Abstract
Background
The objective of this study is to assess the risk of exposure of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines (HCAs) in meat and fish-based products marketed in Malaysia using the margin of exposure (MOE) approach.
Methods
Benchmark Dose (BMD) software was used to model the BMD at a lower end of a one-sided 95% confidence interval with a 10% incremental risk (BMDL10) of PAHs and HCAs from different target organ toxicities. The MOEs of PAHs and HCAs in meat and fish-based products were determined by utilising the calculated BMDL10 values and estimated daily intake of meat and fish-based products from published data.
Results
The calculated BMDL10 values of PAHs (i.e. benzo[a]pyrene [BaP] and fluoranthene [FA]) and HCAs (i.e. 2-amino-3,8,dimethylimidazo[4,5-f]quinoxaline [MeIQx] and 2-amino-1-methyl-6-phenylimidazo[4,5,6]pyridine [PhIP]) ranged from 19 mg/kg bw/day to 71,801 mg/kg bw/day. The MOE of BaP ranged from 41,895 to 71,801 and that of FA ranged from 19 to 1412. As for MeIQx and PhIP, their MOEs ranged from 6,322 to 7,652 and from 2,362 to 14,390, respectively.
Conclusion
The MOEs of FA, MeIQx and PhIP were lower than 10,000, indicating a high concern for human health and therefore demanding effective risk management actions.
Keywords: risk assessment, polycyclic aromatic hydrocarbons, heterocyclic amines, margin of exposure, Benchmark Dose lower confidence limit
Introduction
Meat and fish are subjected to various heat treatments such as roasting, grilling, barbecuing and frying (1). Thermal treatment of meat may generate some undesired compounds, such as food-borne toxicants, despite increasing palatability and reducing microbiological risks (2). During the heat processing stage, the Maillard reaction, thermal decompositions and lipid oxidation reactions are essential chemical transformations that generate the building blocks or precursors of potential toxicants from carbohydrates, amino acids and lipids (3–5). Heterocyclic aromatic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs) are the two thermally generated food toxicants significantly formed during the thermal treatment of meat at high temperatures (6, 7). HCAs, highly mutagenic and potentially carcinogenic by-products, form during Maillard browning reactions, specifically in muscle-rich foods (8–10). With accumulating evidence, the International Agency for Research on Cancer (IARC) (11) has classified 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) as Group 2A (probable human carcinogens) and MeIQ, MeIQx, DiMeIQx and PhIP as Group 2B (possible human carcinogens).
Conversely, PAHs can be formed through the incomplete combustion or pyrolysis (burning) of organic components, including fat, protein and carbohydrates, at a temperature above 200 °C, especially above 400 °C (12, 13). PAHs can be generated in the smoke produced when lipids are dropped onto flames. Consequently, known sources of PAH contamination in thermally treated proteinaceous foods are deposited on the food surface (14). The IARC has classified benzo[a]pyrene (BaP) as a human carcinogen (Group 1) and therefore, exposure to PAHs is a significant health concern (15). Consumption of grilled red meat increases the risk of intestine, breast, bladder, prostate and pancreas cancers, as reported in various epidemiological studies (16).
Risk assessment involves identifying, analysing and characterising a food-related health risk. It estimates the likelihood and severity of an adverse health effect from exposure to a hazard (17). Human exposure studies demonstrated that the magnitude of BaP dietary exposures is 2 ng/day–500 ng/day, which supersedes inhalation exposure of 10 ng/day–50 ng/day (18). Globally, the estimated average intakes of PAHs range from 0.02 μg/person/day to 3.6 μg/person/day, and in countries like India, Nigeria and China, the estimated average intakes of PAHs are 11 μg/person/day, 6 μg/person/day and 3.56 μg/person/day, respectively (19). Jahurul et al. (20) analysed three high-molecular-weight PAHs, namely, fluoranthene (FA), benzo[b]fluoranthene and BaP, in 42 types of meat and fish-based products widely consumed by the Malaysian population. The researchers estimated that the mean dietary intake of the sum of three PAHs was 297.58 ng/day. Earlier, the same authors determined the concentration of six predominant HCAs in meat and fish-based products and reported that the mean dietary intake of HCAs was 553.7 ng/day (21). In addition, the margin of exposure (MOE) approach is used to consider possible safety concerns arising from the presence of toxicants in food that is both genotoxic and carcinogenic. Kirkland et al. (22) updated the recommended lists of genotoxic and non-genotoxic chemicals for the assessment of the performance of genotoxicity tests and stated that PAHs (especially BaP) and HCAs (especially IQ) are both genotoxic and carcinogenic. However, the risk assessment of PAHs and HCAs reported in literature (12, 20, 21) was conducted quantitatively. Benford et al. (23) suggested qualitative risk assessment using the MOE approach for genotoxic and carcinogenic substances such as PAHs and HCAs. Barlow et al. (24) stated that MOE is the ratio of benchmark dose (BMD) at a lower end of a one-sided 95% confidence interval with a 10% incremental risk (BMDL10) supporting the estimated dose. In general, a MOE of 10,000 or higher would be of low concern from a public health point of view if it is based on BMDL10 from an animal study and if the overall uncertainties in the interpretation are taken into account (25). A greater number of MOEs represent a lesser probability of causing risk from exposure to a compound (23).
