Abstract
Although killer Ig-like receptor ligands (KIR-L) mismatch has been associated with alloreactive natural killer cell activity and potent graft-versus-leukemia (GVL) effect among adults with acute myeloid leukemia (AML), its role among children with AML receiving cord blood transplantation (CBT) has not been determined. We conducted a retrospective study using a nationwide registry of the Japanese Society for Transplantation and Cellular Therapy. Patients who were diagnosed with de novo non-M3 AML and who underwent their first CBT in remission between 2000 and 2021 at under 16 years old were included. A total of 299 patients were included; 238 patients were in the KIR-L match group, and 61 patients were in the KIR-L mismatch group. The cumulative incidence rates of neutrophil recovery, platelet engraftment, and acute/chronic graft-versus-host disease did not differ significantly between the groups. The 5-year event-free survival (EFS) rate was 69.8% in the KIR-L match group and 74.0% in the KIR-L mismatch group (p = 0.490). Stratification by CD34 + cell dose into four groups revealed a significant correlation between CD34 + cell dose and EFS in the KIR-L mismatch group (p = 0.006) but not in the KIR-L match group (p = 0.325). According to our multivariate analysis, KIR-L mismatch with a high CD34 + cell dose (≥ median dose) was identified as an independent favorable prognostic factor for EFS (hazard ratio = 0.19, p = 0.029) and for the cumulative incidence of relapse (hazard ratio = 0.09, p = 0.021). Our results suggested that higher CD34 + cell doses are crucial for achieving a potent GVL effect in the context of KIR-L-mismatched CBT.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-024-01548-3.
Keywords: Acute myeloid leukemia, Children, Cord blood cell transplantation, KIR-ligand
To the Editor
In children with acute myeloid leukemia (AML), hematopoietic stem cell transplantation (HSCT) is an essential treatment modality [1], and cord blood transplantation (CBT) is a well-established procedure [2–4]. Although killer Ig-like receptor ligands (KIR-L) mismatch has been associated with alloreactive natural killer (NK) cell activity and potent graft-versus-leukemia (GVL) effect among adults with AML [5, 6], its roles among children with AML receiving CBT has not been determined [7, 8].
We conducted a retrospective study using a nationwide registry in Japan, and explored the associations of KIR-L incompatibility and other clinical factors with patient outcomes in children with AML who received CBT in complete remission. A detailed description of methods can be found in Additional file 1.
Findings
A total of 299 patients were included, consisting of 238 patients in the KIR-L match group and 61 patients in the KIR-L mismatch group (Additional file 1: Figure S1). The background characteristics of two groups were overall similar (Additional file 1: Table S1). The median follow-up period for survivors was 7.2 years (range, 0.1–22.4). The cumulative incidence rates of neutrophil recovery, platelet engraftment, and acute/chronic graft-versus-host disease did not differ significantly between the groups (Additional file 1: Figure S2-4).
The 5-year event-free survival (5y-EFS) rate was 69.8% for the KIR-L match group and 74.0% for the KIR-L mismatch group (p = 0.490; Table 1). The 5-year cumulative incidences of relapse were 22.3% and 15.2% (p = 0.257), and the 5-year cumulative incidences of non-relapse mortality (NRM) were 7.9% and 10.8% (p = 0.605) for the KIR-L match and mismatch groups, respectively, and the causes of death were similar between the groups (Additional file 1: Tables S2 and S3).
Table 1.
