Abstract
OBJECTIVE:
To describe the relationship between severe diminished ovarian reserve (DOR) and assisted reproductive technology outcomes.
STUDY DESIGN:
Retrospective cohort including all United States’ fertility centers reporting to the CDC National ART Surveillance System, 2004–2012. Among women aged <41 (504,266 fresh autologous IVF cycles), we calculated cancellation rate/cycle and pregnancy rate/transfer, stratified by age, by maximum follicle-stimulating hormone (FSH). Cancellation rate per cycle and pregnancy, live birth, and miscarriage rates per transfer were compared among women with and without DOR. We used multivariable log binomial regression, stratified by age, to calculate adjusted relative risk (aRR) for the association between DOR and these outcomes and, within DOR groups, between stimulation type and outcomes.
RESULTS:
Cancellation rate/cycle increased with increasing FSH and with DOR severity. For women aged <35 who underzuent transfer, aRR for pregnancy and live birth indicated slightly reduced likelihood of these outcomes (severe vs. no DOR); confidence intervals approached the null. Among women with severe DOR, stimulation type zuas not associated with likelihood of pregnancy or live birth per transfer in any group except women ages 38–40.
CONCLUSION:
Women with severe DOR are at significantly increased risk of cancellation; however, those who undergo transfer have pregnancy and live birth chances similar to those of women without DOR after controlling for cycle characteristics.
Keywords: assisted reproductive techniques, assisted reproductive technology, fertilization in vitro, ovarian reserve, pregnancy outcome, pregnancy rate
In all women, ovarian reserve declines exponentially with advancing age. Among a subset of women this decrease in ovarian potential occurs at a faster rate, resulting in a diagnosis of diminished ovarian reserve (DOR) as defined by low serum anti-Müllerian hormone (AMH) level, elevated serum follicle-stimulating hormone (FSH) level, or low antral follicle count at a relatively young age. Young infertile women with DOR, particularly if it is severe, pose a unique clinical dilemma: Is an attempt at in vitro fertilization (IVF) using autologous oocytes warranted, or does a threshold exist beyond which, regardless of age, the chance of pregnancy approaches zero? If assisted reproductive technology (ART) has potential for success, which ovarian stimulation protocol is best? If a young woman with DOR produces viable embryos for transfer, is her chance of pregnancy comparable to a female her age without diminished reserve? If pregnancy is achieved, are these women at increased risk of miscarriage?
Several relatively small studies have attempted to answer these questions and revealed conflicting results. While some studies found elevated FSH to be predictive of decreased pregnancy rate and increased aneuploidy and miscarriage risk,1–3 others did not find a significant association.4–6 Several studies have suggested that serum FSH above 14 mIU/mL or AMH <0.2 ng/mL is associated with a nearly 0% chance of live birth after IVF,3,7,8 data which are often cited to endorse donor oocyte use for these women. With regards to obstetric outcomes, evidence suggests that elevated FSH is not associated with increased risk for preterm or low birth weight delivery.9
The objectives of this study were to use National ART Surveillance System (NASS) data encompassing fresh autologous IVF cycles from 2002 through 2012 to calculate cancellation rate and pregnancy rate by maximum serum FSH level and to compare outcomes (cancellation rate per cycle and clinical pregnancy, live birth, and miscarriage rates per transfer) for women with severe or moderate DOR to those without DOR, and to assess effects of stimulation type on outcomes among women with severe and moderate DOR, stratified by age.
Materials and Methods
Study data were obtained from NASS, a federally mandated reporting system which collects patient demographics, medical and obstetric history, infertility diagnoses, detailed parameters of each treatment cycle, and, if applicable, the resultant pregnancy outcome for all ART cycles performed in the United States (Fertility Clinic Success Rate and Certification Act of 1992 [FCSRCA], Public Law No. 102–493, October 24, 1992).10 NASS, for which annual validation of 7–10% randomly selected reporting clinics is performed, is estimated to include data from >97% of all ART cycles completed in the United States.10
Among women <41 years old we calculated cancellation rate per cycle by maximum serum FSH level (n=504,266). We also calculated pregnancy rate per transfer by maximum serum FSH, stratified by patient age. Maximum serum FSH is defined as the maximum early follicular phase FSH value to date for an individual patient. We used linear regression models to evaluate trends in the association between serum FSH level and cancellation and pregnancy rates. Cancellation is defined as any cycle for which stimulation is initiated but for which a transfer does not occur; cancellation can occur prior to retrieval or between retrieval and transfer.
For the subsequent analyses our study population was derived from all reported fresh autologous IVF cycles resulting in retrieval for women <41 years (n=688,257) between 2004 and 2012. During those study years NASS defined DOR as “reduced fecundity related to diminished ovarian function, including high FSH or high estradiol measured in the early follicular phase or during a clomiphene challenge test, reduced ovarian volume related to congenital, medical, surgical or other causes, or advanced maternal age (>40).” We excluded all cycles in which the female partner was ≥41, as some women without true clinical DOR may have been grouped as “DOR” based on age alone rather than based on a clinical indicator such as low AMH, elevated serum FSH, or low antral follicle count.
