Abstract
Background
Acupuncture showed better improvement than sham acupuncture in reducing attack frequency of tension-type headache (TTH), but its effectiveness relative to first-line drugs for TTH is unknown, which impedes the recommendation of acupuncture for patients who are intolerant to drugs for TTH. We aimed to estimate the relative effectiveness between acupuncture and tricyclic antidepressants (TCAs) through indirect treatment comparison (ITC) meta-analysis.
Methods
We searched Ovid Medline, Embase, and Cochrane Library from database inception until April 13, 2023. Randomized controlled trials of TCAs or acupuncture in the prevention of TTH in adults were included. The primary outcome was headache frequency. The secondary outcomes were headache intensity, responder rate, and adverse event rate. Bayesian random-effect models were used to perform ITC meta-analysis, and confidence of evidence was evaluated by using the GRADE approach.
Results
A total of 34 trials involving 4426 participants were included. Acupuncture had similar effect with TCAs in decreasing TTH frequency (amitriptyline: mean difference [MD] -1.29, 95% CI -5.28 to 3.02; amitriptylinoxide: MD -0.05, 95% CI -6.86 to 7.06) and reducing TTH intensity (amitriptyline: MD 2.35, 95% CI -1.20 to 5.78; clomipramine: MD 1.83, 95% CI -4.23 to 8.20). Amitriptyline had a higher rate of adverse events than acupuncture (OR 4.73, 95% CI 1.42 to 14.23).
Conclusion
Acupuncture had similar effect as TCAs in reducing headache frequency of TTH, and acupuncture had a lower adverse events rate than amitriptyline, as shown by very low certainty of evidence.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-024-01776-5.
Keywords: Acupuncture, Tricyclic antidepressants, Tension-type headache, Indirect treatment comparison, Meta-analysis
Highlights
Acupuncture showed better improvement than sham acupuncture in reducing headache frequency of tension-type headache (TTH), but the lack of comparisons between acupuncture and first-line drugs impedes recommendation of acupuncture for TTH.
Our indirect treatment comparison meta-analysis found similar effect between acupuncture and tricyclic antidepressants (TCAs) in reducing TTH frequency with very low certainty of evidence.
Similar effect between acupuncture and TCAs were also observed in reducing headache intensity with very low certainty of evidence.
Acupuncture had a lower rate of adverse events than amitriptyline with very low certainty of evidence.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-024-01776-5.
Background
Tension-type headache (TTH), a common neurological disease, is characterized by recurrent bilateral, tightening or pressing, and mild-to-moderate headache [1, 2]. TTH represents a crucial health issue that affects approximately 26.8% of individuals worldwide and has a female preponderance with a gender incidence ratio of 1.2:1 [3]. The condition contributes to the burden of the economy and lowers the quality of life for the people who suffer from it [3]. Acute medication and lifestyle modifications are the major methods for controlling infrequent TTH (lasting from 30 min to seven days, which occur less than once per month), however frequent episodic TTH (occur on 1–14 days per month) or chronic TTH (on 15 or more days per month) may necessitate prophylactic medications and/or behavioral therapies [1, 2].
Tricyclic antidepressants (TCAs) are recommended for patients who suffer from TTH as the primary prophylactic therapy [1, 4]. Evidence from randomized controlled trials (RCTs) and systematic reviews showed that TCAs, such as amitriptyline and clomipramine, are effective in reducing the headache frequency and intensity of TTH [5–7]. Additionally, the guideline of the European Federation of Neurological Societies suggested that due to the limitation of high side effects of pharmacological prophylactic medications, non-pharmacotherapies also deserve to be considered [4].
Acupuncture is a treatment with a long history [8]. The measure has a regulatory effect on the body by stimulating the acupoints with specific tools (such as needle) [8, 9]. Acupuncture has been applied to manage TTH, and RCTs and systematic reviews suggested acupuncture can reduce the frequency and intensity of TTH [10–13]. However, the most of previous studies of acupuncture were compared with sham acupuncture, and there is a lack of studies to compare with positive drugs in preventing TTH. The rare evidence compared with efficacious drugs might limit the use of acupuncture in the treatment of TTH, as well as hamper doctors from developing more appropriate therapeutic plans.
Indirect treatment comparison (ITC) is a method that can be used to evaluate the relative efficacy of different interventions when there is no direct comparison [14]. This approach can provide evidence of the difference in efficacy between different interventions and can help physicians to select a better therapy. In this study, we performed an ITC analysis of RCTs providing evidence for the comparison of TCAs vs. acupuncture in patients with TTH.
Methods
We designed, performed, and reported the study according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses for Network Meta-Analyses (PRISMA-NMA) guidelines [15].
Literature search
The following databases were searched from inception to April 13, 2023: Ovid Medline, Embase, and Cochrane Library, without any restriction in the language of publication (QF-T). The search was performed using keywords and Medical Subject Heading terms associated with TTH and acupuncture or TCAs (including amitriptyline, amitriptylinoxide, clomipramine, doxepin, imipramine, amoxapine, desipramine, dibenzepine, dosulepin, lofepramine, tianeptine, trimipramine), and the search strategies were provided in eTable 1–3. We also searched clinicaltrials.gov for any potentially missing RCTs. Additionally, the reference lists of previous systematic reviews were screened for eligible studies.
Study selection
The duplicate studies were eliminated firstly. Then, the title and abstract of the searched studies were reviewed by two independent reviewers (Q-FT and YB-H) for potential eligible research. Next, to identify the eligible RCTs further, they read the full text. The disagreements were addressed through discussion and judged by a third reviewer (HZ) finally.
Inclusion and exclusion criteria
The RCT was considered eligible when all of the following conditions were met: (1) The study included adult patients with TTH; (2) The diagnostic standard of the study met the criteria conducted by the International Headache Society or the Ad Hoc Committee on Classification of Headache criteria of TTH; (3) The intervention of the study included acupuncture and/or TCAs; (4) The study measured and reported at least one of the following outcomes: headache frequency (the number of headache days of per month), headache intensity, responder (reduction ≥ 50% in the number of headache days of per month) rate, and adverse event rate; (6) The study was a parallel-design RCT or a crossover-design RCT with data of the first phase. The study was excluded when the research included participants with migraine unless results were presented separately for participants with TTH.
