Modern aquaculture, which produces billions of animals from hundreds of species each year, is generating unprecedented welfare risks.
Abstract
The unprecedented growth of aquaculture involves well-documented environmental and public-health costs, but less is understood about global animal welfare risks. Integrating data from multiple sources, we estimated the taxonomic diversity of farmed aquatic animals, the number of individuals killed annually, and the species-specific welfare knowledge (absence of which indicates extreme risk). In 2018, FAO reported 82.12 million metric tons of farmed aquatic animals from six phyla and at least 408 species—20 times the number of species of farmed terrestrial animals. The farmed aquatic animal tonnage represents 250 to 408 billion individuals, of which 59 to 129 billion are vertebrates (e.g., carps, salmonids). Specialized welfare information was available for 84 species, only 30% of individuals; the remaining 70% either had no welfare publications or were of an unknown species. With aquaculture growth outpacing welfare knowledge, immediate efforts are needed to safeguard the welfare of high-production, understudied species and to create policies that minimize welfare risks.
INTRODUCTION
Aquaculture, the farming of aquatic plants (e.g., seaweeds) and animals (e.g., carps and shrimps), is a fast-growing industry (1) and touted as a necessary sector for a sustainable future (2). With capture fisheries in decline worldwide (3), some also promote aquaculture as a solution to global food insecurity (4). Enthusiasm for aquaculture, however, is tempered by evidence that aquatic animal farming has not relieved fishing pressure on wild animal populations (5) and, as an enterprise that has only recently reached a global scale, involves many unchecked risks. To date, biodiversity loss, ecological damage, pollution, antibiotic overuse, lack of sustainability, and human rights abuses have all been identified and investigated as major areas of concern (6–10). Animal welfare issues in aquaculture are also attracting increased attention (11–17), with ongoing estimates suggesting that the number of individual animals killed each year is likely greater than the approximately 70 billion individuals involved in terrestrial animal agriculture (1, 18). Here, we assess for the first time the overall global scope and nature of the aquaculture welfare risk—the range of species used, the total number of individuals involved, and the state of the knowledge regarding their welfare.
Animal welfare—how well an animal is biologically, behaviorally, and emotionally coping with their environment (19, 20)—has become a priority in countries around the world (21, 22). The moral imperative to consider the experiences of other species is a growing policy agenda worldwide, with national, state, and local legislatures regularly passing stricter animal welfare protections (23). Animal welfare is also increasingly recognized as integral to sustainability, beneficial to the United Nations Sustainable Development Goals for 2030, and a high priority for consumers around the world (24, 25). Food products with animal welfare labeling carry a premium in markets from the European Union to Asia to the Americas (26–28). Moreover, multiple fields are establishing the interdependencies between animal welfare and environmental and human health, e.g., agriculture (29), anthropology (30), conservation biology (31), environmental science (32), human medicine (33) psychology (34), and veterinary science (35). These diverse disciplines and global forces underpin the urgent need to understand, protect, and improve the welfare of all animals; however, consideration of the welfare of aquatic animals in farmed systems has been absent from the discussion until very recently (11, 36, 37).
Ongoing efforts to correct this oversight have been motivated in part by the growing body of evidence that many aquatic species live far more complex social and emotional lives than previously understood (38–40). For example, a 2014 review of the scientific literature on pain found that fish and decapods (e.g., shrimp) display hallmarks of the ability to experience pain (41). Similarly, work in various species of fish has revealed complex cognitive abilities (42), including tool use (43), individual personalities (44), and strong preferences about the environments in which they live (45–47). Recent work with aquatic invertebrates is also uncovering unexpected abilities. In addition to the remarkable and diverse mental capabilities of cephalopods (48, 49), studies have found complex maze learning in shore crabs (50), sophisticated navigation in spiny lobsters (51), and emotional behavior in crayfish (52). While there is no singular, agreed upon cutoff for when welfare protections are ethically necessary, many of the species involved in aquaculture—including finfish and tetrapods, decapod crustaceans, and cephalopods—are now recognized as having the behavioral, cognitive, and affective abilities that meet widely accepted criteria for moral consideration and welfare protection (38–40, 53).
Understanding and ensuring the welfare of aquatic animals is a fundamentally different endeavor than it is for terrestrial animals, however. First, unlike terrestrial animal agriculture, the vast majority of species currently farmed in aquaculture are either wild or only recently domesticated (54, 55). Aquaculture originated thousands of years ago, but traditional practices were restricted to a small number of species, cultivated at a minor scale (56). It is only in the past several decades that concern about global overfishing and scientific advances have propelled an explosion in the number of farmed aquatic species (57). As a result, most of the individuals in modern aquaculture are not biologically adapted to life in captivity, which poses serious welfare risks, especially in the absence of historical and traditional knowledge about how to care for newly farmed species. The negative consequences of these compound risk factors are evident in a recent assessment of 41 species in aquaculture, which determined that under current conditions, welfare for most species is likely to be poor across the life cycle: from the high likelihood of malformations and physiological defects at birth to restricted mobility, high aggression, and poor handling throughout development to “extreme pain and suffering” during slaughter (11).
Second, whereas the scientific literature on terrestrial animal welfare has accumulated across many decades and has only needed to cover approximately 20 species, the literature on the welfare of animals in aquaculture is new and needs to cover an order of magnitude more species [at least 300 (57)]. Species-specific welfare research is necessary to understand an individual’s welfare because even within the same genus, different species can display widely divergent biological and behavioral responses, with different implications for their welfare (58). Without species-specific data, welfare standards, assessment tools, labeling schemes, legislative initiatives, and enforcement will not be supported by reliable knowledge, which leads welfare protections to be weak, ineffective, or nonexistent (59, 60). Thus, while the presence of species-specific welfare publications does not ensure an animal’s welfare, its absence does signal extreme welfare risks.