We divided the remaining cycles into groups using a hierarchical classification where diagnosis of DOR was the primary consideration. In the absence of a diagnosis, a combination of oocyte yield and maximum serum FSH were used to assign groups as follows: “No DOR” was defined as no diagnosis of DOR and serum FSH <10 mIU/mL; “Moderate DOR” was defined as diagnosis of DOR and serum FSH between 10–14 mIU/mL, or diagnosis of DOR and 5–9 oocytes retrieved, or no diagnosis of DOR and serum FSH >10 mIU/mL and 5–9 oocytes retrieved; and “Severe DOR” was defined as diagnosis of DOR and serum FSH 15 mIU/mL or greater, or diagnosis of DOR and <5 oocytes retrieved, or no diagnosis of DOR and <5 oocytes retrieved and serum FSH ≥15 mIU/mL. We also divided the cycles by stimulation type, specifically “natural cycle” defined as no stimulation medication, “mild stimulation” defined as oral medication such as clomiphene citrate combined with gonadotropins, and “traditional stimulation” defined as gonadotropins only for stimulation combined with either standard GnRH agonist downregulation, GnRH agonist flare, or GnRH antagonist. We performed a sensitivity analysis with a narrower definition for the stimulation groups: “mild stimulation” defined as oral medication plus 0–1,500 IU of FSH and “full stimulation” as no oral medication plus ≥ 1,501 IU of exogenous FSH medication. After excluding 218,362 cycles that could not be accurately classified into DOR categories, largely due to missing (176,772 cycles) or out-of-range values for FSH (462 cycles with value <0 or >200), our study population included 469,895 cycles.
We compared the distribution of patient and clinical characteristics between severe, moderate, and no DOR groups using Pearson’s χ2 tests. The primary outcomes of interest were clinical pregnancy, live birth at ≥20 weeks gestational age, and miscarriage at <20 weeks gestational age. Bivariate analyses were conducted to explore the relationship between severity of DOR as compared with no DOR, to explore those outcomes, and to assess possible effects of stimulation type (natural cycle and mild stimulation as compared with traditional stimulation) on outcomes among women with moderate or severe DOR. For both analyses we stratified cycles by maternal age at time of oocyte retrieval (<35, 35–37, and 38–40 years).
We used multivariable log binomial regression, stratified by female age (<35, 35–37, 38–40 years) to compare rates of cancellation, pregnancy, live birth, and miscarriage among women with severe or moderate DOR to those with no DOR, and to assess effects of stimulation type on those outcomes, while adjusting for the following maternal and treatment characteristics: infertility diagnosis, cycle history, prior pregnancies, number of oocytes retrieved, use of intracytoplasmic sperm injection (ICSI), use of assisted hatching, number of embryos transferred, embryo stage, and number of embryos cryopreserved; unadjusted and adjusted relative risks with 95% confidence intervals are reported. Calculating outcomes per transfer allowed for adjustment for cycle characteristics such as use of assisted hatching, number of embryos transferred, embryo stage, and number of embryos cryopreserved that would otherwise have not been possible had outcomes been calculated per cycle start or per retrieval.
Statistical significance was determined using an alpha of 0.05. All analyses were conducted using SAS v. 9.3.
This study was approved by the Institutional Review Board of the Centers for Disease Control and Prevention.
Results
Among 504,266 IVF cycles for women aged <41, the cancellation rate per cycle increased with increasing maximum serum FSH from 4.9% for FSH 0–5 mlU/mL to 18.9% for FSH ≥21 mlU/mL (Figure 1A). For women <35 years old, pregnancy rate per transfer decreased from 53.2% for FSH 0–5 mIU/mL to 43.4% for FSH >21 mIU/mL (Figure 1B).
Figure 1.