Outcome assessments
The primary outcome was headache frequency (the changes of the number of headache days of per month). The secondary outcomes were headache intensity (the changes of the score that was measured on visual analogue scale or numeric rating scale for pain), responder rate, and adverse event rate. We evaluated the outcomes at the end of treatment.
Data extraction
Two reviewers (YB-H and LY) independently extracted the relevant data by standardized extraction forms, involving characteristics of the eligible RCTs, details of intervention and control arm, and data of outcomes. Inconsistencies were resolved by discussion and ultimately by the decision of a third reviewer (HZ).
Risk of bias assessment
Two reviewers (YZ-S and DQ) independently assessed the risk of bias of included RCTs by the Cochrane risk-of-bias tool (version 2) [16]. Several questions involving the following five parts were required to estimate the ROB: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The risk of bias of the eligible study was rated as low, some concerns, or high risk of bias.
The certainty of the evidence
We assessed the certainty of evidence by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) minimally contextualized framework approach that was a method designed for network meta-analysis [17]. The certainty of evidence was assessed in two levels: high (moderate to high certainty) and low (very low to low certainty) certainty. The classification of intervention was assessed into category 0, category 1, and category 2, representing among the least effective, moderately effective, and among the most effective, respectively.
Statistical analysis
We performed the arm-based network meta-analysis (NMA) by using multinma package version 0.5.1 in R 4.3.1 environment and estimated the model in a Bayesian framework using Stan [18, 19]. We set N (0,1002) prior distributions for the treatment effects and study-specific intercepts and utilized half-N (52) prior for the heterogeneity standard deviation of the random-effect (RE) model. The posterior total residual deviance, the number of unconstrained data points, and the deviance information criteria (DIC) of both the RE and fixed-effect (FE) models were calculated to estimate the model fit. A closer posterior total residual deviance to the number of unconstrained data points and a smaller DIC indicates a better model fit. A difference of more than 5 points suggests a significant difference [20]. We assessed the global inconsistency by drawing the dev-dev plots of the consistency model and inconsistency model, there is no evidence of inconsistency if all the points are approximately on the line of equality. Based on pooled data from included RCTs, pair-wise comparison analyses were used to assess the mean difference (MD) of continuous outcomes and the odds ratio (OR) of binary outcomes with 95% confidence intervals (CIs). For each outcome, we conducted category-level and individual-level analyses of TCAs. The heterogeneity among studies was evaluated by tau-squared, and a value of tau-squared greater than 0.36 suggested significant heterogeneity [21]. Further, the surface under the cumulative rank curve (SUCRA) value also was calculated to estimate the ranking of each intervention in the network, with a higher SUCRA value indicating a higher ranking of the intervention [22, 23].
We conducted the following sensitivity analyses for primary outcome to estimate the robustness of results: (1) excluding RCTs at high risk of bias; (2) excluding RCTs with a randomized sample size of less than 50 participants. Further, we also performed subgroup analyses in the difference of type of TTH, classification of acupuncture, and endpoint of treatment to explore the source of heterogeneity.
Results
Characteristics of the included RCTs
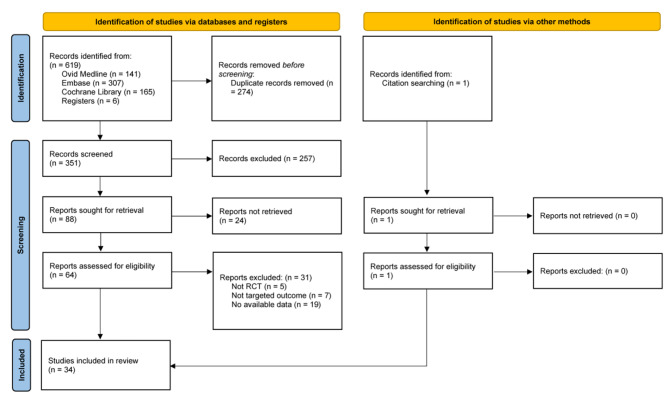
Figure 1 shows the PRISMA flowchart of literature search and study selection. Our study identified 620 articles, and after removing the duplicate articles, 351 articles were screened the titles and abstracts. Subsequently, 66 articles were reviewed in full text. Finally, 34 articles [5, 6, 10, 11, 24–53] with 4426 participants were included in our research.
Fig. 1.
PRISMA flow diagram of literature search and study selection
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses
Table 1 presents the characteristics of the eligible RCTs. These eligible RCTs were performed in 18 countries. The average age of included participants was 41.1 years old, and 70% of participants were female. Eighteen RCTs included acupuncture, and sixteen RCTs included TCAs, of which twelve RCTs included amitriptyline, one RCT included both amitriptyline and doxepin, one RCT included amitriptyline and amitriptylinoxide, one RCT included imipramine, and one RCT included clomipramine. The results of risk of bias assessment in eligible RCTs are presented in eFigure 1. Eight (23.53%) RCTs were evaluated as low risk of bias, eighteen (52.94%) RCTs were evaluated as some concerns, and eight (23.53%) were evaluated as high risk of bias.
Table 1.
