ABSTRACT
Introduction:
Odontogenic keratocyst (OKC) is an aggressive recurrent cyst with intriguing features. Various factors such as the surgical procedure are involved, and certain histological features contribute to its recurrence. We assessed the clinical, radiographic, and histopathological data of OKCs to better comprehend the true nature of this cyst.
Material and Methods:
A total of 58 lesions including four cases in association with nevoid basal cell carcinoma syndrome (NBCCS) were assessed. Radiographic features and histopathological features within the epithelium and capsule were assessed.
Results:
72% of cases were seen in males and 28% in females. 43% of cases were seen in the mandibular ramus, and 65% exhibited unilocular radiolucency. 95% showed true parakeratinization. Cuboidal basal cell morphology was seen in 41.3% of cases and reversal of polarity in 60%. Basal budding, rete pegs, and mitosis were also observed within the epithelium. The epithelium showed separation at the subbasal level and suprabasal levels in 55 (94.9%) cases.
Conclusion:
Features such as basal cell budding, suprabasal mitotic activity, suprabasal split, localized inflammation, subepithelial hyalinization, and satellite cysts were commonly associated with recurrent cysts. Many newer genetic and molecular hypotheses have generated path-breaking contributions to the understanding of the biology of OKC. With the guidance and help of such factors, improved post-surgery results can be anticipated.
Keywords: Clinicopathology, histopathology, keratocyst, odontogenic cyst
INTRODUCTION
Odontogenic keratocyst (OKC) is an aggressive odontogenic cyst with an infiltrative nature due to its origin from the dental lamina remnants or the basal cells.[1] It occurs mainly in the second and third decades, with a predilection for males, within the ramus and angle of the mandible.[2] Radiographically, it is a unilocular or multilocular, well-circumscribed radiolucent lesion with scalloped and corticated margins.[3] It has eight to 10 cell layers of parakeratinized stratified squamous epithelium, which may show budding, satellite microcysts, and odontogenic islands. OKCs exhibit a high proliferative potential, balanced by apoptotic cell death.[3,4]
OKCs have a recurrent potential ranging from 0% to 62%.[5] Woolgar et al.[6] have asserted the major causes of recurrence as incomplete removal of cyst lining; growth from small satellite cysts; or odontogenic epithelial rests left after surgical treatment and development of unrelated cysts in the adjacent region. Such a high recurrence rate may not result solely from the type of surgical treatment but also due to the biological nature of the cyst.[7]
The assessment of histopathological features having a direct impact on the recurrence potential needs more exploration. Therefore, the aim of this study was to assess the histopathological features of OKCs in addition to clinicoradiographical data, to predict factors that might assist in comprehending the true nature of OKCs.
MATERIAL AND METHODS
Ethics
The institutional ethical committee has exempted the retrospective analytical studies from ethical approval.
Study design
We retrospectively reviewed all the OKC patients’ data treated in the Oral and Maxillofacial Surgery Department, Maulana Azad Institute of Dental Sciences between 2015 and 2017. Patients treated surgically via various treatment protocols such as enucleation, marsupialization, or resection, fulfilling the histologic criteria by Pindborg and Hansen, and Brown,[4] were chosen. Hematoxylin and eosin (H and E)-stained sections of all 58 cases were inspected by two oral pathologists who were blinded to the clinical and radiographic information. The cysts were observed in 50 patients, of which six patients exhibited multiple lesions. Clinicoradiographic information and pathologic information were reviewed. The site of involvement was classified as maxilla and mandible; the mandible was further divided into three regions, the symphysis, mandibular body, and the ramus and angle. The association with nevoid basal cell carcinoma syndrome (NBBCS) was confirmed by means of diagnostic criteria.[8] The radiographic appearance was classified as unilocular or multilocular [Table 1].
Table 1.
