Abstract
Background
A substantial proportion of patients with macroscopic stage III melanoma do not benefit sufficiently from adjuvant anti-PD-1 therapy, as they either recur despite therapy or would never have recurred. To better inform adjuvant treatment selection, we have performed translational analyses to identify prognostic and predictive biomarkers.
Patients and methods
Two cohorts of patients with macroscopic stage III melanoma from an ongoing biobank study were included. Clinical data were compared between an observation cohort (cohort 1) and an adjuvant intention cohort (cohort 2). RNA sequencing for translational analyses was performed and treatment subgroups (cohort 1A and cohort 2A) were compared for possible biomarkers, using a cut-off based on the treatment-naïve patients. In addition, two validation cohorts (Melanoma Institute Australia (MIA) and University Medical Centre Utrecht (UMCU)) were obtained.
Results
After a median follow-up of 26 months of the 98 patients in our discovery set, median recurrence-free survival (RFS) was significantly longer for the adjuvant intention cohort (cohort 2, n=49) versus the observation cohort (cohort 1, n=49). Median overall survival was not reached for either cohort, nor significantly different. In observation cohort 1A (n=24), RFS was significantly longer for patients with high interferon-gamma (IFNγ) score (p=0.002); for adjuvant patients of cohort 2A (n=24), a similar trend was observed (p=0.086). Patients with high B cell score had a longer RFS in cohort 1A, but no difference was seen in cohort 2A. The B cell score based on RNA correlated with CD20+ cells in tumor area but was not independent from the IFNγ score. In the MIA validation cohort (n=44), longer RFS was observed for patients with high IFNγ score compared with low IFNγ score (p=0.046), no difference in RFS was observed according to the B cell score. In both the observation (n=11) and the adjuvant (n=11) UMCU validation cohorts, no difference in RFS was seen for IFNγ and B cell.
Conclusions
IFNγ has shown to be a prognostic marker in both patients who were and were not treated with adjuvant therapy. B cell score was prognostic but did not improve accuracy over IFNγ. Our study confirmed RFS benefit of adjuvant anti-PD-1 for patients with macroscopic stage III melanoma.
Keywords: Melanoma, Adjuvant Drug Therapy, Tumor Biomarkers, Immune Checkpoint Inhibitor
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have examined the prognostic and predictive performance of clinicopathologic features such as Breslow thickness and ulceration to improve the stratification for adjuvant therapy. However, sensitive and specific tissue biomarkers have yet to be identified in stage III melanoma.
WHAT THIS STUDY ADDS
Both the interferon-gamma (IFNγ) and B cell scores have prognostic value in stage III melanoma, but a single strong predictive biomarker of adjuvant anti-PD-1 was not determined.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
These results provide a rationale for further investigations of both prognostic and predictive biomarkers of adjuvant anti-PD-1 therapy in stage III melanoma.
Introduction
Until recently, the standard of care for patients with macroscopic stage III melanoma has been surgery only. Due to the high risk of recurrence, adjuvant therapies (eg, interferon alpha-2b) have been investigated over the past decade, many without demonstrating a significant benefit to patients. Recently, both immune checkpoint inhibition (ICI) and BRAF-targeted therapies have been shown to improve recurrence-free survival (RFS) and are now a standard treatment for patients with resected stage III melanoma.1–5 However, approximately 80% of patients do not benefit sufficiently from these adjuvant therapies. This issue is presented by two subsets of patients: first, patients who recur despite adjuvant systemic therapy and second, patients who would never have recurred after surgery (and therefore do not require adjuvant treatment). In terms of the number needed to treat, on average five patients need adjuvant therapy in order to prevent one recurrence in stage III melanoma, illustrating the issue of overtreatment, unnecessary toxicity, and high healthcare costs.
Selection of adjuvant therapy is currently based on the American Joint Committee on Cancer (AJCC) staging system.6 Patients with stage IIIA (sentinel node tumor burden >1 mm), IIIB, and IIIC melanoma, according to the AJCC 7th edition, were included in the randomized clinical trials demonstrating improved outcomes for patients treated with adjuvant systemic therapy compared with placebo or ipilimumab.1 3 Previous studies have examined the prognostic and predictive performance of additional clinicopathologic features, for example, age, Breslow thickness, ulceration, number of positive lymph nodes, and extracapsular extension, to improve the stratification for adjuvant therapy.7–10 However, sensitive and specific tissue biomarkers have yet to be identified in stage III melanoma. Adding tissue biomarkers to these clinical characteristics could lead to a more personalized approach by a more adequate selection of patients, whereby patients are selected to receive adjuvant therapy based on their risk of recurrence and the expected reduction in risk of recurrence from such therapy.
Unraveling the immunologic characteristics of the tumor microenvironment associated with recurrence may reveal insight into possible biomarkers.11 12 Interferon-gamma (IFNγ) plays an important role in the antitumor response in the tumor microenvironment, and signatures related to IFNγ signaling were evaluated as possible biomarkers in previous studies. These studies have shown the IFNγ signature to be predictive of response in both patients with advanced melanoma treated with anti-PD-1 therapy and patients with stage III melanoma treated with combination ICI in a neoadjuvant setting.13–15 Therefore we hypothesized that the IFNγ signature could also be useful as a prognostic and/or predictive marker in patients treated with adjuvant therapy.
The aim of this study was to identify biomarkers prognostic and predictive of recurrence after surgery for macroscopic stage III melanoma, both in patients who did and who did not receive adjuvant systemic therapy, in order to predict in whom adjuvant therapy should be omitted, either because of a very low risk of recurrence or due to a lack of benefit.
Patients and methods
Patients
Patients were selected from an ongoing institutional database and biobank study (collecting tumor material from patients with macroscopic stage III melanoma) at the Netherlands Cancer Institute (NKI). Patients included between October 2017 and June 2020 were eligible for selection, when naïve to systemic therapy, complete resection of macroscopic stage III melanoma was performed and sufficient tumor material was available for RNA isolation. Exception for complete resection was made for patients with in-transit metastases who underwent an isolated limb perfusion (ILP), alone or combined with lymph node dissection (LND). Patients with missing data on adjuvant therapy or follow-up were excluded.