To evaluate the extent and magnitude of the welfare-knowledge gap, we aimed to (i) quantify the taxonomic diversity of the animals currently involved aquaculture, (ii) provide formal estimates of the number of individuals killed for consumption in 2018, and (iii) gauge the extent to which the scientific literature is meeting the diverse needs of the animals in aquaculture. Data from the Food and Agriculture Organization (FAO) of the United Nations (FishStatJ) provided estimates of the amount and diversity of aquatic animals used in farming worldwide in the year 2018. Reflecting the extent of their commodification, animals in aquaculture are reported in production weight, not as number of individuals (as is the norm for terrestrial animal agriculture). To generate estimates of the number of individual aquatic animals used in 2018, therefore, we matched the FAO tonnage data to biometric information in FishBase [fishbase.org; (61)] and slaughter weights from fishcount (fishcount.org.uk; (18)]. Last, we collected bibliometric data from Web of Science (WOS; Clarivate Analytics) to assess the scientific information available on each species. These methods combine to provide a conservative estimate of the welfare risks involved in global aquaculture.
RESULTS
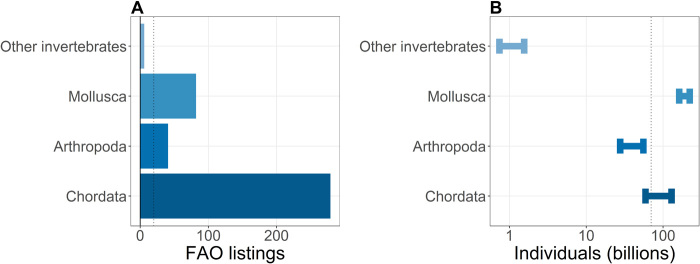
FAO reported that a total of 82.12 million metric tons of animals were produced in aquaculture in 2018, comprising at least 408 species distributed across six phyla, most of which were from the Chordata phylum (68% of listings; Fig. 1A). The other phyla were all invertebrates, with Mollusca (e.g., clams and mussels) and Arthropoda (e.g., shrimps and crabs) having the second and third most FAO listings, respectively. We estimate that the total tonnage corresponds to approximately 250 to 408 billion individual animals: 191 to 279 billion invertebrates and 59 to 129 billion vertebrates (Fig. 1B).
Fig. 1. Diversity and scope of global animal aquaculture production (data FAO, 2018).
(A) At the global level, each FAO listing typically corresponds to the production of a particular species, but a listing may also refer to a higher taxonomic grouping such as a genus, class, or phylum not listed elsewhere in the data. The number of FAO listings is therefore a conservative estimate of the total diversity of species involved in aquaculture production. Bars represent the total number of FAO aquaculture animal listings by phylum and the dotted gray line refers to the total number of FAO listings for all of terrestrial animal agriculture combined. (B) Estimates of the number of individual animals in aquaculture production for 2018. The error bars represent the present analyses’ upper and lower estimates for the number of individuals involved in global animal aquaculture by phylum. The dotted gray line refers to the total number individuals involved across all of terrestrial animal agriculture combined.
Of the 408 listings, 231 species had no welfare publications, 59 had one to four welfare publications, 25 had five or more welfare publications, and 93 of the listing did not contain species-level taxonomic information (Table 1, as of May 2020). In accordance with our estimates using the most specific taxonomic data available, 128 to 183 billion individuals (48% of total aquaculture) are not covered by any welfare literature (105 to 136 billion invertebrates and 23 to 48 billion vertebrates), and an additional 50 to 102 billion individuals (22% of total aquaculture) are of unknown species and thus cannot have any species-specific welfare publications (35 to 69 billion invertebrates and 15 to 33 billion vertebrates); 58 to 91 billion individuals (23% of total aquaculture) are covered by one to four welfare publications (51 to 75 billion invertebrates and 7 to 16 billion vertebrates), and 14 to 32 billion individuals (7% of total aquaculture) were covered by five or more welfare publications (all vertebrates; Table 1).
Table 1. Welfare by phyla.
The number of individuals and minimum number of species involved (FAO listing is an underestimate of species total) by welfare research and phyla.
| Chordata | Arthropoda | Mollusca | Other Invert. | Total | |
| Species unknown | |||||
| Individuals (in billions) | 15–33 | 1–2 | 34–66 | 0–1 | 50– 102 |
| FAO listings | 62 | 11 | 16 | 4 | 93 |
| No welfare publications | |||||
| Individuals (in billions) | 23–48 | 21–42 | 84–93 | 0–1 | 128–183 |
| FAO listings | 144 | 26 | 60 | 1 | 231 |
| Few welfare publications | |||||
| Individuals (in billions) | 7–16 | 8–11 | 45–63 | 0–1 | 58–91 |
| FAO listings | 48 | 4 | 6 | 1 | 59 |
| 5+ welfare publications | |||||
| Individuals (in billions) | 14–32 | 0 | 0 | 0 | 14–32 |
| FAO listings | 25 | 0 | 0 | 0 | 25 |
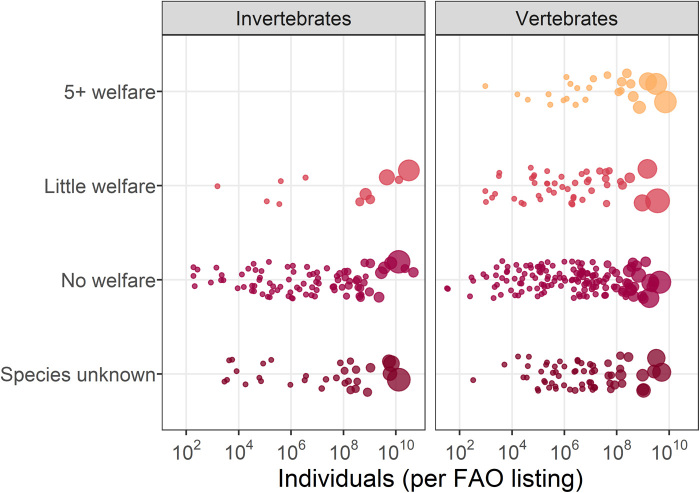
Further examining these data by FAO listing shows the extent of the problem: Billions of individual animals belonging to hundreds of different species are not covered by any animal welfare publications (Fig. 2). Information on the five vertebrates with highest number of individuals by aquatic environment (freshwater versus marine) can be found in Table 2 (see the Supplementary Materials for information on all 2018 FAO listings; table S1).