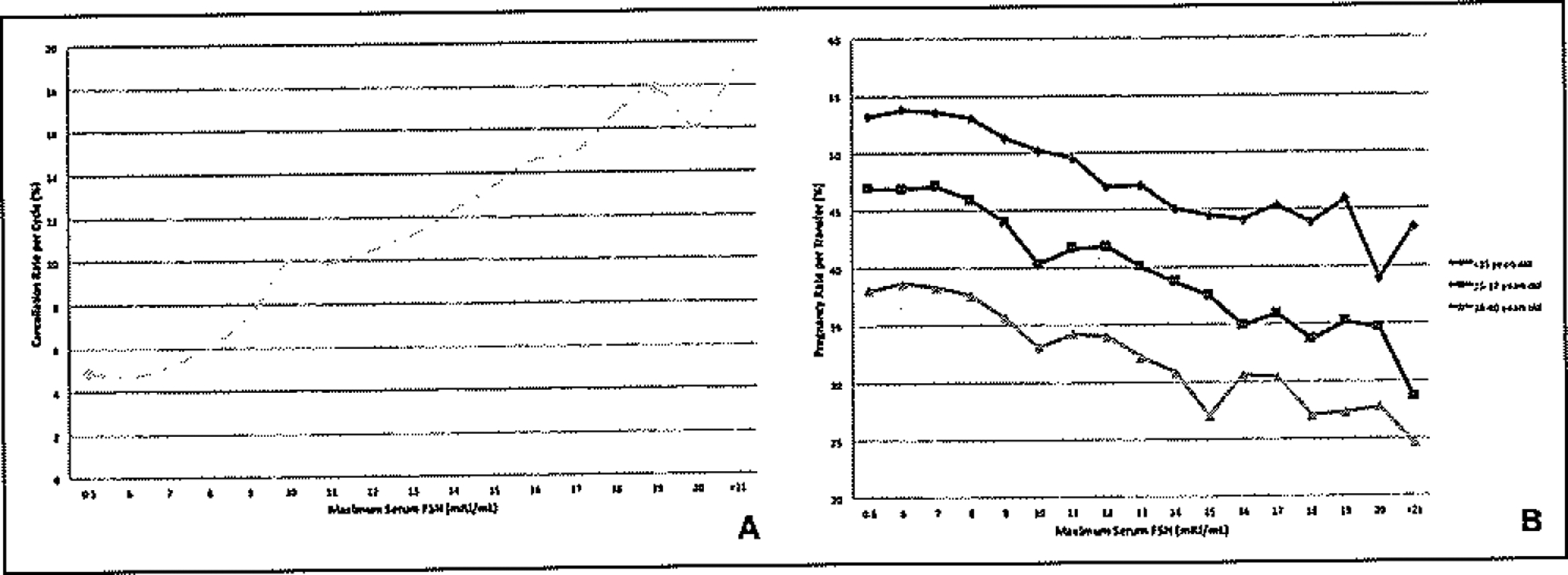
(A) Cancellation rate by maximum serum FSH, fresh IVF cycles, all stimulation regimens, 2004–2012. All p values for trend <0.0001. (B) Clinical pregnancy rate by maximum serum FSH, stratified by patient age, fresh IVF cycles, all stimulation regimens, 2004–2012. All p values for trend <0.0001.
Population Characteristics
Among fresh autologous IVF cycles resulting in retrieval for women aged <41 (n=469,895), 5.8% of cycles (n=27,484) were characterized as severe DOR, 15.2% (n=71,410) as moderate DOR, and 79% (n=371,001) as no DOR (Table I). A higher percentage of older women were in the severe and moderate DOR groups than in the no DOR group. Race and obstetric history were similarly distributed among the 3 groups. BMI values in the overweight and obese ranges were more common in the no DOR group. A concomitant infertility diagnosis of ovulatory dysfunction was less common in the severe and moderate DOR groups versus the no DOR group (4.22% and 5.51% vs. 18.48%). Prior ART with no previous live births was more common in the severe and moderate DOR groups. Cancellation of cycles between retrieval and transfer, use of natural cycle protocol, retrieval of 0–4 oocytes, use of ICSI, use of assisted hatching, transfer of 0–1 embryos, transfer of cleavage stage embryo, and having zero embryos available for cryopreservation were more common in the severe DOR group than in the moderate or no DOR groups.
Table I.