Approval and reimbursement of adjuvant systemic therapy in the Netherlands started in December 2018, resulting in two cohorts of similar high-risk patients: prior to availability of adjuvant therapy (observation cohort 1) and thereafter (adjuvant treatment cohort 2) (online supplemental figure S1). Clinical data regarding patient and tumor characteristics, adjuvant therapy, and outcome were collected with a median follow-up of 24 months for both cohorts.
jitc-2023-008125supp002.pdf (612.8KB, pdf)
As shown by the screening failures in randomized trials evaluating adjuvant therapy,16 a proportion of patients will not receive adjuvant systemic therapy due to an early recurrence that occurs between surgery and start of adjuvant therapy. These patients were present in our study, as they represent patients faced in daily clinical practice and were therefore included in the clinical data analyses. However, in terms of translational research, analyses were performed for treatment groups: patients who did not (observation cohort) versus who did receive adjuvant therapy (cohort 1A and 2A for RNA sequencing data, cohort 1B and 2B for PD-L1 data, respectively). To perform an equal comparison, patients with early recurrence were excluded from these translational analyses in both groups (online supplemental figure S1). The definition of early recurrence is described in online supplemental methods.
jitc-2023-008125supp001.pdf (228.6KB, pdf)
Tumor samples
Tumor samples were derived from surgical resection material in most patients and in some patients from biopsies prior to surgical procedures (eg, ILP). Per biobank protocol both fresh-frozen (FF) and formalin-fixed paraffin-embedded (FFPE) samples were stored, if feasible. From FF samples that contained sufficient tumor material based on the pathologist’s scoring (at least 30% tumor cells of H&E stained cryostat frozen section), RNA was isolated using the AllPrep DNA/RNA/miRNA universal isolation kit (QIAgen, 80224) on the QIAcube, according to the manufacturer’s protocol. Details on RNA sequencing can be found in the online supplemental methods.
Immunohistochemistry (IHC) of FFPE tumor samples was performed on a BenchMark Ultra (PD-L1 clone 22C3) or a Discovery Ultra (CD20-CD3 double stain) automated stainer (Ventana Medical Systems). Detailed methods of IHC are described in online supplemental methods. PD-L1 expression was scored by a pathologist in a blinded fashion: a score of >1% was considered to indicate PD-L1 positivity.1 2 Image analysis of CD20-CD3 double-stained samples was performed using HALO software (Module Multiplex IHC v3.0.3). A MiniNet AI classifier was used to define tissue into tumor and non-tumor. Color thresholds were set for CD20+ B cells (yellow), CD3+ T cells (purple), and Melanin and used in analysis for reliable detection. Cells were segmented using hematoxylin as a nuclear detection.
Validation cohorts
To validate the findings from our cohort, we performed analyses on two validation cohorts.
Validation cohort MIA
A cohort of 44 patients treated with adjuvant anti-PD-1 at Melanoma Institute Australia (MIA) between May 2015 and December 2018 was selected from a retrospective discovery cohort of patients with available resected stage III melanoma tissue.17 FFPE tumor samples were collected for research purposes with Sydney Local Health District Human Ethics Review Committee approval (Protocol no X15-0454 & 2019/ETH06874+X17-0312 & HREC/11/RPHA/32) and informed patient consent from the MIA Biospecimen Tissue Bank. RNA sequencing was performed at MIA, which is described in online supplemental methods. Raw data were made available, on which the same analyses were performed as in the initial cohort.
Validation cohort University Medical Centre Utrecht (UMCU)
The second validation cohort of 25 patients consisted of patients with melanoma treated at the UMCU. This cohort, similar to the Netherlands Cancer Institute (NKI) study population, consisted of patients who did (n=11) and did not (n=11) receive adjuvant treatment after complete resection of stage III melanoma and patients with early recurrence (n=3). FFPE tumor samples were transported to the NKI, RNA was isolated and sent to CeGaT for sequencing. The same protocols for isolation, sequencing, and analyses were performed as on the initial cohort. In this validation cohort, not all non-adjuvantly treated patients were from the preadjuvant treatment era (which is the case in the original study cohort). Patients who decided not to undergo adjuvant treatment despite available adjuvant therapy were included in this cohort.
Statistical analyses
Clinical data
Clinical data were analyzed using IBM SPSS Statistics, V.27. Baseline and treatment characteristics were compared between patients who did not receive adjuvant systemic therapy (observation cohort 1) and patients who intended to receive adjuvant therapy (cohort 2). Follow-up, RFS, and overall survival (OS) were analyzed using Kaplan-Meier estimates and a log-rank test was used to compare cohorts. Follow-up, RFS, and OS were defined as the time between surgery at the time of inclusion in the biobank and recurrence or death, respectively. Patients not experiencing an event were censored at the time of last follow-up. Cox regression analyses were carried out; correlation was assessed by Spearman’s rho test.
RNA sequencing analyses
The previously defined IFNγ,15 Danaher immune cell,18 and micro-environment cell population (MCP counter)19 gene expression signatures were analyzed. Cut-offs were calculated based on receiver operating characteristic (ROC) curves, using only observation patients (including patients with an early recurrence) to exclude a treatment effect on risk of disease recurrence. For the immune cell populations of both the Danaher and MCP-counter, z-score of the immune subsets was compared between the treatment groups. Bar and dot plots were generated in GraphPad Prism (V.9.0.2).
Results
The 98 patients in our discovery set were grouped into two cohorts: cohort 1 included 49 patients who did not receive adjuvant systemic therapy (observation); and cohort 2 included 49 patients who received adjuvant therapy, although some patients did not due to recurrence prior to the planned start (adjuvant intention) (online supplemental figure S1). Patients in cohort 2 were younger (p=0.027), more often had a tumor harboring a BRAF mutation (p=0.031) and a normal lactate dehydrogenase (LDH) level (p=0.009). Also, these patients more frequently underwent an LND only, instead of an LND combined with surgical treatment of in-transit metastases (p=0.002) (table 1). Patients were well balanced between the cohorts for sex and Breslow thickness. In both cohorts, the vast majority of patients had AJCC 8th edition stage IIIC melanoma and S100B levels below the upper limit of normal.
Table 1.
