Abstract
It is known that volatile fatty acids can inhibit growth of species of the family Enterobacteriaceae in vitro. However, whether these volatile fatty acids affect bacterial populations in the ceca of chickens is unknown. Therefore, a study was conducted to investigate if changes in volatile fatty acids in ceca of broiler chickens during growth affect bacterial populations. Results showed that members of the Enterobacteriaceae and enterococci are present in large numbers in 3-day-old broilers and start to decrease when broilers grow older. Lactobacilli are present in large numbers as well in 3-day-old broilers, but they remain stable during the growth of broilers. Acetate, butyrate, and propionate increase from undetectable levels in 1-day-old broilers to high concentrations in 15-day-old broilers, after which they stabilize. Significant negative correlations could be calculated between numbers of Enterobacteriaceae and concentrations of undissociated acetate, propionate, and butyrate. Furthermore, pure cultures of Enterobacteriaceae isolated from the ceca were grown in the presence of volatile fatty acids. Growth rates and maximal optical density decreased when these strains grew in the presence of increasing volatile fatty acid concentrations. It is concluded that volatile fatty acids are responsible for the reduction in numbers of Enterobacteriaceae in the ceca of broiler chickens during growth.
Broiler chickens in commercial farmhouses in The Netherlands are raised for slaughter within 40 days. The microflora in the gastrointestinal tract of broiler chickens plays an important role during this period. Therefore, research has focused on the development of the microflora in the ceca of chickens during growth under laboratory conditions (1, 11, 20). In 0- to 4-day-old broilers, Enterobacteriaceae and enterococci are dominant in the ceca (11). Viable counts of these bacteria decrease in the ceca when broilers grow older, but this decrease seems to be dependent on the diet fed to the broilers (1, 20). The cause for this reduction in numbers of Enterobacteriaceae and enterococci is not known. Lactobacilli are present in large numbers in 2- to 4-day-old broilers and remain stable during the growth of broilers (1, 10, 11, 20). From 7 days of age, the obligate anaerobic microflora dominate the ceca of broilers (1, 10, 11, 19). One study reports that the dominant microflora consists mainly of gram-negative, nonsporing rods (1), while others report that these bacteria are gram-positive, nonsporing rods (11) or a mixture of these two types (19).
Treatment of 1-day-old broilers with cecal microflora from Salmonella-free adult chickens can protect the broilers from infection with Salmonella (14). Research in this area showed that significant reduction in Salmonella numbers in broilers' ceca can be obtained with undefined bacterial mixtures or defined mixtures containing a large number of different bacterial species (n = 25 to 35) isolated from the ceca of chickens (7, 23). Defined mixtures containing a low number of bacterial species (n = 1 to 3) caused a reduction in Salmonella numbers in the ceca of chickens (16). In contrast, others have seen no effect on Salmonella numbers when using defined mixtures with low numbers of bacterial species (22, 23). The exact mechanism behind reduction in Salmonella numbers by the protective cecal microflora is still unknown. Several mechanisms have been suggested: competition for nutrients, competition for receptor sites, immunomodulation, production of antimicrobial substances, or production of acetate, propionate, and butyrate (see reference 6 and references therein). Administration of different defined bacterial mixtures to 1-day-old broilers showed that the concentration of propionate in 3-day-old broilers correlated negatively with Salmonella numbers enumerated in the ceca of 10-day-old broilers (13). In the intestines of mice, increasing concentrations of butyrate were related to decreasing numbers of Enterobacteriaceae (9).
These studies suggest that volatile fatty acids (in particular acetate, propionate, and butyrate) may play a key role in the development of the microflora in the ceca of broiler chickens during growth. To our knowledge, correlations between volatile fatty acids and the development of the normal microflora have not been determined in chicken's ceca yet. Therefore, the objective of this study was to evaluate the role of volatile fatty acids in the establishment of the cecal microflora in broiler chickens reared in a commercial farmhouse. Correlations were calculated between cecal volatile fatty acid concentrations and numbers of different cecal bacterial groups. Furthermore, the effect of these acids on growth of bacterial strains isolated from the ceca was studied in vitro to support our correlation data.
MATERIALS AND METHODS
Chickens and management.