Clinical and radiographic features
| Clinical and radiographic presentations | Description | Number of recurrent cases |
|---|---|---|
| Sex (n=50, four cases of NBBCS) | ||
| • Male | 36 (72%) | 4 |
| • Female (one recurrence) | 14 (28%) | 0 |
| Age (n=50, four cases of NBBCS) | ||
| • <20 years | 9 (18%) | 2 |
| • 3rd | 18 (36%) | 1 |
| • 4th | 12 (24%) | 1 |
| • >40 years | 11 (22%) | 0 |
| Site (n=58, four cases of NBBCS) | ||
| • Symphysis | 8 (13.7%) | 0 |
| • Body | 11 (18.9%) | 1 |
| • Ramus and angle | 25 (43.1%) | 3 |
| • Maxilla | 14 (24%) | 0 |
| Radiographic (n=58, four cases of NBBCS) | ||
| • Multilocular | 20 (34.4%) | 0 |
| • Unilocular | 38 (65%) | 4 |
Various histopathological features were analyzed over the entire section of the tissue at 100x and 200x. The type and thickness of keratinization were observed. The presence of surface corrugations, intracellular edema, epithelial folding, and rete pegs was assessed. The morphology of basal cells along with the presence/absence of reversal of polarity and basal budding was noted. The mitosis was studied at three levels, basal and parabasal, suprabasal, and throughout the epithelium. Three or more mitotic figures were considered significant. The epithelial connective tissue interface was either intact or had a subbasal or suprabasal split or a combination of these two [Table 2].
Table 2.
Histological features within the epithelium
| Histological feature (epithelium) | Total cases (n=58, four cases of NBBCS) |
|---|---|
| Keratin | |
| • Parakeratinized | 55 (95%) |
| • Mixed | 3 (5%) |
| Keratin thickness | |
| • Thin | 17 (29.3%) |
| • Thick | 14 (24.1%) |
| • Mixed | 27 (46.5%) |
| Corrugations | 40 (69%) |
| Intracellular edema | 32 (55%) |
| Basal cells | |
| • Columnar | 13 (22.4%) |
| • Cuboidal | 24 (41.3%) |
| • Mixed | 21 (36.2%) |
| Epithelial folding | 31 (53%) |
| Reversal of polarity | 35 (60%) |
| Rete pegs | 14 (24%) |
| Basal budding | 32 (55%) |
| Mitosis | |
| • Basal and parabasal | 24 (41%) |
| • Suprabasal | 5 (13.8%) |
| • Mixed | 7 (12%) |
| • None | 22 (38%) |
| Epithelium connective tissue interface | |
| • Intact | 3 (5.1%) |
| • Suprabasal split | 5 (8.6%) |
| • Subbasal split | 30 (51.7%) |
| • Mixed | 20 (34.4%) |
Within the connective tissue, the density of collagen was divided into loose, dense, or mixed. Inflammation was assessed as localized or generalized. Cases were categorized as exhibiting generalized inflammation when it altered the epithelial lining from the classic histological appearance of OKC or when it densely covered a quarter of the cyst wall. The hyalinization of collagen was graded as focal, comprising of subepithelial presence/diffusely throughout the thickness in a localized area and diffuse if within the entire tissue. Histological variables such as odontogenic epithelial rests, satellite cysts, calcification, and cholesterol clefts were scored as present or absent [Table 3]. Among the 50 patients, four exhibited recurrence, which included two syndromic patients [Table 1].
Table 3.
Histological features within the connective tissue
| Histological feature (Capsule) | Total cases (n=58, four cases of NBBCS included) |
|---|---|
| Collagen | |
| • Loose | 19 (32.7%) |
| • Dense | 24 (41.3%) |
| • Mixed | 15 (25.8%) |
| Inflammation | |
| • Localized | 41 (71%) |
| • Generalized | 17 (29%) |
| Hyalinization | |
| A) • Focal | 8 (13.7%) |
| • Subepithelial | 11 (19%) |
| • Diffuse B) • Diffuse |
5 (8.6%) |
| Odontogenic rests | 22 (38%) |
| Satellite cysts | 5 (8.6%) |
| Cholesterol clefts | 4 (6.8%) |
RESULTS
A total of 50 patients of which four cases in association with NBCCS were assessed. Thirty-six (72%) patients were males and 14 (28%) were females with a ratio of 2.5:1. The average age at the time of presentation was 31 years with a range from 10 to 72 years [Table 1]. There were a total of 58 lesions in these 50 patients as attributable to multiple lesions in a few patients. Fourteen (24%) lesions were in the maxilla, and 44 lesions were in the mandible. Thirty-eight (65%) cases showed unilocular radiolucency, whereas 20 (34.4%) cases were multilocular [Table 1].