Baseline characteristics of all patients intended for adjuvant therapy versus observation
| All patients (N=98) | Observation cohort 1 (n=49) | Adjuvant intention cohort 2 (n=49) | P value | |
| Age | 0.027 | |||
| Median | 63 | 69 | 59 | |
| IQR | 54–73 | 54–76 | 53–70 | |
| Sex | 0.306 | |||
| Male | 57 (58) | 31 (63) | 23 (47) | |
| Female | 41 (42) | 18 (37) | 26 (53) | |
| Site primary | 0.055 | |||
| Extremities | 50 (51) | 31 (63) | 19 (39) | |
| Trunk | 41 (42) | 15 (31) | 26 (53) | |
| Head and neck | 2 (2) | 0 | 2 (4) | |
| Acral | 1 (1) | 0 | 1 (2) | |
| Mucosal | 2 (2) | 2 (4) | 0 | |
| MUP | 2 (2) | 1 (2) | 1 (2) | |
| Breslow thickness | 0.369 | |||
| ≤1.0 mm | 7 (7) | 2 (4) | 5 (10) | |
| 1.01–2.0 | 25 (26) | 13 (27) | 12 (25) | |
| 2.01–4.0 | 37 (38) | 21 (43) | 16 (33) | |
| >4.0 | 21 (21) | 8 (16) | 13 (27) | |
| Unknown | 8 (8) | 5 (10) | 3 (6) | |
| Ulceration | 0.067 | |||
| No | 53 (54) | 21 (43) | 32 (65) | |
| Yes | 29 (30) | 17 (35) | 12 (25) | |
| Unknown | 16 (16) | 11 (22) | 5 (10) | |
| Stage (AJCC 8th edition) | 0.718 | |||
| IIIB | 14 (14) | 5 (10) | 9 (18) | |
| IIIC | 78 (80) | 41 (84) | 37 (76) | |
| IIID | 4 (4) | 2 (4) | 2 (4) | |
| Unknown | 2 (2) | 1 (2) | 1 (2) | |
| Mutation status | 0.031 | |||
| BRAF | 59 (60) | 24 (49) | 35 (71) | |
| NRAS | 24 (25) | 12 (25) | 12 (25) | |
| cKIT | 1 (1) | 1 (2) | 0 | |
| No driver mutations | 10 (10) | 8 (16) | 2 (4) | |
| Unknown | 4 (4) | 4 (8) | 0 | |
| Type of surgery | 0.002 | |||
| LND | 54 (55) | 18 (37) | 36 (74) | |
| ITM | 20 (20) | 13 (27) | 7 (14) | |
| LND+ITM | 10 (10) | 6 (12) | 4 (8) | |
| ILP±LND | 14 (14) | 12 (25) | 2 (4) | |
| S100b | 0.905 | |||
| ≤ULN | 81 (83) | 39 (80) | 42 (86) | |
| >ULN | 12 (12) | 6 (12) | 6 (12) | |
| Unknown | 5 (5) | 4 (8) | 1 (2) | |
| LDH | 0.009 | |||
| ≤ULN | 85 (87) | 38 (78) | 47 (96) | |
| >ULN | 6 (6) | 6 (12) | 0 | |
| Unknown | 7 (7) | 5 (10) | 2 (4) |
Reported as number (%), percentages may not total 100 due to rounding.
P<0.05 is statistically significant and set in bold.
ILP, isolated limb perfusion; IQR, Interquartile Range; ITM, in-transit metastasis; LND, lymph node dissection; MUP, melanoma of unknown primary; ULN, upper limit of normal.
In cohort 2, 10 patients (20%) developed a recurrence before start of adjuvant therapy, and therefore 39 patients received at least one dose of adjuvant therapy. One patient initially started adjuvant BRAF-targeted therapy due to temporarily adjusted logistics following the outbreak of the COVID-19 pandemic, but switched to anti-PD-1 because of toxicity. All other patients received adjuvant anti-PD-1. The median time between surgery until the start of adjuvant therapy was 9 weeks (IQR 8–12) (online supplemental table S1). At data cut-off, all patients had ceased adjuvant therapy. The main reason for cessation was end of treatment (56%), followed by recurrence (26%). Due to the outbreak of the COVID-19 pandemic, 15 patients (39%) had a break in their adjuvant regimen, skipping one or two cycles of treatment.
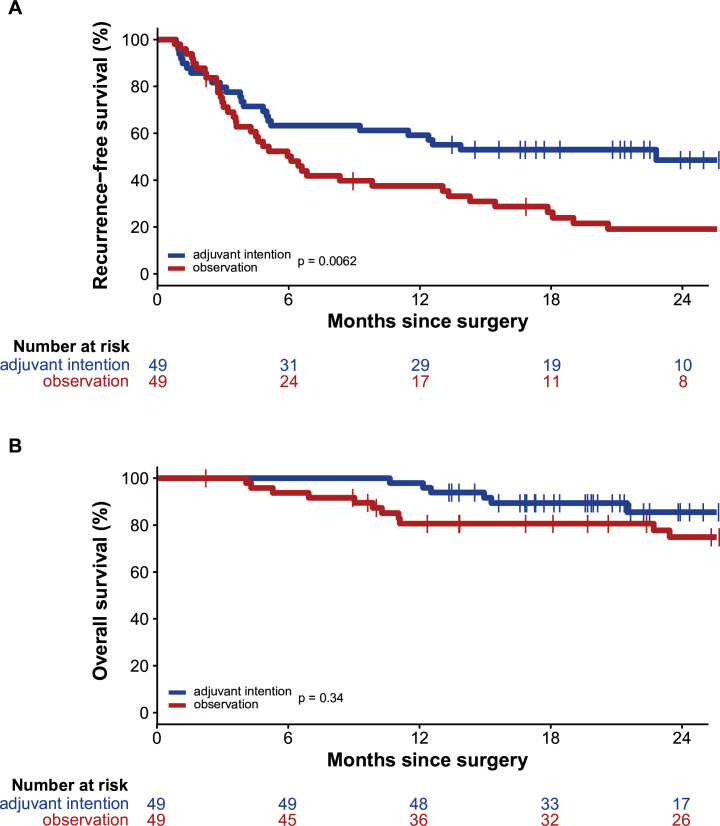
Median follow-up of the total patient cohort was 26 months (95% CI 23 to 28). After a median follow-up of 28 months (95% CI 26 to 30) in cohort 1, median RFS was 6 months (95% CI 4 to 8). After a median follow-up of 22 months (95% CI 19 to 25), the median RFS of 23 months (95% CI not reported (NR)) was significantly longer in cohort 2 (p=0.006) (figure 1A). At 24 months, the RFS rate was 19% for cohort 1 and 49% for cohort 2 (p=0.008). Univariable Cox regression analyses for RFS (online supplemental table S2) demonstrated, besides adjuvant therapy, significance for type of surgery and S100b levels at baseline, but not for LDH levels. In multivariable analyses, S100b and adjuvant therapy remained significant contributors.
Figure 1.
Survival curve. (A) Recurrence-free survival for patients intended for adjuvant therapy versus observation. (B) Overall survival for patients intended for adjuvant therapy versus observation.
Median OS was not reached in both cohorts (figure 1B). At 12 months, there was a significant difference in OS rate in favor of adjuvant therapy (81% vs 98%, p=0.006). The 24 months OS rate was 75% vs 86% in cohort 1 and cohort 2, respectively (p=0.158).