Two experiments with broiler chickens were conducted. In both experiments, broiler chickens were housed in a commercial farmhouse in Leusden, The Netherlands. In the first experiment, the flock contained 15,000 broilers (Ross breed). Three broiler chickens were obtained from this flock on days 10, 24, and 38. In the second experiment, the flock contained 17,000 broilers (Cobb breed). Three broiler chickens were obtained from this flock on days 1, 3, 5, 8, 12, 15, 29, and 37. In both experiments, water and feed were given ad libitum. All broilers received feed with growth-promoting antibiotics. From day 1 to day 10, feed contained nicarbazin (100 mg/kg) and zinc bacitracin (25 mg/kg); from day 11 to day 32, feed contained salinomycin (70 mg/kg) and zinc bacitracin (50 mg/kg); and from day 33 to day 40, feed contained only zinc bacitracin (50 mg/kg). Broilers were euthanized by cervical dislocation after transportation to the laboratory. The gastrointestinal tract was isolated, and the content of the cecum was sampled for bacteriological and volatile fatty acid analyses.
Bacteriological analysis.
Samples for bacteriological analysis were diluted with a reduced physiological salt solution (NaCl, 8 g liter−1; cysteine-HCl, 0.5 g liter−1) in an anaerobic cabinet (80% N2, 15% CO2, 5% H2). For viable counts of total aerobic bacteria, total anaerobic bacteria, lactobacilli, Enterobacteriaceae, enterococci, and Bacteroides spp., dilutions were spread plated on the appropriate selective agar plates and incubated as described by Snel et al. (21). Eubacterium spp. were counted on a selective agar medium as described by Terada et al. (24), in which Columbia blood agar was used as the base agar. Numbers of CFU are expressed as log CFU per gram. At day 37, five colonies from all selective agar plates were randomly selected, and after growth in liquid medium, they were confirmed as the specific bacterial group growing on the respective selective agar medium (12).
Volatile fatty acid and pH analyses.
Approximately 0.4 g of cecal material was resuspended in 1.6 ml of sterile milli-Q water. The pH of the samples was measured according to a procedure described previously (6), and the same samples were stored thereafter at −20°C. Concentrations of acetate, propionate, butyrate, and lactate were determined by high-performance liquid chromatography (HPLC). After thawing, samples were centrifuged (10 min at 18,500 × g) and 12.5 μl of a xylitol solution (0.586 M in 1.5 M HCl; internal standard) was added to 700 μl of sample. Subsequently, samples were centrifuged again (10 min at 18,500 × g) before analysis by HPLC. Twenty microliters of sample was injected into the HPLC with a Spark Holland autosampler (Emmen, The Netherlands). The HPLC was equipped with a model ERC-7510 refractive index detector (Erma Optical Works, Tokyo, Japan) and an organic acid column (30 cm by 6.5 mm) from Chrompack, Inc. (Bridgewater, Mass.). The column was operated at room temperature with 0.005 N H2SO4 at 0.6 ml/min as the eluent. The operating pressure was approximately 95 × 105 Pa.
Concentrations of undissociated volatile fatty acids and lactate were calculated with the Henderson-Hasselbach equation (pH = pKa + 10log[A−]/[HA]) (where A− is dissociated acids and HA is undissociated acids), pH values, total concentration of each volatile fatty acid and lactate, and the respective pKa of acetic (4.75), propionic (4.87), butyric (4.81), and lactic (3.08) acid under standard conditions (25).
In vitro assay.
Five strains of Enterobacteriaceae, four strains of Enterococcus, and one strain of Lactobacillus were randomly isolated from the selective agar plates on day 37 (Cobb breed experiment). Acetate, propionate, butyrate, and lactate were added to brain heart infusion (BHI) broth containing 0.5 g of cysteine-HCl liter−1 with the concentrations of volatile fatty acids equal to the concentration measured in the ceca of 3-, 5-, 8-, and 15-day-old broilers, respectively. The pH of these BHI solutions was adjusted with HCl to 5.8 as measured in the ceca of chickens (Cobb breed). These four BHI solutions and a control without volatile fatty acids (BHI broth, pH 5.8) were inoculated with overnight cultures (BHI broth, pH 5.8) of the 10 strains mentioned above and incubated anaerobically at 37°C. For 12 h, growth was monitored by measuring the change in optical density at 620 nm (OD620). All strains were tested in triplicate.