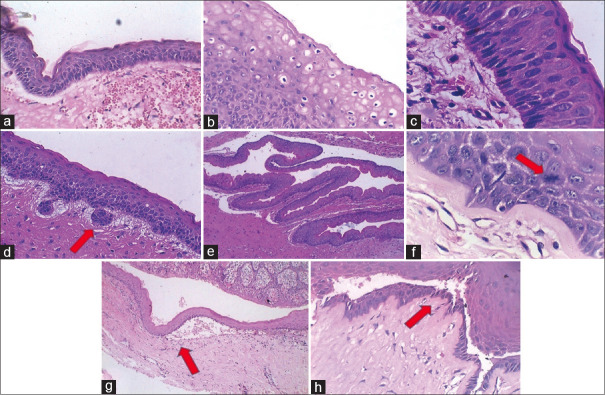
Histologically, 55 cases (95%) exhibited true parakeratinization with three (5%) cases presenting a mixed lining. The keratin thickness was assessed qualitatively and was found as thick in 14 (24.1%) cases, thin in 17 (29.3%), and mixed in 27 (46.5%) cases [Table 2, Figure 1]. The basal cell morphology was predominantly cuboidal in 24 (41.3%) cases with a characteristic reversal of polarity in 35 (60%) cases. Basal budding and rete pegs were noted in 32 (55%) and 14 (24%) cases, respectively. Mitosis was observed, but no epithelial dysplasia was found [Table 2 and Figure 1].
Figure 1.
Representative histological images of the lining epithelium of examined OKCs. (a) Corrugated parakeratin. (b) Intracellular edema. (c) Columnar basal cells with reversal of polarity. (d) Basal cell budding. (e) Epithelial folding. (f) Suprabasal mitosis. (g) Subepithelial separation. (h) Intraepithelial separation (H and E)
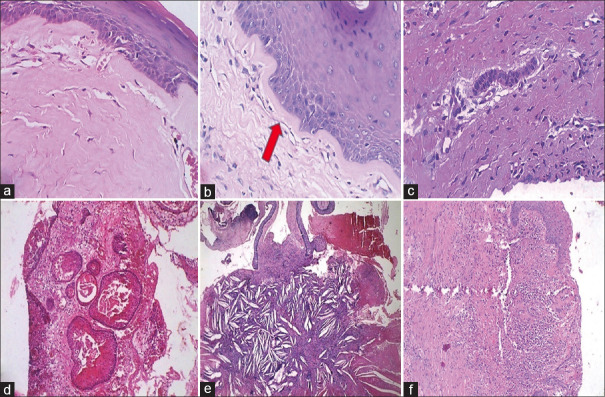
The epithelium showed separation from the underlying connective tissue capsule at subbasal and suprabasal levels in 55 (94.9%) cases. The inflammatory cells comprised a mixture of polymorphs, lymphocytes, plasma cells, and histiocytes in these areas; rete peg formation was often observed. Subepithelial hyalinization was also analyzed. Odontogenic epithelial cell rests in 38% and satellite cysts in 8.6% of cases were seen. Calcification and cholesterol clefts were identified in nine (15.5%) and four (6.8%) cases, respectively [Table 3 and Figure 2].
Figure 2.
Representative histological images of connective tissue of examined OKCs. (a) Diffuse hyalinization. (b) Focal subepithelial hyalinization. (c) Odontogenic epithelial island. (d) Satellite cyst. (e) Cholesterol clefts. (f) Inflammation (H and E)
DISCUSSION
A male predominance was reported, which was in accordance with previous reports.[9,10] The posterior mandible, from molar to the angle–ramus area (75.7%), and also unilocular radiographic appearance were observed in accordance with certain studies.[1,4,11,12] According to Boffano et al.,[4] most multilocular, large, and probably aggressive lesions are found in patients younger than 41 years. However, 78% of cases in the present study below 41 years of age were predominantly unilocular. True parakeratinization was apparent in 95% and is related to higher recurrence.[13] 69% of cases evidenced a corrugated parakeratin and 60% reversal of polarity. A greater difference in hydrostatic pressure between the cyst lining and lumen can affect the lining thickness. Only 29.3% of our cases were thickly lined, mainly in areas of inflammation.