IFNγ score
RNA sequencing data were available for the subset of patients in the discovery set of whom RNA was of sufficient quality for sequencing: 24 observation patients (cohort 1A) and 24 adjuvant treatment patients (cohort 2A) (online supplemental figure S1). To perform an equal comparison, patients with early recurrence were excluded from these translational analyses in both groups. Of the patients in cohort 1A, 18 patients had a recurrence, compared with 9 patients in cohort 2A. Baseline characteristics for these cohorts are described in online supplemental table S3.
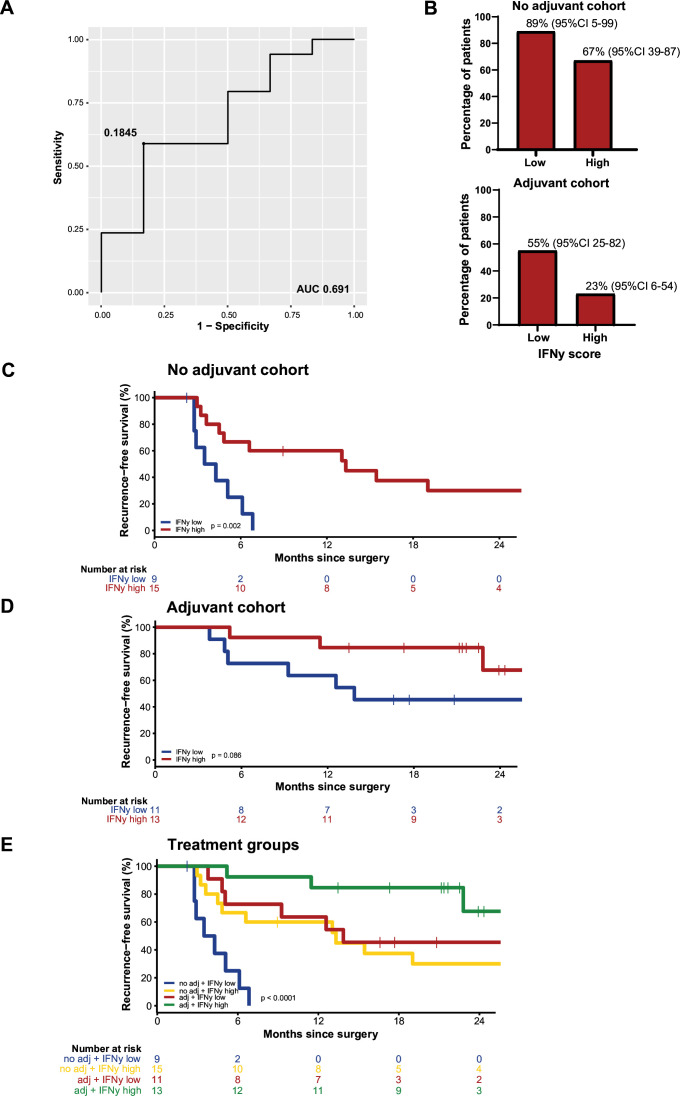
The optimal cut-off for the IFNγ score was determined at 0.1845 with a ROC curve, with an area under the curve (AUC) of 0.691 (figure 2A). In cohort 1A, 9 patients had low IFNγ score of whom 8 (89%, 95% CI 5 to 99) had a recurrence, and 15 had a high IFNγ score of whom 10 (67%, 95% CI 39 to 87) had disease recurrence. In cohort 2A, recurrences occurred in 6/11 patients (55%, 95% CI 25 to 82) with low IFNγ score and in 3/13 (23%, 95% CI 6 to 54) patients with high IFNγ score (figure 2B). In the patients with an early recurrence, more patients had a low IFNγ score (12 vs 4 with a high IFNγ score) (online supplemental figure S2).
Figure 2.
Interferon-gamma (IFNγ). (A) ROC curve determining the cut-off for the IFNγ score. (B) Bar plots with percentage of patients with recurrence, split for low and high IFNγ score. (C) Recurrence-free survival (RFS) for patients with low versus high IFNγ score in the no adjuvant cohort. (D) RFS for patients with low versus high IFNγ score in the adjuvant cohort. (E) RFS for patients with low versus high IFNγ score for both the no adjuvant and adjuvant cohort. AUC, area under the curve; ROC, receiver operating characteristic.
In both cohort 1A (median follow-up of 27 months) and cohort 2A (median follow-up of 22 months), a longer RFS for patients with high IFNγ scores was observed, although not statistically significant in the adjuvant cohort (figure 2C,D). RFS was significantly improved by adjuvant systemic therapy compared with observation, both in patients with high and especially in patients with low IFNγ score (p<0.001) (figure 2E). Patients with low IFNγ score had a median RFS of 4 months (95% CI 2 to 5) in the observation cohort, which increased to 13 months (95% CI 2 to 25) in patients receiving adjuvant therapy. In patients with high IFNγ scores, median RFS was 14 months (95% CI NR) versus not reached for patients receiving adjuvant therapy.
IFNγ score was an independent prognostic parameter, as it did not correlate with other known prognostic clinical factors such as T stage, staging according to AJCC 8th edition, Breslow thickness, ulceration, and S100B and LDH levels at day of surgery (online supplemental table S4). In addition, the observed differences between the cohorts for age, mutation status, and surgical treatment were not correlated with IFNγ score either.
Immune cell infiltration
The subsets of immune cells for both the Danaher signature18 and MCP counter19 did not show significant differences between patients with and without a recurrence within the two treatment cohorts 1A and 2A (online supplemental figures S3 and S4). However, full cohorts 1 and 2, including the patients with an early recurrence, demonstrated a difference (p=0.014) for the B cell subset within the Danaher signature. Therefore, we decided to expand analyses with the B cell score.