Characteristics of the included RCTs
| Study | Country | Diagnostic criteria |
Type of TTH | Interventions | Treatment duration (weeks) |
Follow-up duration (weeks) |
Number of patients | Female (%) |
Mean ages |
|---|---|---|---|---|---|---|---|---|---|
| TCAs | |||||||||
| Bendtsen 1996 [42] | Denmark | IHS | Chronic | Amitriptyline 75 mg vs. placebo | 8 | 0 | 40 | 63 | 40 |
| Bettucci 2006 [47] | Italy | IHS | Chronic | Amitriptyline 20 mg vs. amitriptyline 20 mg plus tizanidine 4 mg | 12 | 0 | 18 | 72 | 35.3 |
| Boline 1995 [52] | USA | IHS | Episodic | Amitriptyline 30 mg vs. spinal manipulation | 6 | 4 | 150 | 61 | 42 |
| Boz 2003 [6] | Turkey | IHS | Chronic | Amitriptyline 25 mg vs. sertraline 50 mg | 12 | 0 | 90 | 88 | 39.1 |
| Damapong 2015 [43] | Thailand | IHS | Chronic | Amitriptyline 25 mg vs. massage | 4 | 2 | 60 | 87 | 49.8 |
| Deodato 2019 [51] | Italy | IHS | Chronic | Amitriptyline 50 mg vs. osteopathic manipulative therapy | 12 | 0 | 24 | 60 | 47 |
| Holroyd 1991 [41] | USA | IHS | Chronic | Amitriptyline 75 mg vs. cognitive-behavioral therapy | 12 | 0 | 41 | 80 | 32.3 |
| Holroyd 2001 [5] | USA | IHS | Chronic | Amitriptyline 100 mg vs. placebo | 8 | 24 | 203 | 76 | 37 |
| Indaco 1988 [45] | Italy | Ad Hoc criteria | Chronic | Amitriptyline 50 mg vs. placebo | 12 | 0 | 36 | 52 | 61.2 |
| Langemark 1990 [24] | Denmark | IHS | Chronic | Clomipramine 150 mg vs. mianserin 60 mg vs. placebo | 6 | 0 | 114 | NA | 41 |
| Mitsikostas 1997 [46] | Greece | IHS | Chronic | Amitriptyline 50 mg vs. buspirone 30 mg | 12 | 0 | 58 | 62 | 42.5 |
| Mousavi 2011 [48] | Iran | IHS | Chronic | Imipramine 50 mg vs. TENS | 12 | 0 | 138 | 46 | 28.2 |
| Okasha 1973 [53] | Egypt | Ad Hoc criteria | Psychogenic | Amitriptyline 30 mg vs. doxepin 30 mg vs. diazepam 6 mg vs. placebo | 8 | 0 | 80 | 28 | NA |
| Pfaffenrath 1994 [50] | Germany, Austria, Switzerland | IHS | Mixed | Amitriptyline 75 mg vs. placebo | 12 | 8 | 197 | 56 | 38 |
| Surbakti 2017 [49] | Indonesia | IHS | Chronic | Amitriptyline 12.5 mg vs. flunarizine 5 mg vs. flunarizine 10 mg | 2 | 0 | 95 | 82 | 44.6 |
| Vernon 2009 [44] | Canada | IHS | Chronic | Amitriptyline 25 mg plus chiropractic vs. Amitriptyline 25 mg plus sham chiropractic vs. placebo plus sham chiropractic | 14 | 12 | 20 | 80 | 33.9 |
| Acupuncture | |||||||||
| Chassot 2015 [36] | Brazil | IHS | Chronic | Acupuncture, twice/week vs. sham acupuncture, twice/week | 5 | 0 | 34 | 100 | 40.3 |
| Ebneshahidi 2005 [40] | Iran | IHS | Chronic | Acupuncture, 3 times/week vs. sham acupuncture, 3 times/week | 4 | 12 | 50 | 80 | 35.8 |
| Endres 2007 [29] | Germany | IHS | Mixed | Acupuncture, twice/week vs. sham acupuncture, twice/week | 6 | 24 | 409 | 78 | 39.1 |
| Gildir 2019 [26] | Turkey | IHS | Chronic | Acupuncture, 3 times/week vs. sham acupuncture, 3 times/week | 2 | 4 | 160 | 43 | 36.4 |
| Guo 2020 [32] | China | IHS | Mixed | Acupuncture, once every other day vs. eperisone hydrochloride 150 mg plus flunarizine hydrochloride | 4 | 0 | 150 | 62 | 33.7 |
| Jena 2008 [30] | Germany | IHS | Mixed | Acupuncture, 15 times/12w vs. usual care | 12 | 12 | 1265 | NA | NA |
| Karst 2001 [38] | Germany | IHS | Mixed | Acupuncture, twice/week vs. sham acupuncture, twice/week | 5 | 20 | 69 | 55 | 48.1 |
| Kawk 2007 [39] | Korea | IHS | Chronic | Acupuncture, twice/week vs. sham acupuncture, twice/week | 4 | 12 | 32 | NA | 81 |
| Melchart 2005 [11] | Germany | IHS | Mixed | Acupuncture, 1–2 times/week vs. sham acupuncture, 1–2 times/week vs. waiting list | 8 | 16 | 270 | 74 | 42.7 |
| Schiller 2021 [33] | Germany | IHS | Mixed | Acupuncture, twice/week vs. usual care vs. medical training therapy | 6 | 18 | 72 | 74 | 38.5 |
| Silva 2012 [28] | Brazil | IHS | Mixed | Acupuncture, 8–12 times/8week vs. usual care | 8 | 0 | 43 | 100 | 27.3 |
| Söderberg 2006 [31] | Sweden | IHS | Chronic | Acupuncture, once/week vs. physical training vs. relaxation training | 10–12 | 24 | 90 | 81 | 37.4 |
| Wang 2007 [34] | Denmark | IHS | Chronic | Acupuncture, 14 times/week vs. sham acupuncture, 14 times/week | 4 | 6 | 40 | 50 | 45.3 |
| White 1996 [25] | UK | IHS | Episodic | Acupuncture, once/week vs. sham acupuncture, once/week | 6 | 3 | 10 | 70 | 57.3 |
| White 2000 [27] | UK | IHS | Episodic | Acupuncture, once/week vs. sham acupuncture, once/week | 5 | 12 | 50 | 76 | 49 |
| Xu 2015 [35] | China | IHS | Mixed | Acupuncture, 3 times/week vs. head acupuncture, 3 times/week | 4 | 0 | 60 | 67 | NA |
| Xue 2004 [37] | Australia | IHS | Mixed | Acupuncture, twice/week vs. sham acupuncture, twice/week | 4 | 0 | 40 | 65 | 42.1 |
| Zheng 2022 [10] | China | IHS | Chronic | Acupuncture, 2–3 times/week vs. sham acupuncture, 2–3 times/week | 8 | 24 | 218 | 79 | 43.1 |
RCT, randomized controlled trial; TTH, tension-type headache; TCAs, tricyclic antidepressants; IHS, International Headache Society
For all the outcomes, we compared the total posterior residual deviance with the number of unconstrained data points and DIC for all RE and FE NMA models. As the results are shown in eTable 4, the RE model had a residual deviance that was closer to the number of unconstrained data points and a lower DIC. Meanwhile, according to the dev-dev plots, all points lie roughly on the line of equality, indicating that there is no evidence for inconsistency (eFigure 2). Therefore, we choose the RE consistency models.