Data analysis.
Correlations between Enterobacteriaceae, lactobacilli, enterococci, Bacteroides spp., Eubacterium spp., and total and undissociated volatile fatty acids were calculated and analyzed statistically by Pearson's correlation with SPSS 7.5 software. We made the assumption in this statistical analysis that neither of the two variables tested for correlation is dependent on any other variable. Furthermore, we used the number of bacteria and the concentrations of volatile fatty acids measured in every individual chicken (n = 24) from the in vivo experiment with the Cobb breed.
RESULTS
In vivo experiments.
The repeatability of the in vivo experiments was tested by using two types of chicken breed (Ross and Cobb). Both experiments gave similar results for the viable counts of lactobacilli, Enterobacteriaceae, total aerobic count, total anaerobic count, enterococci, Bacteroides spp., and Eubacterium spp. (data not shown). The concentrations of acetate, butyrate, and propionate increased in the same manner in both experiments, and only the concentrations of propionate differed in the last 3 weeks of life of the broilers (±17 mM in the Ross breed and ±9 mM in the Cobb breed). Since the results from both experiments are very similar, it was decided to present only the results from the experiments with the Cobb breed in the rest of this paper.
Development of bacterial flora.
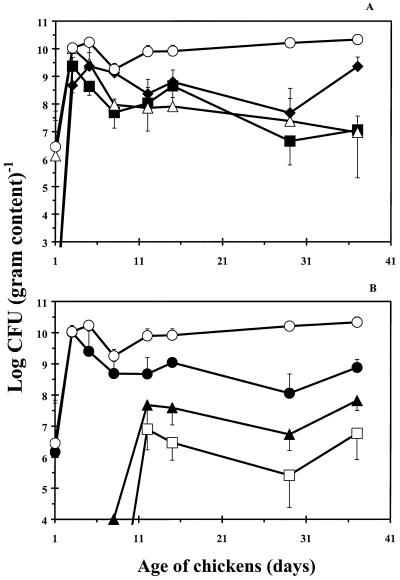
The development of the dominant microflora in the broilers' ceca is presented in Fig. 1. In the early life of broilers, Enterobacteriaceae, enterococci, and lactobacilli were the most important groups of bacteria in the ceca. After 3 days, CFU of Enterobacteriaceae and enterococci started to decrease until the broilers were 15 days old. Thereafter, their numbers stabilized. Viable counts of Bacteroides spp. and Eubacterium spp. were established after 2 weeks at stable levels of 7 and 6 log CFU g−1, respectively. These numbers were 3 to 4 log CFU g−1 lower than the total anaerobic number. This indicates that these groups were not the most dominant groups of obligate anaerobic bacteria. From day 12 onwards, total numbers of anaerobically grown bacteria were approximately 1 to 1.5 log CFU g−1 higher than those of the aerobic bacteria. Microscopic examination of the bacteria from colonies from the anaerobically incubated Columbia blood agar showed that most of these obligate anaerobes were gram-positive, Y-branched bacteria.
FIG. 1.
Numbers of total anaerobic bacteria (○), lactobacilli (⧫), Enterobacteriaceae (■), enterococci (▵), total aerobic bacteria (●), Bacteroides spp. (▴), and Eubacterium spp. (□) given as log CFU per gram in the ceca of broiler chickens during growth. Results are presented as the means of three chickens ± standard deviations.
pH, lactate, and volatile fatty acids.
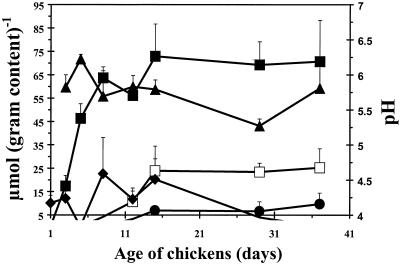
Cecal pH values were in the range of 5.5 to 6.0 during growth of broilers (Fig. 2). Acetate could be detected in the ceca of 3-day-old broilers, and concentrations increased until broilers were 15 days old. From this day on, the acetate concentration stabilized at 70 μmol g−1. Propionate and butyrate were detected in 12- to 15-day-old broilers, and these concentrations remained stable from day 15 onwards (propionate, 8 μmol g−1; butyrate, 24 μmol g−1). Lactate was detected during the first 15 days, and thereafter it could not be detected (Fig. 2).
