Abstract
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 is an important food-borne pathogen in industrialized countries. We developed a rapid and simple test for detecting E. coli O157:H7 using a method based on restriction site polymorphisms. Restriction-site-specific PCR (RSS-PCR) involves the amplification of DNA fragments using primers based on specific restriction enzyme recognition sequences, without the use of endonucleases, to generate a set of amplicons that yield “fingerprint” patterns when resolved electrophoretically on an agarose gel. The method was evaluated in a blinded study of E. coli isolates obtained from environmental samples collected at beef cattle feedyards. The 54 isolates were all initially identified by a commonly used polyclonal antibody test as belonging to O157:H7 serotype. They were retested by anti-O157 and anti-H7 monoclonal antibody enzyme-linked immunosorbent assay (ELISA). The RSS-PCR method identified all 28 isolates that were shown to be E. coli O157:H7 by the monoclonal antibody ELISA as belonging to the O157:H7 serotype. Of the remaining 26 ELISA-confirmed non-O157:H7 strains, the method classified 25 strains as non-O157:H7. The specificity of the RSS-PCR results correlated better with the monoclonal antibody ELISA than with the polyclonal antibody latex agglutination tests. The RSS-PCR method may be a useful test to distinguish E. coli O157:H7 from a large number of E. coli isolates from environmental samples.
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 has received much attention in recent years as the cause of numerous food-borne diarrhea outbreaks in developed countries. First identified in 1982 as an etiologic agent of hemorrhagic colitis (22), E. coli O157:H7 is now a major public health problem, causing an estimated 20,000 infections and 250 deaths per year in the United States (2). While most outbreaks have been associated with the consumption of beef and dairy products, outbreaks related to contaminated apple juice (16), alfalfa sprouts (4), and a water park (13) have been documented. The pathogenesis of E. coli O157:H7 is not clearly understood, but it is believed to involve a number of specialized virulence factors, including Shiga-like toxins (SLTs), adherence factors, and a plasmid-encoded hemolysin (17). Although other bacterial pathogens such as Shigella and Campylobacter spp. also are associated with bloody diarrhea in the United States, E. coli O157:H7 is now the agent most commonly isolated from fecal specimens containing blood (23).
Due to the rising incidence of E. coli O157:H7 infections in the United States and, thus, the need for improved epidemiologic surveillance, the development of simple and rapid E. coli O157:H7 detection methods is of utmost importance. Many assays have been developed for isolating and identifying the organism in food and clinical specimens. Culture methods based on biochemical characteristics, such as the inability of E. coli O157:H7 to ferment sorbitol on sorbitol MacConkey agar, are frequently used in clinical laboratories (17). Serological techniques such as enzyme-linked immunosorbent assay (ELISA) (6, 9, 21), dipstick immunoassays (14), and other antibody-based methods for detection of E. coli O157:H7 have also been developed (1, 5). More recently, the development of molecular approaches and PCR-based methods, which detect E. coli O157:H7 based on the presence or absence of specific virulence genes such as the stx and eaeA genes, have been described (3, 8–10, 33). Oberst et al. developed a PCR-based method that incorporates fluorogenic probes in a 5′ nuclease assay and have shown it to be rapid and specific in the detection of E. coli O157:H7 from environmental samples (18). While these methods have allowed for improved detection, many such techniques are labor-intensive, time-consuming, expensive, or often not specific enough for accurate identification. For example, antibodies often cross-react with various antigens, and many E. coli serotypes other than O157:H7 are known to produce verotoxins (19).
This study reports a simple method called restriction-site-specific-PCR (RSS-PCR) for the detection of E. coli O157:H7 strains. RSS-PCR is a technique that is based on the principle of restriction fragment length polymorphism (RFLP) but which is unique in that it does not require the use of restriction endonucleases. The RSS-PCR method is based on the use of primers that are homologous to specific restriction enzyme recognition sequences that are 10 to 18 bp long. The primers are designed in such a way that they will amplify genomic DNA segments that lie between the restriction site sequences on which the primers are based. The rationale for this procedure is that genetically different bacteria exhibit variations in the numbers and locations of different restriction site sequences throughout the genome. Harris et al. (11) applied the technique to dengue virus for differentiating strains belonging to serotypes 2 and 3 and have shown that RSS-PCR has a level of discriminatory power comparable to a more labor-intensive subtyping method, which involves nucleotide sequencing of the dengue virus envelope (E) gene. The application of this method allows amplification of fragments of various lengths, yielding a unique collection of DNA fragments or “fingerprint” pattern for each different serotype. Thus, the RSS-PCR method can be used as a rapid and specific screening assay for E. coli O157:H7 isolates from food and clinical samples.
MATERIALS AND METHODS
Bacterial strains.
