Abstract
400-m races are based on anaerobic energy metabolism, they induce significant muscle fatigue, muscle fiber damage, and high blood lactate (LA) concentration. Despite extensive research on sprint training, our understanding of the training process that leads to world-class sprint performance is rather limited. This study aimed to determine differences in LA concentration and anaerobic power using jumping tests after an intense glycolytic effort in a group of elite and sub-elite 400-m runners. One hundred thirty male runners were divided into two groups: elite (n = 66, body mass = 73.4 ± 7.8 kg, body height = 182.1 ± 6.2 cm, age = 20.8 ± 4.0 y) running the 400-m dash below 50 s and sub-elite (n = 64, body mass = 72.0 ± 7.1 kg, body height = 182.1 ± 5.2 cm, age = 20.8 ± 4.0 y) with a 400-m personal best above 50 s. The power of the countermovement and the sequential squat jumps was measured in two sets after a warm-up, followed by two intermittent 30-s Wingate tests. LA concentration was measured eight times. It was observed that elite athletes achieved significantly higher power in both types of jumps. The maximum post-exercise LA concentration was significantly lower in the sub-elite group after the 3rd, the 6th, the 9th, and the 20th min after the cessation of two Wingate tests (p < 0.001). The rate of LA accumulation after exercise and the rate of LA utilization did not differ between the groups. It can be concluded that elite and non-elite runners differ in higher LA production but not in LA utilization. Anaerobic power and LA concentration seem to differentiate between 400 elite and sub-elite performance.
Keywords: fatigue, competition, blood lactate, sprinting
Introduction
The 400-m dash is an athletic event requiring a combination of maximum running speed, which must be achieved and maintained throughout the race (Okudaira et al., 2019). During 400-m sprinting, the anaerobic contributions in men average 63% and represent a critical factor in achieving high performance (Hill, 1999; Nummela and Rusko, 1995). In this event, a runner must reach a very high velocity and preserve the optimal velocity despite intense fatigue. Moreover, it is possible to predict 400 m sprint time based on the anaerobic endurance capacity of athletes (Weyand et al., 1994). For these reasons, understanding the physiological responses to this effort and its relationship with power results during biomechanical testing is critical to performance as it allows to identify areas for improvement to achieve world-class performance.
Sprint performance is significantly influenced by genetic characteristics, with variations among individual athletes being less pronounced than the typical variability observed in external conditions such as wind and monitoring methodologies (Eynon et al., 2013; Maciejewska-Skrendo et al., 2019; Siembida et al., 2021; Yang et al., 2017). Despite these genetic predispositions, key performance determinants such as power, technique, and sprint-specific endurance are trainable aspects (Haugen et al., 2019). Sustaining a high relative velocity for as long as possible is essential for success (Jones and Whipp, 2002), but it is assumed that completing the entire 400-m race at maximum effort is not possible (Hanon and Thomas, 2011; Willis et al., 2012). Some recommend a high-velocity start (van Schenau et al., 1994), while others recommend a more contained and progressive first 200 m to delay rapid lactate production and muscle acidosis (Arcelli et al., 2008). The divergence in recommendations raises questions about the optimal sprinting strategy and its impact on physiological responses, such as lactate production and fatigue. Coaches and athletes try to improve the anaerobic energy system through speed endurance training (Rago et al., 2022). Successful performance in a 400-m run necessitates a swift start followed by a reduction in velocity relative to peak velocity during the last 100 m (Hanon et al., 2011). The fast-start procedure induces higher oxygen uptake and blood lactate concentration (Bishop et al., 2002). This significant anaerobic contribution may influence the decline in velocity during the final 100 m of the race (Hanon and Gajer, 2009). The blood lactate (LA) measurements are used to determine the contribution of anaerobic metabolism, exercise intensity, the lactate threshold, and recovery assessment, enabling a better understanding of the metabolic demands of different activities. The measurement of lactate concentration after exercise provides information about the workload and its impact on recovery (Goodwin et al., 2007).
The need for high LA tolerance is a necessary factor influencing the 400-m dash. However, whether LA production, LA utilization, speed, or power is the differentiating factor between elite and sub-elite 400-m dash athletes has not yet been fully explored. The effect of training adaptation, primarily the ability to generate greater power in the lower limbs, should include the ability to accumulate higher lactate production and buffer H+. Therefore, this study aimed to determine differences in lactate concentration in response to maximum anaerobic exercise in a group of 400-m runners, further divided into groups according to their performance. The relationship among maximum power, lactate concentration during exertion and the time of the 400-m run was also investigated.