Fig. 2. Welfare knowledge and number of individuals per FAO listing (2018).
Each FAO listing typically refers to the production tonnage of a particular species, in which case we searched the WOS for species-specific welfare publications and recorded whether there were no publications (no welfare), one to four publications (little welfare), or five or more publications (5+ welfare). We recorded FAO listings that referred to higher taxonomic grouping (such as genus or class) as species unknown. The dots represent the lower-bound estimate of number of individual animals involved for that listing and are jittered vertically to minimize overlap. Dots size corresponds to aquaculture production tonnage.
Table 2. Top vertebrates in global aquaculture by number of individuals and environment (freshwater versus marine).
Although there is a modest body of welfare literature for some of these species (five or more welfare publications), several species have very few or no publications about their welfare, or their species is unknown. In the FAO data, nei stands for “not elsewhere included.” N/A, not applicable.
| FAO listing | Species | Individuals (billions) | Environment | Welfare |
| Nile tilapia | Oreochromis niloticus | 7–16 | Freshwater | 5+ welfare |
| Carassius nei | Carassius spp. | 5–11 | Freshwater | Species unknown |
| Silver carp | Hypophthalmichthys molitrix | 4–10 | Freshwater | No welfare |
| Grass carp | Ctenopharyngodon idellus | 4–8 | Freshwater | Little welfare |
| Common carp | Cyprinus carpio | 3–8 | Freshwater | 5+ welfare |
| Atlantic salmon | Salmo salar | 2–4 | Marine | 5+ welfare |
| Marine fishes nei | N/A | 1–2 | Marine | Species unknown |
| Gilthead seabream | Sparus aurata | 0–1 | Marine | 5+ welfare |
| Large yellow croaker | Larimichthys croceus | 0–1 | Marine | No welfare |
| European seabass | Dicentrarchus labrax | 0–1 | Marine | 5+ welfare |
DISCUSSION
In 2018, 250 to 408 billion individual animals from more than 408 species were farmed in aquaculture. Integrating these estimates with the scientific literature reveals the magnitude of the animal welfare risk. Only 25 species (corresponding to 14 to 32 billion individuals, 7% of total aquaculture) are covered by even a modest body of welfare literature (five or more publications). The remaining 383+ species (corresponding to 236 to 376 billion individuals, 93% of total aquaculture) had few to no welfare publications. By rapidly expanding the cultivation of a wide diversity of undomesticated species, aquaculture is now characterized by the intensive management of billions of individuals in the absence of basic knowledge about how to ensure their welfare.
It is generally understood that animal welfare policy must be grounded in scientific knowledge; in the absence of such information, protections will be weak, misinformed, or lacking. Thus, while species-specific science does not guarantee individual well-being (e.g., billions of chickens still face poor living conditions despite decades of research on their welfare), its absence does signal extreme risk. In aquaculture, that predicament is borne out by recent work systematically evaluating typical aquaculture conditions. For most of the species considered, current practices entail poor welfare across all stages of production (11).
Strategies to address this urgent welfare risk fall roughly into three, complimentary categories, only one of which involves traditional animal welfare science. While more species-specific information is undoubtedly needed, generating reliable and actionable knowledge is extremely resource intensive and slow to translate into policy. In the near term, going from some knowledge about a few species to authoritative knowledge about hundreds of species is simply not feasible. In the meantime, billions of individual animals will continue to face conditions that are likely harming their welfare. Although international support for fish welfare science has increased over the past few decades (62), these investments have not kept pace with the expansion of aquaculture (1, 63). Moreover, animal welfare is just one of multiple factors to consider along with environmental impacts, food security, and others (16, 17).
There is, nevertheless, a hidden advantage to these troubling circumstances. The expansion of aquaculture is a recent enough phenomenon that scientific, civic, and financial input can still play a large role in shaping its future. For example, decades of research has established that the cultivation of carnivorous species, e.g., Atlantic salmon (Salmo salar), pose greater sustainability risks, including welfare, than herbivorous species, e.g., Nile tilapia (Oreochromis niloticus) (7, 64, 65). Going forward, addressing these factors will require that greater emphasis is placed on cultivating species with fewer welfare and environmental risks, e.g., seaweeds and some bivalves (17), rather than those with greater risks, e.g., cephalopods (16). This approach to addressing the welfare risks in aquaculture can be considered a structural change or system change method, growing and developing the cultivation of the lowest risk species while building consensus around prohibitions against further efforts to cultivate and domesticate those of highest risk (16, 17, 66). Future research assessing the sustainability of aquaculture will benefit from incorporating animal welfare parameters to gain a more complete picture of the costs involved in the ongoing attempts to farm wild aquatic animals.