Headache frequency
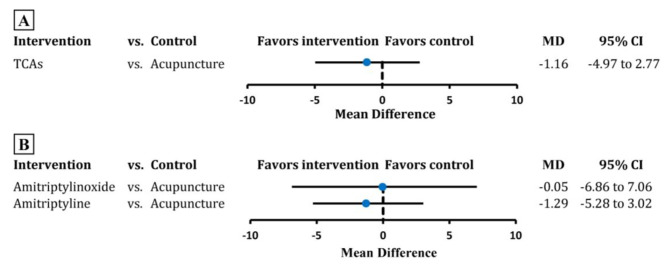
Nineteen RCTs containing 3046 participants were pooled in the analysis on headache frequency at the end of treatment. Evidence from pair-wise comparison indicated no significant difference between TCAs and acupuncture in reducing the TTH frequency (MD -1.16, 95% CI -4.97 to 2.77; tau-squared 11.30, Fig. 2A, eFigure 3). Very low certainty of evidence showed that acupuncture was no significantly different to amitriptyline and amitriptylinoxide (amitriptyline: MD -1.29, 95% CI -5.28 to 3.02; amitriptylinoxide: MD -0.05, 95% CI -6.86 to 7.06; tau-squared 12.15; Fig. 2B, eFigure 4, Table 2, eTable 5). The SUCRA suggested amitriptyline ranked first with a value of 0.67 (eTable 6).
Fig. 2.
Estimate of comparison between acupuncture and TCAs of headache frequency
TCAs, tricyclic antidepressants; MD, mean difference; CI, confidence interval. The black vertical line corresponds to 0。
Table 2.
Final classification of TCAs and acupuncture, based on NMA of intervention for headache frequency
| Certainty of the evidence, and classification* of intervention | Intervention | Certainty of the evidence** |
|---|---|---|
| Low certainty (low to very low certainty evidence) | ||
| Category 0: might be not convincingly different than acupuncture | Amitriptyline | Very low |
| Amitriptylinoxide | Very low | |
TCAs, tricyclic antidepressants; NMA, network meta-analysis. *Categories do not inform value judgements about the importance of the effects; **Certainty of evidence for each intervention when compared with acupuncture
Headache intensity
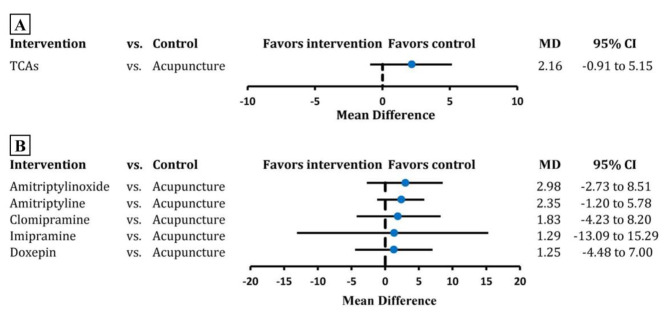
Twenty-four RCTs including 2062 individuals were entered in the analysis on headache intensity. Pair-wise comparisons revealed that acupuncture presented no significant difference with TCAs in reducing the headache intensity (MD 2.16, 95% CI -0.91 to 5.15; tau-squared 7.14; Fig. 3A, eFigure 5). At an individual-level, acupuncture presented a similar effect size with amitriptylinoxide, amitriptyline, and clomipramine with very low certainty of evidence (amitriptylinoxide: MD 2.98, 95% CI -2.73 to 8.51; amitriptyline: MD 2.35, 95% CI -1.20 to 5.78; clomipramine: MD 1.83, 95% CI -4.23 to 8.20; tau-squared 8.78; Fig. 3B, eFigure 6, eTable 5, eTable 7). The results of SUCRA showed acupuncture ranked first (SUCRA 0.77, eTable 6).
Fig. 3.
Estimate of comparison between acupuncture and TCAs of headache intensity
TCAs, tricyclic antidepressants; MD, mean difference; CI, confidence interval. The black vertical line corresponds to 0
Responder rate
Eight RCTs involving 2536 participants were included in the analysis of the responder rate. Evidence from pair-wise comparison suggested that acupuncture performs a similar effect with TCAs (OR 1.19, 95% CI 0.30 to 4.53; tau-squared 0.32; eFigure 7 A, eFigure 8). At an individual-level, there was no significant statistical difference between acupuncture and amitriptyline and amitriptylinoxide (amitriptyline, OR 0.81, 95% CI 0.16 to 4.20; amitriptylinoxide, OR 1.54, 95% CI 0.31 to 8.33; tau-squared 0.34; eFigure 7B, eFigure 9). The GRADE evidence was very low (eTable 5, eTable 8). Amitriptylinoxide ranked first with a SUCRA value of 0.89 (eTable 6).