Methods
The experimental design employed in this study was a cross-sectional case-control approach aimed at investigating the relationship between athletes’ power and their immediate response to a high-intensity glycolytic load with particular emphasis on post-exercise lactate production and clearance. Health questionnaires were administered before the body mass and height measurement and a standardized warm-up. Subsequently, assessments included countermovement jumps (CMJs), sequential squat jumps (SSJs), and a loading protocol including two repeated all-out anaerobic Wingate tests on a bicycle ergometer. Athletes underwent a 5–6-min warm-up at 1 W/kg and a cadence of 60 revolutions per minute (rpm), with two accelerations of 3–5 s, before conducting the jump and Wingate tests.
Participants
The study involved 130 male elite athletes from Poland and the Czech Republic. The exclusion criteria were intense training in the preceding 72 h, injury in the past 3 months, and lack of approval from a sports doctor. The inclusion criteria for the experimental group were running a distance of 400 m below 50 s for the elite group and above 50 s in the sub-elite group in the current season, five workouts per week, and participating in 80% of club training sessions. Those who were eligible were included in the elite group (n = 66, body mass = 73.4 ± 7.8 kg, body height = 182.1 ± 6.2 cm, age = 20.8 ± 4.0 y) with 25 athletes from Poland and 41 from the Czech Republic, and the sub-elite group (n = 64, body mass = 72.0 ± 7.1 kg, body height = 182.1 ± 5.2 cm, age = 20.8 ± 4.0 y) with 23 athletes from Poland and 41 from the Czech Republic. In the season in which the research was performed, the average time of the 400-m race for all runners was 49 ± 1.4 s, and it was in the range of 46 to 49 s in the elite and 50 to 52 s in the sub-elite group. Before the study began, all participants were informed about the purpose of the study, the procedures to be applied, as well as potential risks and benefits. All participants completed a survey questionnaire.
Measures
During both CMJs and SSJs, participants kept their hands on their hips to remove the influence of the arm movement. Participants performed two sets of CMJs and SSJs at their chosen foot position. SSJs were performed in sequences of four squat jumps to a 90° knee joint angle, measured by goniometry. The starting position was marked with a wire behind the participant’s thighs so that participants were aware of the lowest position before jumping upwards. They were instructed to jump dynamically to reach their maximum height. Ground reaction forces were collected using a 600 × 400 mm force plate (Kistler Group, Switzerland, model 9281EA). Force data were collected at 500 Hz. Ground reaction data were used to identify the power of the CMJ and the SSJ during the driving phase using methods recommended previously (Chavda et al., 2018; McMahon et al., 2018). After the evaluations were completed, the raw force plate data were analyzed using Microsoft Excel (Chavda et al., 2018). LA concentration was measured 8 times: at rest, one minute after the warm-up, in the third minute of rest after the first Wingate test, immediately after the second Wingate test, and at the 3rd, the 6th, the 9th, and the 20th min into recovery. Blood was taken from the fingertip. The samples were collected into sodium heparin-treated end-to-end capillaries. LA concentration was assessed with the Biosen apparatus, which uses chip sensor technology and measures LA concentration within the 0.5–40 mmol/l (5–360 mg/dl) range (Biosen C-Line Lactate analyzer, EKF Diagnostics). The following variables of LA kinetics were calculated based on individual LA concentration from the 1st to the 20th min: the peak of LA concentration (LAmax), the ratio of LA accumulation from the 1st to the 3rd min (ΔLA1'–3'), the ratio of LA concentration from the 3rd to the 6th min (ΔLA3'–6') and from the 6th to the 9th min (ΔLA6'–9') and the ratio of LA clearance from the 9th to the 20th min (ΔLA9'–20'). The ratio of LA accumulation was obtained as the slope of linear regression in the proper time range. The power of the Wingate test was measured during the loading protocol for the maximal glycolytic response by two repeated all-out anaerobic Wingate tests on a bicycle ergometer (Monark 894 E peak bike, Sweden). It was crucial to attain the highest possible power output to achieve the most effective results on the muscular system and its response to glycolytic exercise. To this end, maximum power values were used in all measurements for further comparisons between groups and to evaluate any potential correlations.