B cell score
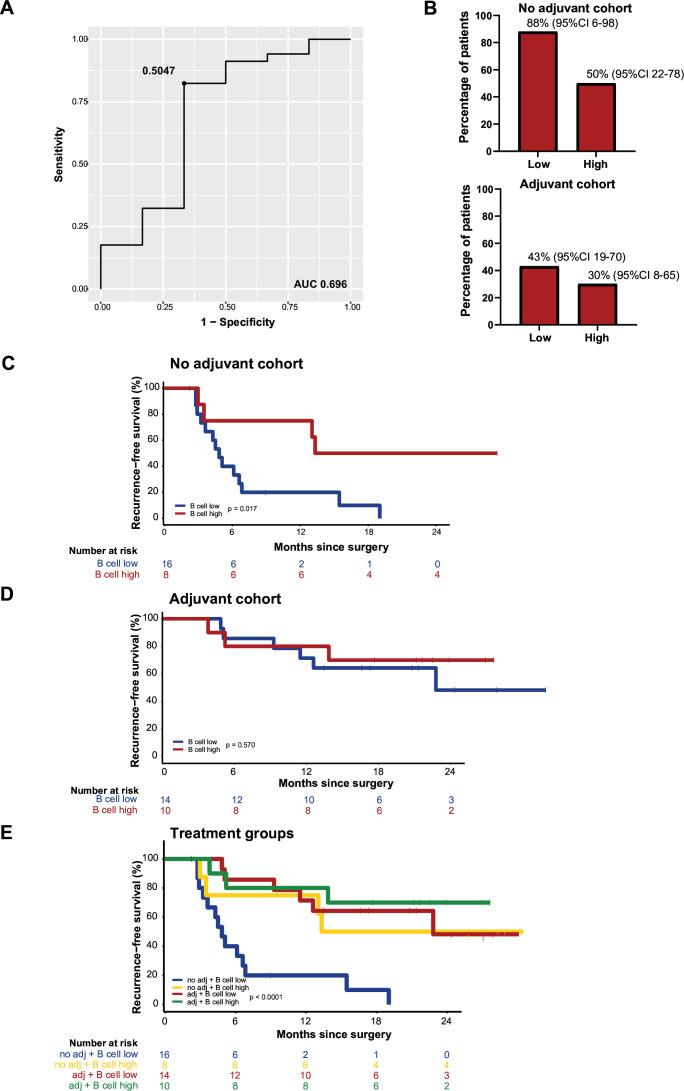
Similar to the IFNγ score, we determined the optimal cut-off at 0.5047 for a low and high B cell score using a ROC curve, with an AUC of 0.696 (figure 3A). In cohort 1A, 16 patients with a low B cell score were identified, of whom 14 (88%, 95% CI 6 to 98) had a recurrence versus 4/8 patients (50%, 95% CI 22 to 78) with a high B cell score. In cohort 2A, 6/14 patients (43%, 95% CI 19 to 70) with a low B cell score had a recurrence, versus 3/10 (30%, 95% CI 8 to 65,) of the patients with a high B cell score (figure 3B). In the patients with an early recurrence, more patients had a low B cell score (10 vs 6 with a high B cell score) (online supplemental figure S5).
Figure 3.
B cell. (A) ROC curve determining the cut-off for the B cell score. (B) Bar plots with percentage of patients with recurrence, split for low and high B cell score. (C) Recurrence-free survival (RFS) for patients with low versus high B cell score in the no adjuvant cohort. (D) RFS for patients with low versus high B cell score in the adjuvant cohort. (E) RFS for patients with low versus high B cell score for both the no adjuvant and adjuvant cohort. AUC, area under the curve; ROC, receiver operating characteristic.
In cohort 1A, RFS was longer in patients with a high B cell score (figure 3C). In cohort 2A, there was no difference in RFS (figure 3D). For patients of cohort 1B, median RFS was 5 months (95% CI 4 to NR) in patients with low and 13 months (95% CI 13 to NR) in patients with high B cell score. Patients with low B cell score had a median RFS of 23 months (95% CI 13 to NR) versus not reached for patients with a high score receiving adjuvant therapy (figure 3E).
To validate if the B cell score based on RNA sequencing data corresponded with B cell presence in the tumor, an IHC CD3/CD20 staining was performed and scored on 46/48 samples used for RNA sequencing (2 samples were not scored due to lack of tumor). In cohort 1A (n=23), the percentage CD20+ cells in tumor region of patients with recurrence was lower, but higher in the stroma region. In cohort 2A (n=23), there was a minimal difference in percentage CD20+ cell in the tumor region, but this was higher for patients without recurrence in the stroma region (online supplemental figure S6A). The B cell score correlated strongly with CD20 staining, the strongest correlation (p<0.001) was seen for the CD20+ cells in the tumor area (online supplemental figure S6B).
The Danaher T cell score correlated strongly (p<0.001) with the CD3 staining as well (online supplemental figure S6C). As the B cell score and IFNγ score were strongly correlated (p<0.001), these are not independent markers for recurrence (online supplemental figure S6D).
Validation cohorts
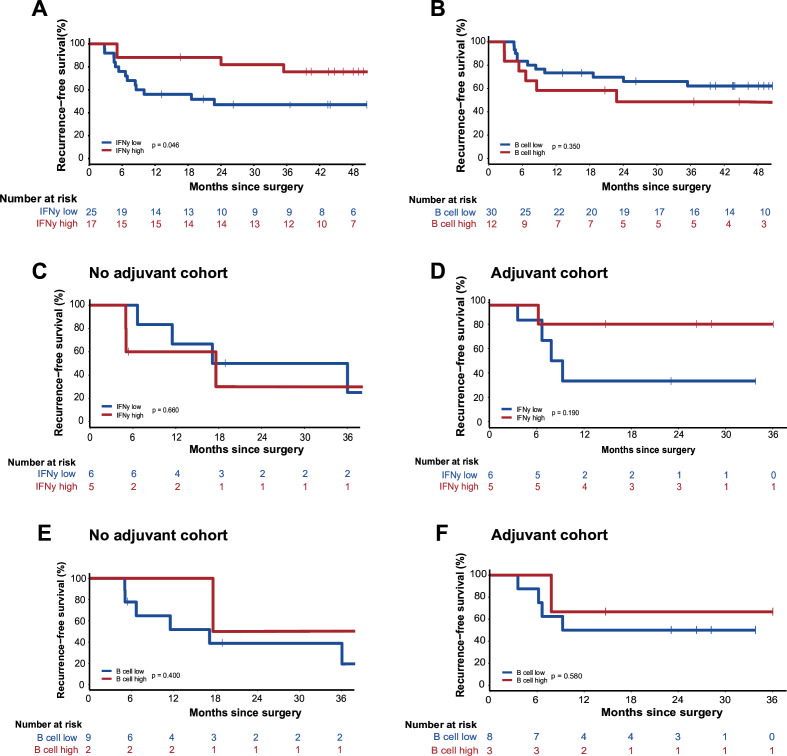
We used two validation cohorts to further test our determined cut-offs. Validation cohort MIA consisted of 44 patients treated with adjuvant therapy: 17 had a high and 27 had a low IFNγ score, 36 had a high and 8 had a low B cell score. After a median follow-up of 46 months (95% CI 44 to 53), RFS was significantly longer for patients with high IFNγ score compared with those with a low score (p=0.046) (figure 4A), but no difference in RFS was observed according to the B cell score (figure 4B).
Figure 4.
