39,166 | 42.7 | 141,859 | 45.1 | |
| 3 | 28,096 | 30.6 | 87,703 | 27.9 | |
| 4+ | 16,234 | 17.7 | 53,876 | 17.1 | |
| eSETa | < .0001 | ||||
| Yes eSET | 1,630 | 1.9 | 7,694 | 2.6 | |
| No eSET | 83,512 | 98.1 | 283,516 | 97.4 | |
| No. of supernumerary embryos cryopreserved | < .0001 | ||||
| 0 | 61,078 | 67.0 | 205,407 | 65.6 | |
| 1–2 | 10,422 | 11.4 | 37,791 | 12.1 | |
| 3–4 | 8,644 | 9.5 | 31,103 | 9.9 | |
| 5+ | 11,028 | 12.1 | 38,875 | 12.4 | |
| No. of infants born among cycles resulting in pregnancy | |||||
| Zero (no live birth) | 6,488 | 16.2 | 23,895 | 17.0 | < .0001 |
| Singleton live birth | 22,246 | 55.5 | 78,995 | 56.1 | |
| Twin live birth | 10,343 | 25.8 | 35,220 | 25.0 | |
| Triplet or higher-order live birth | 1,009 | 2.5 | 2,725 | 1.9 | |
All missing <1% except elective single embryo transfer (eSET) 7.4% missing.
As compared with male factor infertility, endometriosis was associated with a higher mean total FSH dose (3,274.3 IU vs. 3,106.6 IU, adjusted estimate 224.53, 95% CI 179.1–270.0), and with a significantly lower risk of hyperstimulation (1.1% vs. 1.3%, adjusted RR [aRR] 0.82, 95% CI 0.74–0.91) and an increased risk of cancellation (12.9% vs. 10.1%, aRR 1.30, 95% CI 1.25–1.35) (Table 3). Hospitalization rates were not significantly different between the two groups (0.3% for both, aRR 0.97, 95% CI 0.84–1.11).
TABLE 3.
Outcomes among fresh nondonor IVF cycles, endometriosis vs. male factor infertility 2000–2011.
| Outcome | Endometriosis, n (%) | Male factor, n (%) | RR (95% CI) | aRR (95% CI) |
|---|---|---|---|---|
| Hyperstimulationa | 1,187 (1.06) | 4,929 (1.31) | 0.80 (0.72–0.90) | 0.82 (0.74–0.91) |
| Hospitalizationa | 337 (0.3) | 1,175 (0.31) | 0.96 (0.83–1.11) | 0.97 (0.84–1.11) |
| Cancellationa | 14,474 (12.87) | 37,936 (10.10) | 1.27 (1.22–1.33) | 1.30 (1.25–1.35) |
| Pregnancyb | 40,085 (43.74) | 140,835 (44.81) | 0.98 (0.96–0.99) | 0.96 (0.95–0.98) |
| Live birth (≥20 wk)b | Tubal: 5,781 (33.80) | Tubal: 10,177 (32.57) | Tubal: 1.04(1.002–1.08) | Tubal: 1.00 (0.97–1.03) |
| No tubal: 27,694 (37.15) | No tubal: 106,366 (37.58) | No tubal: 0.99 (0.97–1.01) | No tubal: 0.96 (0.94–0.98) | |
| Miscarriage (<20 wk)b | 5,323 (5.83) | 19,793 (6.32) | 0.92 (0.89–0.96) | 0.93 (0.89–0.97) |
| Implantationb,c | 25.28 (34.80) | 26.30 (35.51) | 0.98 (0.95–1.0008) | 0.96 (0.94–0.97) |
Per cycle start.
Per noncancelled cycles for which a transfer was performed.
Implantation is defined as the maximum of the total number of heartbeats on first ultrasound or number of infants born divided by the number of embryos transferred times 100. Implantation rate is calculated per cycle; table reflects mean implantation rates for all cycles in each group.