Wingate Loading Protocol
After the jump tests, participants rested for 5 min. The Wingate test was chosen due to prior research supporting its effectiveness in eliciting maximal engagement of anaerobic metabolism. Previous research has shown that a single 30-s Wingate sprint can reduce muscle glycogen stores in the vastus lateralis muscle by 20–30% (Metcalfe et al., 2012). The applied resistance was set at 7.5% of athletes’ body mass; participants remained seated during the test duration and started from a complete standstill position. Once the load (7.5% of body mass) was preset, the participant’s feet were fixed to the pedals. They were then given a 5-s countdown and began the test with maximal effort. Throughout testing, power was monitored. Participants pedaled as fast as possible for 30 s. Afterwards, they had a 4-min passive rest interval (Lopez et al., 2014) and then performed a second test following the same procedure. After completing the second Wingate test, participants were instructed to take an active rest on a cycling ergometer for 20 min and then LA concentration was determined.
Loading protocols were completed in the preseason period. All testing was carried out in the Physiological Laboratory of the Department of Biomedical Sciences at the University of Physical Education in Warsaw and in the Biomedical Laboratory at the Faculty of Physical Education and Sport at the Charles University.
The study protocol along with the informed consent form were approved by the Bioethics Commission of the District Medical Chamber in Gdansk (approval code: KB-2/21; approval date: 3 February 2021). All the procedures were carried out in accordance with the Declaration of Helsinki developed by the World Medical Association (2013).
Statistical Analysis
All data were examined to ensure they met the requirements of normality and homogeneity of variance using the Kolmogorov-Smirnov and Shapiro-Wilk tests. To examine the intra- and inter-group differences, analysis of variance (ANOVA) was used. The Fisher's Least Significant Difference
(LSD) test was applied post hoc to make adjustments. All calculations were performed using Statistica (version 13.1) and the level of significance was set at p < 0.05.
Results
Maximal power (Pmax) of the Wingate (W) and jump (CMJ and SSJ) tests was significantly higher in the elite group (Table 1). Pmax in the second Wingate test (W2) in both groups was lower (p < 0.001) and non-significantly different (p = 0.140).
Table 1.
Mean (± SD) variables of the Wingate and jump tests in elite and sub-elite groups.
| Group | ANOVA effects | ||||
|---|---|---|---|---|---|
| Elite | Sub-elite | Group | Time | Group x Time | |
| Pmax (W1) [W/kg] | 14.7 (1.7) | 13.5 (2.1) | F(1, 128) = 9.26, η2 = 0.067 |
F(1, 128) = 110.62, p < 0.001, η2 = 0.464 |
F(1, 128) = 4.9, p = 0.028, η2 = 0.038 |
| Pmax (W2) [W/kg] | 12.7 (1.6) | 12.3 (1.7) | |||
| Pmax (CMJ) [W/kg] | 38.4 (5.7) | 34.1 (5.5) | F(1, 128) = 19.81, p < 0.001, η2 = 0.050 |
||
| Pmax (SSJ) [W/kg] | 38.3 (5.6) | 34.8 (5.4) | F(1, 128) = 13.328, p < 0.001, η2 = 0.038 |
||
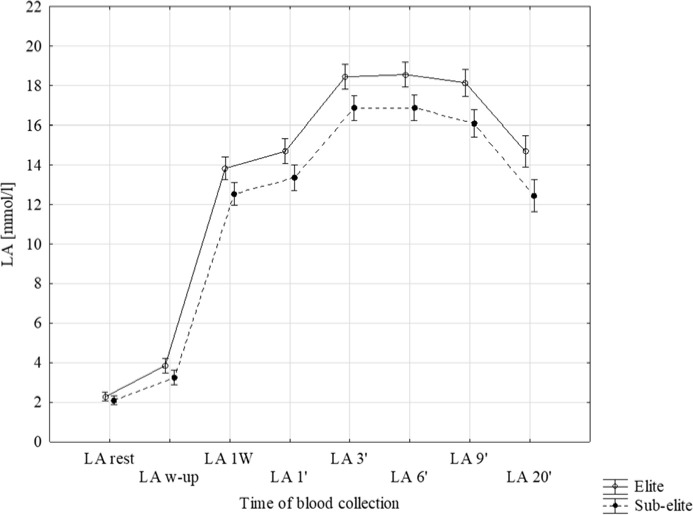
Significantly higher LA concentration in subsequent time points was observed in elite sprinters (p < 0.001), except for rest and post warm-up time (p < 0.05, Figure 1). After the first Wingate test (LA 1'), LA accumulation was approximately 9% higher (p = 0.004) up to the 6th min after exercise in the elite group. The kinetics of LA clearance after the 9th and the 12th min were higher by 3 and 5% after the 9th and the 20th min in the sub-elite group. LA concentration at the 20th min was similar to that at the 1st min in both groups (elite p = 0.974 and sub-elite p = 0.807).