The E. coli O157:H7 strains F4637, F4761, G5244, H2294, H2493, and H2548 from six distinct domestic outbreaks were provided by the Centers for Disease Control and Prevention (CDC), Atlanta, Ga. The 54 E. coli feedlot isolates are environmental sample isolates from beef cattle feedyards in Kansas, obtained from Kansas State University. Representative E. coli strains from each of the other major enteric pathogenic E. coli groups (enteropathogenic E. coli [EPEC] [O55:H7, O111:NM], ETEC [H10407], enteroinvasive E. coli [strain 1], and enteroaggregative E. coli [strain 25-2]) and Salmonella enterica serovar Enteritidis strain phage types 4 and 8 were obtained from the CDC. To grow the bacteria, we picked a loopful of a bacterial colony from a tryptic soy agar slant or plate and inoculated it into Luria broth (LB). All bacterial strains were grown in 2 ml of LB broth overnight at 37°C on a shaker until the bacteria reached stationary phase (optical density at 600 nm [OD600] of 3.0 to 4.0; CFU of ∼108/ml). The overnight culture was pelleted and resuspended in 1 ml of distilled water. The reconstituted bacterial pellet was diluted 10-fold and boiled for 10 to 15 min and immediately frozen for at least 20 min at −70°C. The boiled and frozen bacterial preparation was thawed at room temperature prior to use for PCR.
Primer design.
The primers used in this study were based on the restriction enzyme recognition sequences of BbvI and TaqII found in the chuA gene of E. coli O157:H7 strain EDL933 (GenBank accession U67920). chuA encodes a 69-kDa outer membrane heme receptor that has been shown to be responsible for iron transport and is specific to E. coli (27). We identified four BbvI recognition sites and three TaqII recognition sites within the chuA gene. Primer 1 (EC-1), an 18-mer, was designed to hybridize to the recognition sequence of BbvI. Primer 2 (EC-2), an 18-mer, was designed to hybridize to the recognition sequence of TaqII. The sequences of the two primers are as follows: EC-1, 5′-GGC-AGC-CAG-CAT-TTT-TTA; EC-2, 5′-CAC-CCA-ACA-GAG-AAG-CCA.
PCR amplification.
All PCR assays were performed in 50-μl volumes containing 2.5 mM MgCl2, 10 mM Tris-HCl, 50 mM KCl, 10% dimethyl sulfoxide, 0.8 mM of each deoxynucleoside triphosphate (dATP, dCTP, dGTP, and dTTP), 4 μM concentrations of each primer, 5 U of Taq DNA polymerase (AmpliTaq; Perkin-Elmer/Roche, Foster City, Calif.) and 5 μl of bacterial template DNA. The reactions were performed in an automated DNA Thermal Cycler, Model 480 (PE Biosystems, Foster City, Calif.). Each reaction was carried out with an initial denaturation step of 5 min at 95°C, followed by 35 cycles of denaturation for 1 min at 94°C, 2 min of annealing at 42°C, and 5 min of primer extension at 72°C. A final extension step of 10 min at 72°C was carried out at the end to ensure complete amplification. Each reaction included a negative control, which was a reaction mixture that did not include a template DNA, and positive controls that included purified extracted DNA from a known strain of E. coli O157:H7, as well as E. coli O157:H7 bacterial cells subjected to the same treatment as the test E. coli samples prior to the PCR test. All mixtures were prepared in a UV-irradiated, PCR-dedicated biosafety cabinet, using filtered, aerosol-resistant pipettor tips (ART; Molecular BioProducts) to prevent carryover contaminations.
PCR amplification products were resolved on a 1.5% agarose gel and stained with ethidium bromide for 30 min to 1 h. Each gel was electrophoresed for 10 min at 40 V and then for 45 or 75 min at 100 V. We compared these two time periods of electrophoresis to determine if the generated patterns improved the discriminatory power of the technique. The stained gels were visualized by UV illumination and photographed. Each gel included a DNA molecular weight standard (1-kb ladder; Gibco-BRL, Grand Island, N.Y.). Resulting patterns were compared to each other and to the prototype O157:H7 pattern by visualization.
Serotyping and ELISA for E. coli feedlot strains.
At Kansas State University, the presence of E. coli O157:H7 in environmental samples collected at beef cattle feedyards was determined by using standard procedures, including enrichment, isolation on sorbitol MacConkey agar (Difco), biochemical confirmation by use of API20e strips (BioMierieux Vitek), and serological testing by latex agglutination (Remel). All 54 isolates were identified initially as O157:H7 based on these tests. Subsequently, at U.S. Department of Agriculture (Albany, Calif.), we reanalyzed the isolates in an ELISA using bacterial cells as antigen and anti-O157 and anti-H7 monoclonal antibodies (MAbs). Each of the strains was grown on LB agar overnight at 37°C. Cells were then harvested with a plastic loop and suspended in 10 mM phosphate-buffered saline (pH 7.4) to an OD620 of 0.2 to 0.3 (∼108 cells/ml). The suspension was incubated in a 55°C water bath for 30 min, and then 70 μl was added to microtiter plate wells (Maxisorp; Nalge Nunc, Inc., Naperville, Ill.). The plates were incubated