Abstract
Background:
Despite advances in the treatment of early triple-negative breast cancer (TNBC), patients with residual invasive disease after neoadjuvant therapy have a high risk of disease recurrence and worse survival outcomes than those who have pathological complete response (pCR). Improving outcomes in early TNBC remains an unmet need requiring new adjuvant treatment approaches. Datopotamab deruxtecan (Dato-DXd) is an antibody–drug conjugate comprising a humanized anti-trophoblast cell-surface antigen 2 immunoglobulin G1 (IgG1) monoclonal antibody attached via a plasma-stable, cleavable linker to a potent topoisomerase I inhibitor payload, with activity observed in advanced TNBC.
Objectives:
TROPION-Breast03 is an ongoing phase III study evaluating the efficacy and safety of Dato-DXd alone or combined with durvalumab versus standard-of-care therapy as adjuvant treatment in patients with stage I–III TNBC with residual invasive disease at surgical resection following neoadjuvant treatment.
Methods and design:
Eligible patients, aged ⩾18 years, will be randomized in a 2:1:2 ratio to receive Dato-DXd [6 mg/kg intravenously (IV) every 3 weeks (Q3W); eight cycles] and durvalumab (1120 mg IV Q3W; nine cycles), Dato-DXd monotherapy (6 mg/kg IV Q3W), or investigator’s choice of therapy (ICT; capecitabine, pembrolizumab, or capecitabine and pembrolizumab). The primary endpoint is invasive disease-free survival (iDFS) for Dato-DXd and durvalumab versus ICT. Key secondary endpoints include safety, distant disease-free survival, and overall survival for Dato-DXd and durvalumab versus ICT and iDFS for Dato-DXd monotherapy versus ICT.
Ethics:
TROPION-Breast03 will be approved by the independent ethics committees or institutional review boards at each study site. All study participants will provide written informed consent.
Discussion:
TROPION-Breast03 will help define the potential role of Dato-DXd in the treatment of patients with early-stage TNBC who do not have pCR after neoadjuvant therapy.
Trial registration:
ClinicalTrials.gov identifier: NCT05629585 (registration date: 29 November 2022).
Keywords: adjuvant therapy, antibody–drug conjugates, immunotherapy, programmed cell death ligand-1, triple-negative breast cancer, trophoblast cell-surface antigen 2
Plain language summary
TROPION-Breast03: a clinical trial designed to assess the effectiveness and safety of Dato-DXd, alone or in combination with durvalumab, in patients with triple-negative breast cancer who have cancer cells remaining at the time of surgery after initial systemic therapy
Triple-negative breast cancer (TNBC), in which cells do not have estrogen or progesterone receptors or high levels of human epidermal growth factor receptor 2, is the most aggressive breast cancer subtype. TNBC is difficult to treat and associated with high risk of recurrence despite standard systemic therapy (treatment targeting the entire body), which can include chemotherapy alone or in combination with immunotherapy (treatment targeting the immune system). To reduce the risk of recurrence, standard systemic treatment is often followed by surgical removal of the patient’s tumors and additional systemic treatment. Dato-DXd is an antibody-drug conjugate, which is an anticancer drug (DXd) connected to an antibody (datopotamab) by a stable linker. Datopotamab binds to TROP2, a protein found on breast cancer cells, and is taken into the tumor cell where the linker breaks, releasing DXd to kill the cell. By delivering DXd directly to cancer cells, Dato-DXd reduces exposure in the rest of the body, reducing the risk of side effects. Since Dato-DXd can recruit immune cells to cancer sites, it may work better combined with durvalumab, a drug that blocks the activity of a protein called PD-L1, making cancer cells more susceptible to being killed by immune cells. The TROPION-Breast03 study will compare Dato-DXd, alone or combined with durvalumab, with standard-of-care therapy in patients with TNBC that has not spread to parts of the body away from the original tumor site(s), but with cancer cells remaining at the time of surgery after initial systemic therapy. It will assess how well each treatment works and describe any side effects. We plan to recruit 1,075 eligible adults who will be randomly assigned in a 2:1:2 ratio to: • Dato-DXd + durvalumab • Dato-DXd alone • Standard-of-care therapy • Patients will receive treatment until they complete the planned course of therapy (8 or 9 cycles), their cancer returns, side effects become unacceptable, or they choose to stop.
Introduction
Breast cancer has the highest incidence of any cancer worldwide, accounting for significant global morbidity and mortality; it was diagnosed in almost 2.3 million people and accounted for approximately 685,000 deaths in 2020. 1 Triple-negative breast cancer (TNBC), which is characterized by the absence of the estrogen receptor (ER) and progesterone receptor (PR) and lack of amplification/overexpression of human epidermal growth factor receptor 2 (HER2), accounts for approximately 15% of breast cancer diagnoses and is the most aggressive subtype. 2 While most patients with TNBC present without overt metastatic disease, approximately 30% of patients with early-stage disease will relapse with distant metastasis, with a median time to distant metastasis of approximately 2 years from diagnosis.2–6 Once patients develop advanced or metastatic TNBC, median overall survival with recommended therapies ranges from 8.1 to 24.2 months and the rate of survival at 5 years is approximately 13%.7,8
TNBC is challenging to treat due to a relative lack of actionable molecular targets such as ER, PR, and HER2.9–11 Standard recommended treatment for early-stage TNBC includes neoadjuvant chemotherapy with an anthracycline- and taxane-containing regimen followed by surgical resection and pathologic response guided adjuvant therapy.12–14 Patients with TNBC who have pathologic complete response (pCR), defined as lack of invasive and in situ residual cancer cells in breast tissue and lymph nodes, have improved event-free survival [EFS; hazard ratio (HR), 0.18] and overall survival (OS; HR, 0.20) compared with those with residual disease; 15 however, pCR has been reported in only 22–51% of patients with TNBC treated with neoadjuvant chemotherapy alone.16–18 Patients with TNBC with residual disease have 5- to 10-year recurrence rates ranging from 40% to 60%.19–21 In this setting, guidelines recommend adjuvant therapy, as it has been demonstrated to reduce the risk of recurrence and distant metastasis.12,22 The ECOG-ACRIN EA 1131 study showed that in patients with stage II or III TNBC and residual disease after neoadjuvant chemotherapy, adjuvant capecitabine was associated with improved outcomes compared with observation, with 3-year OS of 69.4%, recurrence-free survival of 53.4%, and invasive disease-free survival (iDFS) of 53.5%. 23 Furthermore, the CREATE-X study showed that in patients with stage I–IIIB TNBC and residual disease after neoadjuvant chemotherapy, adjuvant capecitabine improved 5-year disease-free survival (69.8% versus 56.1%) and overall survival (78.8% versus 70.3%) compared with no further systemic treatment. 24
Several clinical trials have shown that the addition of immunotherapy to chemotherapy regimens for early-stage TNBC is associated with improvement in both pCR and prognosis. 25 Many immunotherapies block the interaction of programmed cell death-1 (PD-1) with its ligand (PD-L1), reducing the ability of cancer cells to avoid anti-cancer immune responses. 26 Pembrolizumab, an immunotherapy targeting PD-1, is one such agent approved in early-stage TNBC. 27 Based on the results of the KEYNOTE-522 trial, the approved regimen comprises neoadjuvant therapy with both pembrolizumab and chemotherapy prior to surgery followed by adjuvant pembrolizumab monotherapy.27,28 This regimen is now the guideline-recommended treatment for high-risk early-stage TNBC. 29 Compared with neoadjuvant chemotherapy and surgery without adjuvant treatment, the addition of neoadjuvant/adjuvant pembrolizumab improved pCR rate from 56.2% to 63.4% and improved EFS at 3 years from 76.8% to 84.5% among patients with stage II or III TNBC.28,30 Despite these improvements, distant metastatic recurrence rates ranged from 8.7% to 35.0% among patients with residual disease depending on the extent of the disease. 30 Thus, new therapeutic options are still needed to improve outcomes in patients with early-stage TNBC who have residual disease after neoadjuvant therapy even when pembrolizumab is included in the regimen.