FIG. 2.
Lactate (⧫), acetate (■), propionate (●), and butyrate (□) concentrations and pH (▴) in the ceca of broiler chickens during growth. Results are presented as the means of three chickens ± standard deviations.
Correlations between bacterial numbers and volatile fatty acids.
The correlations between lactate, acetate, propionate, or butyrate and bacterial numbers (log CFU per gram) and their significance (P < 0.05) were calculated (Table 1). In the ceca, significant negative correlations were observed between numbers of Enterobacteriaceae and acetate, as well as the undissociated form of acetate, propionate, and butyrate. Numbers of enterococci showed only a negative correlation with total and undissociated acetate. No significant correlations could be detected between numbers of lactobacilli and volatile fatty acids or among the different bacterial groups in the ceca (data not shown).
TABLE 1.
Statistical data for the correlations between bacterial numbers (log CFU per gram) and concentrations of lactate or volatile fatty acids in the ceca of broilers
| Volatile fatty acid concn (μmol g−1) | Statistical correlation fora:
|
||||
|---|---|---|---|---|---|
| Lactobacilli (P) |
Enterobacteriaceae
|
Enterococci
|
|||
| P | R | P | R | ||
| Total | |||||
| Lactate | NS | NS | NS | ||
| Acetate | NS | <0.05 | −0.593 | <0.05 | −0.765 |
| Propionate | NS | NS | NS | ||
| Butyrate | NS | NS | NS | ||
| Undissociated | |||||
| Lactate | NS | NS | NS | ||
| Acetate | NS | <0.05 | −0.712 | <0.05 | −0.610 |
| Propionate | NS | <0.05 | −0.609 | NS | |
| Butyrate | NS | <0.05 | −0.656 | NS | |
Results are presented as P values of <0.05 (significant) or >0.05 (NS, not significant) and R values for the significant correlations.
In vitro studies.
The correlations may suggest an effect of volatile fatty acids on the development of the microflora. To study this effect in more detail, we used an in vitro test system. Therefore, Enterobacteriaceae, enterococci, and a Lactobacillus strain were grown in the presence of volatile fatty acid concentrations, as determined on specific days, in ceca of broiler chickens (Fig. 3). These in vitro experiments show that strains of Enterobacteriaceae isolated from the chicken's ceca are more susceptible to volatile fatty acids than enterococci or lactobacilli. For Enterobacteriaceae, increasing concentrations of volatile fatty acids caused a gradual decrease in the maximal specific growth rate (μmax) and the OD after 12 h of growth (Fig. 3 and Table 2). Enterococcal strains were also affected by the volatile fatty acids, but this was mainly related to μmax, whereas the ODs after 12 h of growth were similar for all incubations. However, there was a reduction in OD after 12 h of growth in the presence of volatile fatty acids compared to control BHI broth. Furthermore, the relative decrease in μmax was less for enterococci than for Enterobacteriaceae (Table 2). Growth of the Lactobacillus strain was not reduced by the addition of volatile fatty acids.
FIG. 3.
Average growth curves of five strains of Enterobacteriaceae (A), four strains of Enterococcus (B), and one strain of Lactobacillus (C) in control BHI broth (⧫) and BHI broth with volatile fatty acid concentrations mimicking the ceca of 3-day-old (■), 5-day-old (□), 8-day-old (●), and 15-day-old (○) broilers. Results are presented as means of five different strains of Enterobacteriaceae and four different strains of Enterococcus ± standard deviations, as well as one strain of Lactobacillus. All strains were tested in triplicate.
TABLE 2.