Patient and Cycle Characteristics by Degree of DOR Among Women Age <41, Fresh Autologous IVF Cycles, 2004–2012
| Patient characteristic | Severe DOR N = 27,484 No. (%) |
Moderate DOR N = 71,410 No. (%) |
No DOR N = 371,001 No. (%) |
|---|---|---|---|
| Female age | |||
| < 35 | 6,394 (23.26) | 21,113 (29.57) | 205,901 (55.60) |
| 35–37 | 7,824 (28.47) | 20,626 (28.88) | 95,400 (25.76) |
| 38–40 | 13,266 (48.27) | 29,671 (41.55) | 67,027 (18.64) |
| Race/ethnicity | |||
| Non-Hispanic White | 12,415 (70.28) | 34,638 (73.67) | 180,560 (72.50) |
| Non-Hispanic Black | 1,109 (6.28) | 2,645 (5.63) | 16,707 (6.71) |
| Asian/Pacific Islander | 2,682 (15.18) | 6,238 (13.27) | 28,859 (11.59) |
| Hispanic | 1,424 (8.06) | 3,409 (7.25) | 22,361 (8.98) |
| Other | 36 (0.20) | 86 (0.18) | 558 (0.22) |
| BMI categories (kg/m2) | |||
| < 18.5 (underweight) | 607 (4.14) | 1,390 (3.61) | 557,364 (2.89) |
| 18.5–24.9 (normal) | 8,949 (61.04) | 23,580 (61.25) | 10,284 (54.57) |
| 25.0–29.9 (overweight) | 3,086 (21.05) | 8,339 (21.66) | 45,062 (23.36) |
| ≥ 30 (obese) | 2,020 (13.78) | 5,187 (13.47) | 37,019 (19.19) |
| Missing (not collected prior to 2007) | 12,822 | 32,914 | 178,063 |
| Concomitant infertility diagnosis | |||
| Male factor | 8,279 (30.12) | 24,635 (34.50) | 154,126 (41.54) |
| Endometriosis | 3,192 (11.61) | 8,527 (11.94) | 47,129 (12.70) |
| Ovulatory dysfunction | 11,960 (4.22) | 3,938 (5.51) | 68,569 (18.48) |
| Tubal factor | 3,821 (13.90) | 10,505 (14.71) | 71,854 (19.37) |
| Uterine factor | 1,318 (4.80) | 3,527 (4.94) | 17,661 (4.76) |
| Unexplained | 391 (1.42) | 3,200 (4.48) | 58,545 (15.78) |
| Cycle history | |||
| First IVF, no previous LB | 10,091 (36.83) | 28,433 (39.93) | 180,208 (48.71) |
| First IVF, ≥ 1 previous LB | 2,848 (10.39) | 8,222 (11.55) | 47,689 (12.89) |
| ≥ 1 previous IVF, no previous LB | 9,884 (36.07) | 22,887 (32.14) | 90,316 (24.41) |
| ≥ 1 previous IVF, ≥ 1 previous LB | 4,577 (16.70) | 11,664 (16.38) | 51,764 (13.99) |
| No. of prior spontaneous abortions | |||
| 0 | 19,078 (69.63) | 48,808 (68.58) | 264,198 (71.53) |
| 1 | 5,415 (19.76) | 14,503 (20.38) | 68,304 (18.49) |
| 2+ | 2,905 (10.60) | 7,857 (11.04) | 36,865 (9.98) |
| No. of prior preterm births | |||
| 0 | 26,730 (97.73) | 69,428 (97.73) | 359,034 (97.41) |
| 1 | 567 (2.07) | 1,440 (2.04) | 8,486 (2.30) |
| 2+ | 55 (0.20) | 167 (0.24) | 1,066 (0.29) |
| No. of prior full term births | |||
| 0 | 20,425 (74.52) | 52,551 (73.76) | 277,451 (75.00) |
| 1 | 5,410 (19.74) | 14,752 (20.71) | 69,315 (18.74) |
| 2+ | 1,574 (5.74) | 3,940 (5.53) | 3,189 (6.27) |
| Cycle cancellation between retrieval and transfer | |||
| Yes | 5,973 (21.73) | 5,970 (8.36) | 24,278 (6.54) |
| No | 21,511 (78.27) | 65,440 (91.64) | 346,723 (93.46) |
| Stimulation type | |||
| None (natural cycle) | 823 (3.05) | 244 (0.34) | 1,096 (0.30) |
| Mild stimulation | 1,484 (5.50) | 1,655 (2.33) | 2,418 (0.66) |
| Traditional stimulation | 24,676 (91.45) | 69,065 (97.32) | 365,545 (99.05) |
| No. of oocytes retrieved | |||
| 0–4 | 22,087 (80.37) | 8,081 (11.32) | 23,736 (6.40) |
| 5–9 | 3,814 (13.88) | 54,371 (76.14) | 88,476 (23.85) |
| ≥ 10 | 1,581 (5.75) | 8,958 (12.54) | 258,788 (69.75) |
| No. of embryos transferred | |||
| 0–1 | 7,331 (34.06) | 8,560 (13.08) | 39,339 (11.34) |
| 2 | 8,521 (39.58) | 27,046 (41.32) | 194,940 (56.21) |
| 3 | 4,464 (20.74) | 20,415 (31.19) | 81,512 (23.5) |
| > 3 | 1,210 (5.62) | 9,430 (14.41) | 31,011 (8.94) |
| Use of ICSI* | |||
| Yes | 16,167 (75.29) | 47,789 (73.08) | 253,779 (73.23) |
| No | 5,307 (24.71) | 17,604 (26.92) | 92,774 (26.77) |
| Use of assisted hatching* | |||
| Yes | 13,539 (62.94) | 36,607 (55.94) | 131,326 (37.88) |
| No | 7,972 (37.06) | 28,833 (44.06) | 215,395 (62.12) |
| Embryo stage at transfer* | |||
| Days 2/3 | 18,234 (84.92) | 49,585 (75.85) | 196,913 (56.86) |
| Days 5/6 | 2,506 (11.67) | 13,891 (21.25) | 139,875 (40.39) |
| Other days | 733 (3.41) | 1,893 (2.9) | 9,522 (2.75) |
| No. of supernumerary embryos cryopreserved* | |||
| 0 | 19,921 (92.61) | 54,050 (82.59) | 19,6501 (56.67) |
| 1–2 | 970 (4.51) | 7,000 (10.7) | 54,044 (15.59) |
| 3–4 | 279 (1.3) | 2,848 (4.35) | 42,644 (12.3) |
| 5+ | 341 (1.59) | 1,542 (2.36) | 53,532 (15.44) |
Per noncancelled cycles for which a transfer was performed. χ2 p value <0.0001 for all comparisons except uterine factor.