Adverse event rate
Nineteen RCTs containing 2052 individuals were pooled in the analysis of adverse events at the end of treatment. The main adverse events due to acupuncture were hematoma and pain; the main adverse events associated with amitriptyline were dry mouth and drowsiness. There was no significant difference between acupuncture and TCAs in adverse events rate (OR 3.37, 95% CI 0.90 to 12.99; tau-squared 0.80; eFigure 10 A, eFigure 11). At an individual-level, very low certainty of evidence suggested that amitriptyline had a significantly higher adverse event rate than acupuncture, but amitriptylinoxide was similar to acupuncture with very low GRADE evidence (amitriptyline: OR 4.73, 95% CI 1.42 to 14.23; amitriptylinoxide: OR 1.15, 95% CI 0.25 to 7.05; tau-squared 0.39; eFigure 10B, eFigure 12, eTable 5, eTable 9). The results of SUCRA suggested acupuncture ranked first (SUCRA 0.37, eTable 6).
Sensitivity analysis
To evaluate the robustness of the results, we conducted two sensitivity analyses of the primary outcome. When RCTs at high risk of bias were excluded, we found acupuncture showed a similar effect size with TCAs in decreasing the frequency of headache (14 RCTs with 1685 participants; amitriptylinoxide: MD 0.37, 95% CI -6.67 to 7.95; amitriptyline: MD -0.80, 95% CI -6.19 to 4.69; tau-squared 14.26; eFigure 13), indicating the results were stable. After excluding RCTs with a randomized sample size of less than 50 participants, acupuncture also presented a similar effect with TCAs (13 RCTs with 2886 participants; amitriptylinoxide: MD -1.05, 95% CI -6.26 to 3.86; amitriptyline: MD -2.18, 95% CI -5.63 to 1.26; tau-squared 5.56; eFigure 14), suggesting the results were stable.
Subgroup analysis
We performed subgroup analyses of the primary outcome in the subtype of TTH, classification of acupuncture, and different endpoints of treatment to explore the source of heterogeneity. We found these elements did not significantly influence the heterogeneity (eFigure 15–17). Meanwhile, we also found no significant difference between acupuncture and TCAs in reducing the frequency of chronic TTH (14 RCTs with 1130 participants, amitriptylinoxide: MD -0.88, 95% CI -6.94 to 5.24; amitriptyline: MD -2.18, 95% CI -6.47 to 2.11; tau-squared 7.78; eFigure 15). In addition, there was no statistical difference of the effectiveness between acupuncture and TCAs in managing TTH when subgroup analyses were performed with acupuncture classification (eFigure 16) and endpoints of treatment (eFigure 17).
Discussion
Main finding
In our ITC meta-analyses, we found very low evidence demonstrating similar effectiveness of acupuncture and TCAs in preventing TTH attacks. There was no significant statistical difference between acupuncture and TCAs in reducing frequency and intensity, and in responder rate. We found amitriptyline had a higher rate of adverse events than acupuncture with an OR of 4.73 (95% CI 1.42 to 14.23).
Our results showed no significant variation between acupuncture and TCAs, which may be attributed to the fact that both interventions are effective in preventing TTH. TCAs are the first-line drugs that have been recommended for preventing TTH [4, 54]. Amitriptyline was the first-choice drug for preventing TTH and has been assessed as evidence of level A [4, 55]. Acupuncture was also effective in managing TTH [10]. As the results of previous systematic review and meta-analysis [12, 56], both TCAs (SMD 1.29) and acupuncture (SMD − 1.49) had a high effect size in reducing the number of headache days per month compared to placebo or sham acupuncture [57]. Moreover, TCAs (OR 1.41) and acupuncture (OR 1.29) presented a similar high effect size in decreasing at least 50% of headache frequency per mouth [13, 56]. Therefore, acupuncture was presented as effective as TCAs with no statistically significant difference.
Evidence from studies of TCAs and acupuncture also demonstrated that they could exert analgesic effects through various mechanisms, respectively. Amitriptyline may exert analgesic effects by inhibiting norepinephrine reuptake, antagonizing n-methyl-d-aspartate receptors, blocking muscarinic receptors and ion channels, and modulating noradrenergic and serotonergic downstream pain inhibitory systems [58, 59]. Additionally, amitriptyline can prevent TTH attacks by relieving the central sensitization [60]. The mechanisms of acupuncture analgesia involve signal molecules such as adenosine [61], γ-aminobutyric acid [62], serotonin [63], opioid peptide [64], and endocannabinoid [65]. Furthermore, recent studies have shown that acupuncture can also exert analgesic effects by recruiting β-END-containing ICAM-1 + /CD11b + immune cells [66].
Furthermore, we found amitriptyline presented a higher adverse event rate than acupuncture (OR 4.73), and the value of SUCRA also suggested amitriptyline had the lowest probability of being safe (SUCRA 0.08). Previous studies demonstrated that acupuncture is a safe therapy with few adverse events [13]. Adverse events associated with acupuncture were pain and hematoma near the needle, these symptoms were usually transient and mild. In contrast, compared with placebo, amitriptyline was more likely to contribute to dry mouth and drowsiness [56]. Acupuncture seems to be safer than amitriptyline.
Implication for practice and research
Based on our ITC analyses, acupuncture was as effective as first-line positive drugs in decreasing TTH frequency and intensity, and had lower adverse events rate than amitriptyline. Therefore, acupuncture can be an alternative therapy for managing TTH, especially for patients who are reluctant to take medications and who have severe adverse reactions to them. Meanwhile, it is necessary to conduct head-to-head trials between acupuncture and positive drugs. Our study was an indirect comparison analysis, and we found no head-to-head study had compared the effect of acupuncture and positive drugs. Endres and colleagues attempted to design an RCT in which a proportion of participants were randomly assigned to take amitriptyline [29]. However, due to participants unwilling to receive amitriptyline, the arm was dropped. Hence head-to-head studies still should be conducted for more evidence of the effectiveness of acupuncture.