Datopotamab deruxtecan (Dato-DXd) is a trophoblast cell-surface antigen 2 (TROP2)-directed antibody–drug conjugate (ADC) composed of a humanized anti-TROP2 immunoglobulin G1 monoclonal antibody attached to a highly potent topoisomerase I inhibitor payload (an exatecan derivative, DXd) via a plasma-stable, tumor-selective, cleavable linker. 31 ADCs are a class of anticancer agents that selectively deliver a cytotoxic payload to tumor cells, thereby minimizing systemic toxicity, and benefit from a robust local bystander effect with payloads like DXd that have high membrane permeability.32–35 TROP2 is a type one transmembrane glycoprotein expressed on several normal tissues 36 ; notably, it is of clinical interest as it is broadly expressed in several solid tumors, including TNBC, and has been associated with poor prognosis.37–39 Another TROP2-directed ADC, sacituzumab govitecan, has been approved as second-line and beyond therapy in advanced/metastatic TNBC based on the results of the phase III ASCENT trial,40,41 and is under evaluation as adjuvant therapy in the phase III SASCIA trial in patients with primary HER2-negative breast cancer and residual disease after standard neoadjuvant treatment.42,43
Preclinical evidence indicates that topoisomerase I inhibitors like DXd can enhance antitumor immunity by recruiting immune cells.44–46 Given that many solid tumors, including TNBC, are known to evade the immune system, 47 combination therapy with both Dato-DXd and an immune cell activating agent may be more effective than either therapeutic agent alone. The immune checkpoint inhibitor durvalumab is a human monoclonal antibody of the immunoglobulin G1 kappa subclass that blocks the interaction of PD-L1, but not programmed cell death ligand-2, with PD-1 on T cells and CD80 on immune cells. 48 Blockade of the interaction of PD-L1 with PD-1 and CD80 releases the inhibition of immune responses, including those that may result in tumor elimination. 48 Thus, the combination of Dato-DXd and durvalumab may lead to improved clinical responses through complementary pharmacodynamic effects: Dato-DXd increases the recruitment of immune cells to tumor sites, and durvalumab increases the activation of immune cells.44–46,48
Dato-DXd monotherapy has shown encouraging preliminary safety and efficacy in a variety of advanced solid tumors in TROPION-PanTumor01 (NCT03401385), an ongoing phase I first-in-human study. 49 In a subset of patients with advanced/metastatic TNBC who had not received prior topoisomerase I inhibitor treatment, Dato-DXd resulted in a confirmed overall response rate of 44% and a manageable safety profile. 50 Dato-DXd has also been investigated in combination with durvalumab in the phase Ib/II BEGONIA study (NCT03742102). 51 Preliminary data from BEGONIA suggest that the combination has clinical activity in TNBC, with an objective response rate of 79%, median duration of response of 15.5 months, and median progression-free survival of 13.8 months as first-line therapy for locally advanced or metastatic TNBC.51,52 In addition to results from TROPION-PanTumor01 and BEGONIA, the ongoing TROPION-Breast02 trial (NCT05374512) is evaluating Dato-DXd as first-line monotherapy in advanced/metastatic TNBC 53 ; however, Dato-DXd has yet to be evaluated as adjuvant treatment in TNBC. Evaluation of Dato-DXd with or without durvalumab in early-stage TNBC is warranted given the unmet need to prevent relapse and disease progression.
Here we describe the design of TROPION-Breast03, which is investigating whether Dato-DXd, alone or in combination with durvalumab, has clinical benefit as adjuvant treatment in patients with stage I–III TNBC who do not have pCR at the time of surgery following neoadjuvant therapy.