Validation cohorts. (A) Recurrence-free survival (RFS) of patients of validation cohort MIA with a low versus high IFNγ score. (B) RFS of patients of validation cohort MIA with a low versus high B cell score. (C) RFS of patients of validation cohort UMCU with a low versus high IFNγ score in the no adjuvant cohort. (D) RFS of patients of validation cohort UMCU with a low versus high IFNγ score in the adjuvant cohort. (E) RFS of patients of validation cohort UMCU with a low versus high B cell score in the no adjuvant cohort. (F) RFS of patients of validation cohort UMCU with a low versus high B cell score in the adjuvant cohort. IFNγ, interferon-gamma; MIA, Melanoma Institute Australia; UMCU, University Medical Centre Utrecht.
For validation cohort UMCU, in line with our own cohort, three patient groups were determined: patients not receiving adjuvant therapy (n=11), patients receiving adjuvant therapy (n=11), and patients with early recurrence (n=3). 11 patients had high and 14 had a low IFNγ score, 7 had high and 18 had a low B cell score. In both the no adjuvant and the adjuvant cohort, no difference in RFS was seen for the IFNγ (figure 4C,D) or B cell score (figure 4E,F).
PD-L1
PD-L1 staining was performed and scored on 73 tumor samples of the 98 patients in the discovery set; 28 from observation cohort 1 (cohort 1B), 28 from adjuvant treatment cohort 2 (cohort 2B), and 16 patients with an early recurrence (online supplemental figure S1). Samples of 24 patients were not scored due to either not enough tumor in the stained slide or too much pigment present for reliable scoring. Baseline characteristics are displayed in online supplemental table S5.
For patients who received adjuvant therapy (cohort 2B), 23 (82%) had a high and 5 (18%) had a low PD-L1 score. Fewer patients with a positive PD-L1 score developed a recurrence compared with patients with a negative PD-L1 score (30%, 95% CI 14 to 53 vs 80%, 95% CI 30 to 99). In cohort 1B, this was not as distinct: 71% (95% CI 48 to 88) of 21 patients with PD-L1 positive score developed a recurrence versus 86% (95% CI 42 to 99) of 7 patients with PD-L1 negative score (online supplemental figure S7A). In cohort 1B, 21 patients (75%) had high and 7 (25%) had low PD-L1 score. In the patients with an early recurrence, more patients scored negative for PD-L1 (9 vs 7 patients with PD-L1 positive score) (online supplemental figure S7B).
After a median follow-up of 28 months (95% CI 25 to 30), median RFS did not differ for patients with PD-L1 positive score versus PD-L1 negative score in cohort 1B (p=0.285) (online supplemental figure S7C). In cohort 2B, RFS was significantly longer (p=0.032) for patients with PD-L1 positive tumors (median RFS not reached), versus 13 months (95% CI 0 to 29) for PD-L1 negative tumors (online supplemental figure S7D). Both PD-L1 low and PD-L1 high cohorts had an improved RFS when receiving adjuvant therapy in the treatment cohorts (online supplemental figure S7E).
Discussion
Our study, focusing on identifying biomarkers for adjuvant treatment selection in macroscopic stage III melanoma, demonstrated IFNγ to be of prognostic value. The lack of predictive value, however, limits its sole use in clinical decision making.
Furthermore, our study demonstrated a significant RFS benefit for adjuvant anti-PD-1 therapy. These real-world data confirm the improved RFS seen with pembrolizumab in the EORTC-1325/Keynote-054 and nivolumab in the Checkmate-238 trials.1 2 Additionally, our study showed a significantly better 1-year and numerically higher (although not statistically significant) 2-year OS rate for adjuvant therapy, in slightly imbalanced cohorts with a possible selection bias due to the retrospective nature of the study. This is in line with data from the EORTC-1325/Keynote-054 and Checkmate-238 trial, which as of last analyses have not shown an OS benefit.20
As reflected in the number needed to treat five for adjuvant anti-PD-1, there is a clear need to identify patients with a higher risk of disease recurrence and a higher chance of benefit of anti-PD-1 prior to commencing adjuvant therapy, as well as those with lower risk and lower benefit. Accurate biomarkers would enable patient selection, so only patients truly in need and with high chance of treatment benefit would receive adjuvant therapy. Furthermore, patients destined to recur despite adjuvant anti-PD-1 therapy could be directed to alternative adjuvant therapies such as BRAF/MEK inhibitors or novel clinical trials, while those would never recur after surgery regardless of any adjuvant therapy can be spared treatment and the risk of toxicities altogether.
PD-L1 has previously been shown to correlate with response and outcome in advanced melanoma and non-small-cell lung cancer, therefore PD-L1 was analyzed in our cohorts.21–26 PD-L1 did show a predictive value in our study, although this should be interpreted with caution as the PD-L1 low group consisted of very few patients. Our results are in line with previous data: in the EORTC-1325/Keynote-054 trial, in both the PD-L1 positive and negative subgroups, adjuvant pembrolizumab demonstrated a significantly longer RFS than placebo.1 In the Checkmate-238 trial, RFS was longer for patients with PD-L1 ≥1% treated with either adjuvant nivolumab or adjuvant ipilimumab.2 Expression of PD-L1 is a controversial biomarker, as both trials demonstrated activity of ICI in PD-L1 low tumors. Moreover, the assessment of PD-L1 is heterogeneous because of different assays used, different cut-offs, and the staining is subject to interobserver variability.