LB = live birth.
Outcomes: Severe Versus No DOR
Among women <35 years old, severe DOR was associated with a significantly higher chance of cancellation after cycle start as compared to no DOR (37.7% vs. 5.8%, aRR=6.4, CI 6.2–6.6) (Table II). Among women <35 years old who made it to transfer, absolute pregnancy and live birth rates were lower in those with severe DOR than in those without DOR; upper confidence limits of adjusted relative risk estimates approached the null value (aRR, 95% CI=0.94, 0.91–0.98 and 0.95, CI 0.91–0.99 for pregnancy and live birth, respectively). A similar pattern was noted for moderate DOR, as well as for ages 35–37 and 38–40 years, although among the older age groups fewer differences were statistically significant. In all age groups, no significant relationship was noted between diagnosis and miscarriage.
Table II.
Pregnancy Outcomes per Transfer Stratified by Age in Severe DOR and Moderate DOR Versus No DOR Among Fresh Autologous IVF Cycles, 2004–2012
| Outcome by age group | Severe DOR | Moderate DOR | No DOR | ||||
|---|---|---|---|---|---|---|---|
| No. (%) | RR (95% CI) | aRR (95% CI) | No. (%) | RR (95% CI) | aRR (95% CI) | No. (%) | |
| < 35 years old | |||||||
| Cancellationa | 3,874 (37.73) | 6.47 (6.27–6.66) | 6.42 (6.21–6.63) | 1,442 (6.39) | 1.10 (1.04–1.16) | 1.10 (1.04–1.16) | 12,779 (5.83) |
| Pregnancyb | 1,876 (36.72) | 0.68 (0.66–0.71) | 0.94 (0.91–0.98) | 8,843 (45.16) | 0.84 (0.83–0.86) | 0.95 (0.94–0.97) | 103,677 (53.62) |
| Live birth (≥ 20 wks)b | 1,598 (31.28) | 0.67 (0.65–0.70) | 0.95 (0.91–0.99) | 7,621 (38.92) | 0.84 (0.82–0.85) | 0.96 (0.94–0.97) | 89,879 (46.48) |
| Miscarriage (< 20 wks)c | 237 (12.63) | 1.12 (0.99–1.26) | 0.96 (0.84–1.09) | 1,072 (12.12) | 1.07 (1.01–1.14) | 1.01 (0.94–1.07) | 11,696 (11.28) |
| 35–37 years old | |||||||
| Cancellationa | 5,077 (39.35) | 4.96 (4.81–5.11) | 4.82 (4.66–4.98) | 1,846 (8.21) | 1.03 (0.98–1.08) | 1.02 (0.97–1.07) | 8,244 (7.94) |
| Pregnancyb | 1,961 (31.63) | 0.67 (0.65–0.70) | 0.97 (0.93–1.01) | 7,616 (40.14) | 0.85 (0.84–0.87) | 0.97 (0.95–0.99) | 42,149 (47.12) |
| Live birth (≥ 20 wks)b | 1,540 (24.84) | 0.64 (0.61–0.67) | 0.97 (0.92–1.02) | 6,258 (32.98) | 0.85 (0.83–0.87) | 0.98 (0.96–1.00) | 34,678 (38.77) |
| Miscarriage (< 20 wks)c | 367 (18.71) | 1.21 (1.10–1.34) | 0.98 (0.88–1.10) | 1,218 (15.99) | 1.04 (0.98–1.10) | 0.96 (0.90–1.03) | 6,496 (15.41) |
| 38–40 years old | |||||||
| Cancellationa | 9,000 (40.42) | 4.18 (4.07–4.30) | 4.06 (3.94–4.18) | 3,180 (9.68) | 1.00 (0.96–1.04) | 0.98 (0.94–1.02) | 7,396 (11.45) |
| Pregnancyb | 2,454 (24.05) | 0.61 (0.59–0.63) | 0.95 (0.91–0.99) | 8,848 (32.91) | 0.84 (0.82–0.85) | 0.96 (0.94–0.99) | 25,152 (39.35) |
| Live birth (≥ 20 wks)b | 1,696 (16.62) | 0.56 (0.54–0.59) | 0.95 (0.90–1.00) | 6,390 (23.77) | 0.81 (0.79–0.83) | 0.95 (0.92–0.98) | 18,832 (29.46) |
| Miscarriage (< 20 wks)c | 680 (27.71) | 1.23 (1.15–1.32) | 1.02 (0.94–1.11) | 2,199 (24.85) | 1.11 (1.06–1.15) | 1.05 (0.99–1.10) | 5,666 (22.53) |
Per all cycles initiated. Cancellation models adjusted for infertility diagnosis, cycle history, and prior pregnancies.