Methods
Study design
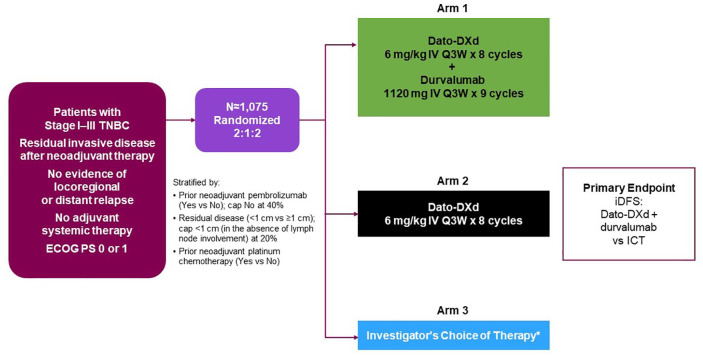
TROPION-Breast03 (NCT05629585) is an ongoing phase III, global, multicenter, randomized, open-label, active-controlled, three-arm study (Figure 1) investigating the efficacy and safety of Dato-DXd with or without durvalumab compared with investigator’s choice of treatment (ICT; capecitabine and/or pembrolizumab) as adjuvant therapy in patients with stage I–III TNBC who have residual invasive disease at the time of surgery after neoadjuvant therapy. Eligible patients enrolled from approximately 300 sites in 16 countries across Asia, Europe, and the Americas will be randomized 2:1:2 among three study arms: combination therapy with Dato-DXd and durvalumab (Arm 1), Dato-DXd monotherapy (Arm 2), or ICT (Arm 3). Randomization will be stratified based on receipt of prior neoadjuvant pembrolizumab, receipt of prior neoadjuvant platinum chemotherapy, and extent of residual disease at the time of surgery (<1 cm in the absence of lymph node involvement versus ⩾1 cm or any lymph node involvement). A recruitment cap of 40% will be applied to patients who have not received neoadjuvant pembrolizumab, and a cap of 20% will be applied to patients who have residual disease <1 cm in the absence of lymph node involvement. The treatment assigned to patients will be determined by a randomization scheme administered via an Interactive Response Technology/Randomization and Trial Supply Management system. The randomization scheme will be software-generated and comprise a blocked randomization list for each stratum, with randomization codes assigned strictly sequentially.
Figure 1.
TROPION-Breast03 study design.
*Capecitabine (1000 or 1250 mg/m2 BID for eight cycles) and/or pembrolizumab (200 mg IV Q3W for nine cycles); only patients who have received prior pembrolizumab in the neoadjuvant setting should receive pembrolizumab as part of their adjuvant therapy in the Investigator’s Choice of Therapy arm.
BID, twice per day; Dato-DXd, datopotamab deruxtecan; ECOG PS, Eastern Cooperative Oncology Group performance status; ICT, investigator’s choice of therapy; iDFS, invasive disease-free survival; IV, intravenous; Q3W, every 3 weeks; TNBC, triple-negative breast cancer.
Eligibility criteria
Key inclusion and exclusion criteria are presented in Tables 1 and 2. Eligible patients include adults aged ⩾18 years with stage I–III TNBC with residual invasive disease in the breast and/or axillary lymph nodes at surgical resection following neoadjuvant therapy. Diagnosis of TNBC will be based on local laboratory results and defined according to the American Society of Clinical Oncology-College of American Pathologists guidelines:54,55 ER expression of <1% of tumor cells by immunohistochemistry (IHC), PR expression of <1% of tumor cells by IHC, and HER2 negative (IHC 0/1+, or 2+ intensity and in situ hybridization non-amplified). Eligible patients must have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; 56 adequate organ function; completed at least six cycles of neoadjuvant therapy containing an anthracycline and/or a taxane, with or without carboplatin, with or without pembrolizumab; and undergone surgical removal of all clinically evident disease in the breast and lymph nodes with no evidence of locoregional or distant relapse; radiological scans before treatment are not required and should be obtained as per local institutional practice. If radiotherapy is indicated, it must be completed at least 2 weeks prior to randomization. Eligible patients must not have received any adjuvant systemic therapy, have no known germline breast cancer gene 1/2 (BRCA1/2) mutations, and must be eligible for at least one of the ICT treatment options. A formalin-fixed paraffin-embedded tumor sample from residual invasive disease at the time of surgical resection must be available.
Table 1.
Key inclusion criteria.
| Inclusion criteria |
|---|
| • Documented informed consent • Aged ⩾18 years at time of screening; male or female • Histologically confirmed invasive TNBC (per ASCO/CAP guidelines; based on local results) ○ Negative for ER with <1% of tumor cells positive for ER on IHC ○ Negative for PR with <1% of tumor cells positive for PR on IHC ○ Negative for HER2 with 0 or 1+ intensity on IHC, or 2+ intensity on IHC and no evidence of amplification on in situ hybridization • Residual invasive disease in the breast and/or axillary lymph node(s) at surgical resection following neoadjuvant therapy • Completed at least six cycles of neoadjuvant therapy containing anthracycline and/or a taxane with or without carboplatin, with or without pembrolizumab • No evidence of locoregional or distant relapse • Surgical removal of all clinically evident disease in the breast and lymph nodes • ECOG PS of 0 or 1 • Documented availability of FFPE tumor sample from residual invasive disease at surgery • No adjuvant systemic therapy • Radiotherapy (if indicated) delivered before the start of the study intervention • LVEF ⩾ 50% by either ECHO or MUGA scan within 28 days prior to randomization • Eligible for one of the therapy options listed as the investigator’s choice of therapy • No known germline BRCA1 or BRCA2 mutation • Adequate bone marrow reserve and organ function within 7 days before randomization • For women of childbearing potential, a negative serum pregnancy test |
ASCO/CAP, American Society of Clinical Oncology/College of American Pathologists; BRCA1/2, breast cancer gene 1/2; ECHO, echocardiogram; ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; FFPE, formalin-fixed paraffin-embedded; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; LVEF, left ventricular ejection fraction; MUGA, multigated acquisition; PR, progesterone receptor; TNBC, triple-negative breast cancer.
Table 2.
Key exclusion criteria.