Univariate and multivariate analysis for event-free survival
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| n | 5y EFS (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age at HSCT, years old | 0–4 | 144 | 73.3 (65.1–79.9) | 0.918 | ref | |
| 5–9 | 78 | 68.2 (55.9–77.7) | 0.83 (0.44–1.57) | 0.575 | ||
| 10–15 | 77 | 68.2 (56.0–77.6) | 0.77 (0.38–1.55) | 0.466 | ||
| TNC* | < median | 147 | 69.8 (61.4–76.8) | 0.772 | ||
| ≥ median | 147 | 71.2 (62.9–78.1) | ||||
| CD34 + cells* | < median | 143 | 65.1 (56.3–72.6) | 0.093 | ||
| ≥ median | 143 | 76.3 (68.1–82.7) | ||||
| KIR-L | match | 238 | 69.8 (63.2–75.4) | 0.490 | ||
| mismatch | 61 | 74.0 (60.4–83.5) | ||||
| KIR-L and CD34 | KIR-L match-CD34 low | 112 | 67.0 (57.1–75.2) | 0.096 | ref | |
| KIR-L mismatch-CD34 low | 31 | 58.0 (37.9–73.7) | 1.22 (0.59–2.50) | 0.590 | ||
| KIR-L match-CD34 high | 115 | 73.4 (63.9–80.7) | 0.77 (0.44–1.35) | 0.355 | ||
| KIR-L mismatch-CD34 high | 28 | 89.1 (70.0–96.4) | 0.19 (0.04–0.85) | 0.029 | ||
| CR status at HSCT | CR1 | 212 | 73.4 (66.6–79.0) | 0.255 | ref | |
| CR2 | 87 | 63.9 (52.5–73.3) | 1.35 (0.81–2.23) | 0.249 | ||
| HSCT Year | 2000–2009 | 97 | 66.6 (56.2–75.1) | 0.393 | ref | |
| 2010–2021 | 202 | 72.4 (65.2–78.3) | 1.48 (0.86–2.56) | 0.158 | ||
| HCT-CI | 0 | 219 | 72.9 (66.2–78.5) | 0.666 | ||
| 1 | 20 | 56.2 (29.2–76.4) | ||||
| 2 | 3 | 66.7 (5.4–94.5) | ||||
| 3 | 1 | NA | ||||
| 6 | 1 | NA | ||||
| Conditioning regimen | chemo-MAC | 120 | 79.3 (70.7–85.6) | 0.004 | ref | |
| TBI-MAC | 129 | 58.6 (49.3–66.8) | 1.99 (1.13–3.50) | 0.017 | ||
| RIC | 50 | 82.4 (67.7–90.9) | 0.65 (0.26–1.60) | 0.350 | ||
| GVHD prophylaxis | CSA-based | 86 | 67.5 (56.2–76.4) | 0.347 | ||
| TAC-based | 208 | 71.8 (64.8–77.6) | ||||
| ATG | No | 288 | 70.6 (64.8–75.7) | 0.957 | ||
| Yes | 11 | 70.0 (32.9–89.2) | ||||
| ECOG PS | 0–1 | 262 | 71.1 (65.0–76.4) | 0.954 | ||
| 2–4 | 13 | 75.0 (40.8–91.2) | ||||
| Recipient CMV serostatus | Negative | 95 | 72.9 (62.2–81.1) | 0.877 | ref | |
| Positive | 175 | 70.3 (62.7–76.7) | 0.99 (0.61–1.62) | 0.978 | ||
| Donor recipient sex mismatch | Match | 115 | 74.3 (64.6–81.7) | 0.631 | ||
| F to M | 75 | 67.1 (55.0–76.6) | ||||
| M to F | 65 | 68.1 (54.4–78.5) | ||||
| Cytogenetic risk | Favorable | 51 | 74.0 (59.4–84.0) | 0.639 | ref | |
| Intermediate | 192 | 70.0 (62.7–76.2) | 1.34 (0.66–2.70) | 0.414 | ||
| Adverse | 56 | 69.7 (55.4–80.3) | 1.48 (0.65–3.35) | 0.349 | ||
| grade II–IV acute GVHD** | No | [-] | ref | |||
| Yes | [-] | 1.10 (0.68–1.78) | 0.705 | |||
*The median total nucleated cell and CD34 + cell doses were 6.7 × 107/kg (range, 0.01–12.3) and 1.9 × 105/kg (range, 0.01–59.4), respectively
**GVHD was treated as a time-dependent covariate in the multivariate analysis
Abbreviations: EFS, event-free survival; CI, confidence interval; HSCT, hematopoietic stem cell transplantation; TNC, total nucleated cell count; CR, complete remission; KIR, killer cell immunoglobulin-like receptor; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; TBI, total body irradiation; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; CSA, cyclosporine A; TAC, tacrolimus; ATG, antithymocyte globulin; ECOG, Eastern Cooperative Oncology Group; PS, performance status; CMV, cytomegalovirus; F, female; M, male; GVHD, graft-versus-host disease.