Per noncancelled cycles for which a transfer was performed.
Per cycles that resulted in pregnancy.
Models adjusted for infertility diagnosis, cycle history, prior pregnancies, number of oocytes retrieved, use of intracytoplasmic sperm injection, use of assisted hatching, number of embryos transferred, embryo stage at transfer, and number of embryos cryopreserved.
Outcomes: By Stimulation Type
Among women in all age groups with severe DOR who underwent embryo transfer, traditional stimulation (as compared to mild stimulation or natural cycle) was associated with a higher absolute percentage of pregnancy and live birth; however, after adjusting for cycle characteristics, the only significant difference between different stimulation types was noted for women ages 38–40, for whom natural cycle was associated with a significantly increased likelihood of pregnancy (aRR=1.68, CI 1.37–2.07), but not live birth (aRR=1.35, CI 0.99–1.83) (Table III). Among women with moderate DOR, mild stimulation was associated with a lower chance of live birth for women <35 years of age (aRR=0.84,95% CI 0.71–0.99), and lower chance of pregnancy (aRR=0.86, 95% CI 0.75–0.97) and live birth (aRR=0.84, 95% CI 0.71–0.99) for women aged 38–40.
Table III.
Pregnancy Outcomes by Stimulation Type (Natural and Mild Stimulation Versus Traditional) Among Moderate DOR and Severe DOR, Stratified by Age, Fresh Autologous IVF Cycles, 2004–2012
| Outcome by age group | Natural cycle | Mild stimulation | Traditional stimulation | ||||
|---|---|---|---|---|---|---|---|
| No. (%) | RR (95% CI) | aRR (95% CI) | No. (%) | RR (95% CI) | aRR (95% CI) | No. (%) | |
| Severe DOR | |||||||
| < 35 years old | |||||||
| Pregnancya | 27 (26.73) | 0.72 (0.52–0.99) | 1.10 (0.80–1.52) | 73 (30.17) | 0.81 (0.66–0.98) | 1.00 (0.82–1.21) | 1,753 (37.34) |
| Live birth (≥ 20 wks)a | 23 (22.77) | 0.72 (0.50–1.03) | 1.16 (0.81–1.67) | 62 (25.62) | 0.81 (0.65–1.00) | 1.02 (0.82–1.26) | 1,492 (31.78) |
| Miscarriage (< 20 wks)b | < 5 | n/a | n/a | 8 (10.96) | n/a | n/a | 223 (12.72) |
| 35–37 years old | |||||||
| Pregnancya | 36 (25.35) | 0.79 (0.59–1.05) | 1.14 (0.85–1.52) | 70 (24.82) | 0.77 (0.63–0.95) | 0.95 (0.78–1.16) | 1,826 (32,15) |
| Live birth (≥ 20 wks)a | 27 (19.01) | 0.75 (0.53–1.06) | 1.14 (0.80–1.61) | 57 (20.21) | 0.80 (0.63–1.01) | 0.99 (0.78–1.24) | 1,436 (25.28) |
| Miscarriage (< 20 wks)b | 8 (22.22) | 1.19 (0.64–2.21) | 0.95 (0.48–1.89) | 10 (14.29) | 0.75 (0.42–1.35) | 0.72 (0.40–1.29) | 341 (18.67) |
| 38–40 years old | |||||||
| Pregnancya | 74 (25.78) | 1.06 (0.87–1.29) | 1.68 (1.37–2.07) | 97 (20.12) | 0.83 (0.69–0.99) | 0.90 (0.75–1.08) | 2,254 (24.31) |
| Live birth (≥ 20 wks)a | 39 (13.59) | 0.82 (0.65–1.03) | 1.35 (0.99–1.83) | 67 (13.90) | 0.82 (0.65–1.03) | 0.91 (0.73–1.14) | 1,574 (16.98) |
| Miscarriage (< 20 wks)b | 26 (35.14) | 1.29 (0.94–1.76) | 1.06 (0.75–1.50) | 27 (27.84) | 1.02 (0.73–1.41) | 0.99 (0.71–1.38) | 616 (27.33) |
| Moderate DOR | |||||||
| < 35 years old | |||||||
| Pregnancya | 12 (41.38) | 0.91 (0.59–1.41) | 1.05 (0.70–1.58) | 127 (36.92) | 0.82 (0.71–0.94) | 0.93 (0.82–1.07) | 8,653 (45.28) |
| Live birth (≥ 20 wks)a | 11 (37.93) | 0.97 (0.61–1.55) | 1.13 (0.73–1.74) | 98 (28.49) | 0.73 (0.62–0.86) | 0.84 (0.71–0.99) | 7,470 (39.09) |
| Miscarriage (< 20 wks)b | < 5 | n/a | n/a | 25 (19.69) | n/a | n/a | 1,038 (12.00) |
| 35–37 years old | |||||||
| Pregnancya | 15 (37.50) | 0.93 (0.64–1.39) | 1.40 (0.98–1.99) | 115 (33.24) | 0.83 (0.71–0.96) | 0.96 (0.83–1.11) | 7,432 (40.22) |
| Live birth (≥ 20 wks)a | 11 (27.50) | 0.83 (0.50–1.38) | 1.30 (0.82–2.08) | 98 (28.32) | 0.86 (0.72–1.02) | 1.01 (0.86–1.19) | 6,103 (33.03) |
| Miscarriage (< 20 wks)b | < 5 | n/a | n/a | 15 (13.04) | n/a | n/a | 1,194 (16.07) |
| 38–40 years old | |||||||