Limitations of the study
Our study also had some limitations that should be noted when interpreting the findings. First, we only searched three specific databases and clinicaltrials.gov, and we excluded 24 studies due to the unavailability of full-text copies. Therefore, there may be relevant literature that was not included. However, we tried to avoid having eligible articles overlooked by scanning previous studies. Second, we evaluated the relative effectiveness between acupuncture and TCAs by ITC meta-analyses, and head-to-head trials are still needed to obtain direct evidence. Third, eight of thirteen-four included RCTs were assessed at high risk of bias, which might affect the quality and stability of our results. Therefore, we excluded the high risk of bias RCTs to verify the robustness of our results. Fourth, the studies we included were highly heterogeneous, and we attempted to explore the sources of heterogeneity through subgroup analyses of the TTH subtype, acupuncture classification, and endpoint of treatment. However, we found that these factors did not significantly contribute to the heterogeneity.
Conclusions
Our indirect comparison meta-analysis suggested very low evidence that acupuncture was as effective as TCAs in reducing the frequency and intensity of TTH, and acupuncture had a lower rate of adverse events than amitriptyline. Acupuncture can be an alternative option for TTH, and head-to-head studies are warranted for more direct evidence in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CI
confidence interval
- DIC
deviance information criteria
- FE
fixed-effect
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ITC
Indirect treatment comparison
- MD
mean difference
- NMA
network meta-analysis
- OR
odds ratio
- PRISMA-NMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses for Network Meta-Analyses
- RCT
randomized controlled trial
- RE
random-effect
- TCA
Tricyclic antidepressant
- TTH
Tension-type headache
Author contributions
Qing-Feng Tao: study selection, analyzed and interpreted the data, drafted the manuscript for intellectual content. Yan-Bing Huang: study selection, data extraction, and critical revision of the manuscript for important intellectual content. Lu Yuan: data extraction, and critical revision of the manuscript for important intellectual content. Yun-Zhou Shi, Di Qin: quality assessment, critical revision of the manuscript for important intellectual content. Kun Y, Wen-Yan Peng, Chao-Rong Xie: critical revision of the manuscript for important intellectual content. Hui Zheng: study design and conceptualization, critical revision of the manuscript for important intellectual content. All authors had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors have also agreed to submit for publication.
Funding
HZ received a grant from the Sichuan Youth Science and Technology Innovation Research Team (No.2021JDTD0007). HZ also received a grant from Chengdu University of TCM for evidence based medicine research (No. XKTD2021004). These sponsors did not participate in the design of the study, the analyzed and interpretation the data, and the decision process to submit the manuscript for publication.
Data availability
The data that supports our study are shown in the article and supplementary material, further inquiries can be directed to the corresponding author.
Declarations
Ethical approval and consent to participate
Our study was a systematic review and indirect treatment comparison meta-analysis of published studies and was exempt from review and approval by the research ethics committee. Ethical approval and consent to participate were acquired by each included study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ashina S, Mitsikostas DD, Lee MJ, et al. Tension-type headache. Nat Rev Dis Primers. 2021;7:24. doi: 10.1038/s41572-021-00257-2. [DOI] [PubMed] [Google Scholar]
- 2.(2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211. 10.1177/0333102417738202 [DOI] [PubMed]
- 3.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendtsen L, Evers S, Linde M, et al. EFNS guideline on the treatment of tension-type headache - report of an EFNS task force. Eur J Neurol. 2010;17:1318–1325. doi: 10.1111/j.1468-1331.2010.03070.x. [DOI] [PubMed] [Google Scholar]
- 5.Holroyd KA, O’Donnell FJ, Stensland M, et al. Management of chronic tension-type headache with tricyclic antidepressant medication, stress management therapy, and their combination: a randomized controlled trial. J Am Med Assoc. 2001;285:2208–2215. doi: 10.1001/jama.285.17.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boz C, Altunayoglu V, Velioglu S, Ozmenoglu M. Sertraline versus Amitriptyline in the prophylactic therapy of non-depressed chronic tension-type headache patients. J Headache pain. 2003;4:72–78. doi: 10.1007/s10194-003-0034-9. [DOI] [Google Scholar]
- 7.Jackson JL, Mancuso JM, Nickoloff S, et al. Tricyclic and tetracyclic antidepressants for the Prevention of frequent episodic or chronic tension-type headache in adults: a systematic review and Meta-analysis. J Gen Intern Med. 2017;32:1351–1358. doi: 10.1007/s11606-017-4121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374–383. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- 9.Meakins A. Acupuncture: what’s the point? Br J Sports Med. 2017;51:484. doi: 10.1136/bjsports-2016-096248. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Gao T, Zheng Q-H, et al. Acupuncture for patients with chronic tension-type headache: a Randomized Controlled Trial. Neurology. 2022;99:E1560–E1569. doi: 10.1212/WNL.0000000000200670. [DOI] [PubMed] [Google Scholar]
- 11.Melchart D, Streng A, Hoppe A, et al. Acupuncture in patients with tension-type headache: randomised controlled trial. BMJ. 2005;331:376–382. doi: 10.1136/bmj.38512.405440.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of tension-type headache. Cochrane Database Syst Rev. 2016;4:CD007587. doi: 10.1002/14651858.CD007587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao Q-F, Wang X-Y, Feng S-J, et al. Efficacy of acupuncture for tension-type headache prophylaxis: systematic review and meta-analysis with trial sequential analysis. J Neurol. 2023;270:3402–3412. doi: 10.1007/s00415-023-11695-1. [DOI] [PubMed] [Google Scholar]