overnight (12 to 20 h) at 40°C in a drying oven. The wells were rinsed twice with distilled and deionized (DD) water (18 mohm resistance) to remove salts and unbound cells. Then, 200 μl of a blocking solution (0.5% casein, 10 mM Tris-HCl, 150 mM NaCl, 5 mM MgCl2, 0.05% Tween 20, 30 mM sodium azide; pH 7.4) (modified from reference 15) was added to each well, and the plates were incubated for 1 h. The wells were emptied, washed with DD water, and used immediately or dried, placed in a plastic bag, and stored at 4°C for up to 3 weeks. The anti-O157 MAb 13B3 (30) and anti-H7 MAb 2B7 (12) were diluted in 10 mM Tris-HCl–150 mM NaCl–1% bovine serum albumin–0.05% Tween 20 (pH 7.4; TBS-BSA); a 70-μl aliquot of this solution was then added to the wells, and they were incubated for 1 h at room temperature (RT). The wells were washed three times with DD water, and 70 μl of alkaline phosphatase-conjugated rabbit anti-mouse immunoglobulin G (H+L; Zymed, South San Francisco, Calif.) diluted 1:1,000 in TBS-BSA was added to wells; the wells were then incubated 1 h at RT. The wells were washed three times with DD water, and 70 μl of a 1-mg/ml p-nitrophenylphosphate substrate mixture (Sigma, St. Louis, Mo.) diluted in 1 M diethanolamine–0.5 mM MgCl2 (pH 9.8) was added to the wells. The OD405 was measured after 30 min. Strains producing an OD405 of >0.15 at an MAb dilution of 1:4,800 were designated tentatively as positive for that epitope. Of 54 strains (39%), 21 were found to be O157:H7 in the first assay. The H7 antigen may be expressed weakly depending upon growth medium or other conditions (12, 24). Passage of potential H7+ strains on a blood-containing medium can enhance the expression of H7 (24). Each of the 54 strains was passaged three times on successive days on LB medium containing 5% sheep blood and then retested for both O157 and H7 epitope expression as described above. Of 54 strains, 31 strains were found to be O157+ before and after passage on blood medium; no additional O157+ strains were identified. However, seven strains designated as O157:H7− after the first assay were found to be H7+ after passage on blood (28 of total O157:H7 strains [52%]).
Comparison of RSS-PCR and serological data.
All of the assays were performed independent of each other, and the monoclonal serotyping results were not disclosed to those who performed the RSS-PCR procedure. Thus, the study was carried out in a blinded fashion to prevent biased observation of PCR patterns. The RSS-PCR patterns for the 54 feedlot strains were designated as either an O157:H7 pattern, possibly an O157:H7 pattern, or a non-O157:H7 pattern, based on visual comparison of the electrophoretic patterns. Each unique non-O157:H7 pattern was given an alphabetical designation (B to K) to further discriminate the patterns. The RSS-PCR pattern data were compared with the serotyping and ELISA results for concordance.
RESULTS
Prototype E. coli O157:H7 “fingerprint” pattern.
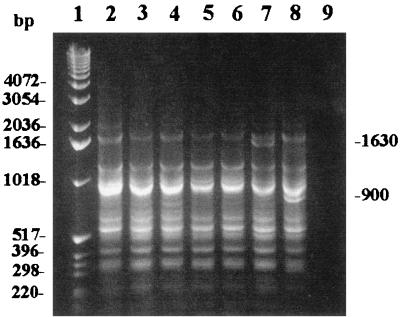
The RSS-PCR method with primers EC-1 and EC-2 was applied to six unrelated E. coli O157:H7 isolates (strains F4637, F4761, G5244, H2294, H2493, and H2548). PCR amplification of all six strains generated identical band patterns comprising 11 bands for each sample on an agarose gel (Fig. 1, lanes 2 to 7). An extra band of approximately 1,630 bp was observed from strain H2548. To determine the stability of these patterns, we performed the PCR on the same six strains multiple times on different days for a period of 6 months. While there appeared to be some day-to-day variation in the intensity of two of the lower-molecular-weight bands (ca. 360 and 320 bp) and a high-molecular-weight band of approximately 2,000 bp on the agarose gel, the patterns remained consistent over multiple tests. The most common pattern associated with E. coli O157:H7 was designated pattern A, and the pattern with the extra 1,630-bp band (in strain H2548) was designated pattern A2. In the analysis of 54 test isolates, another variant pattern from RSS-PCR of E. coli O157:H7 samples was recognized (Fig. 1, lane 8). This pattern had a band of approximately 900 bp in addition to all of the other bands seen in pattern A and was designated E. coli O157:H7 pattern A1. E. coli O157:H7 pattern A was hereafter considered the prototype pattern and was used as a positive control for the PCR assays.
FIG. 1.
RSS-PCR patterns of seven different E. coli O157:H7 isolates from various domestic outbreaks. Lane 1 is a 1-kb molecular-weight DNA ladder (Gibco-BRL). Lanes 2 to 6 are, in order, E. coli O157:H7 strains F4637, F4761, G5244, H2294, and H2493. All represent E. coli O157:H7 pattern A. Lane 7 is E. coli O157:H7 strain H2548 which represents E. coli O157:H7 pattern A2 (extra band of 1,630 bp). Lane 8 shows a pattern for a KSU E. coli O157:H7 strain (strain 34) which represents E. coli O157:H7 pattern A1. Note the extra band of approximately 900 bp. Lane 9 is a negative control (no template DNA).
RSS-PCR on selected pathogenic E. coli serotypes and Salmonella serovar Enteritidis.