| Pregnancya | 23 (25.84) | 0.78 (0.55–1.11) | 1.10 (0.79–1.54) | 167 (25.30) | 0.76 (0.67–0.87) | 0.86 (0.75–0.97) | 8,584 (33.09) |
| Live birth (≥ 20 wks)a | 14 (15.73) | 0.66 (0.41–1.06) | 1.00 (0.63–1.61) | 116 (17.58) | 0.73 (0.62–0.87) | 0.84 (0.71–0.99) | 6,202 (23.91) |
| Miscarriage (< 20 wks)b | 8 (34.78) | 1.40 (0.80–2.45) | 1.10 (0.62–1.96) | 45 (26.95) | 1.08 (0.84–1.40) | 1.01 (0.79–1.31) | 2,133 (24.85) |
Per noncancelled cycles for which a transfer was performed.
Per cycles that resulted in pregnancy.
Models adjusted for infertility diagnosis, cycle history, prior pregnancies, number of oocytes retrieved, use of ICSI, use of assisted hatching, number of embryos transferred, embryo stage, and number of embryos cryopreserved.
In a subgroup sensitivity analysis with more stringent definitions for stimulation type, the findings were similar—traditional stimulation was associated with a higher absolute chance of pregnancy and live birth per transfer; however, after adjusting for cycle characteristics, no significant differences in pregnancy or live birth were found between different stimulation types in any age group. Additionally, a similar pattern was noted when results were tabulated per retrieval rather than per transfer.
Discussion
Although increasing maximum serum FSH is associated with increasing risk of cancellation, an FSH threshold was not found at which pregnancy rate approaches zero. These results are in contrast to several smaller studies that have suggested serum FSH >14 mIU/mL or AMH <0.2 ng/mL is associated with a nearly 0% chance of live birth after IVF.3,7,8 Overall, our results show that, among women who avoid cancellation and make it to transfer, only minimal differences in the likelihood of live birth exist between women with and without DOR, regardless of severity.
While cancellation rate was higher and absolute chance of pregnancy was lower in the severe DOR group, adjusted rate ratio estimates for pregnancy, live birth, and miscarriage outcomes overall suggest little difference between women with and without severe DOR who undergo embryo transfer. This supports prior studies that did not find a significant association between elevated FSH and decreased pregnancy rate4–6 and suggests that those who found elevated FSH to be predictive of decreased pregnancy rate and increased aneuploidy and miscarriage risk1–3 may not have controlled for confounding factors such as age. The large sample size and inclusion of all reporting U.S. clinics allowed for an analysis of a larger portion of young women with severe DOR and, as a result, an ability to investigate outcomes specifically among young women with severe DOR.
Among women of all ages <41 who proceed with autologous IVF and reach embryo transfer, traditional stimulation (as compared to mild stimulation or natural cycle) was associated with a higher absolute percentage of cycles resulting in live birth; however, after adjusting for cycle characteristics, no significant association was noted between stimulation type and live birth among women with severe DOR. The absolute pregnancy rates among natural cycles may initially appear higher than one would expect; however, the rates are reported per transfer rather than per cycle start. Among patients with severe DOR able to successfully complete an embryo transfer, the stimulation protocols were not associated with a lesser or greater chance of pregnancy overall. Admittedly, our data lack detail regarding nuance of stimulation protocols such as use of adjunct medications like growth hormone and dehydroepiandrosterone (DHEAS). However, it is somewhat reassuring that no one class of protocols consistently demonstrates clear advantage over another as long as the patient is able to reach embryo transfer.