Average μmax for strains of Enterobacteriaceae enterococci, and Lactobacillus growing in the presence of volatile fatty acids
| Mediuma | Result forb:
|
|||||
|---|---|---|---|---|---|---|
|
Enterobacteriaceae(n = 5)
|
Enterococci (n = 4)
|
Lactobacillus(n = 1)
|
||||
| μmax (h−1) | % of control μmax | μmax (h−1) | % of control μmax | μmax (h−1) | % of control μmax | |
| Control | 0.77 | 100 | 0.56 | 100 | 0.26 | 100 |
| Day 3 | 0.59 | 76.6 | 0.44 | 80.0 | 0.32 | 123 |
| Day 5 | 0.34 | 44.2 | 0.45 | 80.4 | 0.35 | 135 |
| Day 8 | 0.23 | 29.9 | 0.37 | 66.1 | 0.33 | 127 |
| Day 15 | 0.15 | 19.5 | 0.31 | 55.4 | 0.33 | 127 |
The control is BHI broth (pH 5.8); days 3, 5, 8, and 15 are BHI broth (pH 5.8) with volatile fatty acids added at the concentrations measured in the ceca of 3-, 5-, 8-, and 15-day-old broilers, respectively.
All strains were tested in triplicate.
DISCUSSION
The trends observed for the development of the microbial groups in the ceca of broiler chickens during growth in a commercial farmhouse were similar to the development observed for broiler chickens reared under laboratory conditions (1, 11). In contrast, our study shows that numbers of lactobacilli, Enterobacteriaceae, and enterococci are higher in the ceca than those in a previous study (1). We observed gram-positive, Y-branched bacteria to be the dominant microflora in the ceca of 15- to 40-day-old chickens. These observations are in agreement with those of the study of Ochi et al. (15), whereas others found gram-negative or gram-positive straight, nonsporing rods to be the dominant anaerobes in the ceca of broilers (1, 11, 19). The reasons for these differences are unknown, but may be explained by the differences in rearing conditions, chicken breed, diet, or bacterial enumeration methods used in these studies.
This study shows the presence of high concentrations of acetate, propionate, and butyrate in the ceca. High concentrations of volatile fatty acids are indicative that fermentations by obligate anaerobic bacteria are important (2, 9). This is in agreement with the 10- to 50-times-higher number of anaerobic bacteria than aerobic bacteria observed in our study. The concentrations of volatile fatty acids in this study are similar to results obtained in other studies (2, 6). However, those studies focused on volatile fatty acids alone and not on the microflora. Therefore, correlations were calculated between volatile fatty acids and the log CFU of different bacterial groups per gram. A significant negative correlation was observed between Enterobacteriaceae and acetate and the undissociated form of acetate, propionate, and butyrate. We made the assumption with our statistical correlation analysis that the two variables used (bacterial numbers against concentrations of volatile fatty acids) are independent of any other variable. However, the decrease in numbers of Enterobacteriaceae can be dependent on many other variables (e.g., competition for attachment sites, competition for substrates, immunomodulation, and production of antimicrobial substances). Similarly, the production of acetate is dependent on other variables (e.g., bacterial metabolism). Therefore, it remains uncertain whether these significant correlations are influenced by other variables and thus if these significant correlations are causal. However, they may be an indication that the undissociated form of volatile fatty acids reduced the numbers of Enterobacteriaceae in vivo. This hypothesis is supported by a proposed mechanism for volatile fatty acid toxicity. That mechanism states that the undissociated form of these acids can diffuse freely across the bacterial membrane into the cell. Inside the bacterial cell, the acid dissociates, thereby reducing the internal pH, which will cause internal cell damage. However, the anion itself may damage the cell as well (3, 5, 18).
Reports concerning correlations between volatile fatty acids and Enterobacteriaceae have mainly focused on the intestines of mice (4, 8, 9, 17). Unfortunately, in these reports, some contradictory results have been shown. In some of the studies, it was observed that higher concentrations of butyrate (9) or total volatile fatty acids (4) are related to reduced numbers of Enterobacteriaceae, but the correlations and their significance have not been calculated. Furthermore, pH values were not shown, and therefore correlations between undissociated volatile fatty acids and Enterobacteriaceae cannot be deduced. In contrast, Freter and Abrams (8) did not observe any relationship between volatile fatty acids and Enterobacteriaceae in mice. The pH values for the cecum of mice in their study ranged from 6.5 to 7.0. At these pH values, the concentrations of undissociated volatile fatty acids are very low. This could be an explanation for the absence of a correlation between volatile fatty acids and Enterobacteriaceae. In our study, pH values are around 5.5 to 6.0, resulting in 10 times as much of the dissociated acid at the same total volatile fatty acid concentration. This might very well result in the significant correlations we observed in the ceca of chickens, in contrast to what was observed in the cecum of mice.