BMD modelling is the state of the science for determining the point of departure for risk assessment. The modelling accounts for all of the data for a particular effect from a particular experiment, increased consistency and better accounting for statistical uncertainties (26). The European Food Safety Authority (EFSA) Scientific Committee (2012) reiterated that an effective and practical method to assess the risk of genotoxic and carcinogenic substances is by MOE and agreed that BMD acts as a better practice that signifies the point of departure in the observable dose–response range (24). BMD is the dose that signifies a low but calculable response, with a lower confidence limit of 95%, which is identified as BMDL (27).
Studies on human exposure to PAHs and HCAs in meat and fish-based products are limited. Most of existing literature has mainly reported limited studies of PAHs or HCAs and human exposure separately (12, 20, 21). This establishes a knowledge gap due to the insufficient details that reported human exposure to both PAHs and HCAs in meat and fish-based products that are widely consumed by the Malaysian population. Therefore, this research was conducted to model the BMDL10 of PAH and HCA using BMD software and to calculate the MOEs of PAHs and HCAs by utilising the modelled BMDL10.
Methods
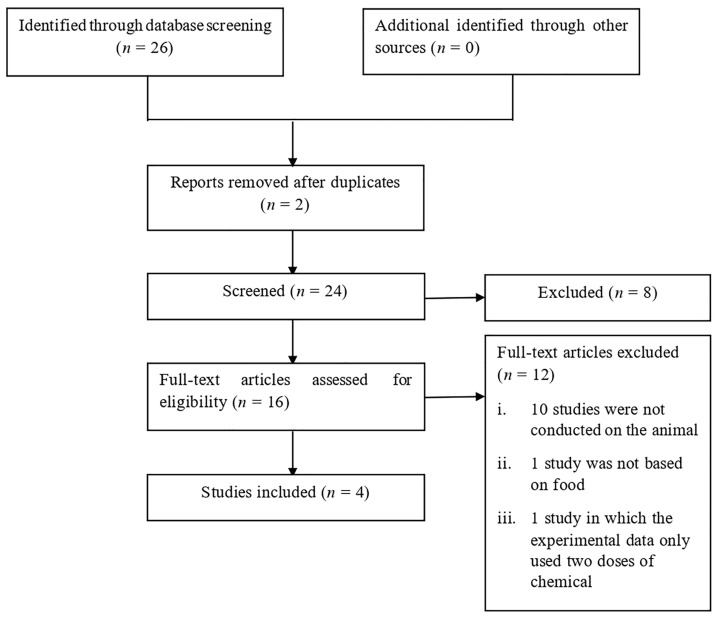
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to aid the collection of relevant articles. PRISMA is a systematic review and meta-analysis that contains 27 checklists and four-phase flow diagrams, which assist an author in making a better report. This method was carried out by reviewing reports where data from various studies were extracted to attain the aim of this study: to calculate the MOEs of PAHs and HCAs by utilising the BMDL10 data. The executed systematic review was specified in animal studies on the toxicity of PAHs and HCAs in meat and fish-based products that include the amount of the chemical used, type of animals used, number of animals used, duration of the study and type of cell study. Studies involving humans were excluded as there was no sufficient amount of data reported.