Limitations of our study include cycle- rather than patient-based data collection, lack of embryo quality data, exclusion of a large proportion of data as a result of our classification method and limited available cycle characteristics that pertain to DOR, and the retrospective observational nature of the study. To minimize the effects of these limitations, we were able to control for number of prior failed IVF cycles and for number of supernumerary embryos cryopreserved, which has been shown to correlate with embryo quality.11 Additionally, randomly selected clinics undergo annual validation that has shown overall discrepancy rates <5%. Admittedly, the DOR discrepancy rate was 6.5% in 201210; part of the rationale for our defining characteristics was to more accurately capture patients’ ovarian reserve. Some of the classification limitations have been described previously12 and may be improved in the future; while the current NASS collection system lacks characteristics such anti-Müllerian hormone level and considers age >40 to be diagnostic of DOR, revisions to the collection questionnaire that address these weaknesses are in progress.
Strengths of our study include its large sample size and generalizability resultant from its inclusion of clinics throughout the United States, the proven validity of the reported data, and the ability to control for demographic and cycle characteristics, namely age, concomitant infertility diagnosis, ART cycle history, obstetric history, number of oocytes retrieved, use of ICSI, use of assisted hatching, number of embryos transferred, embryo stage, and number of embryos cryopreserved.
Providing the option of IVF using autologous oocytes to young women with severe DOR who desire a biologically related child is likely warranted; while risk of cancellation increases with increasing serum FSH, no clear serum FSH threshold was found beyond which the chance of pregnancy with IVF approaches zero, suggesting that ART has potential for success even among women with severe DOR. In this population no particular ovulation protocol was consistently associated with improved outcomes. Overall, our results indicate that the chance of pregnancy among young women with severe DOR who are able to complete embryo transfer is comparable to that of women of the same age and with similar characteristics but without diminished reserve. Moreover, once pregnancy was achieved, women with DOR were not at increased risk of miscarriage as compared to women of similar age without DOR.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Presented as a poster at the American Society for Reproductive Medicine National Meeting, Baltimore, Maryland, October 17–21, 2015.
Financial Disclosure: The authors have no connection to any companies or products mentioned in this article.
References
- 1.Stem JE, Brown MB, Wantman E, et al. : Live birth rates and birth outcomes by diagnosis using linked cycles from the SART CORS database. J Assist Reprod Genet 2013;30:1445–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz-Jaffe MG, Surrey ES, Minjarez DA, et al. : Association of abnormal ovarian reserve parameters with a higher incidence of aneuploid blastocysts. Obstet Gynecol 2013;121:71–77 [DOI] [PubMed] [Google Scholar]
- 3.Levi AJ, Raynault MF, Bergh PA, et al. : Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril 2001;76:666–669 [DOI] [PubMed] [Google Scholar]
- 4.Szafarowska M, Molinska-Glura M, Jerzak MM: Anti-Mullerian hormone concentration as a biomarker of pregnancy success or failure. Neuro Endocrinol Lett 2014;35:322–326 [PubMed] [Google Scholar]
- 5.Massie JA, Burney RO, Miiki AA, et al. : Basal follicle-stimulating hormone as a predictor of fetal aneuploidy. Fertil Steril 2008;90:2351–2355 [DOI] [PubMed] [Google Scholar]
- 6.De Sutter P, Dhont M: Poor response after hormonal stimulation for in vitro fertilization is not related to ovarian aging. Fertil Steril 2003; 79:1294–1298 [DOI] [PubMed] [Google Scholar]
- 7.Joiner LL, Robinson RD, Bates W, et al. : Establishing institutional critical values of follicle-stimulating hormone levels to predict in vitro fertilization success. Mil Med 2007;172:202–204 [DOI] [PubMed] [Google Scholar]
- 8.Merhi Z, Zapantis A, Berger DS, et al. : Determining an anti-Mullerian hormone cutoff level to predict clinical pregnancy following in vitro fertilization in women with severely diminished ovarian reserve. J Assist Reprod Genet 2013;30:1361–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calhoun KC, Fritz MA, Steiner AZ: Examining the relationship between ovarian reserve, as measured by basal FSH levels, and the risk of poor obstetric outcome in singleton IVF gestations. Hum Reprod 2011;26:3424–3430 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention ASRM, Society for Assisted Reproductive Technology: 2010 Assisted Reproductive Technology Fertility Clinic Success Rates Report. Atlanta, U.S. Department of Health and Human Services, 2012 [Google Scholar]
- 11.Hill MJ, Richter KS, Heitmann RJ, et al. : Number of supernumerary vitrified blastocysts is positively correlated with implantation and live birth in single-blastocyst embryo transfers. Fertil Steril 2013;99: 1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devine K, Mumford SL, Wu M, et al. : Diminished ovarian reserve in the United States assisted reproductive technology population: Diagnostic trends among 181,536 cycles from the SART Clinic Outcomes Reporting System. Fertil Steril 2015;104:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]