E 1Among noncancelled cycles for which either a day-3 or day-5 transfer was performed, the average implantation rate in the endometriosis group was lower than in the male factor group (25.3% vs. 26.3%, aRR 0.96, 95% CI 0.94–0.97). Also for cycles resulting in either a day-3 or day-5 transfer, endometriosis was associated with a decreased chance of pregnancy (43.7% vs. 44.8%, aRR 0.96, 95% CI 0.95–0.98), a decreased chance of miscarriage (5.8% vs. 6.3%, aRR 0.93, 95% CI 0.89–0.97), and, among couples who did not have tubal factor infertility, a decreased chance of live birth (37.2% vs. 37.6%, aRR 0.96, 95% CI 0.94–0.98).
DISCUSSION
The percentage of endometriosis-associated ART cycles decreased over the 12-year study period. The decreasing trend in endometriosis-associated cycles may reflect not only a decrease in diagnosis of endometriosis among women using ART but also an increase in alternate indications for ART. The decrease in diagnosis may reflect the tendency to perform IVF immediately rather than after diagnostic laparoscopy among women with unexplained infertility.
As compared with male factor infertility, endometriosis is associated with increased total medication dose and cancellation risk and with decreased oocyte yield and hyperstimulation risk. An initial assumption may be that the lower oocyte yield, increased medication requirement, increased cancellation, and decreased ovarian hyperstimulation rates may reflect diminished ovarian reserve among patients with endometriosis (17). However, the fact that the diagnosis of diminished ovarian reserve was more prevalent among the male factor group and the fact that the association with cancellation persists after adjusting for age and concomitant diagnoses including diminished ovarian reserve suggests that endometriosis itself may confer an altered response to high-dose gonadotropins.
Among cycles for which transfer was performed, the likelihood of live birth is statistically decreased among cycles associated with endometriosis in the absence of tubal factor as compared with male factor infertility but is likely of limited clinical significance. Although statistically significant owing to the large number of cycles in this study, the clinical impact of endometriosis on chance of pregnancy and live birth is likely minimal, with the percentage of cycles leading to pregnancy or live birth differing only by 1% or less between the two groups, and point estimates and confidence intervals approaching 1. Previous studies investigating ART pregnancy outcomes among women with endometriosis have yielded conflicting results: several have found no association between endometriosis and pregnancy outcomes (8, 9, 13, 14), whereas a prior systematic review and meta-analysis found an association between severe stage III/IV endometriosis and decreased implantation and clinical pregnancy rates as compared with women without endometriosis (6, 12, 18). This study supports prior findings suggesting limited clinically significant impact of endometriosis on pregnancy outcomes among women undergoing ART. The lack endometriosis stage within the collected variables prevents us from drawing a conclusion about the impact of severe-stage endometriosis.
The study is limited by the lack of patient surgical history; however, the mean serum FSH and presence or absence of a diagnosis of diminished ovarian reserve indirectly reflect the potential deleterious impact of prior ovarian surgery on ovarian reserve. The study is also limited by the fact that data are cycle-, rather than patient-based, and that embryo quality is not a collected variable. We were also not able to account for time between endometriosis diagnosis and treatment, method of diagnosis, and treatment before initiation of IVF. Moreover, this is a retrospective cohort study that uses surveillance system data that rely on the input accuracy of individual clinics.
The study is among the first of its size to investigate ART outcomes specifically among those with an endometriosis diagnosis; it also offers additional analysis of medication requirements and cancellation and ovarian hyperstimulation syndrome risks. Additionally, we were able to control for potential confounders, including age, obstetric history, ART history, concomitant infertility diagnoses, stimulation type, laboratory procedures, number and stage of embryo transfer, and time.
In conclusion, when counseling infertility patients with endometriosis who are considering ART, clinicians now have additional information regarding slightly increased medication requirements and chance of cancellation and slightly decreased risk of hyperstimulation compared with those without endometriosis. Although statistically significant, the magnitude of effect detected for the decrease in implantation, live birth, and pregnancy rates was small, and confidence intervals for these measures approached the null value, suggesting that any difference in these outcomes between women who do and who do not have endometriosis is likely to be minimal.