Eight electronic databases were used (Google Scholar, ScienceDirect, Research Gate, BMC Cancer, Taylor & Francis Online, Oxford Academic, Springer Link and Wiley Online Library) to search for previous studies from September 1986 to March 2019 using the following search terms: (Margin of Exposure OR MOE) AND (rats OR mouse OR mice OR animal) AND (chicken OR poultry OR meat OR fish OR Polycyclic Aromatic Hydrocarbons OR PAHs OR Heterocyclic Aromatic Amines OR HCAs). Broad search terms were used to avoid overlooking in any publications. The search was restricted only to the English language. After the first screening, the articles were assessed for acceptance and a few were excluded because of the following reasons: i) the studies were not conducted on animals, ii) the studies were not based on food and iii) the experimental data only used two doses of chemical (Figure 1). A sheet containing extracted data was revised to ensure that all essential information was included and sufficient. The extracted data were then used to model BMDL10 using BMD Software version 3.1.2 (https://www.epa.gov/). BMD modelling displayed data of BMD10, BMDL10, Akaike information criterion (AIC), BMD software recommendation and BMD software notes that were used in calculating MOE. Data on the lowest and highest BMDL10 values from each compound were extracted.
Figure 1.
Study selection for inclusion in systematic review
The MOE was calculated by dividing BMDL10 by the estimated dietary intake (EDI) of the food for human consumption. The present study used the EDI of the sum of three PAHs and the six predominant HCAs in meat and fish-based products reported by Jahurul et al. (20) and Jahurul et al. (21), respectively. These studies reported the dietary exposure of PAHs and HCAs in meat and fish-based products among the adult Malaysian population.
Results
In the present study, PRISMA was used to collect articles that reported studies on the toxicity of PAHs and HCAs in animals from 1993 to 2013. A total of four published journals were selected (28–31). Subsequently, BMD software was used to model the BMDL10 of four different compounds, i.e. FA, benzo[a]pyrene (BaP), 2-amino-3,8,dimethylimidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo[4,5,6]pyridine (PhIP). Table 1 shows the BMDL10 value of the recommended model, P-value and AIC of different types of PAHs and HCAs of different genders, duration of administration and target organs. The BMD analysis was conducted using default settings based on the assumption of equal potency of the selected PAHs and HCAs. The results showed that the BMDL10 of BaP (2.90 mg/kg bw/day) in females with liver as the target organ was the highest. As for PhIP, the BMDL10 of PhIP in males with colon as the target organ was the highest (1.40 mg/kg bw/day).
Table 1.
Results from a BMD analysis of the data for different organs exposed to different type of PAHs and HCAs using BMD software version 3.1.2, a BMD of 10% and default settings based on assumption of equal potency of the selected PAHs and HCAs
| Chemical | Gender | Duration | Type of disease | Model | BMDL10 (unit) | P-value | AIC |
|---|---|---|---|---|---|---|---|
| Fluoranthene (FA) | Ma | 6 months | Lung adenoma | Logistic | 0.175 | 0.46 | 47.03 |
| Fa | Gamma | 0.17 | 0.70 | 48.25 | |||
| Ma | 9 months | Dichotomous Hill | 0.00 | 65535 | 68.08 | ||
| Fa | – | – | – | – | |||
| Ma | 6 months | Lung adenocarcinoma | Gamma | 0.21 | 0.55 | 25.20 | |
| Fa | Weibull | 0.52 | 1.00 | 2.00 | |||
| Ma | 9 months | Logistic | 0.20 | 0.84 | 27.01 | ||
| Fa | Logistic | 0.29 | 0.99 | 15.77 | |||
|
| |||||||
| Benzo[a]pyrene (BaP) | Mb | 104 weeks | Stomach cancer | – | – | – | – |
| Fb | Quantal linear | 2.68 | 0.99 | 147.33 | |||
| Mb | Liver cancer | Log-logistic | 2.36 | 0.51 | 127.85 | ||
| Fb | Multistage degree 3 | 2.90 | 0.99 | 92.25 | |||
|
| |||||||
| 2-amino-3,8,dimethylimidazo[4,5-f]quinoxaline (MeIQx) | Mc | 56 weeks | Liver cancer | Weibull | 1.12 | 1.00 | 50.32 |
|
| |||||||
| 2-amino-1-methyl-6-phenylimidazo[4,5,6]pyridine (PhIP) | Md | 104 weeks | Mammary cancer | – | – | – | – |
| Fd | Logistic | 0.77 | 0.75 | 124.42 | |||
| Md | Mammary cancer | Quantal linear | 0.48 | 0.98 | 94.59 | ||
| Fd | Quantal linear | 1.28 | 0.30 | 73.80 | |||
| Md | Colon cancer | Weibull | 1.40 | 1.00 | 43.05 | ||
| Fd | Dichotomous hill | 0.82 | 0.99 | 27.56 | |||
The highest and lowest modelled BMDL10 values were used to calculate a range of MOEs. The BMDL10 values on each compound are tabulated in Table 2 based on gender, duration and target organ. The BMDL10 of a male administrated with FA for 9 months that had been recognised to have lung adenoma had the lowest BMDL10, which was 0.01 mg/kg bw/day. In comparison, the highest BMDL10 value (2.90 mg/kg bw/day) was found in a female with colon cancer administrated with PhIP. FA had only been detected to cause tumourigenicity in the lungs.