| Exclusion criteria |
|---|
| • Stage IV (metastatic) TNBC • History of prior invasive breast cancer or evidence of recurrent disease following preoperative therapy and surgery • Any evidence of disease which, in the investigator’s opinion, makes it undesirable for the participant to participate in the study or that would jeopardize compliance with the protocol • History of another primary malignancy except for adequately resected basal cell carcinoma of the skin or squamous cell carcinoma of the skin, in situ disease that has undergone potentially curative therapy, or other solid malignancy treated with curative intent with no known active disease within 5 years before randomization and of low potential risk for recurrence • Persistent toxicities caused by previous anticancer therapy not yet improved to grade ⩽1 or baseline - Patients with irreversible toxicity that is not reasonably expected to be exacerbated by the study intervention may be included • Patients who have received prior pembrolizumab: - Must not have experienced toxicity that led to permanent discontinuation of prior immunotherapy - All AEs while receiving prior immunotherapy must have completely resolved or returned to baseline prior to screening for this study - Must not have experienced a grade ⩾3 immune-related AE or an immune-related neurologic or ocular AE of any grade while receiving prior immunotherapy - Must not have required the use of additional immunosuppression other than corticosteroids for the management of an AE, not have experienced recurrence of an AE if rechallenged, and not currently require maintenance doses of >10 mg prednisone or equivalent per day • Active or prior documented autoimmune or inflammatory disorders, except vitiligo or alopecia, hypothyroidism stable on hormone replacement, celiac disease controlled by diet, or any chronic skin condition that does not require systemic therapy • Clinically significant corneal disease • Active or uncontrolled hepatitis B or C infection • Known to have tested positive for HIV (positive HIV 1/2 antibodies) • Uncontrolled infection requiring IV antibiotics, antivirals, or antifungals; suspected infections; or inability to rule out infections • Mean resting corrected QTcF > 470 ms • Uncontrolled or significant cardiac disease • History of non-infectious ILD/pneumonitis that required steroids, current ILD/pneumonitis • Clinically severe pulmonary function compromise resulting from intercurrent pulmonary illnesses • Any known active liver disease • Grade ⩾2 peripheral neuropathy • Prior exposure to chloroquine/hydroxychloroquine without an adequate treatment washout period of >14 days prior to randomization • Receipt of a live, attenuated vaccine within 30 days prior to the first dose of study intervention • Prior exposure to the following anticancer therapies without an adequate treatment washout period prior to randomization: - Chemotherapy, radiation therapy, endocrine therapy, immunotherapy (non-antibody-based therapy), retinoid therapy, targeted therapy: ⩾2 weeks or five times the terminal elimination half-life of the therapeutic agent, whichever is longer; ⩾6 weeks for nitrosoureas or mitomycin C - Antibody-based anticancer therapy: ⩾4 weeks • Prior exposure to a PD-(L)1 inhibitor other than pembrolizumab • Any concurrent anticancer treatment • Concurrent systemic hormone replacement therapy • Concomitant or prior use of immunosuppressive medication within 14 days prior to randomization, with the following exceptions: - Intranasal, inhaled, topical steroids, or local steroid injections (e.g. intra-articular injection) - Systemic corticosteroids at physiologic doses not exceeding 10 mg/day of prednisone or its equivalent - Steroids as pre-medication for hypersensitivity reactions or as an anti-emetic (e.g. CT scan pre-medication) • Major surgical procedure or significant traumatic injury within ⩽4 weeks of Cycle 1, Day 1 or an anticipated need for major surgery during the study • Prior treatment with any agent including ADCs containing a chemotherapeutic agent targeting topoisomerase I • Prior treatment with TROP2-targeting therapy • Previous randomization in the current study • Participation in another clinical study with a study intervention or investigational medicinal device administered in the last 4 weeks prior to first dosing, randomization into a prior Dato-DXd, T-DXd, or durvalumab study, or concurrent enrolment in another clinical study, unless it is an observational clinical study or during the follow-up period of an interventional study • Known severe hypersensitivity to Dato-DXd or any of the excipients of the product • Known severe hypersensitivity to any monoclonal antibody • Known severe hypersensitivity to PD-(L)1 inhibitors |
AE, adverse event; CT, computed tomography; Dato-DXd, datopotamab deruxtecan; ILD, interstitial lung disease; PD-(L)1, programmed cell death (ligand)-1; QTcF, corrected QT interval by Fredericia’s formula; T-DXd, trastuzumab deruxtecan; TNBC, triple-negative breast cancer; TROP2, trophoblast cell-surface antigen 2.
Endpoints
The study endpoints are summarized in Tables 3 and 4.
Table 3.
Primary and secondary endpoints.
| TROPION-Breast03 primary and secondary endpoints |
|---|
| Primary endpoint • iDFS* for Dato-DXd + durvalumab versus ICT Key secondary endpoints • DDFS $ and OS for Dato-DXd + durvalumab versus ICT • iDFS for Dato-DXd monotherapy versus ICT Secondary endpoints • DDFS and OS for Dato-DXd monotherapy versus ICT • iDFS and DDFS for Dato-DXd + durvalumab versus Dato-DXd monotherapy • TTD in physical function via PROMIS Physical Function Short Form 8c • TTD in GHS/QoL via GHS/QoL scale from EORTC IL172 • Participant-reported fatigue via PROMIS Fatigue Short Form 7a • Pharmacokinetics of Dato-DXd • Immunogenicity of Dato-DXd Safety • AEs graded by CTCAE version 5.0 • ECOG PS • Vital signs, body weight, physical examination • Clinical chemistry, hematology, and urinalysis assessments • Ophthalmologic assessments |
iDFS is defined as the time from randomization until the date of the first occurrence of one of the following events: ipsilateral invasive breast tumor (local) recurrence, regional invasive breast cancer recurrence (axilla, regional lymph nodes, chest wall, and skin of ipsilateral breast), or distant recurrence (metastatic breast cancer that has either been biopsy-confirmed or clinically diagnosed as recurrent invasive breast cancer); contralateral invasive breast cancer; second primary non-breast invasive cancer (other than squamous or basal cell skin cancer); or death from any cause.
DDFS is defined as the time from randomization to the date of the first distant recurrence, occurrence of second primary non-breast invasive cancer (other than squamous or basal cell skin cancer), or death from any cause.
AE, adverse event; CTCAE, common terminology criteria for adverse events; Dato-DXd, datopotamab deruxtecan; DDFS, distant disease-free survival; ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC, European Organisation for Research and Treatment of Cancer; GHS, global health status; HIV, human immunodeficiency virus; ICT, investigator’s choice of therapy; iDFS, invasive disease-free survival; OS, overall survival; PROMIS, Patient-Reported Outcomes Measurement Information System; QoL, quality of life; TTD, time to deterioration.
Table 4.