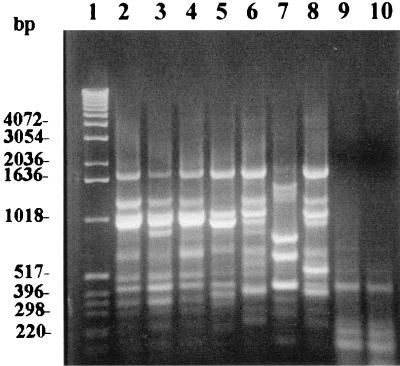
To determine the discriminatory power of the RSS-PCR method in differentiating serotypes, we tested several representative strains from each of the major diarrheagenic E. coli groups and two strains of Salmonella serovar Enteritidis (phage types 4 and 8). With the exception of E. coli O157:H7 and E. coli O55:H7, amplification with primers EC-1 and EC-2 generated unique patterns for each different E. coli serotype (Fig. 2). Interestingly, E. coli O55:H7 generated a pattern that is identical to the E. coli O157:H7 pattern A. As expected, both Salmonella serovar Enteritidis strains (phage types 4 and 8) did not generate any interpretable patterns.
FIG. 2.
RSS-PCR patterns of selected diarrheagenic E. coli isolates and Salmonella serovar Enteritidis strains. Lane 1, 1-kb molecular-weight ladder; lane 2, EHEC (E. coli O157:H7 pattern A [F4761]); lane 3, EHEC (E. coli O157:H7 pattern A1 [KSU 34]); lane 4, EPEC (O55:H7); lane 5, EPEC (O111:NM); lane 6, ETEC (strain H10407); lane 7, enteroaggregative E. coli (strain 25-2); lane 8, enteroinvasive E. coli (strain 11); lane 9, Salmonella serovar Enteritidis phage type 4; lane 10, Salmonella serovar Enteritidis phage type 8. Note the similarity between the patterns for E. coli O157:H7 and E. coli O55:H7 (lanes 2 and 4).
RSS-PCR of feedlot strains.
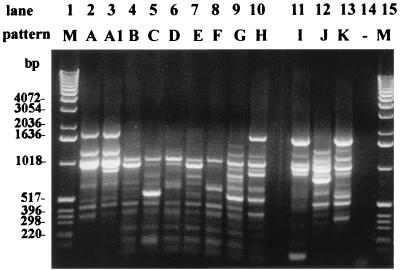
To validate RSS-PCR as a method for detecting E. coli O157:H7, we assayed 54 E. coli strains isolated from Kansas cattle feedlot samples and without prior knowledge of their serotypes or other serological characteristics. Based on visual comparison of the generated patterns with the prototype E. coli O157:H7 pattern (E. coli O157:H7 pattern A), 15 strains were determined to have the O157:H7 pattern, and 21 strains were determined to possibly have the O157:H7 pattern. The designation of “possibly O157:H7” were given to those patterns which looked almost identical to the prototype O157:H7 pattern except for 1 band (E. coli O157:H7 pattern A1). The remaining 18 strains were determined to have patterns that appeared to be completely different from the O157:H7 prototype pattern and were thus designated non-O157:H7 patterns (“−” in RSS-PCR result column, Table 1). The non-O157:H7 patterns were further compared to each other, and each unique pattern was given a letter designation (B to K). There were 10 distinct patterns represented among the 18 strains found to have non-O157:H7 patterns. A representative example of each non-O157:H7 pattern among the feedlot strains is shown in Fig. 3.
TABLE 1.
Results of serology and PCR assays with 54 feedlot strains
| Feedlot strain no. | Binding of MAba to bacteria passaged:
|
H7 serotype changed after passage | MAb-defined serotypec | RSS-PCR result (PCR pattern type) | Agreement between ELISA and RSS-PCR | |||
|---|---|---|---|---|---|---|---|---|
| On LB agar
|
Three times on 5% blood agarb
|
|||||||
| Anti-O157 | Anti-H7 | Anti-O157 | Anti-H7 | |||||
| 1 | No | Neg:Neg | − (B) | Yes | ||||
| 2 | No | Neg:Neg | − (C) | Yes | ||||
| 3 | ++++ | ++++ | No | O157:Neg | − (D) | Yes | ||
| 4 | No | Neg:Neg | − (E) | Yes | ||||
| 5 | ++++ | ++++ | No | Neg:H7 | − (E) | Yes | ||
| 6 | No | Neg:Neg | − (F) | Yes | ||||
| 7 | ++++ | ++++ | +++ | Yes | O157:H7 | O157:H7 | Yes | |
| 8 | ++++ | ++++ | + | Yes | O157:H7 | O157:H7 | Yes | |
| 9 | ++ | ++++ | + | ++++ | No | O157:H7 | O157:H7 | Yes |
| 10 | No | Neg:Neg | − (E) | Yes | ||||
| 11 | ++ | ++++ | No | Neg:H7 | − (E) | Yes | ||
| 12 | ++++ | ++++ | No | Neg:H7 | − (E) | Yes | ||
| 13 | No | Neg:Neg | − (G) | Yes | ||||
| 14 | + | +++ | No | Neg:H7 | − (I) | Yes | ||
| 15 | No | Neg:Neg | − (H) | Yes | ||||
| 16 | ++++ | ++++ | No | O157:Neg | − (I) | Yes | ||
| 17 | No | Neg:Neg | − (G) | Yes | ||||
| 18 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 19 | +++ | + | + | ++++ | No | O157:H7 | O157:H7 | Yes |
| 20 | ++++ | ++ | ++++ | +++ | No | O157:H7 | O157:H7 | Yes |
| 21 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 22 | ++++ | ++++ | ++ | Yes | O157:H7 | O157:H7 | Yes | |
| 23 | ++++ | ++++ | ++ | Yes | O157:H7 | O157:H7 | Yes | |
| 24 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 25 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 26 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 27 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 28 | ++ | ++ | + | ++++ | No | O157:H7 | O157:H7 | Yes |
| 29 | ++++ | ++++ | + | Yes | O157:H7 | O157:H7 | Yes | |