- 14.Song F, Altman DG, Glenny A-M, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.Brignardello-Petersen R, Florez ID, Izcovich A, et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ. 2020;371:m3900. doi: 10.1136/bmj.m3900. [DOI] [PubMed] [Google Scholar]
- 18.Phillippo DM, Dias S, Ades AE, et al. Multilevel network meta-regression for population-adjusted treatment comparisons. J R Stat Soc Ser Stat Soc. 2020;183:1189–1210. doi: 10.1111/rssa.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter B, Gelman A, Hoffman MD, et al. Stan: a Probabilistic Programming Language. J Stat Softw. 2017;76:1. doi: 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support document 4: inconsistency in networks of evidence based on randomised controlled trials. London: National Institute for Health and Care Excellence (NICE); 2014. [PubMed] [Google Scholar]
- 21.da Costa BR, Juni P. Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J. 2014;35:3336–3345. doi: 10.1093/eurheartj/ehu424. [DOI] [PubMed] [Google Scholar]
- 22.Daly CH, Neupane B, Beyene J, et al. Empirical evaluation of SUCRA-based treatment ranks in network meta-analysis: quantifying robustness using Cohen’s kappa. BMJ Open. 2019;9:e024625. doi: 10.1136/bmjopen-2018-024625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Langemark M, Loldrup D, Bech P, Olesen J. Clomipramine and mianserin in the treatment of chronic tension headache. A double-blind, controlled study. HEADACHE. 1990;30:118–121. doi: 10.1111/j.1526-4610.1990.hed3003118.x. [DOI] [PubMed] [Google Scholar]
- 25.White A, Eddleston C, Hardie R, et al. A pilot study of acupuncture for tension headache, using a novel placebo. Acupunct Med. 1996;14:11–15. doi: 10.1136/aim.14.1.11. [DOI] [Google Scholar]
- 26.Gildir S, Tuzun EH, Eroglu G, Eker L. A randomized trial of trigger point dry needling versus sham needling for chronic tension-type headache. Med (Baltim) 2019;98:e14520. doi: 10.1097/MD.0000000000014520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White AR, Resch K-L, Chan JCK, et al. Acupuncture for episodic tension-type headache: a multicentre randomized controlled trial. Cephalalgia. 2000;20:632–637. doi: 10.1046/j.1468-2982.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 28.da Silva JBG, Nakamura MU, Cordeiro JA, Kulay L. Acupuncture for tension-type headache in pregnancy: a prospective, randomized, controlled study. Eur J Integr Med. 2012;4:E366–E370. doi: 10.1016/j.eujim.2012.04.002. [DOI] [Google Scholar]
- 29.Endres HG, Bowing G, Diener H-C, et al. Acupuncture for tension-type headache: a multicentre, sham-controlled, patient-and observer-blinded, randomised trial. J HEADACHE PAIN. 2007;8:306–314. doi: 10.1007/s10194-007-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jena S, Witt CM, Brinkhaus B, et al. Acupuncture in patients with headache. Cephalalgia. 2008;28:969–979. doi: 10.1111/j.1468-2982.2008.01640.x. [DOI] [PubMed] [Google Scholar]
- 31.Söderberg E, Carlsson J, Stener-Victorin E. Chronic tension-type headache treated with acupuncture, physical training and relaxation training. Between-group differences. Cephalalgia. 2006;26:1320–1329. doi: 10.1111/j.1468-2982.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 32.Guo NN, Shen HQ. Clinical effect of head-nine-needle therapy on tension headache. Zhen Ci Yan Jiu. 2020;45:148–151. doi: 10.13702/j.1000-0607.1908196. [DOI] [PubMed] [Google Scholar]
- 33.Schiller J, Karst M, Kellner T, et al. Combination of acupuncture and medical training therapy on tension type headache: results of a randomised controlled pilot study. Cephalalgia. 2021;41:879–893. doi: 10.1177/0333102421989620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K, Svensson P, Arendt-Nielsen L. Effect of acupuncture-like electrical stimulation on chronic tension-type headache: a randomized, double-blinded, placebo-controlled trial. Clin J Pain. 2007;23:316–322. doi: 10.1097/AJP.0b013e318030c904. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Lu W, Zhang D, et al. Eight acupuncture points in treatment of tension-type headache and cerebral blood flow condition. Liaoning J Traditional Chin Med [liao ning Zhong Yi Za Zhi] 2015;42:1509–1511. [Google Scholar]
- 36.Chassot M, Dussan-Sarria J, Sehn F, et al. Electroacupuncture analgesia is associated with increased serum brain-derived neurotrophic factor in chronic tension-type headache: a randomized, sham controlled, crossover trial. BMC Complement Altern Med. 2015;15:144. doi: 10.1186/s12906-015-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue CCL, Dong L, Polus B, et al. Electroacupuncture for tension-type headache on distal acupoints only: a Randomized, controlled, crossover trial. Headache. 2004;44:333–341. doi: 10.1111/j.1526-4610.2004.04077.x. [DOI] [PubMed] [Google Scholar]
- 38.Karst M, Reinhard M, Thum P, et al. Needle acupuncture in tension-type headache: a randomized, placebo-controlled study. Cephalalgia. 2001;21:637–642. doi: 10.1046/j.1468-2982.2001.00198.x. [DOI] [PubMed] [Google Scholar]
- 39.Kwak BM, Kim MJ, Kim YM, et al. Persisting effects of acupuncture method for chronic tension-type headache;a Randomized Controlled Trial. J Korean Acupunct Moxibustion Soc = Taehan Chimgu Hakhoe chi. 2008;25:165–177. [Google Scholar]
- 40.Ebneshahidi NS, Heshmatipour M, Moghaddami A, Eghtesadi-Araghi P. The effects of laser acupuncture on chronic tension headache - A randomised controlled trial. Acupunct Med. 2005;23:13–18. doi: 10.1136/aim.23.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Holroyd KA, Nash JM, Pingel JD, et al. A comparison of pharmacological (Amitriptyline HCL) and nonpharmacological (cognitive-behavioral) therapies for chronic tension headaches. J CONSULT CLIN PSYCHOL. 1991;59:387–393. doi: 10.1037//0022-006X.59.3.387. [DOI] [PubMed] [Google Scholar]