Exploratory endpoints.
| TROPION-Breast03 exploratory endpoints |
|---|
| Exploratory endpoints • IBCFS* of Dato-DXd monotherapy versus ICT • IBCFS of Dato-DXd + durvalumab versus ICT • Patient-reported outcomes • Fertility status • Association of TROP2, PD-L1, or other tumor-derived biomarkers with response and tolerability to investigative regimens • Association of exploratory biomarkers in the tumor, plasma, whole blood, or serum collected before, during treatment, or at disease progression with disease status and/or response and tolerability to Dato-DXd ± durvalumab • Assessment of ctDNA dynamic changes as an indicator for recurrence on Dato-DXd ± durvalumab • Assessment of molecular and genomic determinants of response/resistance to Dato-DXd ± durvalumab in tumor and blood • To explore the impact of treatment and disease on healthcare resource use |
IBCSF is defined as time from randomization until the date of the first occurrence of one of the following: ipsilateral invasive breast tumor (local) recurrence, regional invasive breast cancer recurrence (axilla, regional lymph nodes, chest wall, and skin of ipsilateral breast), or distant recurrence (metastatic breast cancer that has either been biopsy-confirmed or clinically diagnosed as recurrent invasive breast cancer); contralateral invasive breast cancer; or death from any cause.
ctDNA, circulating tumor DNA; Dato-DXd, datopotamab deruxtecan; IBCFS, invasive breast cancer free survival; ICT, investigator’s choice of therapy; PD-L1, programmed cell death ligand-1; TROP2, trophoblast cell-surface antigen 2.
Primary and key secondary endpoints
The primary endpoint is invasive disease-free survival (iDFS) for treatment Arm 1 (Dato-DXd + durvalumab) versus Arm 3 (ICT). iDFS is defined as time from randomization until the date of the first occurrence of one of the following events: ipsilateral invasive breast tumor (local) recurrence, regional invasive breast cancer recurrence (axilla, regional lymph nodes, chest wall, and skin of ipsilateral breast), or distant recurrence (metastatic breast cancer that has either been biopsy-confirmed or clinically diagnosed as recurrent invasive breast cancer); contralateral invasive breast cancer; second primary non-breast invasive cancer (other than squamous or basal cell skin cancer); or death from any cause.
Key secondary endpoints include distant disease-free survival (DDFS) and OS for Arm 1 versus Arm 3 and iDFS for Arm 2 (Dato-DXd monotherapy) versus Arm 3. DDFS is defined as the time from randomization to the date of the first distant recurrence, occurrence of second primary non-breast invasive cancer (other than squamous or basal cell skin cancer), or death from any cause. Note that DDFS excludes potentially non-lethal events including ipsilateral invasive breast tumor recurrence, regional invasive breast cancer recurrence, contralateral invasive breast cancer, and all in situ carcinomas. OS is defined as the time from randomization until the date of death due to any cause.
Additional secondary endpoints
Additional secondary endpoints include DDFS and OS for Arm 2 versus Arm 3; and iDFS and DDFS for Arm 1 versus Arm 2, as well as pharmacokinetics and immunogenicity of Dato-DXd. Patient-reported outcomes are also being investigated, including time to deterioration (TTD; defined as the time from the date of randomization to the date of deterioration) in physical function assessed by the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function Short Form 8c;57,58 TTD in Global Health State (GHS)/Quality of Life (QoL) measured by the European Organisation for Research and Treatment of Cancer (EORTC) IL172 scale, which comprises 12 items for the GHS/QoL and role, emotional, cognitive, and social function scales from the EORTC QLQ-C30; 59 and fatigue measured via the PROMIS Fatigue Short Form 7a.60,61 Safety and tolerability of Dato-DXd with or without durvalumab relative to ICT will be assessed in terms of adverse events (AEs) graded by Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (v5.0), 62 ECOG PS, clinical examination, and laboratory assessment.
Study procedures and assessments
Patients will receive treatment until they complete the planned course of therapy, their cancer returns, side effects become unacceptable, or they choose to stop. Disease recurrence will be determined per investigator assessment. Efficacy assessments, including medical history and physical examination, will be performed every 3 months during the first 2 years, every 6 months during years 3 through 5, and annually thereafter. Bilateral mammogram or magnetic resonance imaging will be performed annually timed from the last assessment prior to randomization, with additional imaging assessments for recurrent or metastatic disease at the discretion of the treating physician based on the local standard of care. If no breast tissue remains in patients who underwent bilateral mastectomy, a physical examination is sufficient, and imaging will not be required. Patients will be regularly assessed for at least 5 years of follow-up regardless of discontinuation of study treatment or initiation of subsequent anti-cancer therapy. Following an iDFS event, patients will be followed for survival until at least 5 years after the last participant is randomized.
Continuous assessment for safety and tolerability will begin at screening and continue up to 90 days after the last dose of the study drug. An independent data monitoring committee comprised of independent experts will review safety data and make recommendations to continue, amend, or stop the study based on safety findings. AEs will be graded by CTCAE v5.0 and toxicities treated according to Dato-DXd and durvalumab toxicity management guidelines provided to each study site. Collected information will include ECOG PS, vital signs, body weight, and physical examinations; laboratory assessments, ophthalmologic assessments, and cardiac testing will also be performed. If new or worsening pulmonary symptoms or radiological abnormality suggestive of interstitial lung disease (ILD) or pneumonitis is observed, Dato-DXd will be delayed, and a full investigation will be required. A daily oral care protocol for stomatitis prophylaxis will be given to all patients; steroid-containing mouthwash is highly recommended for participants randomized to Dato-DXd or Dato-DXd in combination with durvalumab, and prophylactic cryotherapy (e.g. ice chips or ice water help in the mouth) during Dato-DXd infusion should also be considered. For nausea prevention, prophylactic anti-emetic agents are recommended prior to Dato-DXd infusion and on subsequent days as needed.
Patient-reported outcomes will be recorded every week from Cycle 1, Day 1 for the first 12 weeks, then every 3 weeks thereafter until the end of treatment. Blood samples will be collected at various time points throughout the study and be used for pharmacokinetic, immunogenicity, and biomarker analysis.
Statistical methods
An intention-to-treat (ITT) population comprising all patients who are randomized to study intervention, regardless of treatment actually received, will be used for all efficacy analyses. The safety analysis set will include all patients who received any amount of randomized treatment.
Approximately 1400 participants will be screened/enrolled to achieve approximately 1075 participants randomized to study treatment. The study is powered to demonstrate the superiority of combination therapy with Dato-DXd and durvalumab (Arm 1) versus ICT (Arm 3) as measured by the primary endpoint of iDFS in the ITT population (Figure 1) at 2-sided 5% alpha level.
iDFS, DDFS, and OS will be assessed using a log-rank test adjusting for stratification factors at randomization. A stratified Cox proportional hazards model will be used to estimate the HR and associated 95% confidence intervals for each endpoint, and Kaplan–Meier plots will be presented by the treatment group. Safety data will be summarized descriptively.