In 40% of participants from the elite and sub-elite groups, the peak lactate concentration (LAmax) was obtained 3 min after the second Wingate test. The remaining 32% from the elite and 38% from sub-elite groups achieved LAmax in the 6th min. However, the time of LAmax onset was not found to differentiate between both groups (F(2, 124) = 0.714, p = 0.492). The kinetics of LA concentration were assessed in three intervals (Figure 1). The kinetics of LA accumulation after the second Wingate tests (ΔLA1'–3') and clearance from the 9th to the 20th min (ΔLA9'–20') were similar in both groups (Table 2). Non-significant differences were only observed from the 3rd to the 9th min.
Figure 1.
Average LA values in particular minutes of collection. LA rest: at rest, LA w-up: after the warm-up, LA 1W: after the first Wingate test, LA 1' to LA 20': subsequent minutes after the end of the second Wingate test.
Table 2.
Mean (± SD) variables of LA kinetics in elite and sub-elite groups.
| Group | ANOVA effect | ||
|---|---|---|---|
| elite | sub-elite | Group | |
| LAmax [mmol/l] | 19.1 (2.4) | 17.5 (2.8) | F(1, 128) = 12.069, p< 0.001 |
| ΔLA1'–3' [mmol/l/min] | 1.2 (0.7) | 1.2 (0.7) | F(1, 128) = 0.387, p= 0.535 |
| ΔLA3'–6' [mmol/l/min] | 0.04 (0.30) | 0.006 (0.43) | F(1, 128) = 0.279, p= 0.597 |
| ΔLA6'–9' [mmol/l/min] | −0.14 (0.41) | −0.26 (0.41) | F(1, 128) = 2.621, p= 0.108 |
| ΔLA9'–20' [mmol/l/min] | −0.36 (0.1) | −0.36 (0.1) | F(1, 128) = 0.066, p= 0.799 |
LAmax significantly correlated with LA kinetics except for the 3rd to 6th and 6th to 9th min intervals, which did not significantly differentiate between the two groups (Table 3). Significant relationships were also observed between maximal power for all tests except the second Wingate test and LAmax. We also found a significant correlation among running time (t400) and maximum power for all tests, LAmax, and LA kinetics from the 1st to the 3rd min (ΔLA1'–3'), except Pmax of the second Wingate test (Pmax (W2)) and LA kinetics from the 3rd to the 6th, the 6th to the 9th, and the 9th to the 20th min (Table 3).
Table 3.
Pearson correlation coefficients and the level of significance (p) for lactate kinetics, maximal power, and time of the 400-m race for all tested athletes.
| LAmax | ΔLA 1'–3' | ΔLA 3'–6' | ΔLA 6'–9' | ΔLA 9'–20' | Pmax(W1) | Pmax(W2) | Pmax(CMJ) | Pmax(SSJ) | |
|---|---|---|---|---|---|---|---|---|---|
| LAmax | 0.33 p= 0.001 |
0.08 p= 0.381 |
0.02 p=0.829 |
−0.19 p= 0.027 |
0.35 p= 0.001 |
0.20 p= 0.024 |
0.25 p= 0.004 |
0.30 p= 0.001 |
|
| t400 | −0.29 p< 0.001 |
−0.18 p= 0.040 |
0.03 p= 0.721 |
−0.12 p= 0.187 |
0.08 p= 0.339 |
−0.20 p= 0.022 |
−0.12 p= 0.159 |
−0.36 p= 0.001 |
−0.32 p= 0.001 |
Discussion
This study aimed to determine differences in lactate concentration as the effect of a maximum anaerobic exercise load in 400-m runners divided into two groups according to their performance. Moreover, the association among maximum power, lactate concentration, and the 400-m run time was estimated. When exercise is repeated over weeks, numerous adaptations occur within the muscle, such as metabolic pathways of energy, which are associated with the ability to remove the accumulation of metabolites after fatigue-inducing efforts (Hanon et al., 2012). It is interesting to note that there are contradictory findings in the literature about the effects of sprint training on adaptation. Although sprint training has a significant anaerobic component, one study discovered that anaerobic metabolism indices were not enhanced during exhaustive exercise after training, suggesting aerobic adaptations (Harmer et al., 2000). However, this might result from prolonged exercise applied in that study considering that a post-training sprint test occurred after the initial sprint. Elite 400-m sprinters reach LA concentration of approximately 20 mmol/l following a competition. This indicates the predominance of anaerobic metabolism and fast twitch muscle fiber recruitment during a prolonged sprint. Thus, elite athletes have a higher glycolytic capacity and can produce more ATP via anaerobic metabolism, which results in higher post-exercise LA concentration. Lower-level athletes have a limited glycolytic capacity and a lower buffering ability of the blood and muscles (Hirvonen et al., 1992; Ohkuwa and Miyamura, 1984). Other studies suggest that adaptation to sprint training is dependent on the duration of sprinting, recovery between sprint repetitions, total volume, and frequency of training bouts, and these variables have profound effects on the metabolic, structural, and performance adaptations induced by a sprint-training program (Iskra et al., 2021; Ross and Leveritt, 2001). It was also proven that in the 400-m race, differences in race strategy might affect physiological reactions (Hanon and Gajer, 2009). Elite athletes can achieve higher absolute and relative speeds (a percentage of their best 200-m result). In addition, world-class athletes pesent a more significant loss of speed in the second half of the race. From a training point of view, this means that elite runners need to increase the rate of ATP delivery from anaerobic metabolism (Hanon et al., 2012; Hirvonen et al., 1992). Our results confirm the above