Work aimed at tackling the aquaculture welfare research gap can seek to identify, benchmark, standardize, and improve upon welfare best practices within current aquaculture settings, including leveraging historical knowledge. For example, promising recent studies have investigated traditional practices of providing fish with a temperature gradient to allow for behavioral thermoregulation (67) and the effects of increasing structural complexity in modern aquaculture settings (68). When working within production systems, it is especially important to recognize that welfare is not synonymous with production optimization (69, 70), a notion that some of the current work on fish and aquatic invertebrate welfare overlooks. Many of the articles counted as welfare publications in the present analysis applied a production-oriented lens to welfare, e.g., “Effects of different stunning/slaughter methods on frozen fillets quality of cobia (Rachycentron canadum).” Healthy biological functioning is a critical component of welfare, and across aquaculture, disease is a primary concern (71). On its own, however, biological health is not sufficient to ensure welfare and can, at times, be too easily be conflated with economic and production optimization (72). Instead, a more wholistic approach to welfare, including attention to psychological health and ecologically relevant behaviors, is required (20).
Last, an “animal-first” approach to welfare research is needed (73). Some welfare-relevant information can only be garnered by studying animals for their own sake, outside the constraints of the current farming systems, and by adhering to principles of avoiding harm, providing benefits, and respecting an individual animal’s agency and autonomy (74–76). This approach to welfare research is an extension of recent discussions of “positive welfare” and the calls to provide animals with a “good life,” both of which acknowledge that minimizing suffering does not ensure a decent quality of life and that mere survival is not the same as acceptable welfare (77–80). One of the advantages of the animal-first approach is that it speaks to the concerns of multiple stakeholders simultaneously. It can provide unique “within-system” solutions and fill gaps regarding baseline behavioral expectations [e.g., current industry standards markedly increase aggression in coho salmon (45)]. By prioritizing the interests of the individual, the animal-first approach also provides basic science data and answers foundational questions regarding the reasons for attending to a particular species’ welfare in the first place, e.g., their capacities, sapience, and sentience. Although relatively new for research programs with farmed fishes and especially aquatic invertebrates, indications of the utility of this approach for aquatic animals already exist [e.g., (38, 81, 82)].
The modern expansion of aquaculture is remarkable for its size and scope and the untracked harms faced by the billions of aquatic animals it produces each year. Addressing these welfare risks in concert with other risks—e.g., environmental degradation, human rights abuses, food security, and fisheries depletion—will require a multipronged strategy. First, risk can be mitigated structurally with investment, research, policy, and advocacy to support the infrastructure to cultivate species with low welfare and environmental concerns (e.g., seaweeds) and disincentivize the cultivation of species with high welfare and environmental concerns (e.g., cephalopods). Second, to align aquaculture with current scientific knowledge and civic expectations, research tackling species-specific welfare questions will need to address all aspects of welfare (biological, psychological, and ecological). Last, to generate a complete picture of these complex, fascinating, and mostly wild species, high-quality information about their lives outside the current systems of production is also needed. Although aquaculture has been around for thousands of years, its current expansion is not only unprecedented, posing great threats, but also has the opportunity to make scientifically grounded and wiser choices going forward. This work contributes to the mounting evidence of the urgency of that obligation.
MATERIALS AND METHODS
Number of species and tonnage: FAO listings of aquaculture production
Using FishStatJ, the FAO’s dataset on global aquaculture production, we exported information on all the aquatic animals farmed in all geographical regions for 2018 (the most recently available year as of May 2020). Each listing in this dataset corresponds to a species, a hybrid, or an aggregation of multiple species not elsewhere included in the dataset, e.g., “Snappers nei (not elsewhere included)” and tonnage for 2018. The FAO uses the Aquatic Sciences and Fisheries Information System (ASFIS) to code the common names of the aquatic animals. We used the ASFIS scientific name listings to match the common names of the aquatic animals listed in the FAO aquaculture production dataset with their scientific names (genus and species) and higher taxonomic classifications (family, order, class, and phylum). Some listings indicate that tonnage as “not available” or “known to be nil or zero.” The original data contained 745 listings, but after removing those with no reported tonnage, 408 positive tonnage listings remained as a minimum estimate of the number of species involved in aquaculture in 2018.
Estimating the number of individuals: Biometric data (FishBase) and harvest weights (FishCount)
FAO tracks aquaculture as production weights for each listing, not as individuals (as is done in terrestrial animal agriculture). Calculating the number of individuals farmed in aquaculture thus requires estimating, for each species, the typical weight of an individual at processing and using that figure to back-calculate the number of individuals farmed that year. Using a variety of sources, the website FishCount has identified and compiled reliable upper and lower harvest weights for 48 aquatic species (18). We used these data (publicly available at fishcount.org.uk) in combination with a two-step process to generate an upper-bound and a lower-bound estimate of likely harvest weights for each listing. With these harvest-weight estimates, we were able to generate plausible range of individuals farmed for each listing.
As the first step, we estimated maximum growth capacities for each listing. Maximum growth weight of a species can be calculated using the classic equation: W = a*Lb, which describes the relationship between an individual’s weight (W) and length (L). The parameters a, b, and Lmax (maximum length) are constant and well described for many aquatic species (83). Of the 408 listings in 2018 with positive tonnage data, we were able to match 234 listings to all the necessary biometric parameters a, b, and Lmax from FishBase (61). For the remaining 174 listings, we used the closest taxonomic median information to impute the likely maximum weight of an individual of that group. In other words, if we did not have biometric information for a certain species listing, we sought the median information for other species in its genus. If we lacked the necessary information for the genus, we used median information for other members of the family, then order, class, phylum. This procedure generated estimates for the maximum weight (Wmax) of each listing in our dataset.