Discussion
Patients with early TNBC who do not have pCR at the time of surgery following neoadjuvant therapy have a substantial risk of recurrence/progression and limited treatment options; TROP2-directed ADCs may represent an opportunity to expand treatment options in this setting. The phase III TROPION-Breast03 trial is evaluating the efficacy and safety of Dato-DXd with and without durvalumab as adjuvant treatment in patients with early-stage TNBC with the residual invasive disease at the time of surgery after neoadjuvant therapy. Enrollment began in November 2022.
TROPION-Breast03 complements the ongoing TROPION-Breast02 and BEGONIA studies which are evaluating Dato-DXd in patients with advanced/metastatic TNBC. While TROPION-Breast02 and BEGONIA will define the role of Dato-DXd, with or without durvalumab, in advanced/metastatic TNBC, the results of TROPION-Breast03 will help define the potential role of Dato-DXd, with or without durvalumab, in the treatment of patients with early-stage TNBC who do not have pCR after neoadjuvant therapy.
Acknowledgments
SWOG Cancer Research Network Clinical Trials Partnerships (SQOG CTP) is the lead academic group for the trial and was a key collaborator in its protocol development. Medical writing support for the development and submission of this manuscript, under the direction of the authors, was provided by Eric Exner of Ashfield MedComms (Milwaukee, WI, USA), an Inizio company, and was funded by AstraZeneca.
Footnotes
ORCID iDs: Aditya Bardia  https://orcid.org/0000-0003-4885-1157
https://orcid.org/0000-0003-4885-1157
Nadia Harbeck  https://orcid.org/0000-0002-9744-7372
https://orcid.org/0000-0002-9744-7372
Contributor Information
Aditya Bardia, Jonsson Comprehensive Cancer Center, University of California Los Angeles, Los Angeles, CA, USA.
Lajos Pusztai, Yale University, New Haven, CT, USA.
Kathy Albain, Loyola University Chicago Stritch School of Medicine, Cardinal Bernardin Cancer Center, Maywood, IL, USA.
Eva Maria Ciruelos, Hospital Universitario 12 de Octubre, Madrid, Spain and HM Hospitales, Madrid, Spain.
Seock-Ah Im, Seoul National University College of Medicine, Cancer Research Institute, Seoul National University, Seoul, Republic of Korea.
Dawn Hershman, Herbert Irving Comprehensive Cancer Center at Columbia University, New York, NY, USA.
Kevin Kalinsky, Winship Cancer Institute at Emory University, Atlanta, GA, USA.
Claudine Isaacs, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA.
Delphine Loirat, Institut Curie, Paris, France.
Laura Testa, Instituto D’Or de Pesquisa e Ensino (IDOR), São Paulo, Brazil.
Eriko Tokunaga, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan.
Jiong Wu, Shanghai Cancer Center, Fudan University, Shanghai, China.
Hannah Dry, AstraZeneca, Waltham, MA, USA.
William Barlow, Cancer Research and Biostatistics, Seattle, WA, USA.
Robert Kozarski, Biostatistics, AstraZeneca, Cambridge, UK.
Micah Maxwell, Late Development – Oncology R&D, AstraZeneca, Gaithersburg, MD, USA.
Nadia Harbeck, Breast Center, Department of Obstetrics and Gynecology and Comprehensive Cancer Center (CCC) Munich, LMU University Hospital, Munich, Germany.
Priyanka Sharma, The University of Kansas Medical Center, Kansas City, KS, USA.
Declarations
Disclaimer: Authors Aditya Bardia, Nadia Harbeck, and Priyanka Sharma are Associate Editors (AB and NH) or Editorial Board Members (PS) of Therapeutic Advances in Medical Oncology; therefore, the peer-review process was managed by alternative members of the board and the submitting editors were not involved in the decision-making process.
Ethics approval and consent to participate: This study is being performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with the International Conference on Harmonisation/Good Clinical Practice, and applicable regulatory requirements, and will be approved by the independent ethics committees or institutional review boards at each study site. All study participants will provide written informed consent before any study-specific procedures are performed.
Consent for publication: Not applicable.
Author contributions: Aditya Bardia: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing.
Lajos Pusztai: Data curation; Resources; Writing – review & editing.
Kathy Albain: Investigation; Methodology; Project administration; Writing – review & editing.
Eva Maria Ciruelos: Conceptualization; Investigation; Writing – review & editing.
Seock-Ah Im: Investigation; Writing – review & editing.
Dawn Hershman: Conceptualization; Investigation; Writing – review & editing.
Kevin Kalinsky: Investigation; Writing – review & editing.
Claudine Isaacs: Investigation; Writing – review & editing.
Delphine Loirat: Investigation; Writing – review & editing.
Laura Testa: Investigation; Writing – review & editing.
Eriko Tokunaga: Investigation; Writing – review & editing.
Jiong Wu: Investigation; Writing – review & editing.
Hannah Dry: Data curation; Formal analysis; Methodology; Project administration; Validation; Writing – review & editing.
William Barlow: Conceptualization; Writing – review & editing.
Robert Kozarski: Methodology; Writing – review & editing.
Micah Maxwell: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Nadia Harbeck: Conceptualization; Investigation; Writing – review & editing.
Priyanka Sharma: Conceptualization; Investigation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The TROPION-Breast03 trial (NCT05629585) is sponsored by AstraZeneca. In July 2020, Daiichi-Sankyo entered into a global development and commercialization collaboration with AstraZeneca for datopotamab deruxtecan (Dato-DXd).