Acknowledgments:
The NASS Working Group also includes the following members, all of whom have no financial disclosures and gave written permission to be included in the manuscript: Sheree Boulet, Dr.P.H., M.P.H., Jeani Chang, M.P.H., Aniket Kulkarni, M.B.B.S., M.P.H., Allison Mneimneh, M.P.H., C.P.M., Mithi Sunderam, Ph.D., and Yujia Zhang, Ph.D.
Footnotes
J.F.K. has nothing to disclose. S.C. has nothing to disclose. D.R.S. has nothing to disclose. D.M.K. has nothing to disclose. D.J.J. has nothing to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/kawwassj-endometriosis-art-trends-outcomes/
REFERENCES
- 1.Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet 2010;27:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC. Endometriosis. Lancet 2004;364:1789–99. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril 2008;90:247–57. [DOI] [PubMed] [Google Scholar]
- 4.Filippi F, Benaglia L, Paffoni A, Restelli L, Vercellini P, Somigliana E, et al. Ovarian endometriomas and oocyte quality: insights from in vitro fertilization cycles. Fertil Steril 2014;101:988–93.e1. [DOI] [PubMed] [Google Scholar]
- 5.Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility. Fertil Steril 2006;86(5 Suppl 1):S156–60. [DOI] [PubMed] [Google Scholar]
- 6.Harb HM, Gallos ID, Chu J, Harb M, Coomarasamy A. The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta-analysis. BJOG 2013;120:1308–20. [DOI] [PubMed] [Google Scholar]
- 7.Surrey ES. Endometriosis and assisted reproductive technologies: maximizing outcomes. Semin Reprod Med 2013;31:154–63. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa MA, Teixeira DM, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2014;44:261–78. [DOI] [PubMed] [Google Scholar]
- 9.Matalliotakis IM, Cakmak H, Mahutte N, Fragouli Y, Arici A, Sakkas D. Women with advanced-stage endometriosis and previous surgery respond less well to gonadotropin stimulation, but have similar IVF implantation and delivery rates compared with women with tubal factor infertility. Fertil Steril 2007;88:1568–72. [DOI] [PubMed] [Google Scholar]
- 10.Muzii L, Di Tucci C, Di Feliciantonio M, Marchetti C, Perniola G, Panici PB. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod 2014;29:2190–8. [DOI] [PubMed] [Google Scholar]
- 11.Stern JE, Brown MB, Wantman E, Kalra SK, Luke B. Live birth rates and birth outcomes by diagnosis using linked cycles from the SART CORS database. J Assist Reprod Genet 2013;30:1445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril 2002;77:1148–55. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Lata K, Naha M, Malhotra N, Tiwari A, Vanamail P. Effect of endometriosis on implantation rates when compared to tubal factor in fresh non donor in vitro fertilization cycles. J Hum Reprod Sci 2014;7:143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamdan M, Omar SZ, Dunselman G, Cheong Y. Influence of endometriosis on assisted reproductive technology outcomes: a systematic review and meta-analysis. Obstet Gynecol 2015;125:79–88. [DOI] [PubMed] [Google Scholar]
- 15.Adashi EY, Wyden R. Public reporting of clinical outcomes of assisted reproductive technology programs: implications for other medical and surgical procedures. JAMA 2011;306:1135–6. [DOI] [PubMed] [Google Scholar]
- 16.Fertility Clinic Success Rate and Certification act of 1992 (FCSRCA). Public Law 102–493; October 24, 1992. [PubMed] [Google Scholar]
- 17.Pacchiarotti A, Frati P, Milazzo GN, Catalano A, Gentile V, Moscarini M. Evaluation of serum anti-Mullerian hormone levels to assess the ovarian reserve in women with severe endometriosis. Eur J Obstet Gynecol Reprod Biol 2014;172:62–4. [DOI] [PubMed] [Google Scholar]
- 18.Pop-Trajkovic S, Popovic J, Antic V, Radovic D, Stavanovic M, Vukomanovic P. Stages of endometriosis: does it affect in vitro fertilization outcome. Taiwan J Obstet Gynecol 2014;53:224–6. [DOI] [PubMed] [Google Scholar]