conclusions, by higher lactate concentration after exhausting anaerobic exercise in the elite group of sprinters and the ability to generate higher maximum power in each test. Thus, we confirmed the research hypothesis. A surprising result concerns the rate of LA accumulation after exercise. It has been observed that the accumulation rate is related to speed and distance (Hirvonen et al., 1992). In our research, the rate of LA accumulation did not differ between the two groups (ΔLA1'–3'). Such differences were only observed from the 3rd to the 6th and the 6th to the 9th min. During these time intervals, lactate concentration did not change significantly, yet the rate of LA clearance was different. This shows that the rate of lactate accumulation was similar, however, differences in peak LA concentration influenced the initial rate of LA clearance. This rate until the 9th min was non-significantly lower in the elite group. However, from the 9th min, we did not observe differences in the rate of LA clearance. Similar conclusions can be found in a study on African and Caucasian athletes (Bret et al., 2013). Mean blood lactate concentration was significantly higher in Africans than Caucasians from the 5th min. Furthermore, blood lactate concentration rapidly increased to peak LA between the 2nd and the 10th min. However, the estimated LA clearance time was significantly lower at the beginning of rest, yet higher in Africans from the 5th to the 12th min compared to Caucasians (Bret et al., 2013). Therefore, the similarities within these results concern only the accumulation rate, but they are inconsistent with the rate of LA clearance. During a 400-m run, the intensity of exercise will be much higher than during standardized exercise, and it is known that the greater the intensity of exercise, the lower the ability to transport lactate between muscles and blood (Chatel et al., 2016). Previous research has shown that the ability to transport lactate from muscles to blood was related to performance during high-intensity exercise (Bret et al., 2013). This should explain the differences among the results from tests carried out under different conditions despite using a highly strenuous anaerobic protocol. Additionally, Tomschi et al. (2018) found no difference in the rate of lactate accumulation by red blood cells in trained and untrained people. However, other lactate clearance mechanisms may contribute to differences in the clearance rate, such as improved hepatic gluconeogenesis (Sumida et al., 1993), which alters lactate removal in athletes compared to recreationally active individuals. Thus, training status may contribute to a difference in the rate of LA clearance. The accumulation rate is directly related to speed or maximum power, as indicated by significant correlations between power and the rate of LA accumulation. According to the research conducted by Okudaira et al. (2019), the ability to transport lactate into capillaries during sprint interval training protocols with very short recovery time varies among sprinters based on their performance level. Maemura et al. (2004) indicate that the capillary-to-muscle volume ratio is closely linked to anaerobic performance. Therefore, it can be speculated that capillaries will be well developed in the elite group, allowing for more effective lactate clearance even with short recovery periods (Maemura et al., 2004). It should also be noted that the time taken by all sprinters in our research to run 400 m correlated significantly only with the rate of lactate accumulation, suggesting anaerobic influence, and not with the rate of lactate clearance. It is worth mentioning that there is significant variability among individuals in how quickly they clear lactate, which could be influenced by various factors such as individual physiology, training status, metabolic efficiency, and genetics. Generally, LA concentration is considered an effective determinant of running results (Nummela et al., 1992; Okudaira et al., 2019). Fatigue affects a decrease in power (Nummela et al., 1992), and therefore, an increase in LA concentration can be postulated, and a decrease in speed is a consequence of extreme fatigue. It may also be the result of the choice of running tactics. Based on the literature, the contribution of glycolysis to the 400 m may explain the relationship between blood lactate concentration and less aggressive running strategies (Hanon and Gajer, 2009). Therefore, sub-elite athletes exhibiting slower performance in the 400-m race demonstrated lower lactate concentration post exercise and diminished power in all jump tests. This is likely a consequence of employing a different running strategy, potentially impacting the efficacy of both the Wingate tests and jump assessments. This could result from weaker mental commitment or a diminished capacity to perform under fatigue. Moreover, it is noteworthy that the deceleration in speed observed in the last 50 and 100 m is more pronounced in world-class athletes than in athletes at lower performance levels (Hanon and Gajer, 2009). This means that elite runners must be physiologically and mentally able to adopt a more risky strategy than their less experienced counterparts.