A.B. reports consulting fees from Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics/Gilead, Sanofi, Daiichi Pharma/AstraZeneca, Phillips, Eli Lilly, and Foundation Medicine; grants or funds from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, Immunomedics/Gilead, Daiichi Pharma/AstraZeneca, and Eli Lilly. L.P. reports membership on the board of directors for the HOPE Foundation; consulting fees from Pfizer, AstraZeneca, Merck, Novartis, Bristol Myers Squibb, GlaxoSmithKline, Roche/Genentech, Personalis, Daiichi, Natera, and Exact Sciences; and institutional research funding from Seagen, GlaxoSmithKline, AstraZeneca, Merck, Pfizer, and Bristol Myers Squibb. K.A. reports institutional research funding from Quantum Leap (I-SPY 2); and non-financial conflicts with IDMC (member) and Seattle Genetics. EMC reports consulting fees from Pfizer, MSD, Gilead, Roche, Eli Lilly, Daiichi-Sankyo, Novartis, and AstraZeneca; and institutional research funding from Roche. S.-A.I. reports advisory council or committee participation with AstraZeneca, Novartis, Hanmi, Pfizer, Eisai, Roche, Eli Lilly, GlaxoSmithKline, MSD, Daiichi-Sankyo, Idience, and Bertis; and grants or funds from AstraZeneca, Pfizer, Eisai, Roche, Daewoong Pharm, Boryung Pharm, and Daiichi-Sankyo. D.H. declares no conflicts of interest. K.K. reports consulting fees from Merck, Eli Lilly, Novartis, AstraZeneca, Roche/Genentech, Immunomedics, Seattle Genetics, Oncosec, 4D pharma, Daiichi-Sankyo, Puma Biotechnology, Mersna, Menarini Silicon Biosystems, Myovant Sciences, and Takeda; and grants or funds from Novartis, Ascentage, Roche/Genentech, Eli Lilly, Seattle Genetics, AstraZeneca, and Daiichi-Sankyo. C.I. reports consulting fees from Genentech, Puma Biotechnology, Seattle Genetics, AstraZeneca, Novartis, Pfizer, ION, and Gilead; institutional research funding from Tesaro/GlaxoSmithKline, Seattle Genetics, Pfizer, AstraZeneca, Bristol Myers Squibb, Genentech, and Novartis; and other financial relationships with Wolters Kluwer (UptoDate) and McGraw Hill (Goodman and Gillman). D.L. reports advisory council or committee participation with MSD, Seagen, Novartis, Eli Lilly, AstraZeneca, and Exact Sciences; honoraria from MSD, Seagen, Pfizer, Novartis, Eli Lilly, AstraZeneca, Roche, Exact Sciences, and Daiichi-Sankyo; and consulting fees from MSD and AstraZeneca. L.T. reports advisory council or committee participation with AstraZeneca, Daichii-Sankyo, MSD, Eli Lilly, Novartis, and Pfizer; consulting fees from AstraZeneca, Daiichi-Sankyo, MSD, Novartis, and Pfizer; grants or funds from Novartis; and provision of educational support for Roche, Pfizer, AstraZeneca, and Gilead. E.T. reports honoraria from Daiichi-Sankyo, AstraZeneca, and Eli Lilly. J.W. reports research funding from the National Natural Science Foundation and F. Hoffmann-La Roche Ltd; committee participation with Eli Lilly, AstraZeneca; honoraria from Novartis. H.D. reports employment by AstraZeneca and ownership of stock/shares in AstraZeneca. W.B. declares no conflicts of interest. R.K. reports employment by AstraZeneca. M.M. reports employment by AstraZeneca and ownership of stock/shares in AstraZeneca. N.H. reports membership on the board of directors of the West German Study Group (WSG); status as the ESMO Director of Education; honoraria from Amgen, AstraZeneca, Daiichi-Sankyo, EPG Communication, Gilead, Eli Lilly, Medscape, MSD, Novartis, Pierre-Fabre, Pfizer, Roche, Sandoz, Sanofi, Seagen, Springer, Viatris, and Zuellig Pharma; consulting fees from Gilead, Roche, Sandoz, Sanofi, and Seagen. P.S. reports consulting fees from Pfizer, Merck, Gilead, Genzyme (Sanofi), Novartis, AstraZeneca, and GlaxoSmithKline; institutional research funding from Novartis, Merck, and Gilead; and royalties from UpToDate.
Availability of data and materials: This is a clinical trial protocol manuscript, and no data are being reported. On completion of the trial, data will be available in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of Incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. O’Reilly D, Sendi MA, Kelly CM. Overview of recent advances in metastatic triple negative breast cancer. World J Clin Oncol 2021; 12: 164–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Asad S, Weber Z, et al. Genomic features of rapid versus late relapse in triple negative breast cancer. BMC Cancer 2021; 21: 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao Y, Chu Y, Xu B, et al. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci Rep 2019; 39: BSR20190288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13: 4429–4434. [DOI] [PubMed] [Google Scholar]
- 6. Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 2008; 113: 2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Institutes of Health - National Cancer Institute (NIH NCI). National Institutes of Health, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer subtypes, https://seer.cancer.gov/statfacts/html/breast-subtypes.html (2023, accessed 30 August 2023). [Google Scholar]
- 8. Li CH, Karantza V, Aktan G, et al. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res 2019; 21: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363: 1938–1948. [DOI] [PubMed] [Google Scholar]
- 10. Palma G, Frasci G, Chirico A, et al. Triple negative breast cancer: looking for the missing link between biology and treatments. Oncotarget 2015; 6: 26560–26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakhjavani M, Hardingham JE, Palethorpe HM, et al. Druggable molecular targets for the treatment of triple negative breast cancer. J Breast Cancer 2019; 22: 341–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 1674. [DOI] [PubMed] [Google Scholar]
- 13. Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 2021; 39: 1485–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pusztai L, Foldi J, Dhawan A, et al. Changing frameworks in treatment sequencing of triple-negative and HER2-positive, early-stage breast cancers. Lancet Oncol 2019; 20: e390–e396. [DOI] [PubMed] [Google Scholar]
- 15. Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 2020; 26: 2838–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 17. Caparica R, Lambertini M, Ponde N, et al. Post-neoadjuvant treatment and the management of residual disease in breast cancer: state of the art and perspectives. Ther Adv Med Oncol 2019; 11: 1758835919827714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020; 382: 810–821. [DOI] [PubMed] [Google Scholar]
- 19. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 20. Sikov WM, Polley M-Y, Twohy E, et al. CALGB (Alliance) 40603: long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/- carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J Clin Oncol 2019; 37(15_suppl): 591. [Google Scholar]
- 21. Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 2017; 35: 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denduluri N, Chavez-MacGregor M, Telli ML, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 2018; 36: 2433–2443. [DOI] [PubMed] [Google Scholar]
- 23. Mayer IA, Zhao F, Arteaga CL, et al. Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. J Clin Oncol 2021; 39: 2539–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376: 2147–2159. [DOI] [PubMed] [Google Scholar]
- 25. Tarantino P, Corti C, Schmid P, et al. Immunotherapy for early triple negative breast cancer: research agenda for the next decade. NPJ Breast Cancer 2022; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol 2021; 16: 223–249. [DOI] [PubMed] [Google Scholar]
- 27. United States Food and Drug Administration (FDA). FDA approves pembrolizumab for high-risk early-stage triple-negative breast cancer, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer (2021, accessed 30 August 2023).