Table 2.
The highest and lowest value of BMDL10 (mg/kg bw/day) of different types of PAHs and HCAs on different genders at different durations of exposure
| Chemical | Fluoranthene | Benzo[a]pyrene | MeIQx | PhIP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Ma | Fa | Ma | Fa | Mb | Fb | Mc | Md | Fd | |
|
|
||||||||||
| Duration | 6 months | 9 months | 104 weeks | 56 weeks | 104 weeks | |||||
| Lung adenoma | Highest BMDL10 | 0.17 | 0.22 | 0.05 | - | - | - | - | - | - |
| Lowest BMDL10 | 0.08 | 0.09 | 0.01 | - | - | - | - | - | - | |
|
| ||||||||||
| Lung adenocarcinoma | Highest BMDL10 | 0.30 | 0.52 | 0.19 | 0.29 | - | - | - | - | - |
| Lowest BMDL10 | 0.16 | 0.52 | 0.12 | 0.25 | - | - | - | - | - | |
|
| ||||||||||
| Stomach cancer | Highest BMDL10 | - | - | - | - | - | 2.68 | - | - | - |
| Lowest BMDL10 | - | - | - | - | - | 1.69 | - | - | - | |
|
| ||||||||||
| Liver cancer | Highest BMDL10 | - | - | - | - | 2.42 | 2.90 | 1.33 | - | - |
| Lowest BMDL10 | - | - | - | - | 2.03 | 2.56 | 1.09 | - | - | |
|
| ||||||||||
| Mammary cancer | Highest BMDL10 | - | - | - | - | - | - | - | 0.77 | |
| Lowest BMDL10 | - | - | - | - | - | - | - | - | 0.55 | |
|
| ||||||||||
| Leukemia | Highest BMDL10 | - | - | - | - | - | - | - | 0.95 | 2.01 |
| Lowest BMDL10 | - | - | - | - | - | - | - | 0.48 | 1.28 | |
|
| ||||||||||
| Colon cancer | Highest BMDL10 | - | - | - | - | - | - | - | 1.91 | 2.90 |
| Lowest BMDL10 | - | - | - | - | - | - | - | 0.96 | 0.82 | |
Table 3 shows the type of compound and the estimated daily intake (mg/kg bw/day) of each compound through processed and cooked meat and fish-based products. The EDI was adapted from a published journal (20, 21) that includes a list of Malaysian dishes. BaP had the lowest EDI whereas PhIP had the highest, which were 0.000040450 mg/kg bw/day and 0.000201836 mg/kg bw/day, respectively.
Table 3.
The daily intake (mg/kg bw/day) of PAHs (FA and BaP) and HCAs (MeIQx and PhIP) through processed and cooked meat and fish-based products
| Chemical | Daily intake (mg/kg bw/day) | |
|---|---|---|
| PAHs | Fluoranthene | 0.000365330 |
| Benzo[a]pyrene | 0.000040450 | |
| HCAs | MeIQx | 0.000173406 |
| PhIP | 0.000201836 | |
Table 4 shows the MOEs of two PAH compounds and two HCA compounds. The MOE of FA on both lung adenoma and lung adenocarcinoma was the lowest, which ranged from 19 to 1,412 and the MOE of BaP was the highest, ranging from 41,895 to 71,801. Researchers suggested that a MOE value lower than 10,000 indicates a high concern regarding causing adverse health effects (23). Hence, as shown in Table 4, FA, MeIQx and PhIP had a high concern regarding causing toxicity whereas BaP had a low concern. The MOE of FA on lung adenoma was much lower than that on lung adenocarcinoma.
Table 4.