Conclusions
Elite athletes exhibit greater maximum power and greater LA concentration after the Wingate efforts, likely stemming from their adaptation to the predominance of anaerobic metabolism and fast twitch muscle fiber recruitment during a prolonged sprint and more audacious running strategy compared to their less experienced counterparts in the sub-elite group. Despite these differences, the kinetics of LA accumulation and clearance did not differ between the groups studied. Moreover, the time of 400-m running significantly correlated with the rate of LA accumulation from the 1st to the 3rd min after effort and had no relationship with the rate of LA clearance.
Author Contributions
Conceptualization: E.M.; methodology: E.M., P.S., A.M. (Anna Mróz) and M.P.; validation: A.M. (Andrzej Mastalerz) and P.S.; formal analysis: A.M. (Andrzej Mastalerz) and E.M.; investigation: E.M.; data collection: E.M., D.K., M.J., P.V. and A.P.; writing—original draft preparation: A.M. (Andrzej Mastalerz) and E.M.; writing—review and editing: E.M., A.B. and P.S.; visualization: A.M. (Andrzej Mastalerz) and E.M.; supervision: E.M. and A.M. (Andrzej Mastalerz); project administration: A.M. (Andrzej Mastalerz) and E.M.; funding acquisition: E.M. All the authors have read and agreed to the published version of the manuscript.
Funding Information
This research was funded by the National Science Centre MINIATURA 6 entitled “Polygenetic determinants of lactic acid utilization in elite athletes” in 2022/2023 and the Ministry of Education and Science in 2023/2024 as part of the University Research Project of the University of Physical Education in Warsaw – UPB No. 1.
Institutional Review Board Statement:
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the District Medical Chamber in Gdansk (approval code: KB-2/21; approval date: 3 February 2021) for studies involving humans.
Informed Consent:
Informed consent was obtained from all participants included in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arcelli, E., Marina, M., Cimadoro, G., & Alberti, G. (2008). The aerobic mechanism in the 400 metres. New Studies in Athletics, 23(2), 15–23. [Google Scholar]
- Bishop, D., Bonetti, D., & Dawson, B. (2002). The influence of pacing strategy on VO2 and supramaximal kayak performance. Medicine and Science in Sports and Exercise, 34(6), 1041–1047. 10.1097/00005768-200206000-00022 [DOI] [PubMed] [Google Scholar]
- Bret, C., Lacour, J. R., Bourdin, M., Locatelli, E., De Angelis, M., Faina, M., Rahmani, A., & Messonnier, L. (2013). Differences in lactate exchange and removal abilities between high-level African and Caucasian 400-m track runners. European Journal of Applied Physiology, 113(6), 1489–1498. 10.1007/s00421-012-2573-8 [DOI] [PubMed] [Google Scholar]
- Chatel, B., Bret, C., Edouard, P., Oullion, R., Freund, H., & Messonnier, L. A. (2016). Lactate recovery kinetics in response to high-intensity exercises. European Journal of Applied Physiology, 116(8), 1455–1465. 10.1007/s00421-016-3420-0 [DOI] [PubMed] [Google Scholar]
- Chavda, S., Bromley, T., Jarvis, P., Williams, S., Bishop, C., Turner, A. N., Lake, J. P., & Mundy, P. D. (2018). Force-time characteristics of the countermovement jump: Analyzing the curve in excel. Strength and Conditioning Journal, 40(2), 67–77. 10.1519/SSC.0000000000000353 [DOI] [Google Scholar]
- Eynon, N., Hanson, E. D., Lucia, A., Houweling, P. J., Garton, F., North, K. N., & Bishop, D. J. (2013). Genes for elite power and sprint performance: ACTN3 leads the way. Sports Medicine, 43(9), 803–817. 10.1007/s40279-013-0059-4 [DOI] [PubMed] [Google Scholar]