We have investigated the IFNγ signature as it is known to be predictive of response of neoadjuvant combination ICI in macroscopic stage III melanoma.13–15 In the discovery cohort, IFNγ was an independent prognostic marker in both patients who were and were not treated with adjuvant anti-PD-1 therapy, as patients with low IFNγ score have an inferior RFS compared with patients with high IFNγ score in both cohorts. Comparable, in the COMBI-AD trial, an IFNγ signature score above median was prognostic for prolonged RFS in both patients treated with adjuvant dabrafenib plus trametinib and placebo.27 In the Checkmate-915, patients receiving adjuvant nivolumab±ipilimumab with an IFNγ score above median had a longer RFS than patients with an IFNγ score below median.28 Additionally, the Checkmate-238 trial demonstrated favorable RFS and OS outcomes in IFNγ high tumors in both patients treated with adjuvant nivolumab or ipilimumab.29 In the Checkmate-76K trial of adjuvant nivolumab versus placebo in resected stage IIB/C melanoma, IFNγ was shown to be both prognostic (IFNγ high associated with prolonged RFS regardless of therapy) and predictive (IFNγ high associated with prolonged RFS within the nivolumab group).30 We are the first, however, to use a cut-off based on a cohort of patients with untreated stage III melanoma and to study the risk of disease recurrence, uninfluenced by treatment effects, since above mentioned trials did not use the cut-off of the placebo group in treatment groups. Our data suggest that patients with low IFNγ score benefit from adjuvant therapy especially: without adjuvant therapy, nearly all develop a disease recurrence within 6 months of surgery, and with adjuvant treatment RFS is improved to the level of IFNγ high patients not receiving adjuvant therapy. This was also shown in the COMBI-AD trial.27 However, both the IFNγ low and high groups in our cohort have RFS benefit from adjuvant anti-PD-1 therapy, which makes IFNγ a prognostic and less a predictive biomarker. In the neoadjuvant OpACIN-neo trial, the IFNγ signature did show strong predictive value as it was associated with pathologic response to neoadjuvant ipilimumab plus nivolumab, and responding patients had a significantly longer RFS than patients without pathologic response.14
As IFNγ was a strong prognostic factor, we explored possible additional biomarkers. B cells were the strongest marker we could find of the subsets of immune cells defined by Danaher18 and MCP counter,19 but the IFNγ and B cell scores were strongly correlated with one another in our cohort. Thus, adding B cell analyses to the IFNγ score did not provide an additional predictive effect. It has been shown that B cells can facilitate an antitumor response by releasing pro-inflammatory cytokines, such as IFNγ.31 This could partly explain the correlation found.
Unfortunately, the validation cohorts lacked similar patient cohorts or patient numbers to validate our data. Validation cohort MIA did show a longer RFS in IFNγ high patients but consisted only of patients who had received adjuvant therapy. B cell score was not a significant prognostic factor in this cohort. Validation cohort UMCU did have similar patient groups, but the patient numbers were very low and no differences were seen in RFS for both IFNγ and B cell scores. Additionally, this validation cohort included patients deciding not to receive adjuvant therapy, despite availability. This may be based on, for example, comorbidities or risk of recurrence, and may therefore partly explain differences in results.
To our knowledge, our study is the first to use biomarkers with a cut-off identified on a patient population naïve for systemic therapy, in both patients treated with and without adjuvant systemic therapy in the setting faced in daily clinical practice. However, limitations of our study are the retrospective design, the limited patient numbers both in the original dataset, and even more distinctly, in the validation cohorts. Due to the retrospective design, cohorts were defined by the timeframe in which patients were treated, rather than by randomization. This may account for the imbalances between the treatment cohorts as previously described and therefore limits the definitive conclusions drawn from our study.
Another important observation is the rate of early recurrences after surgery, 20% within 12 weeks, usually on the first postoperative scan prior to commencement of adjuvant systemic therapy. This is in line with the previous observations of Bloemendal et al 16 and is an important consideration when comparing data from neoadjuvant and adjuvant trials, as none of the adjuvant trials have included these early recurrences, because they were considered screen failures. All patients with such aggressive biology are included in neoadjuvant trials and this makes the results of the neoadjuvant SWOG-1801 trial that much more impressive.32
In conclusion, we demonstrated that both the IFNγ and B cell scores have prognostic value in stage III melanoma, but we failed to find a single strong predictive biomarker of response. Our study confirmed the high rate of early recurrences in patients with high-risk stage III melanoma, who are intended to start adjuvant therapy. We confirmed the RFS benefit of adjuvant anti-PD-1 in melanoma versus observation and showed at least a numerical OS benefit when looking at all patients.
Acknowledgments
We thank all patients for participating in the biobank study. We would like to acknowledge the NKI-AVL Core Facility Molecular Pathology & Biobanking (CFMPB) for supplying NKI-AVL Biobank material and /or lab support. GVL is supported by an NHMRC Investigator Grant and the University of Sydney Medical Foundation. RAS and JSW are supported by NHMRC Investigator Grants and funding from the Cancer Institute New South Wales (2021/TPG2114). AMM is supported by an NHMRC Investigator Grant, Nicholas and Helen Moore, and Melanoma Institute Australia.
Footnotes
@Twitter @ProfRScolyerMIA
ACJvA and CUB contributed equally.
JMV and SAB contributed equally.
Contributors: JMV, SAB, ACJvA, and CB designed this study. JMV and SAB collected patient and translational data, analyzed and interpreted clinical and translational data, and wrote the first draft of the manuscript. WvH, YMS, MWJMW, and ACJvA included patients in the biobank study. JSW, WAMB, AMM, KPMS, and GVL provided patient data and material of the validation cohorts. PD performed bioinformatics analyses. AB was responsible for the RNA isolations and performance of the staining of the biopsies. RE scored the CD3/CD20 stainings, JS scored the PD-1 stainings. All authors interpreted the data, reviewed the manuscript, and approved the final version. ACJvA and CB functioned as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PD reported financial interest in Signature Oncology and will receive some possible revenues if the IFN-γ signature is being developed as a clinical companion diagnostic. AMM is a consultant advisor for BMS, MSD, Novartis, Roche, Pierre-Fabre, and QBiotics. RAS has received fees for professional services from MetaOptima Technology Inc., F. Hoffmann-La Roche Ltd, Evaxion, Provectus Biopharmaceuticals Australia, QBiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN Inc., Bristol-Myers Squibb, Myriad Genetics, GlaxoSmithKline. KPMS is consult advisor for Bristol-Myers Squibb, Merck Sharp and Dome, Abbvie, Pierre Fabre, Novartis, and Sairopa; has received honoraria from Novartis, Roche, Merck Sharp, and Dome; and has received research funding from TigaTx, Bristol Myers Squibb, and Philips; all paid to the institute. GVL is a consultant advisor for Agenus, Amgen, Array Biopharma, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA, Merck Sharpe & Dohme, Novartis, OncoSec, PHMR Ltd, Pierre Fabre, Provectus, QBiotics, and Regeneron. ACJvA has received advisory board and consultancy honoraria from Amgen, Bristol-Myers Squibb, Novartis, MSD-Merck, Merck-Pfizer, Pierre Fabre, Provectus, Sanofi, and 4SC, all paid to the institute; and research grants received from Amgen, Bristol-Myers Squibb, Merck-Pfizer, and Novartis, all paid to the institute. CB received compensation (all paid to the institute except TRV) for advisory roles for Bristol-Myers Squibb, MSD, Roche, Novartis, GSK, AZ, Pfizer, Lilly, GenMab, Pierre Fabre, and Third Rock Ventures; received research funding (all paid to the institute) from Bristol-Myers Squibb, Novartis, and NanoString, and declares stockownership in Immagene BV, where he is cofounder. All remaining authors have declared no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request for academic use and within the limitations of the provided informed consent. Every request will be reviewed by the institutional review board of the NKI; the researcher will need to sign a data access agreement with the NKI after approval.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Patients were selected from an ongoing institutional database and biobank study (collecting tumor material from patients with macroscopic stage III melanoma) at the Netherlands Cancer Institute (NKI), after local institutional review board approval (IRBdm20-188), in accordance with national privacy and ethics guidelines.