A probable cause of recurrence is the presence of basal cell budding.[9,14] All four recurrent cases exhibited basal cell budding. Abnormal mitosis and mitotic figures were noted in 36 cases (62%), and 41% located basally. According to Brown,[3] the recurrent cysts showed an increased mitosis in suprabasal layers, but Cottom et al.[14] demonstrated more basal location within recurrent cysts. Suprabasal mitotic activity was observed within two syndromic and one sporadic recurrent OKC emphasizing its role in relapses. Another possible factor in recurrence has been the separation between the epithelial lining and the underlying capsule.[3,15,16] Brannon[16] reported epithelial separation in 94% of cases, similar to our study. Suprabasal splitting was noted in 53%.[3] Single syndromic and sporadic recurrent cases exhibited suprabasal splitting, whereas the other two recurrent OKCs showed subbasal split. Tenascin was demonstrated in the capsule of OKC and led to the cyst lining becoming fragile and weak. Increased expression of the bone morphogenetic protein was also detected among OKCs, which in turn is associated with PTCH.[17]
The fragile epithelial lining when left behind postoperatively leads to recurrence. A direct relation between proliferative activity and inflammation in OKC was reported by Nickolaychuk et al.[18] Few cases exhibited generalized inflammation with the formation of cholesterol crystals within four OKCs, exhibiting disruption of epithelium.[6,9,14] All four recurrent cases manifested localized inflammation in our study. Myoung et al.[9] suggested the absence of any such correlation, and we propose the same.
Subepithelial hyalinization was detected in 13.7% of OKCs. Our study revealed subepithelial hyalinization among two recurrent syndromic OKCs, thus supporting its role in relapse. Hyalinization has been observed in patients of higher age group.[9,14] Satellite cysts were evident in five (8.6%) of the OKCs reviewed. Daughter cyst formation has been significantly associated with high recurrence rates[13] although it is not completely accepted.[9] Also, a high frequency of allelic loss in tumor suppressor genes, suggesting a neoplastic nature, has been postulated.[19,20] Both recurrent syndromic OKCs displayed the presence of satellite cysts. Also, 38% of OKCs exhibited the presence of odontogenic islands. These epithelial remnants or residual tissue are seemingly the foremost factors leading to recurrence, as stated by Brannon.[16]
The four recurrent cases in this study underwent enucleation and curettage, which consists of the removal of the lesion by shelling it off the bone. These cases also showed the presence of daughter cysts and epithelial budding, which are significantly associated with high recurrence. The cases were enucleated again when presented with recurrence and were followed by placing the fixative Carnoy’s solution within the cyst cavity. Carnoy’s solution eliminates the epithelial residues from cyst walls that may have been left behind after enucleation.
Highly variable recurrence rates ranging between 2.5% and as high as 62.5% have been observed in various studies.[21,22] Habibi et al.[23] reported a higher recurrence rate in young children and adults. As younger patients are more likely to undergo conservative management, a greater chance of recurrence is predicted for them. Most commonly recurrence occurs in the mandibular molar and post-molar region. Scharfetter dictated two dissimilar areas within OKC, areas exhibiting proliferation at a mild rate and others with a faster proliferation. An increased fibrinolytic activity within the cyst wall, high mitotic activity, epithelial proliferations, intraluminal hyperosmolality in the capsule, and residual dental lamina with associated new cyst formation have been hypothesized to increase the recurrent potential. Thus, ultimately the type of management depends on the site, size, extent of the lesion, perforation of overlying mucosa, and age of the patient.[24] Molecular factors such as the synthesis of interleukin-1 and interleukin-6 by keratinocytes,[25] increased expression of parathyroid hormone-related protein, and the greater frequency of proliferating cell nuclear antigen (PCNA), Ki-67, p53, Bcl-1, and Gp-38 positivity are also quoted. Few studies have found p16, p53, PTCH, MCC, TSLC 1, LTAS2, and FHT genes, exhibiting allelic losses in heterozygosity as causes of recurrence.[26]
Some previous studies attempted to discover the main causal factor for cyst reduction after marsupialization or decompression. One such study showed that older patients were associated with a smaller reduction percentage in lesion size. Cases that do not show a response to decompression may require a more aggressive procedure. Other studies show that the epithelial lining of OKC changes from a parakeratinized to a nonkeratinized lining together with the fibrous capsule enlargement.[13] The odds of relapses owing to the preservation of the tooth after the cyst enucleation are ambiguous. According to Cunha et al. 2016,[13] the location of OKC between the roots of a tooth may increase the risk of recurrence, suggesting that the involved tooth should be removed if it is associated with the cyst.