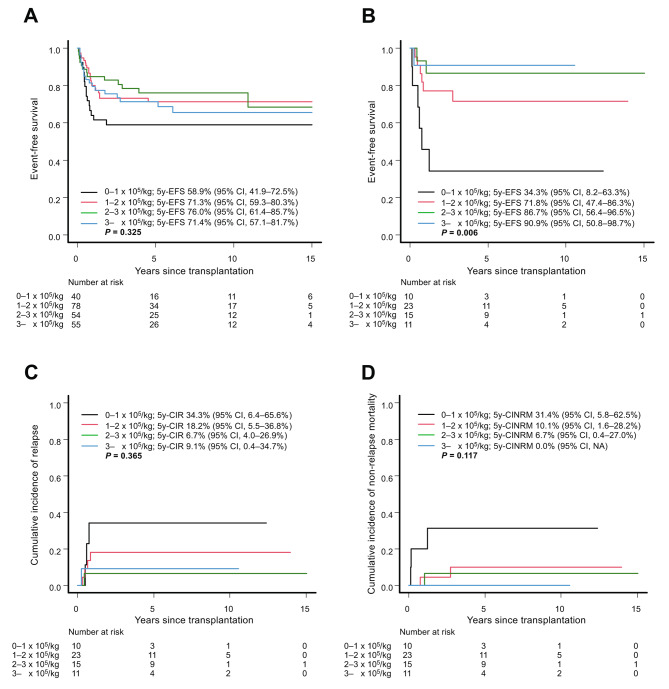
As a number of previous studies have suggested that CD34 + cell doses have an impact on the survival and/or engraftment [9–11], we stratified patients by CD34 + cell dose, and univariate analysis revealed a significant correlation between higher CD34 + cell dose and better EFS in the KIR-L mismatch group, as 5y-EFS was 34.3% for those with less than 1 × 105/kg, 71.8% for those with 1–2 × 105/kg, 86.7% for those with 2–3 × 105/kg, and 90.9% for those with ≥ 3 × 105/kg CD34 + cell doses (Fig. 1B, p = 0.006). On the other hand, this correlation was not detected in the KIR-L match group (Fig. 1A; p = 0.325). The impacts of CD34 + cell doses on EFS in the KIR-L mismatch group was attributed not only to the lower NRM but also to the lower relapse rate among those receiving higher doses, although neither was significant according to univariate analysis (Fig. 1C and D). These results were similar when we classified the patients into two groups: one with CD34 + cell doses less than the median (referred to as CD34low) and the other with CD34 + cell doses equal to or greater than the median (CD34high) (Additional file 1: Figure S5).
Fig. 1.
Event-free survival according to the infused CD34 + cell dose in the (A) KIR-ligand match group and (B) KIR-ligand mismatch group. The cumulative incidence of (C) relapse and (D) non-relapse mortality according to the infused CD34 + cell dose in the KIR-ligand mismatch group is also shown. EFS, event-free survival; CIR, cumulative incidence of relapse; CINRM, cumulative incidence of non-relapse mortality; CI, confidence interval; NA, not available; KIR, killer immunoglobulin-like receptor
In the multivariate analysis for EFS, the KIR-Lmismatch–CD34high (≥ median dose) subgroup was identified as an independent favorable prognostic factor (hazard ratio = 0.19, p = 0.029; Table 1). We also performed multivariate analysis for the incidence of relapse, and the KIR-Lmismatch–CD34high subgroup was identified as an independent favorable prognostic factor (hazard ratio = 0.09, p = 0.021; Additional file 1: Table S4).
Discussion
In this study, CD34 + cell doses were associated with outcomes in the KIR-L mismatch group but not in the KIR-L match group. Consequently, children who received KIR-L-mismatched CBT with high CD34 + cell doses had the best outcome.
To our knowledge, this was the first study in which higher CD34 + cell doses were associated with not only the lower NRM but also the lower relapse rate in the setting of KIR-L-mismatched CBT for children with AML. One interesting study showed that higher infused CD34 + cell doses promoted early reconstitution of NK cells. This, in turn, was associated with a reduced relapse rate and improved survival [12]. These results are compatible with our finding that infusion of higher CD34 + cell doses is important when we expect relapse-reducing effects from NK cells. Moreover, the association between cell dose and survival was strengthened, as we observed a dose-response relationship, as shown in Fig. 1B. Notably, there was no clear association between CD34 + cell dose and survival among those receiving KIR-L-matched CBT (Fig. 1A). This was thought to be a reasonable result, as in the setting of KIR-L-matched CBT, we could not expect a GVL effect from NK cells even when the number of NK cells was greater. As the number of patients in the KIR-L match group was limited (n = 61), a clinical trial including a larger number of patients is warranted to verify the results of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AML
Acute myeloid leukemia
- HSCT
Hematopoietic stem cell transplantation
- CBT
Cord blood cell transplantation
- KIR-L
Killer immunoglobulin-like receptors ligand
- NK
Natural killer
- GVL
Graft-versus-leukemia
- GVHD
Graft-versus-host disease
- 5y-EFS
5-year event-free survival
- NRM
Non-relapse mortality
Author contributions
HI: Conceputalization, methodology, data curation, formal analysis, funding aquisition, and writing–original draft. YKawahara: Methodology, formal analysis and writing–reviewing and editing. DT: Methodology, formal analysis and writing–reviewing and editing. YO: Methodology, formal analysis and writing–reviewing and editing. AH: Methodology, formal analysis and writing–reviewing and editing. YC: Investigation and writing–reviewing and editing. KKoh: Investigation and writing–reviewing and editing. YKoga: Investigation and writing–reviewing and editing. NY: investigation and writing–reviewing and editing. MS: Investigation and writing–reviewing and editing. KTerui: Investigation and writing–reviewing and editing. NM: Investigation and writing–reviewing and editing. AW: Investigation and writing–reviewing and editing. JT: Investigation and writing–reviewing and editing. RK: Investigation and writing–reviewing and editing. MY: Investigation and writing–reviewing and editing. KW: Investigation and writing–reviewing and editing. KO: Investigation and writing–reviewing and editing. KKato: Data curation and writing–reviewing and editing. KM: Data curation and writing–reviewing and editing. MH: Data curation and writing–reviewing and editing. KTabuchi: Data curation and writing–reviewing and editing. HS: Methodology, formal analysis and writing–reviewing and editing. All authors read and approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number JP23K14978 (Grant-in-Aid for Early-Career Scientists).