| 30 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 31 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 32 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 33 | ++++ | ++++ | ++ | Yes | O157:H7 | O157:H7 | Yes | |
| 34 | ++++ | +++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 35 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 36 | ++ | + | + | +++ | No | O157:H7 | O157:H7 | Yes |
| 37 | ++++ | ++++ | + | Yes | O157:H7 | O157:H7 | Yes | |
| 38 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 39 | ++++ | ++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 40 | ++++ | +++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 41 | No | Neg:Neg | − (I) | Yes | ||||
| 42 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
| 43 | No | Neg:Neg | − (I) | Yes | ||||
| 44 | No | Neg:Neg | − (I) | Yes | ||||
| 45 | No | Neg:Neg | − (I) | Yes | ||||
| 46 | No | Neg:Neg | − (I) | Yes | ||||
| 47 | No | Neg:Neg | − (J) | Yes | ||||
| 48 | ++ | No | O157:Neg | − (I) | Yes | |||
| 49 | No | Neg:Neg | − (F) | Yes | ||||
| 50 | No | Neg:Neg | − (J) | Yes | ||||
| 51 | No | Neg:Neg | − (K) | Yes | ||||
| 52 | ++++ | ++++ | No | O157:Negd | O157:H7 | No | ||
| 53 | No | Neg:Neg | − (K) | Yes | ||||
| 54 | ++++ | ++++ | ++++ | ++++ | No | O157:H7 | O157:H7 | Yes |
MAbs were added to wells at a 1:4,800 dilution: negative (i.e., no entry), OD405 < 0.150; +, OD405 = 0.151 to 0.400; ++, OD405 = 0.401 to 0.800; +++, OD405 = 0.801 to 1.200; ++++, OD405 > 1.200.
Bacteria were subcultured for 3 successive days on LB agar containing 5% sheep blood.
Neg, designates that the strain did not bind the anti-O157 or the anti-H7 MAb. The strains were not assayed for other O and H serotypes.
This strain was positive for SLTII, intimin, and flicC in a multiplex PCR (data not shown).
FIG. 3.
RSS-PCR patterns of representative non-O157:H7 strains among KSU strains. Lanes 1 and 15 are 1-kb DNA ladders (Gibco-BRL). Lanes 2 and 3 represent E. coli O157:H7 patterns A and A1, respectively. Lanes 4 to 13 represent non-O157:H7 patterns B to K, respectively. Lane 14 is a negative control (no template DNA).
Serotyping and ELISA of cattle feedlot strains.
All 54 feedlot isolates were initially classified as belonging to the O157:H7 serotype by the commercial latex agglutination test based on polyclonal antibodies (Remel). Of the 54 isolates passaged three times on LB 5% blood agar, 28 (52%) reacted strongly with both anti-O157 and anti-H7 MAbs. Four strains reacted strongly with anti-O157 antibody but not with anti-H7 antibody. Four strains reacted strongly with anti-H7 antibody but not with anti-O157 antibody. Eighteen strains reacted with neither anti-O157 antibody nor anti-H7 antibody and were classified as non-O157:H7 strains.
Comparison of RSS-PCR with MAb-ELISA serology results.
Of the 54 feedlot strains, 28 strains were found to be of serotype O157:H7, based on ELISA. Among these 28 strains, the RSS-PCR method identified all 28 strains as O157:H7 strains. Thus, the sensitivity of the RSS-PCR method compared to the monoclonal ELISA was 100%. Based on the ELISA serology results, the remaining 26 feedlot strains were determined to be non-O157:H7 strains, or strains that had either the O157 lipopolysaccharide antigen or the H7 flagellar antigen, but not both. The RSS-PCR method identified 18 of these 26 strains as non-O157:H7 strains. However, the remaining eight strains were falsely identified as O157:H7 strains by the RSS-PCR method. Therefore, the specificity of the RSS-PCR method was 69%. Overall, the RSS-PCR method yielded 46 results that were concordant with the serological-ELISA tests. The positive predictive value and negative predictive value of the RSS-PCR method compared with the monoclonal ELISA test as the “gold standard” were 78 and 100%, respectively.
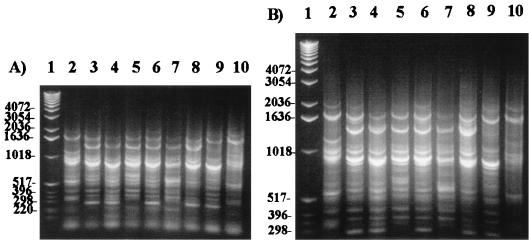
The eight strains for which there was discrepancy between the ELISA and RSS-PCR data were retested by RSS-PCR. The amplified products were electrophoresed for 75 min for better band separation. Seven strains generated patterns that were clearly different from the prototype E. coli O157:H7 pattern A (Fig. 4, lanes 3 to 9). One strain which had the O157 antigen but not the H7 flagellar antigen still generated a pattern identical to the prototype O157:H7 pattern (Fig. 4, lane 10). Based on this reevaluation, the sensitivity and the specificity of the RSS-PCR method were 100 and 96%, respectively. The positive predictive value and the negative predictive value were 97 and 100%, respectively.