CONCLUSION
OKCs have shown delayed recurrence potential, making it mandatory to follow up with the patients both clinically and radiographically even after many years of treatment. Our institute is a tertiary health center and caters to a large number of OKC patients from across India. Our study revealed four recurrences among the 50 patients. Basal cell budding, suprabasal mitotic activity, suprabasal split, localized inflammation, subepithelial hyalinization, and satellite cysts were among the features observed variably among the relapses. Features such as parakeratinized lining epithelium, odontogenic epithelial islands, and satellite cysts are observed more in NBCCS[21,22] along with a higher recurrence rate. Markers known to be rapidly induced in response to growth factors, tumor promoters, cytokines, bacterial endotoxins, oncogenes, hormones, and shear stress, such as COX-2, also might play roles in biological mechanisms involved in the development of this aggressive lesion of the jaws. The clarity of reasons influencing the prognosis related to the clinicopathologic findings still remains unclear. With the guidance and help of such factors, improved post-surgery results can be anticipated. In the future, the chief constituent in the management of such aggressive cyst will be provided with a comprehensive understanding of their biological basis and customizing a strategic treatment plan.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kuroyanagi N, Sakuma H, Miyabe S, Machida J, Kaetsu A, Yokoi M, et al. Prognostic factors for keratocystic odontogenic tumor (odontogenic keratocyst): Analysis of clinico-pathologic and immunohistochemical findings in cysts treated by enucleation. J Oral Pathol Med. 2009;38:386–92. doi: 10.1111/j.1600-0714.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 2.Maurette PE, Jorge J, de Moraes M. Conservative treatment protocol of odontogenic keratocyst: A preliminary study. J Oral Maxillofac Surg. 2006;64:379–83. doi: 10.1016/j.joms.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Browne RM. The odontogenic keratocyst. Histological features and their correlation with clinical behaviour. Br Dent J. 1971;131:249–59. doi: 10.1038/sj.bdj.4802732. [DOI] [PubMed] [Google Scholar]
- 4.Boffano P, Ruga E, Gallesio C. Keratocystic odontogenic tumor (odontogenic keratocyst): Preliminary retrospective review of epidemiologic, clinical, and radiologic features of 261 lesions from University of Turin. J Oral Maxillofac Surg. 2010;68:2994–9. doi: 10.1016/j.joms.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 5.Pazdera J, Kolar Z, Zboril V, Tvrdy P, Pink R. Odontogenic keratocysts/keratocystic odontogenic tumours: Biological characteristics, clinical manifestation and treatment. Biomed J. 2014;158:170–4. doi: 10.5507/bp.2012.048. [DOI] [PubMed] [Google Scholar]
- 6.Woolgar JA, Rippin JW, Browne RM. A comparative study of the clinical and histological features of recurrent and non-recurrent odontogenic keratocysts. J Oral Pathol. 1987;16:124–8. doi: 10.1111/j.1600-0714.1987.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 7.Mendes RA, Carvalho JF, van der Waal I. Characterization and management of the keratocystic odontogenic tumor in relation to its histopathological and biological features. Oral Oncol. 2010;46:219–25. doi: 10.1016/j.oraloncology.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Lyon: IARC; 2005. Pathology and genetics of head and neck tumours; pp. 306–7. [Google Scholar]
- 9.Myoung H, Hong SP, Hong SD, Lee JI, Lim CY, Choung PH, et al. Odontogenic keratocyst: Review of 256 cases for recurrence and clinicopathologic parameters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:328–33. doi: 10.1067/moe.2001.113109. [DOI] [PubMed] [Google Scholar]