- Goodwin, M. L., Harris, J. E., Hernández, A., & Gladden, L. B. (2007). Blood lactate measurements and analysis during exercise: A guide for clinicians. Journal of Diabetes Science and Technology, 1(4), 558–569. 10.1177/193229680700100414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanon, C., Bernard, O., Rabate, M., & Claire, T. (2012). Effect of two different long-sprint training regimens on sprint performance and associated metabolic responses. Journal of Strength and Conditioning Research, 26(6), 1551–1557. 10.1519/JSC.0b013e318231a6b5 [DOI] [PubMed] [Google Scholar]
- Hanon, C., & Gajer, B. (2009). Velocity and stride parameters of world-class 400-meter athletes compared with less experienced runners. Journal of Strength and Conditioning Research, 23(2), 524–531. 10.1519/JSC.0b013e318194e071 [DOI] [PubMed] [Google Scholar]
- Hanon, C., Rabate, M., & Thomas, C. (2011). Effect of expertise on postmaximal long sprint blood metabolite responses. Journal of Strength and Conditioning Research, 25(9), 2503–2509. 10.1519/JSC.0b013e3182001807 [DOI] [PubMed] [Google Scholar]
- Hanon, C., & Thomas, C. (2011). Effects of optimal pacing strategies for 400-, 800-, and 1500-m races on the VO2 response. Journal of Sports Sciences, 29(9), 905–912. 10.1080/02640414.2011.562232 [DOI] [PubMed] [Google Scholar]
- Harmer, A. R., McKenna, M. J., Sutton, J. R., Snow, R. J., Ruell, P. A., Booth, J., Thompson, M. W., Mackay, N. A., Stathis, C. G., Crameri, R. M., Carey, M. F., & Eager, D. M. (2000). Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. Journal of Applied Physiology, 89(5), 1793–1803. 10.1152/jappl.2000.89.5.1793 [DOI] [PubMed] [Google Scholar]
- Haugen, T., Seiler, S., Sandbakk, Ø., & Tønnessen, E. (2019). The Training and Development of Elite Sprint Performance: an Integration of Scientific and Best Practice Literature. Sports Medicine–Open, 5(1), 44. 10.1186/s40798-019-0221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, D. W. (1999). Energy system contributions in middle-distance running events. Journal of Sports Sciences, 17(6), 77–483. 10.1080/026404199365786 [DOI] [PubMed] [Google Scholar]
- Hirvonen, J., Nummela, A., Rusko, H., Rehunen, S., & Härkönen, M. (1992). Fatigue and changes of ATP, creatine phosphate, and lactate during the 400-m sprint. Canadian Journal of Sport Sciences, 17(2), 141–144 [PubMed] [Google Scholar]
- Iskra, J., Matusiński, A., Otsuka, M., & Guex, K. J. (2021). Pacing Strategy in Men’s 400 m Hurdles Accounting for Temporal and Spatial Characteristics of Elite Athletes. Journal of Human Kinetics, 79(1), 175–186). 10.2478/hukin-2021-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A. M., & Whipp, B. J. (2002). Bioenergetic constraints on tactical decision making in middle distance running. British Journal of Sports Medicine, 36(2), 102–104. 10.1136/bjsm.36.2.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, E. I. D., Smoliga, J. M., & Zavorsky, G. S. (2014). The effect of passive versus active recovery on power output over six repeated Wingate sprints. Research Quarterly for Exercise and Sport, 85(4), 519–526. 10.1080/02701367.2014.961055 [DOI] [PubMed] [Google Scholar]
- Maemura, H., Suzuki, Y., Mukai, N., &Takamatsu, K. (2004). Factors influencing excess CO2 output during and after short duration-intensive exercise: Focusing on skeletal muscle characteristics. International Journal of Sport and Health Science, 2, 129–135 [Google Scholar]
- Maciejewska-Skrendo, A., Buryta, M., Czarny, W., Król, P., Spieszny, M., Stastny, P., Petr, M., Safranow, K., & Sawczuk, M. (2019). The Polymorphisms of the Peroxisome-Proliferator Activated Receptors’ Alfa Gene Modify the Aerobic Training Induced Changes of Cholesterol and Glucose. Journal of Clinical Medicine, 8(10), 1043. 10.3390/jcm8071043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, J. J., Suchomel, T. J., Lake, J. P., & Comfort, P. (2018). Understanding the key phases of the countermovement jump force-time curve. Strength and Conditioning Journal, 40(4), 96–106. 10.1519/SSC.0000000000000375 [DOI] [Google Scholar]