- 42.Bendtsen L, Jensen R, Olesen J. A non-selective (amitriptyline), but not a selective (citalopram), serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension-type headache. J Neurol Neurosurg Psychiatry. 1996;61:285–290. doi: 10.1136/jnnp.61.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damapong P, Kanchanakhan N, Eungpinichpong W et al (2015) A Randomized Controlled Trial on the effectiveness of Court-Type Traditional Thai Massage versus Amitriptyline in patients with chronic tension-type headache. Evid-Based Complement Altern Med 2015(930175). 10.1155/2015/930175 [DOI] [PMC free article] [PubMed]
- 44.Vernon H, Jansz G, Goldsmith C, McDermaid C. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manip Physiol Ther. 2009;32:344–351. doi: 10.1016/j.jmpt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Indaco A, Carrieri PB. Amitriptyline in the treatment of headache in patients with Parkinson’s disease: a double-blind placebo-controlled study. Neurology. 1988;38:1720–1722. doi: 10.1212/wnl.38.11.1720. [DOI] [PubMed] [Google Scholar]
- 46.Mitsikostas DD, Gatzonis S, Thomas A, Ilias A. Buspirone vs Amitriptyline in the treatment of chronic tension-type headache. ACTA NEUROL SCAND. 1997;96:247–251. doi: 10.1111/j.1600-0404.1997.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 47.Bettucci D, Testa L, Calzoni S, et al. Combination of tizanidine and Amitriptyline in the prophylaxis of chronic tension-type headache: evaluation of efficacy and impact on quality of life. J HEADACHE PAIN. 2006;7:34–36. doi: 10.1007/s10194-005-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mousavi SA, Mirbod SM, Khorvash F. Comparison between efficacy of imipramine and transcutaneous electrical nerve stimulation in the prophylaxis of chronic tension-type headache: a randomized controlled clinical trial. J Res Med Sci. 2011;16:923–927. [PMC free article] [PubMed] [Google Scholar]
- 49.Surbakti KP, Sjahrir H, Juwita-Sembiring R, Mutiara E. Effect of flunarizine on serum glutamate levels and its correlation with headache intensity in chronic tension-type headache patients. Open Access Maced J Med Sci. 2017;5:757–761. doi: 10.3889/oamjms.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaffenrath V, Diener H, Isler H, et al. Efficacy and tolerability of amitriptylinoxide in the treatment of chronic tension-type headache: a multi-centre controlled study. Cephalalgia. 1994;14:149–155. doi: 10.1046/j.1468-2982.1994.1402149.x. [DOI] [PubMed] [Google Scholar]
- 51.Deodato M, Guolo F, Monticco A, et al. Osteopathic Manipulative Therapy in patients with chronic tension-type headache: a pilot study. J Am Osteopath Assoc. 2019 doi: 10.7556/jaoa.2019.093. [DOI] [PubMed] [Google Scholar]
- 52.Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs. Amitriptyline for the treatment of chronic tension-type headaches: a randomized clinical trial. J Manipulative Physiol Ther. 1995;18:148–154. [PubMed] [Google Scholar]
- 53.Okasha A, Ghaleb HA, Sadek A. A double blind trial for the clinical management of psychogenic headache. Br J Psychiatry. 1973;122:181–183. doi: 10.1192/bjp.122.2.181. [DOI] [PubMed] [Google Scholar]
- 54.Robbins MS. Diagnosis and management of Headache: a review. JAMA. 2021;325:1874–1885. doi: 10.1001/jama.2021.1640. [DOI] [PubMed] [Google Scholar]
- 55.Smitherman TA, Walters AB, Maizels M, Penzien DB. The Use of antidepressants for Headache Prophylaxis. CNS Neurosci Ther. 2010;17:462–469. doi: 10.1111/j.1755-5949.2010.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson JL, Shimeall W, Sessums L, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: 10.1136/bmj.c5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb) 2021;31:010502. doi: 10.11613/BM.2021.010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashina S, Bendtsen L, Jensen R. Analgesic effect of Amitriptyline in chronic tension-type headache is not directly related to serotonin reuptake inhibition. Pain. 2004;108:108–114. doi: 10.1016/j.pain.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Exposto FG, Bendixen KH, Ernberg M, et al. Characterization and predictive mechanisms of experimentally induced tension-type headache. Cephalalgia. 2019;39:1207–1218. doi: 10.1177/0333102419840779. [DOI] [PubMed] [Google Scholar]
- 60.Bendtsen L. Central sensitization in tension-type headache–possible pathophysiological mechanisms. Cephalalgia. 2000;20:486–508. doi: 10.1046/j.1468-2982.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 61.Dai Q-X, Geng W-J, Zhuang X-X, et al. Electroacupuncture-induced neuroprotection against focal cerebral ischemia in the rat is mediated by adenosine A1 receptors. Neural Regen Res. 2017;12:228–234. doi: 10.4103/1673-5374.200806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mawla I, Ichesco E, Zöllner HJ, et al. Greater Somatosensory Afference with acupuncture increases primary somatosensory connectivity and alleviates Fibromyalgia Pain via Insular γ-Aminobutyric acid: a Randomized Neuroimaging Trial. Arthritis Rheumatol. 2021;73:1318–1328. doi: 10.1002/art.41620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Zhang RX, Zhang M, et al. Electroacupuncture inhibition of hyperalgesia in an inflammatory pain rat model: involvement of distinct spinal serotonin and norepinephrine receptor subtypes. Br J Anaesth. 2012;109:245–252. doi: 10.1093/bja/aes136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen S, Wang S, Rong P, et al. Acupuncture for visceral pain: neural substrates and potential mechanisms. Evid Based Complement Alternat Med. 2014;2014:609594. doi: 10.1155/2014/609594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, He S, Hu Y, Zheng H. Antagonism of cannabinoid receptor 1 attenuates the anti-inflammatory effects of electroacupuncture in a rodent model of migraine. Acupunct Med. 2016;34:463–470. doi: 10.1136/acupmed-2016-011113. [DOI] [PubMed] [Google Scholar]
- 66.Shi J-T, Cao W-Y, Zhang X-N, et al. Local analgesia of electroacupuncture is mediated by the recruitment of neutrophils and released β-endorphins. Pain. 2023;164:1965–1975. doi: 10.1097/j.pain.0000000000002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports our study are shown in the article and supplementary material, further inquiries can be directed to the corresponding author.