- 10.Bello IO. Keratocystic odontogenic tumor: A biopsy service's experience with 104 solitary, multiple and recurrent lesions. Med Oral Patol Oral Cir Bucal. 2016;21:e538–46. doi: 10.4317/medoral.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirapathomsakul D, Sastravaha P, Jansisyanont P. A review of odontogenic keratocysts and the behavior of recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:5–9. doi: 10.1016/j.tripleo.2005.03.023. discussion 10. [DOI] [PubMed] [Google Scholar]
- 12.Simiyu BN, Butt F, Dimba EA, Wagaiyu EG, Awange DO, Guthua SW, et al. Keratocystic odontogenic tumours of the jaws and associated pathologies: A 10-year clinicopathologic audit in a referral teaching hospital in Kenya. J Craniomaxillofac Surg. 2013;41:230–4. doi: 10.1016/j.jcms.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Cunha JF, Gomes CC, de Mesquita RA, Goulart EMA, de Castro WH, Gomez RS. Clinicopathologic features associated with recurrence of the odontogenic keratocyst: A cohort retrospective analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:629–35. doi: 10.1016/j.oooo.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Cottom HE, Bshena FI, Speight PM, Craig GT, Jones AV. Histopathological features that predict the recurrence of odontogenic keratocysts. J Oral Pathol. 2012;41:408–14. doi: 10.1111/j.1600-0714.2011.01113.x. [DOI] [PubMed] [Google Scholar]
- 15.Shear M, Speight P. Cysts of the oral and maxillofacial regions. John Wiley & Sons; New Jersey: 2008. [Google Scholar]
- 16.Brannon RB. The odontogenic keratocyst. A clinicopathologic study of 312 cases. Part II. Histologic features. Oral Surg Oral Med Oral Pathol. 1977;43:233–55. doi: 10.1016/0030-4220(77)90161-x. [DOI] [PubMed] [Google Scholar]
- 17.Shear M. The aggressive nature of the odontogenic keratocyst: Is it a benign cystic neoplasm? Part 2. Proliferation and genetic studies. Oral Oncol. 2002;38:323–31. doi: 10.1016/s1368-8375(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 18.Nickolaychuk B, McNicol A, Gilchrist J, Birek C. Evidence for a role of mitogen-activated protein kinases in proliferating and differentiating odontogenic epithelia of inflammatory and developmental cysts. Oral Surg Oral Med Oral Pat Oral Radiol Endod. 2002;93:720–9. doi: 10.1067/moe.2002.123496. [DOI] [PubMed] [Google Scholar]
- 19.Agaram NP, Collins BM, Barnes L, Lomago D, Aldeeb D, Swalsky P, et al. Molecular analysis to demonstrate that odontogenic keratocysts are neoplastic. Arch Pathol Lab Med. 2004;128:313–7. doi: 10.5858/2004-128-313-MATDTO. [DOI] [PubMed] [Google Scholar]
- 20.Kuroyanagi N, Machida J, Sakuma H, Miyabe S, Hashimoto O, Yokoi M, et al. p53 mutations in keratocystic odontogenic tumour. Oral Surg. 2009;2:64–70. [Google Scholar]
- 21.González-Alva P, Tanaka A, Oku Y, Yoshizawa D, Itoh S, Sakashita H, et al. Keratocystic odontogenic tumor: A retrospective study of 183 cases. J Oral Sci. 2008;50:205–12. doi: 10.2334/josnusd.50.205. [DOI] [PubMed] [Google Scholar]
- 22.Pitak-Arnnop P, Chaine A, Oprean N, Dhanuthai K, Bertrand J-C, Bertolus C. Management of odontogenic keratocysts of the jaws: A ten-year experience with 120 consecutive lesions. J Craniomaxillofac Surg. 2010;38:358–64. doi: 10.1016/j.jcms.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Habibi A, Saghravanian N, Habibi M, Mellati E, Habibi M. Keratocystic odontogenic tumor: A 10-year retrospective study of 83 cases in an Iranian population. J Oral Sci. 2007;49:229–35. doi: 10.2334/josnusd.49.229. [DOI] [PubMed] [Google Scholar]
- 24.Belgal PG, Pathak B, Shastry L. Updates in the surgical management of odontogenic keratocyst. J Adv Clin Res Insights. 2019;6:116–8. [Google Scholar]
- 25.Deshmukh SB, Sonawane K. Odontogenic keratocysts to keratocystic odontogenic tumor. Int J Appl Dent Sci. 2019;5:09–15. [Google Scholar]
- 26.Kshirsagar RA, Bhende RC, Raut PH, Mahajan V, Tapadiya VJ, Singh V. Odontogenic keratocyst: Developing a protocol for surgical intervention. Ann Maxillofac Surg. 2019;9:152–7. doi: 10.4103/ams.ams_137_18. [DOI] [PMC free article] [PubMed] [Google Scholar]