Data availability
The data of this study are not publicly available due to ethical restrictions that it exceeds the scope of the recipient/donor’s consent for research use in the registry.
Declarations
Ethics approval and consent to participate
The study was approved by the Data Management Committee of the TRUMP and the institutional ethics committee of Okayama University (2305-004). Patients or their parents provided written consent to undergo transplantation and for the use of medical records for research, in accordance with the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zwaan CM, Kolb EA, Reinhardt D, Abrahamsson J, Adachi S, Aplenc R, et al. Collaborative efforts driving Progress in Pediatric Acute myeloid leukemia. J Clin Oncol. 2015;33:2949–62. doi: 10.1200/JCO.2015.62.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 3.Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. 2016;375:944–53. doi: 10.1056/NEJMoa1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keating AK, Langenhorst J, Wagner JE, Page KM, Veys P, Wynn RF, et al. The influence of stem cell source on transplant outcomes for pediatric patients with acute myeloid leukemia. Blood Adv. 2019;3:1118–28. doi: 10.1182/bloodadvances.2018025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socié G, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoyama H, Kanda J, Kawahara Y, Uchida N, Tanaka M, Takahashi S, et al. Reduced leukemia relapse through cytomegalovirus reactivation in killer cell immunoglobulin-like receptor-ligand-mismatched cord blood transplantation. Bone Marrow Transpl. 2021;56:1352–63. doi: 10.1038/s41409-020-01203-8. [DOI] [PubMed] [Google Scholar]
- 7.Davies SM, Iannone R, Alonzo TA, Wang Y-C, Gerbing R, Soni S, et al. A phase 2 trial of KIR-Mismatched unrelated Donor Transplantation using in vivo T cell depletion with Antithymocyte Globulin in Acute Myelogenous Leukemia: children’s Oncology Group AAML05P1 Study. Biol Blood Marrow Transplant. 2020;26:712–7. doi: 10.1016/j.bbmt.2019.12.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verneris MR, Miller JS, Hsu KC, Wang T, Sees JA, Paczesny S, et al. Investigation of donor KIR content and matching in children undergoing hematopoietic cell transplantation for acute leukemia. Blood Adv. 2020;4:1350–6. doi: 10.1182/bloodadvances.2019001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 10.Konuma T, Kato S, Oiwa-Monna M, Tanoue S, Ogawa M, Isobe M, et al. Cryopreserved CD34 + cell dose, but not total nucleated cell dose, influences hematopoietic recovery and extensive chronic graft-versus-host disease after single-unit cord blood transplantation in adult patients. Biol Blood Marrow Transplant. 2017;23:1142–50. doi: 10.1016/j.bbmt.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Nakasone H, Tabuchi K, Uchida N, Ohno Y, Matsuhashi Y, Takahashi S, et al. Which is more important for the selection of cord blood units for haematopoietic cell transplantation: the number of CD34-positive cells or total nucleated cells? Br J Haematol. 2019;185:166–9. doi: 10.1111/bjh.15418. [DOI] [PubMed] [Google Scholar]
- 12.Zhao F, Shi Y, Chen X, Zhang R, Pang A, Zhai W, et al. Higher dose of CD34 + cells promotes early reconstitution of natural killer cells and is Associated with Better outcomes after unmanipulated hematopoietic stem cell transplantation for myeloid malignancies. Transplantation Cell Therapy. 2022;28:e5891–58910. doi: 10.1016/j.jtct.2022.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are not publicly available due to ethical restrictions that it exceeds the scope of the recipient/donor’s consent for research use in the registry.