- Metcalfe, R. S., Babraj, J. A., Fawkner, S. G., & Vollaard, N. B. J. (2012). Towards the minimal amount of exercise for improving metabolic health: Beneficial effects of reduced-exertion high-intensity interval training. European Journal of Applied Physiology, 112(7), 2767–2775. 10.1007/s00421-011-2254-z [DOI] [PubMed] [Google Scholar]
- Nummela, A., & Rusko, H. (1995). Time course of anaerobic and aerobic energy expenditure during short-term exhaustive running in athletes. International Journal of Sports Medicine, 16(8), 522–527. 10.1055/s-2007-973048 [DOI] [PubMed] [Google Scholar]
- Nummela, A., Vuorimaa, T., & Rusko, H. (1992). Changes in force production, blood lactate and emg activity in the 400-m sprint. Journal of Sports Sciences, 10(3), 217–228. 10.1080/02640419208729920 [DOI] [PubMed] [Google Scholar]
- Okudaira, M., Kuki, S., Yoshida, T., Fukuda, D. H., & Tanigawa, S. (2019). Load Characteristics of Sprint Interval Training According to 400m Running Performance: Competitive Level Comparison. International Journal of Sport and Health Science, 17, 148–154. 10.5432/ijshs.201833 [DOI] [Google Scholar]
- Ohkuwa, T. & Miyamura, M. (1984). Peak Blood Lactate after 400 m Sprinting in Sprinters and Long-Distance Runners. Japanese Journal of Physiology, 34(3), 553–556. 10.2170/jjphysiol.34.553 [DOI] [PubMed] [Google Scholar]
- Rago, V., Krustrup, P., & Mohr, M. (2022). Performance and Submaximal Adaptations to Additional Speed-Endurance Training vs. Continuous Moderate-Intensity Aerobic Training in Male Endurance Athletes. Journal of Human Kinetics, 83(1), 277–285. 10.2478/hukin-2022-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, A., & Leveritt, M. (2001). Long-term metabolic and skeletal muscle adaptations to short-sprint training: Implications for sprint training and tapering. Sports Medicine 31(15), 1063–1082. 10.2165/00007256-200131150-00003 [DOI] [PubMed] [Google Scholar]
- Siembida, P., Zawadka, M., & Gawda, P. (2021). The relationship between vertical jump performance during different training periods and results of 200m-sprint. Balt J Health Phys Activ, 13(3), 1-9. 10.29359/BJHPA.13.3.01 [DOI] [Google Scholar]
- Sumida, K. D., Urdiales, J. H., & Donovan, C. M. (1993). Enhanced gluconeogenesis from lactate in perfused livers after endurance training. Journal of Applied Physiology, 74(2), 782–787. 10.1152/jappl.1993.74.2.782 [DOI] [PubMed] [Google Scholar]
- Tomschi, F., Bizjak, D. A., Predel, H. G., Bloch, W., & Grau, M. (2018). Lactate distribution in red blood cells and plasma after a high intensity running exercise in aerobically trained and untrained subjects. Journal of Human Sport and Exercise, 13(2), 384–392. 10.14198/jhse.2018.132.10 [DOI] [Google Scholar]
- van Schenau, G. J. I., de Koning, J. J., & de Groot, G. (1994). Optimisation of Sprinting Performance in Running, Cycling and Speed Skating. Sports Medicine: An International Journal of Applied Medicine and Science in Sport and Exercise 17(4), 259–275. 10.2165/00007256-199417040-00006 [DOI] [PubMed] [Google Scholar]
- Weyand, P. G., Cureton, K. J., Conley, D. S., Sloniger, M. A., & Liu, Y. L. (1994). Peak oxygen deficit predicts sprint and middle-distance track performance. Medicine and Science in Sports and Exercise, 26(9), 1174–1180. 10.1249/00005768-199409000-00016 [DOI] [PubMed] [Google Scholar]
- Willis, R., Burkett, B., & Sayers, M. (2012). 400 metre race pace strategies: How your 200 metre personal best influences performance. Journal of Fitness Research, 1(1), 40–49. [Google Scholar]
- Yang, R., Shen, X., Wang, Y., Voisin, S., Cai, G., Fu, Y., Xu, W., Eynon, N., Bishop, D. J., & Yan, X. (2017). ACTN3 R577X Gene Variant Is Associated with Muscle-Related Phenotypes in Elite Chinese Sprint/Power Athletes. Journal of Strength and Conditioning Research, 31(4), 1107–1115. 10.1519/JSC.0000000000001558 [DOI] [PubMed] [Google Scholar]