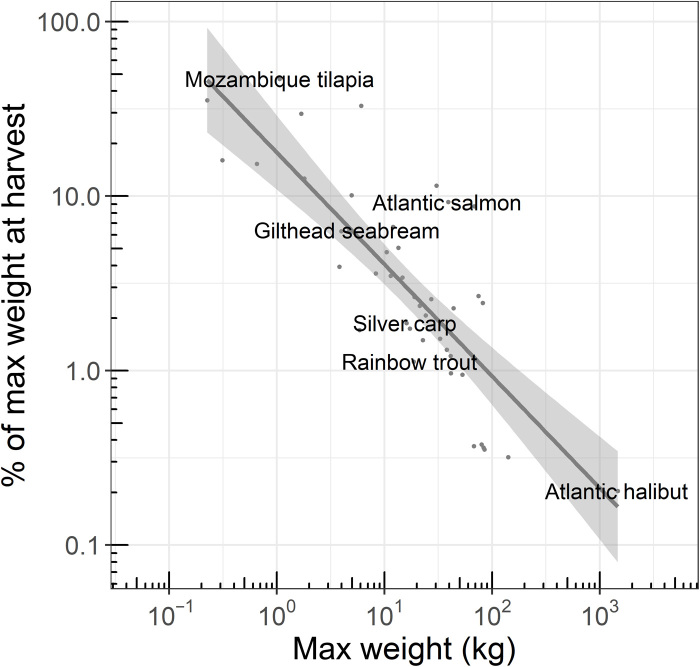
Second, we determined the likely harvest weight for each species. In farming, individuals are typically taken for slaughter at a size well below their growth maximum. Merging the FishBase maximum weight data with the FishCount harvest weight data revealed a strong relationship: Larger species are harvested at a tinier fraction of their maximum capacity than smaller bodied species (r > 0.79, P < 0.0001; Fig. 3; see Table 3 for model information). For example, rainbow trout (Oncorhynchus mykiss) can reach weights of more than 18 kg (40 lb) but are marketed at weights of up to 5 kg (11 lb) or about 27% of capacity. Mozambique tilapia (Oreochromis mossambicus), on the other hand, reach a maximum weight of around 1 kg (2.2 lb) but are marketed at weights up to 0.8 kg (1.8 lb) or about 75% of capacity. Thus, the maximum weight of a species determines the percentage of maximum at which individuals are typically taken for slaughter (Fig. 3). Applying this relationship across all listings and capping percentage of maximum at 100%, we imputed likely lower and upper harvest weights for each listing.
Fig. 3. Maximum weight (from FishBase) determines the percentage at which aquatic animals are harvested (from FishCount).
In aquaculture, smaller-bodied species are typically harvested at weights nearer their biological maximum, i.e., at a higher percentage of their maximum weight. Larger-bodied species, on the other hand, are harvested at a smaller percentage of their biological maximum. Note the large range of percentages from less than 1 to more than 70%. Dots represent individual species with several representative species identified by their common name. Dark-gray line is the best fit line and shading represents the 95% confidence interval of the fit (R2 = 0.64, P < 0.0001).
Table 3. Association between percentage of maximum weight at harvest and maximum weight for lower-bound harvest weight estimates and upper-bound harvest weight estimates.
***P < 0.0001 with 46 degrees of freedom.
| Outcome variable | Linear least squares equation | R 2 |
| Percentage of maximum weight (lower-bound harvest estimate) |
log(pc_lower) = 7.31–0.64*log(Wmax) | 0.64*** |
| Percentage of maximum weight (upper-bound harvest estimate) |
log(pc_upper) = 7.72–0.60*log(Wmax) | 0.62*** |
| Models include 48 observations | ||
Last, to generate a plausible range of the number of individuals farmed in 2018, we divided the total tonnage reported by FAO for each listing by its upper-bound harvest weight estimate (to create the lower estimate of total number of individuals) and by its lower-bound harvest weight estimate (to create the upper estimate of total number of individuals).
Animal welfare literature coverage: WOS bibliometric analysis
Using bibliometric methods, we determined the volume of empirical welfare work for each of the listings in the FAO aquaculture production database. We performed a search (May 2020) for scientific papers (over all time periods) for each scientific name in each of the main groups of aquatic animals in our dataset using Clarivate Analytics WOS, which covers >12,000 scholarly journals, including all major science, medicine, and technology journals. Although the database has shortcomings (e.g., non-English language journals are underrepresented), it is considered to provide a satisfactory representation of international mainstream scientific research (84).
For each listing associated with a species or hybrid, we searched for the scientific name or common hybrid names in combination with the word “welfare.” If no papers were returned, then we coded the listing as “no welfare papers.” When a search did return an article or more, we looked further into the content. Only papers that contained primary research on the welfare of the species in question were included for further analysis. If the authors used the word welfare to refer to some measure of biology, health, behavior, or psychological/emotional state of the species in question, we included the article. We excluded review papers and empirical papers that focused on something other than the welfare of individuals of the species in question (e.g., papers that studied a different species or studied the welfare of the fishery). We then categorized each of the 408 FAO listings in accordance with welfare potential: “No welfare” (i.e., species-level welfare information is not available, although it could be), “little welfare” (i.e., 1 to 4 primary research papers have been published on the welfare of the species in question), “5+ welfare” (i.e., five or more primary research papers have been published on the welfare of the species in question), or “species unknown” (i.e., no species-level or hybrid-level welfare information is possible because the species is unknown/not specified by FAO).