FIG. 4.
Electrophoresis for 55 (A) and 75 (B) min. Comparison of the eight KSU strains that gave discrepant results with the RSS-PCR and serological tests. Lane 1 represents the 1-kb DNA ladder (Gibco-BRL). Lane 2 represents the E. coli O157:H7 pattern A (positive control). Lanes 3 to 8 represent non-O157:H7 strains with pattern H (KSU strains 14, 41, 43, 45, 46, and 48). Lane 9 represents a non-O157:H7 strain with pattern E (KSU strain 49). Lane 10 represents KSU strain 52 which was found to be missing the H7 antigen but which still gave a pattern similar to that of E. coli O157:H7 pattern A. A longer separation time yielded better discrimination of the patterns.
DISCUSSION
An effective bacterial detection method that relies on molecular techniques can be used to efficiently screen a large number of environmental samples. We have developed a simple diagnostic method that relies on restriction site polymorphisms found in the E. coli genome. Other PCR-based methods for the detection of verotoxin-producing E. coli exist. Gannon et al. (9) have described a multiplex PCR method, which uses two pairs of primers that are directed toward SLTI and SLTII genes. The method was shown to be specific in that the two primer pairs amplified the respective toxin genes they were designed to target. Another multiplex PCR method described by Fratamico et al. (7) used three pairs of primers specific for the eaeA gene, conserved regions of the SLTI and SLTII genes, and the 60-MDa plasmid for simultaneous amplification in one PCR reaction. Recently, Gannon et al. (8) introduced a new multiplex PCR method that uses primers directed to the H7 flagellar gene, flicC, in addition to primers for the verotoxin and eaeA genes to improve specificity for EHEC strains. All of these methods are designed to specifically detect verotoxin-producing E. coli strains. However, one common limitation of all of these methods is that they are not specific enough to differentiate individual serotypes among the verotoxin-producing group of E. coli.
The RSS-PCR method described herein is a rapid E. coli O157:H7 detection assay that differentiates genotypes on the basis of multiple band patterns. Most bacterial detection methods that have been developed thus far are mainly based on unique biochemical characteristics (26), immunogenic properties (1, 5, 6, 14, 20, 21), or the presence or absence of genes that are unique to the organism of interest (3, 8–10, 33). Although these methods vary significantly in terms of the specificity in identifying the organism, all of them are limited by the fact that they only give either a “positive” or “negative” result (e.g., generating a single PCR amplicon). This can be problematic when certain strains of E. coli exhibit similar or identical phenotypes while belonging to different serotypes (i.e., expression of SLTs). One major advantage of the RSS-PCR method is that it generates “fingerprint” patterns that are distinct for different serotypes of E. coli. Thus, the method can potentially reduce the ambiguity often encountered in identifying and differentiating serotypes of E. coli among clinical isolates using more conventional diagnostic techniques. The detection of electrophoretic patterns rather than a single amplicon helps to reduce false-positive results, increases specificity, and therefore confidence in the interpretation of the results. While other PCR methods to generate “fingerprint” patterns for E. coli exist (REP, ERIC, and BOX), they often rely on repetitive DNA elements that are not specific to E. coli (28, 29). The RSS-PCR method is based on an outer membrane heme receptor gene chuA that is specific to E. coli (27). Hence, as shown here (Fig. 2), no discernible pattern was generated with another member of the Enterobacteriaceae, S. enterica serovar Enteritidis. The genotypic analysis also precludes the problems encountered with serologic tests that require expression of proteins, such as that observed with differential expression of the H7 antigen by E. coli O157:H7.
We showed that the RSS-PCR method generates a genotypic pattern for strains of E. coli O157:H7 that is distinct from that of other E. coli serotypes. As in other tests used to differentiate E. coli O157:H7 from other E. coli serotypes, this test is designed to differentiate E. coli strains only after the bacterial organism has been shown to be E. coli. It is not designed to differentiate E. coli from non-E. coli bacterial organisms. Our RSS-PCR results correlated well with the MAb ELISA results. Of concern is the discrepancy between results obtained with the MAb ELISA and the RSS-PCR results compared to the polyclonal antibody latex agglutination test. All 54 isolates were initially characterized as O157:H7 by the latex agglutination test following the manufacturer's protocol. Both the MAb ELISA and RSS-PCR tests identified only 28 (52%) of the isolates to be O157:H7. Therefore, there was complete agreement between the MAb ELISA test and RSS-PCR results in the differentiation of E. coli O157:H7 strains. While the latex agglutination test may be simpler to perform, it appears to give a relatively high rate of false-positive results.