Further evidence that the significant correlations observed are causal came from our in vitro assay. In this assay, the situation in the cecum was mimicked by adding volatile fatty acids to BHI broth at concentrations and a pH value corresponding to the pH and concentrations on a specific day of age of the broilers. Under these conditions, the OD and the growth rate of five strains of Enterobacteriaceae decreased. This strongly suggests that undissociated concentrations of volatile fatty acids are responsible for the in vivo reduction in numbers of Enterobacteriaceae in the ceca of broilers. Enterococcal strains are less affected by the volatile fatty acids, since the OD was not affected and the decrease in μmax was not as pronounced as with the strains of Enterobacteriaceae. This could be the reason that we found a significant negative correlation only between acetate and numbers of enterococci. It should be noted, however, that volatile fatty acids are not necessarily the only mechanism behind the reduction of Enterobacteriaceae and enterococci. The Lactobacillus strain was not affected during growth in BHI broth with volatile fatty acids, which is in agreement with the lack of correlations between lactobacilli and concentrations of volatile fatty acids in the ceca of broiler chickens.
One of the mechanisms by which the intestinal microflora may reduce Enterobacteriaceae (including Salmonella) is the bacteriostatic effect of volatile fatty acids in the ceca (6). The data from our study are the first to show that volatile fatty acids are one of the mechanisms responsible for the decrease in numbers of Enterobacteriaceae in the ceca of broiler chickens during growth. This result is not only important for understanding the interactions occurring in the ceca of broiler chickens, but can also be useful in studies focusing on reduction of Salmonella numbers in the ceca of broiler chickens by using competitive exclusion cultures.
ACKNOWLEDGMENTS
We thank David Keuzenkamp for technical assistance, Paul Westers from the Centre for Biostatistics for advice on the statistical analysis, and Len Lipman and Jos Huis in 't Veld for critically reading the manuscript.
REFERENCES
- 1.Barnes E M, Mead G C, Barnum D A, Harry E G. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci. 1972;13:311–326. doi: 10.1080/00071667208415953. [DOI] [PubMed] [Google Scholar]
- 2.Barnes E M, Impey C S, Stevens B J H. Factors affecting the incidence and anti-Salmonella activity of the anaerobic cecal flora of the young chick. J Hyg. 1979;82:263–283. doi: 10.1017/s0022172400025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bearson S, Bearson B, Foster J W. Acid stress responses in enterobacteria. FEMS Microbiol Lett. 1997;147:173–180. doi: 10.1111/j.1574-6968.1997.tb10238.x. [DOI] [PubMed] [Google Scholar]
- 4.Byrne B M, Dankert J. Volatile fatty acids and aerobic flora in the gastrointestinal tract of mice under various conditions. Infect Immun. 1979;23:559–563. doi: 10.1128/iai.23.3.559-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherrington C A, Hinton M, Chopra I. Effects of short-chain organic acids on macromolecular synthesis in Escherichia coli. J Bacteriol. 1990;68:69–74. doi: 10.1111/j.1365-2672.1990.tb02550.x. [DOI] [PubMed] [Google Scholar]
- 6.Corrier D E, Hinton A, Jr, Ziprin R L, DeLoach J R. Effect of dietary lactose on Salmonella colonization of market-age broiler chickens. Avian Dis. 1990;34:668–676. [PubMed] [Google Scholar]
- 7.Corrier D E, Nisbet D J, Scanlan C M, Hollister A G, DeLoach J R. Control of Salmonella typhimurium colonization in broiler chicks with a continuous-flow characterized mixed culture of cecal bacteria. Poult Sci. 1995;74:916–924. doi: 10.3382/ps.0740916. [DOI] [PubMed] [Google Scholar]