References
- 1. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–801. 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 2. Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (Checkmate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol 2020;21:1465–77. 10.1016/S1470-2045(20)30494-0 [DOI] [PubMed] [Google Scholar]
- 3. Dummer R, Hauschild A, Santinami M, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med 2020;383:1139–48. 10.1056/NEJMoa2005493 [DOI] [PubMed] [Google Scholar]
- 4. Blankenstein SA, van Akkooi ACJ. Adjuvant systemic therapy in high-risk melanoma. Melanoma Res 2019;29:358–64. 10.1097/CMR.0000000000000604 [DOI] [PubMed] [Google Scholar]
- 5. Michielin O, van Akkooi ACJ, Ascierto PA, et al. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1884–901. 10.1093/annonc/mdz411 [DOI] [PubMed] [Google Scholar]
- 6. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:472–92. 10.3322/caac.21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madu MF, Franke V, Van de Wiel BA, et al. External validation of the American joint committee on cancer 8th edition melanoma staging system: who needs adjuvant treatment. Melanoma Res 2020;30:185–92. 10.1097/CMR.0000000000000643 [DOI] [PubMed] [Google Scholar]
- 8. Madu MF, Schopman JHH, Berger DMS, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol 2017;116:244–51. 10.1002/jso.24635 [DOI] [PubMed] [Google Scholar]
- 9. Madu MF, Wouters MWJM, Klop WMC, et al. Clinical prognostic markers in stage IIIB melanoma. Ann Surg Oncol 2016;23:4195–202. 10.1245/s10434-016-5396-8 [DOI] [PubMed] [Google Scholar]
- 10. Verver D, van Klaveren D, van Akkooi ACJ, et al. Risk stratification of sentinel node-positive melanoma patients defines surgical management and adjuvant therapy treatment considerations. Eur J Cancer 2018;96:25–33. 10.1016/j.ejca.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 11. Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol 2020;6:519–27. 10.1001/jamaoncol.2019.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blank CU, Haanen JB, Ribas A, et al. The "cancer immunogram. Science 2016;352:658–60. 10.1126/science.aaf2834 [DOI] [PubMed] [Google Scholar]
- 13. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 2018;24:1655–61. 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 14. Rozeman EA, Hoefsmit EP, Reijers ILM, et al. Survival and biomarker analyses from the opacin-neo and opacin neoadjuvant immunotherapy trials in stage III melanoma. Nat Med 2021;27:256–63. 10.1038/s41591-020-01211-7 [DOI] [PubMed] [Google Scholar]
- 15. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-Γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. 10.1172/JCI91190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bloemendal M, van Willigen WW, Bol KF, et al. Early recurrence in completely resected IIIB and IIIC melanoma warrants restaging prior to adjuvant therapy. Ann Surg Oncol 2019;26:3945–52. 10.1245/s10434-019-07274-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Attrill GH, Owen CN, Ahmed T, et al. Higher proportions of Cd39+ tumor-resident cytotoxic T cells predict recurrence-free survival in patients with stage III melanoma treated with adjuvant immunotherapy. J Immunother Cancer 2022;10:e004771. 10.1136/jitc-2022-004771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Danaher P, Warren S, Dennis L, et al. Gene expression markers of tumor infiltrating leukocytes. J Immunother Cancer 2017;5:18. 10.1186/s40425-017-0215-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Becht E, Giraldo NA, Lacroix L, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 2016;17:218. 10.1186/s13059-016-1070-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eggermont AMM, Kicinski M, Blank CU, et al. Five-year analysis of adjuvant pembrolizumab or placebo in stage III melanoma. NEJM Evid 2022;1:EVIDoa2200214. 10.1056/EVIDoa2200214 [DOI] [PubMed] [Google Scholar]
- 21. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 2021;39:2339–49. 10.1200/JCO.21.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jassem J, de Marinis F, Giaccone G, et al. Updated overall survival analysis from Impower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol 2021;16:1872–82. 10.1016/j.jtho.2021.06.019 [DOI] [PubMed] [Google Scholar]
- 23. Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021;397:592–604. 10.1016/S0140-6736(21)00228-2 [DOI] [PubMed] [Google Scholar]
- 24. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 25. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 27. Dummer R, Brase JC, Garrett J, et al. Adjuvant dabrafenib plus trametinib versus placebo in patients with resected, BRAF(V600)-mutant, stage III melanoma (COMBI-AD): exploratory biomarker analyses from a randomised, phase 3 trial. Lancet Oncol 2020;21:358–72. 10.1016/S1470-2045(20)30062-0 [DOI] [PubMed] [Google Scholar]
- 28. Long GV, Desai K, Tang T, et al. Association of pre-treatment ctDNA with disease recurrence and clinical and translational factors in patients with stage IIIB-D/IV melanoma treated with adjuvant immunotherapy (Checkmate 915). Ann Oncol 2022;33:S904. 10.1016/j.annonc.2022.07.914 [DOI] [Google Scholar]
- 29. Weber J. Five-year outcomes with adjuvant nivolumab versus ipilimumab in resected stage IIIB–C or IV melanoma (Checkmate 238). Proceedings of the 2021 SMR Virtual Congress, Clifton Park, NY, USA; 2021:28–31. [Google Scholar]
- 30. Long GV, Kirkwood JMM, Hoeller C, et al. Association of biomarkers (Bms) with efficacy of adjuvant nivolumab (NIVO) vs placebo (PBO) in patients with resected stage IIB/C melanoma (Ca209-76K). JCO 2023;41:9504. 10.1200/JCO.2023.41.16_suppl.9504 [DOI] [Google Scholar]
- 31. Griss J, Bauer W, Wagner C, et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun 2019;10:4186. 10.1038/s41467-019-12160-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel S, Othus M, Prieto V, et al. Neoadjuvant versus adjuvant pembrolizumab for resected stage III-IV melanoma (SWOG S1801). Ann Oncol 2022;33:S1408. 10.1016/j.annonc.2022.08.039 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-008125supp002.pdf (612.8KB, pdf)
jitc-2023-008125supp001.pdf (228.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available on reasonable request for academic use and within the limitations of the provided informed consent. Every request will be reviewed by the institutional review board of the NKI; the researcher will need to sign a data access agreement with the NKI after approval.