Of the 26 MAb ELISA-confirmed non-O157:H7 strains, 8 strains were initially found to give discordant results by the RSS-PCR method. Because these strains yielded patterns that appeared on first inspection to be similar to the prototype O157:H7 pattern, they were initially classified as O157:H7 strains. Upon further analysis of the serological data, we noticed that two of the eight strains were those that had retained the O157 antigen but had lost the H7 antigen, and 1 strain had the H7 antigen but did not have the O157 antigen. These eight discordant strains were retested with the RSS-PCR method to attempt to generate more discriminating patterns for better comparison. When we extended the electrophoresis time from 55 to 75 min, the pattern differences became clearly apparent (Fig. 4). This underscores the limitation of visual analysis of PCR patterns. Although we observed gel-to-gel variation in the intensity of a 2,000-bp band and two other low-molecular-weight bands (360 and 320 bp), the patterns overall were shown to remain stable and reproducible when the test was repeated over a period of 6 months (data not shown). We believe that due to the high molecular weight of the 2,000-bp fragment, it is not always efficiently amplified by the conditions we use. As in other PCR-based methods to generate “fingerprint” patterns, this method is likely to show laboratory-to-laboratory variability and, therefore, there is a need to always include in every gel a positive control sample that will generate the expected O157:H7 RSS-PCR patterns for comparison to the test patterns.
The E. coli serotype, O157:H7, comprises a group of closely related verotoxin-producing strains, which are widely distributed throughout North America (31). While there has been considerable debate concerning the evolution of these strains, based on genetic studies of E. coli O157:H7, it is now widely recognized that O157:H7 serotype represents a group of strains that were derived from a single ancestral clone (31, 32). Multilocus enzyme electrophoresis studies conducted by Whittam et al. have shown that the electrophoretic profiles of four different enzymes among E. coli O157:H7 strains are distinctly similar to each other but clearly different from that of other E. coli isolates from diverse sources in the natural environment (31). Further studies have suggested that E. coli O157:H7 strains are distantly related to other EHEC serotypes and have actually evolved from E. coli O55:H7, a serotype classified under the EPEC group (32). Our results of the RSS-PCR on E. coli serotypes O157:H7 and O55:H7 show that both organisms generate identical patterns (pattern A) that are distinct from patterns generated by other serotypes, which may indicate that the two are highly similar genetically and support the observation made by Whittam et al. Another study (8) that evaluated an EHEC detection assay also showed identical patterns for both E. coli O157:H7 and O55:H7 when the amplified PCR products were treated with a specific restriction enzyme. These observations reflect the clonality of E. coli O157:H7 and lend credence to the theory that E. coli O157:H7 is derived from E. coli O55:H7. They also further support the RSS-PCR technique as a valid method to differentiate E. coli serotypes.
RSS-PCR is a simple and rapid method that requires minimal pieces of equipment and time. The entire procedure can be completed in less than 2 days and does not require the laborious extraction and purification of DNA from organisms. Furthermore, unlike conventional restriction fragment length polymorphism analyses, the procedure does not require the use of restriction enzymes. Another major advantage is that the procedure does not require the use of multiple pairs of primers like the multiplex PCR procedures described previously. Hence, this test may serve as a rapid way to test a large number of E. coli samples, such as those derived from environmental sources.
ACKNOWLEDGMENTS
We thank Tim Barrett at the CDC for providing the reference E. coli O157:H7 strains. We also thank Regina Giraldi for her guidance in the initial stages of the study, and Michele Barocchi, Amee Manges, and Susan Yamanishi for helpful discussions. We also thank James Keen for providing anti-O157 and anti-H7 MAbs and Felicidad Bautista for her excellent technical assistance.
This study was supported in part by a U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service grant (95-37201-2127) and the California Department of Justice Applied Research Program.
REFERENCES
- 1.Bitzan M, Karch H. Indirect hemagglutination assay for diagnosis of Escherichia coli O157 infection in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1992;30:1174–1178. doi: 10.1128/jcm.30.5.1174-1178.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyce T G, Swerdlow D L, Griffin P M. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333:364–368. doi: 10.1056/NEJM199508103330608. [DOI] [PubMed] [Google Scholar]
- 3.Cebula T A, Payne W L, Feng P. Simultaneous identification of strains of Escherichia coli serotype O157:H7 and their Shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J Clin Microbiol. 1995;33:248–250. doi: 10.1128/jcm.33.1.248-250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Outbreaks of Escherichia coli O157:H7 infection associated with eating alfalfa sprouts—Michigan and Virginia. Morbid Mortal Weekly Rep. 1997;46:741–744. [PubMed] [Google Scholar]