Acknowledgments
We wish to thank D. Pauly, R. Froese, E. Lara, N. Boyland, and P. Brooke for their generous time and insights during the development of this research. Funding: B.F. gratefully acknowledges the Fish and Marine Animal Fund and Open Philanthropy for support. J.J.’s research funds from NYU provided support to C.E. Author contributions: B.F. and J.J. conceived and designed the research. B.F. and C.E. collected the data. B.F. analyzed the data. B.F. and C.E. wrote the paper. B.F., C.E., and J.J. reviewed and edited the paper. B.F. and J.J. provided funding acquisition. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/14/eabg0677/DC1
REFERENCES AND NOTES
- 1.FAO, The State of World Fisheries and Aquaculture - Meeting the Sustainable Development Goals (FAO, 2018). [Google Scholar]
- 2.T. Searchinger, R. Waite, T. Beringer, A. Forslund, H. Guyomard, C. Le Mouël, S. Manceron, E. Marajo-Petitzon, World Resources Report: Creating a Sustainable Food Future (World Resources Institue, 2018), p. 96. [Google Scholar]
- 3.Pauly D., Christensen V., Guénette S., Pitcher T. J., Sumaila U. R., Walters C. J., Watson R., Zeller D., Towards sustainability in world fisheries. Nature 418, 689–695 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Duarte C. M., Holmer M., Olsen Y., Soto D., Marbà N., Guiu J., Black K., Karakassis I., Will the oceans help feed humanity? Bioscience 59, 967–976 (2009). [Google Scholar]
- 5.Longo S. B., Clarak B., York R., Jorgenson A. K., Aquaculture and the displacement of fisheries captures. Conserv. Biol. 33, 832–841 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Diana J. S., Aquaculture production and biodiversity conservation. Bioscience 59, 27–38 (2009). [Google Scholar]
- 7.Naylor R. L., Goldburg R. J., Primavera J. H., Kautsky N., Beveridge M. C., Clay J., Folke C., Lubchenco J., Mooney H., Troell M., Effect of aquaculture on world fish supplies. Nature 405, 1017–1024 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Naylor R. L., Hardy R. W., Bureau D. P., Chiu A., Elliott M., Farrell A. P., Forster I., Gatlin D. M., Goldburg R. J., Hua K., Nichols P. D., Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. 106, 15103–15110 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpe J. P., Gee J. L. M., Ethier V. A., Beck M., Wilson A. J., Stoner J. M. S., Global aquaculture performance index (GAPI): The first global environmental assessment of marine fish farming. Sustainability 5, 3976–3991 (2013). [Google Scholar]
- 10.Guillen J., Natale F., Carvalho N., Casey J., Hofherr J., Druon J. N., Fiore G., Gibin M., Zanzi A., Martinsohn J. T., Global seafood consumption footprint. Ambio 48, 111–122 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saraiva J. L., Arechavala-Lopez P., Castanheira M. F., Volstorf J., Studer B. H., A global assessment of welfare in farmed fishes: The FishEthoBase. Fishes 4, 30 (2019). [Google Scholar]
- 12.Ashley P. J., Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 104, 199–235 (2007). [Google Scholar]
- 13.Huntingford F. A., Adams C., Braithwaite V. A., Kadri S., Pottinger T. G., Sandøe P., Turnbull J. F., Current issues in fish welfare. J. Fish Biol. 68, 332–372 (2006). [Google Scholar]
- 14.Brown C., Dorey C., Pain and Emotion in Fishes–Fish welfare implications for fisheries and aquaculture. Anim. Stud. J. 8, 175–201 (2019). [Google Scholar]
- 15.Martins C., Galhardo L., Noble C., Damsgård B., Spedicato M. T., Zupa W., Beauchaud M., Kulczykowska E., Massabuau J.-C., Carter T., Planellas S. R., Kristiansen T., Behavioural indicators of welfare in farmed fish. Fish Physiol. Biochem. 38, 17–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacquet J., Franks B., Godfrey-Smith P., Sanchez-Suarez W., The case against octopus farming. Issues Sci. Technol. 35, 37–44 (2019). [Google Scholar]
- 17.Jacquet J., Sebo J., Elder M., Seafood in the future: Bivalves are better. Solutions 8, 27–32 (2017). [Google Scholar]
- 18.Mood A., Brooke P., Estimating the number of farmed fish killed in global aquaculture each year. FishCount 2012, 40 (2012). [Google Scholar]
- 19.Broom D. M., Animal-welfare - concepts and measurement. J. Anim. Sci. 69, 4167–4175 (1991). [DOI] [PubMed] [Google Scholar]
- 20.Fraser D., Weary D. M., Pajor E. A., Milligan B. N., A scientific conception of animal welfare that reflects ethical concerns. Anim. Welf. 6, 187–205 (1997). [Google Scholar]
- 21.P. Singer, The Expanding Cirle: Ethics, Evolution, and Moral Progress (Princeton Univ. Press, 2011); http://ridum.umanizales.edu.co:8080/jspui/bitstream/6789/377/4/Muñoz_Zapata_Adriana_Patricia_Artículo_2011.pdf.