- 28. Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med 2022; 386: 556–567. [DOI] [PubMed] [Google Scholar]
- 29. Korde LA, Somerfield MR, Hershman DL, et al. Use of immune checkpoint inhibitor pembrolizumab in the treatment of high-risk, early-stage triple-negative breast cancer: ASCO guideline rapid recommendation update. J Clin Oncol 2022; 40: 1696–1698. [DOI] [PubMed] [Google Scholar]
- 30. Pusztai L, Denkert C, O’Shaughnessy J, et al. Event-free survival by residual cancer burden after neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy for early TNBC: exploratory analysis from KEYNOTE-522. J Clin Oncol 2022; 40: 503. [Google Scholar]
- 31. Okajima D, Yasuda S, Maejima T, et al. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther 2021; 20: 2329–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Criscitiello C, Morganti S, Curigliano G. Antibody-drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol 2021; 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolska-Washer A, Robak T. Safety and tolerability of antibody-drug conjugates in cancer. Drug Saf 2019; 42: 295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakada T, Sugihara K, Jikoh T, et al. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo) 2019; 67: 173–185. [DOI] [PubMed] [Google Scholar]
- 35. Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016; 107: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stepan LP, Trueblood ES, Hale K, et al. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J Histochem Cytochem 2011; 59: 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018; 9: 28989–29006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeng P, Chen MB, Zhou LN, et al. Impact of TROP2 expression on prognosis in solid tumors: a systematic review and meta-analysis. Sci Rep 2016; 6: 33658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeon Y, Jo U, Hong J, et al. Trophoblast cell-surface antigen 2 (TROP2) expression in triple-negative breast cancer. BMC Cancer 2022; 22: 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. United Stated Food and Drug Administration (FDA). TRODELVY prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761115s009lbl.pdf (2021, accessed 30 August 2023).
- 41. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 2021; 384: 1529–1541. [DOI] [PubMed] [Google Scholar]
- 42. Marmé F, Stickeler E, Furlanetto J, et al. Phase III postneoadjuvant study evaluating sacituzumab govitecan, an antibody drug conjugate in primary HER2-negative breast cancer patients with high relapse risk after standard neoadjuvant treatment: SASCIA. J Clin Oncol 2021; 39 (15_suppl): TPS602. [Google Scholar]
- 43. Marmé F, Hanusch C, Furlanetto J, et al. 58O Safety interim analysis (SIA) of the phase III postneoadjuvant SASCIA study evaluating sacituzumab govitecan (SG) in patients with primary HER2-negative breast cancer (BC) at high relapse risk after neoadjuvant treatment. Ann Oncol 2022; 33(Suppl. 3): S148–S149. [Google Scholar]
- 44. Yum S, Li M, Chen ZJ. Old dogs, new trick: classic cancer therapies activate cGAS. Cell Res 2020; 30: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKenzie JA, Mbofung RM, Malu S, et al. The effect of topoisomerase I inhibitors on the efficacy of T-cell-based cancer immunotherapy. J Natl Cancer Inst 2018; 110: 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang KC, Chiang SF, Yang PC, et al. Immunogenic cell death by the novel topoisomerase I inhibitor TLC388 enhances the therapeutic efficacy of radiotherapy. Cancers (Basel) 2021; 13: 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bates JP, Derakhshandeh R, Jones L, et al. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018; 18: 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Faiena I, Cummings AL, Crosetti AM, et al. Durvalumab: an investigational anti-PD-L1 monoclonal antibody for the treatment of urothelial carcinoma. Drug Des Devel Ther 2018; 12: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meric-Bernstam F, Spira AI, Lisberg AE, et al. TROPION-PanTumor01: dose analysis of the TROP2-directed antibody-drug conjugate (ADC) datopotamab deruxtecan (Dato-DXd, DS-1062) for the treatment (Tx) of advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2021; 39(Suppl. 15): 9058. [Google Scholar]
- 50. Bardia A, Krop IE, Meric-Bernstam F, et al. Datopotamab deruxtecan (Dato-DXd) in advanced triple-negative breast cancer (TNBC): updated results from the phase 1 TROPION-PanTumor01 study [Abstract P6-10-03 presented at the San Antonio Breast Cancer Symposium (SABCS) 2022]. Cancer Res 2023; 83(Suppl. 5): P6-10-03. [Google Scholar]
- 51. Schmid P, Wysocki P, Ma CX, et al. Abstract PD11-09: PD11-09 Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): updated results from BEGONIA, a phase 1b/2 study Cancer Res 2023; 83(Suppl. 5): PD11-09. [Google Scholar]
- 52. Schmid P, Wysocki PJ, Ma CX, et al. 379MO Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): Updated results from BEGONIA, a phase Ib/II study. Ann Oncol 2023; 34(Suppl. 2): S337. [Google Scholar]
- 53. Dent RA, Cescon DW, Bachelot T, et al. TROPION-Breast02: datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative breast cancer. Future Oncol 2023; 19: 2349–2359. [DOI] [PubMed] [Google Scholar]
- 54. Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 2020; 38: 1346–1366. [DOI] [PubMed] [Google Scholar]
- 55. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med 2018; 142: 1364–1382. [DOI] [PubMed] [Google Scholar]
- 56. Azam F, Latif MF, Farooq A, et al. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol 2019; 12: 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schalet BD, Kaat A, Vrahas M, et al. Extending the ceiling of an item bank: development of above-average physical function items for PROMIS. Qual Life Res 2016; 25(Suppl. 1): 109. [Google Scholar]
- 58. Rose M, Bjorner JB, Gandek B, et al. The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol 2014; 67: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 60. Lai JS, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil 2011; 92: S20–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schneider S, Choi SW, Junghaenel DU, et al. Psychometric characteristics of daily diaries for the Patient-Reported Outcomes Measurement Information System (PROMIS®): a preliminary investigation. Qual Life Res 2013; 22: 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. US Department of Health and Human Services. Common terminology criteria for adverse events. Version 5, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf (2017, accessed 30 August 2023).