- 5.Chart H, Scotland S M, Rowe B. Serum antibodies to Escherichia coli serotype O157:H7 in patients with hemolytic uremic syndrome. J Clin Microbiol. 1989;27:285–290. doi: 10.1128/jcm.27.2.285-290.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dylla B L, Vetter E A, Hughes J G, Cockerill F R., III Evaluation of an immunoassay for direct detection of Escherichia coli O157 in stool specimens. J Clin Microbiol. 1995;33:222–224. doi: 10.1128/jcm.33.1.222-224.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fratamico P M, Sackitey S K, Weidemann M, Deng M Y. Detection of Escherichia coli O157:H7 by multiplex PCR. J Clin Microbiol. 1995;33:2188–2191. doi: 10.1128/jcm.33.8.2188-2191.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gannon V P J, D'Souza S, Graham T, King R K, Rahn K, Read S. Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J Clin Microbiol. 1977;35:656–662. doi: 10.1128/jcm.35.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gannon V P J, King R K, Kim J Y, Golsteyn-Thomas E J. Rapid and sensitive method for detection of Shiga-like toxin producing Escherichia coli in ground beef using the polymerase chain reaction. Appl Environ Microbiol. 1992;58:3809–3815. doi: 10.1128/aem.58.12.3809-3815.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon V P J, Rashed M, King R K, Golsteyn-Thomas E J. Detection and characterization of the eae gene of Shiga-like toxin-producing Escherichia coli using polymerase chain reaction. J Clin Microbiol. 1993;31:1268–1274. doi: 10.1128/jcm.31.5.1268-1274.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris E, Sandoval E, Xet-Mull A M, Johnson M, Riley L W. Rapid subtyping of dengue viruses by restriction site-specific (RSS)-PCR. Virology. 1999;253:86–95. doi: 10.1006/viro.1998.9481. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Keen J E, Westerman R B, Littledike E T, Kwang J. Monoclonal antibodies for detection of the H7 antigen of Escherichia coli. Appl Environ Microbiol. 1996;62:3325–3332. doi: 10.1128/aem.62.9.3325-3332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keene W E, McAnulty J M, Hoesly F C, et al. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7 and Shigella sonnei. N Engl J Med. 1994;331:579–584. doi: 10.1056/NEJM199409013310904. [DOI] [PubMed] [Google Scholar]
- 14.Kim M S, Doyle M P. Dipstick immunoassay to detect enterohemorrhagic Escherichia coli O157:H7 in retail ground beef. Appl Environ Microbiol. 1992;58:1764–1767. doi: 10.1128/aem.58.5.1764-1767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandrell R E, Zollinger W D. Use of a switterionic detergent for the restoration of the antibody binding capacity of electroblotted meningococcal outer membrane proteins. J Immunol Methods. 1984;67:1–11. doi: 10.1016/0022-1759(84)90080-2. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy M. E. coli O157:H7 outbreak in USA traced to apple juice. Lancet. 1996;348:1299. [Google Scholar]
- 17.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberst R D, Hays M P, Bohra L K, et al. PCR-based DNA amplification and presumptive detection of Escherichia coli O157:H7 with an internal fluorogenic probe and the 5′ nuclease (Taqman) assay. Appl Environ Microbiol. 1998;64:3389–3396. doi: 10.1128/aem.64.9.3389-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien A D, Holmes R K. Shiga and Shiga-like toxins. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padhye N V, Doyle M P. Rapid procedure for detecting enterohemorrhagic Escherichia coli O157:H7 in food. Appl Environ Microbiol. 1991;57:2693–2698. doi: 10.1128/aem.57.9.2693-2698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park C H, Vandel N M, Hixon D L. Rapid immunoassay for detection of Escherichia coli O157 directly from stool specimens. J Clin Microbiol. 1996;34:988–990. doi: 10.1128/jcm.34.4.988-990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley L W, Remis R S, Helgerson S D, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 23.Slutsker L, Ries A A, Greene K D, Wells J G, Hutwagner L, Griffin P M. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med. 1997;126:505–513. doi: 10.7326/0003-4819-126-7-199704010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Sowers E G, Wells J G, Strockbine N A. Evaluation of commercial latex reagents for identification of O157 and H7 antigens of Escherichia coli. J Clin Microbiol. 1996;34:1286–1289. doi: 10.1128/jcm.34.5.1286-1289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su C, Brandt L J. Escherichia coli O157:H7 infection in humans. Ann Intern Med. 1995;123:698–714. doi: 10.7326/0003-4819-123-9-199511010-00009. [DOI] [PubMed] [Google Scholar]
- 26.Thompson J S, Hodge D S, Borczyk A A. Rapid biochemical test to identify verotoxin-positive strains of Escherichia coli serotype O157. J Clin Microbiol. 1990;28:2165–2168. doi: 10.1128/jcm.28.10.2165-2168.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres, A. G., and S. M. Payne. Haem iron-transport system in enterohemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 23:825–833. [DOI] [PubMed]
- 28.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Versalovic J, Schneider M, de Bruijn F J, Lupski J R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol. 1994;5:25–40. [Google Scholar]
- 30.Westerman R B, He Y S, Keen J E, Littledike E T, Kwang J. Production and characterization of monoclonal antibodies specific for the lipopolysaccharide of Escherichia coli. J Clin Microbiol. 1997;35:679–684. doi: 10.1128/jcm.35.3.679-684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittam T S, Wachsmuth I K, Wilson R A. Genetic evidence of clonal descent of Escherichia coli O157:H7 associated with hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1988;157:1124–1133. doi: 10.1093/infdis/157.6.1124. [DOI] [PubMed] [Google Scholar]
- 32.Whittam T S, Wolfe M L, Wachsmuth I K, Orskov F, Orskov I, Wilson R A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993;61:1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willshaw G A, Scotland S M, Smith H R, Cheasty T, Thomas A, Rowe B. Hybridization of strains of Escherichia coli O157 with probes derived from the eaeA gene of enteropathogenic E. coli and the eaeA homolog from a vero cytotoxin-producing strain of E. coli O157. J Clin Microbiol. 1994;32:897–902. doi: 10.1128/jcm.32.4.897-902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]