- 8.Freter R, Abrams G D. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun. 1972;6:119–126. doi: 10.1128/iai.6.2.119-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee A, Gemmell E. Changes in the mouse intestinal microflora during weaning: role of volatile fatty acids. Infect Immun. 1972;5:1–7. doi: 10.1128/iai.5.1.1-7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mead G C. Bacteria in the intestinal tract of birds. In: Mackie R I, White B A, Isaacson R E, editors. Gastrointestinal microbiology. 2. Gastrointestinal microbes and host interactions. New York, N.Y: Chapman & Hall; 1997. pp. 216–240. [Google Scholar]
- 11.Mead G C, Adams B W. Some observations on the cecal microflora of the chick during the first two weeks of life. Br Poult Sci. 1975;16:169–176. doi: 10.1080/00071667508416174. [DOI] [PubMed] [Google Scholar]
- 12.Mossel D A A, Jacobs-Reitsma W F, editors. Microbiologisch onderzoek van levensmiddelen. Strategie, principes en methoden. 3rd ed. Zeist, The Netherlands: Uitgeverij P.C. Noordervliet B.V.; 1990. [Google Scholar]
- 13.Nisbet D J, Corrier D E, Ricke S C, Hume M E, Byrd II J A, DeLoach J R. Cecal propionic acid as a biological indicator of the early establishment of a microbial ecosystem inhibitory to Salmonella in chicks. Anaerobe. 1996;2:345–350. [Google Scholar]
- 14.Nurmi E, Rantala M. New aspects of Salmonella infection in broiler production. Nature. 1973;241:210–211. doi: 10.1038/241210a0. [DOI] [PubMed] [Google Scholar]
- 15.Ochi Y, Mitsuoka T, Sega T. Untersuchungen über die Darmflora des Huhnes. III. Mitteilung: Die Entwicklung der Darmflora von Küken bis zum Huhn. Zentbl Bakteriol Parasitenkd Abst 1. 1964;193:80–95. [PubMed] [Google Scholar]
- 16.Pascual M, Hugas M, Badiola J I, Monfort J M, Garriga M. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl Environ Microbiol. 1999;65:4981–4986. doi: 10.1128/aem.65.11.4981-4986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pongpech P, Hentges D J. Inhibition of Shigella sonnei and enterotoxigenic Escherichia coli by volatile fatty acids in mice. Microbiol Ecol Health Dis. 1989;2:153–161. [Google Scholar]
- 18.Russel J B, Diez-Gonzalez F. The effects of fermentation acids on bacterial growth. Adv Microb Physiol. 1998;39:205–234. doi: 10.1016/s0065-2911(08)60017-x. [DOI] [PubMed] [Google Scholar]
- 19.Salanitro J P, Blake I G, Muirhead P A. Studies on the cecal microflora of commercial broiler chickens. Appl Microbiol. 1974;28:439–447. doi: 10.1128/am.28.3.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro S K, Sarles W B. Microorganisms in the intestinal tract of normal chickens. J Bacteriol. 1949;58:531–544. doi: 10.1128/jb.58.4.531-544.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snel J, Van den Brink M E, Bakker M H, Poelma F G J, Heidt P J. The influence of indigenous segmented filamentous bacteria on small intestinal transit in mice. Microbiol Ecol Health Dis. 1996;9:207–214. [Google Scholar]
- 22.Soerjardi A S, Stehman S M, Snoeyenbos G H, Weinack O M, Smyser C F. The influence of lactobacilli on the competitive exclusion of paratyphoid salmonellae in chickens. Avian Dis. 1981;25:1027–1033. [PubMed] [Google Scholar]
- 23.Stavric S. Defined cultures and prospects. Int J Food Microbiol. 1992;15:245–263. doi: 10.1016/0168-1605(92)90056-9. [DOI] [PubMed] [Google Scholar]
- 24.Terada A, Hara H, Sakamoto J, Sato N, Takagi S, Mitsuoka T, Mino R, Hara K, Fujimori I, Yamada T. Effects of dietary supplementation with lactosucrose (4G-β-D-galactosylsucrose) on cecal flora, cecal metabolites, and performance in broiler chickens. Poult Sci. 1994;73:1663–1672. doi: 10.3382/ps.0731663. [DOI] [PubMed] [Google Scholar]
- 25.Weast R C, editor. CRC handbook of chemistry and physics. 52nd ed. Cleveland, Ohio: Chemical Rubber Co.; 1971. [Google Scholar]