- 22.Lu J., Bayne K., Wang J., Current status of animal welfare and animal rights in China. Altern. Lab. Anim. 41, 351–357 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Lundmark F., Berg C., Schmid O., Behdadi D., Röcklinsberg H., Intentions and values in animal welfare legislation and standards. J. Agric. Environ. Ethics. 27, 991–1017 (2014). [Google Scholar]
- 24.Keeling L., Tunón H., Olmos Antillón G., Berg C., Jones M., Stuardo L., Swanson J., Wallenbeck A., Winckler C., Blokhuis H., Animal welfare and the United Nations Sustainable development goals. Front. Vet. Sci. 6, 336 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broom D. M., Animal welfare complementing or conflicting with other sustainability issues. Appl. Anim. Behav. Sci. 219, 104829 (2019). [Google Scholar]
- 26.Janssen M., Rödiger M., Hamm U., Labels for animal husbandry systems meet consumer preferences: Results from a meta-analysis of consumer studies. J. Agric. Environ. Ethics. 29, 1071–1100 (2016). [Google Scholar]
- 27.Xu L., Yang X., Wu L., Chen X., Chen L., Tsai F. S., Consumers’ willingness to pay for food with information on animal welfare, lean meat essence detection, and traceability. Int. J. Environ. Res. Public Health 16, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranda-de la Lama G. C., Estévez-Moreno L. X., Villarroel M., Rayas-Amor A. A., María G. A., Sepúlveda W. S., Consumer attitudes toward animal welfare-friendly products and willingness to pay: Exploration of mexican market segments. J. Appl. Anim. Welf. Sci. 22, 13–25 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Hansen B. G., Østerås O., Farmer welfare and animal welfare- Exploring the relationship between farmer’s occupational well-being and stress, farm expansion and animal welfare. Prev. Vet. Med. 170, 104741 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Bussolini J., Recent French, Belgian and Italian work in the cognitive science of animals: Dominique Lestel, Vinciane Despret, Roberto Marchesini and Giorgio Celli. Soc. Sci. Inf. 52, 187–209 (2013). [Google Scholar]
- 31.Ramp D., Bekoff M., Compassion as a practical and evolved ethic for conservation. Bioscience 65, 323–327 (2015). [Google Scholar]
- 32.Scherer L., Behrens P., Tukker A., Opportunity for a dietary win-win-win in nutrition, environment, and animal welfare. One Earth 1, 349–360 (2019). [Google Scholar]
- 33.Nimer J., Lundahl B., Animal-assisted therapy: A meta-analysis. Anthrozoos 20, 225–238 (2007). [Google Scholar]
- 34.Amiot C. E., Bastian B., Toward a psychology of human-animal relations. Psychol. Bull. 141, 6–47 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Tarazona A. M., Ceballos M. C., Broom D. M., Human relationships with domestic and other animals: One health, one welfare, one biology. Animals. 10, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker M., Diez-Leon M., Mason G. J., Animal welfare science: Recent publication trends and future research priorities. Int. J. Comp. Psychol. 27, 80–100 (2014). [Google Scholar]
- 37.Freire R., Nicol C., A bibliometric analysis of past and emergent trends in animal welfare science. Anim. Welf. 28, 465–485 (2019). [Google Scholar]
- 38.Brown C., Fish intelligence, sentience and ethics. Anim. Cogn. 18, 1–17 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Elwood R. W., Pain and suffering in invertebrates? ILAR J. 52, 175–184 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Broom D. M., Cognitive ability and sentience: Which aquatic animals should be protected? Dis. Aquat. Organ. 75, 99–108 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Sneddon L. U., Elwood R. W., Adamo S. A., Leach M. C., Defining and assessing animal pain. Anim. Behav. 97, 201–212 (2014). [Google Scholar]
- 42.Salwiczek L. H., Prétôt L., Demarta L., Proctor D., Essler J., Pinto A. I., Wismer S., Stoinski T., Brosnan S. F., Bshary R., Adult cleaner wrasse outperform capuchin monkeys, chimpanzees and orang-utans in a complex foraging task derived from cleaner – client reef fish cooperation. PLOS ONE 7, e49068 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown C., Tool use in fishes. Fish Fish. 13, 105–115 (2012). [Google Scholar]
- 44.Croft D. P., Krause J., Darden S. K., Ramnarine I. W., Faria J. J., James R., Behavioural trait assortment in a social network: Patterns and implications. Behav. Ecol. Sociobiol. 63, 1495–1503 (2009). [Google Scholar]
- 45.Gaffney L. P., Franks B., Weary D. M., von Keyserlingk M. A. G., Coho Salmon (Oncorhynchus kisutch) prefer and are less aggressive in darker environments. PLOS ONE 11, e0151325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maia C. M., Volpato G. L., Preference index supported by motivation tests in Nile tilapia. PLOS ONE 12, e0175821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerqueira M., Millot S., Castanheira M. F., Félix A. S., Silva T., Oliveira G. A., Oliveira C. C., Martins C. I. M., Oliveira R. F., Cognitive appraisal of environmental stimuli induces emotion-like states in fish. Sci. Rep. 7, 13181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnell A. K., Clayton N. S., Cephalopod cognition. Curr. Biol. 29, R726–R732 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Mather J. A., Dickel L., Cephalopod complex cognition. Curr. Opin. Behav. Sci. 16, 131–137 (2017). [Google Scholar]
- 50.Davies R., Gagen M. H., Bull J. C., Pope E. C., Maze learning and memory in a decapod crustacean. Biol. Lett. 15, 20190407 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boles L. C., Lohmann K. J., True navigation and magnetic maps in spiny lobsters. Nature 421, 60–63 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Fossat P., Bacqué-Cazenave J., De Deurwaerdère P., Delbecque J.-P., Cattaert D., Anxiety-like behavior in crayfish is controlled by serotonin. Science 344, 1293–1297 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Fraser D., Animal ethics and animal welfare science: Bridging the two cultures. Appl. Anim. Behav. Sci. 65, 171–189 (1999). [Google Scholar]
- 54.J. L. Saraiva, M. F. Castanheira, P. Arechavala-López, J. Volstorf, B. Heinzpeter Studer, Domestication and welfare in farmed fish, in Animal Domestication (Intech Open, 2019). [Google Scholar]
- 55.Teletchea F., Fontaine P., Levels of domestication in fish: Implications for the sustainable future of aquaculture. Fish Fish. 15, 181–195 (2014). [Google Scholar]
- 56.Stickney R. R., Treece G. D., History of aquaculture. Aquac. Prod. Syst. 2012, 15–50 (2012). [Google Scholar]
- 57.Metian M., Troell M., Christensen V., Steenbeek J., Pouil S., Mapping diversity of species in global aquaculture. Rev. Aquac. 12, 1090–1100 (2020). [Google Scholar]
- 58.Mckenzie D. J., Palstra A. P., Planas J., Mackenzie S., Thorarensen H., Vandeputte M., Mes D., Rey S., De Boeck G., Domenici P., Skov P. V., Aerobic swimming in intensive finfish aquaculture: Applications for production , mitigation and selection. Rev. Aquac. 13, 138–155 (2021). [Google Scholar]
- 59.Caporale V., Alessandrini B., Villa P. D., Del Papa S., Global perspectives on animal welfare: Europe. Rev. Sci. Tech. 24, 567–577 (2005). [PubMed] [Google Scholar]
- 60.Jenks E., The bear necessities: Why Captive exhibited animals need stronger regulation based on their species-specific biological needs. Michigan State Law Rev. 2019, 1081 (2020). [Google Scholar]
- 61.R. Froese, D. Pauly, FishBase (2000); www.fishbase.org.
- 62.Kristiansen T. S., Bracke M. B. M., A brief look into the origins of fish welfare science. Welf. Fish. 20, 1–17 (2020). [Google Scholar]
- 63.Love D. C., Gorski I., Fry J. P., An analysis of nearly one billion dollars of aquaculture grants made by the US federal government from 1990 to 2015. J. World Aquac. Soc. 48, 689–710 (2017). [Google Scholar]
- 64.Lund V., Mejdell C. M., Röcklinsberg H., Anthony R., Håstein T., Expanding the moral circle: Farmed fish as objects of moral concern. Dis. Aquat. Organ. 75, 109–118 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Broom D. M., Galindo F. A., Murgueitio E., Sustainable, efficient livestock production with high biodiversity and good welfare for animals. Proc. R. Soc. B Biol. Sci. 280, 20132025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacquet J., Franks B., Godfrey-smith P., The octopus mind and the argument against farming it. Anim. Sci. 271, 1–6 (2019). [Google Scholar]
- 67.Huntingford F., Rey S., Quaggiotto M. M., Behavioural fever, fish welfare and what farmers and fishers know. Appl. Anim. Behav. Sci. 231, 105090 (2020). [Google Scholar]
- 68.Arechavala-Lopez P., Caballero-Froilán J. C., Jiménez-García M., Capó X., Tejada S., Saraiva J. L., Sureda A., Moranta D., Enriched environments enhance cognition, exploratory behaviour and brain physiological functions of Sparus aurata. Sci. Rep. 10, 11252 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayek M. N., Garrett R. D., Nationwide shift to grass-fed beef requires larger cattle population. Environ. Res. Lett. 13, 084005 (2018). [Google Scholar]
- 70.Ritter C., Beaver A., von Keyserlingk M. A. G., The complex relationship between welfare and reproduction in cattle. Reprod. Domest. Anim. 54, 29–37 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Segner H., Sundh H., Buchmann K., Douxfils J., Sundell K. S., Mathieu C., Ruane N., Jutfelt F., Toften H., Vaughan L., Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 38, 85–105 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Broom D. M., The scientific assessment of animal-welfare. Appl. Anim. Behav. Sci. 20, 5–19 (1988). [Google Scholar]
- 73.Franks B., What do animals want? Anim. Welf. 28, 1–10 (2019). [Google Scholar]
- 74.Van Patter L. E., Blattner C., Advancing ethical principles for non-invasive, respectful research with nonhuman animal participants. Soc. Anim. 28, 171–190 (2020). [Google Scholar]
- 75.Ferdowsian H., Johnson L. S. M., Johnson J., Fenton A., Shriver A., Gluck J., A belmont report for animals? Camb. Q. Healthc. Ethics 29, 19–37 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Špinka M., Animal agency, animal awareness and animal welfare. Anim. Welf. 28, 11–20 (2019). [Google Scholar]
- 77.Rault J.-L., Hintze S., Camerlink I., Yee J., Positive welfare and the like: Distinct views and a proposed framework. Front. Vet. Sci. 7, 370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeates J. W., Main D. C. J., Assessment of positive welfare: A review. Vet. J. 175, 293–300 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Webb L. E., Veenhoven R., Harfeld J. L., Jensen M. B., What is animal happiness? Ann. N. Y. Acad. Sci. 1438, 62–76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mellor D. J., Updating animal welfare thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 6, 21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fife-Cook I., Franks B., Positive welfare for fishes: Rationale and areas for future study. Fishes 4, 31 (2019). [Google Scholar]
- 82.Sánchez-Suárez W., Franks B., Torgerson-White L., From land to water: Taking fish welfare seriously. Animals 10, 1585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Froese R., Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 22, 241–253 (2006). [Google Scholar]
- 84.H. F. Moed, Citation Analysis in Research Evaluation (Springer, 2005), vol. 9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/14/eabg0677/DC1