MOE of PAHs (FA and BaP) and HCAs (MeIQx and PhIP) based on their daily intake in processed and cooked meat and fish-based products, and the highest and lowest BMDL10
| Type of disease | Chemical | |||
|---|---|---|---|---|
|
| ||||
| Fluoranthene | Benzo[a]pyrene | MeIQx | PhIP | |
| Lung adenoma | 19*–614* | - | - | - |
| Lung adenocarcinoma | 328*–1412* | - | - | - |
| Stomach cancer | - | 41895–66185 | - | - |
| Liver cancer | - | 50118–71801 | 6322*–7652* | - |
| Mammary cancer | - | - | - | 2711*–3797* |
| Leukemia | - | - | - | 2362*–9969* |
| Colon cancer | - | - | - | 4045*–14390 |
Note:
The MOE that is lower than 10,000 indicate its possible toxicity towards human health
Discussion
This study assessed the risk of PAHs and HCAs in meat and fish-based products marketed in Malaysia using the MOE approach and utilising the calculated BMDL10 values and estimated daily intake of meat and fish-based products from published data. The calculated BMDL10 value of BaP (2.90 mg/kg bw/day) in females with liver as the target organ was the highest. In addition, the highest BMDL10 of PhIP (1.40 mg/kg bw/day) was determined in males with colon as the target organ. The BMD software provided the P-value and AIC of the BMDL10 of the recommended model. Haber et al. (32) stated that besides the best fit of the P-value, AIC was also applied to differentiate the model outputs from the dataset as an additional guideline in considering the best fit. US EPA also explained the two-step model selection process. The goodness of fit P-value, visual fit and scaled residuals were the criteria that primarily need to be considered in identifying the accepted models. In addition, the model was selected among the acceptable fit left. If the estimated BMDL from each model was undoubtedly close, then the one with the lowest AIC could be used (32). However, Haber et al. (32) also stated that the recommended value of AIC had not been reported or suggested. Hence, other measures were considered, instead of choosing only based on AIC, such as P-value, scaled residuals, the visual fit and the evaluation of model influence. At the same time, there were a few sets of data that could not be modelled. The BMD cannot be computed on account of the inconsistency of raw data from a published paper. Haber et al. (32) claimed that a few older research papers might only address observed effects, excluding the incidence data. Some studies might state the mean values and not the overall sets of data that are required to model continuous data. Although these data were not relevant to use, perhaps there were no other choices that could be used to carry out the risk assessment (32).
The BaP BMDL10 values that ranged from 1.69 mg/kg bw/day to 2.90 mg/kg bw/day were among the highest. Benford et al. (23) also reported that BMD and BMDL modelling shows higher values on BaP from data of in vivo studies. The BMDL10 values in females were much higher than those in males in most types of diseases, except for mammary cancer. Majek et al. (33) reported that the differences between males and females could be affected by sex hormones. Also reported that colon cancer is one of the illnesses strongly affected by gender, with the incidence rates of males being higher than females (34).
BaP had the lowest EDI at 0.000040450 mg/kg bw/day, whereas PhIP had the highest at 0.000201836 mg/kg bw/day. Oz et al. (35) claimed that MeIQx and PhIP are the most common HCAs produced in meat products. In addition, the quantity and frequency of the food consumed might affect human exposure to HCAs (35). BaP was shown to induce stomach and liver cancer (36). In 2006, FAO/WHO claimed that BaP causes tumours of the gastrointestinal tract, liver, lungs, mammary glands and other tissues. Even though BaP has been known to be a carcinogenic PAH marker in food, this compound often cannot be identified in food (16). This might be because each PAH has different metabolism steps or a mixture of PAHs may alter the pathways and their target organ (23).
FA, MeIQx and PhIP had a high concern regarding causing toxicity because their MOE values were less than 10,000. The MOE values of PAHs (i.e. FA) that were less than 10,000 are consistent with those from different researchers (37) who conducted a risk assessment of PAH4 in grilled meat and fish in Turkey (37), Baltic states (38) and Denmark (39). However, the MOE values of HCAs (i.e. MeIQx and PhIP) that were less than 10,000 are in contrast with those from Lee et al. (40), who reported that the MOE of PhIP based on a Korean total diet study was 2,349,000. Manan et al. (41) stated that the difference in exposure may be caused by variations in meal patterns, economic growth, culture, lifestyle and eating habits. Moreover, Pouzou et al. (42) performed a probabilistic assessment of dietary exposure to heterocyclic amines and PAHs from the consumption of meats and bread in the United States but did not calculate the MOE values.
The MOE value of MeIQx in liver cancer ranged from 6,322 to 7,652 which indicates high concern regarding causing toxicity in humans. Zimmerli et al. (43) claimed that the mutagenicity of MeIQx in the liver was related to the large dose range. The researchers (43) also stated that the liver is the primary target organ of most HCA compounds, except for PhIP and MeIQ. In Table 4, PhIP, with the lowest value, was for mammary cancer. It was supported by Zimmerli et al. (43), who stated that the PhIP compound causes tumours in the mammary glands of female rats and causes prostate carcinomas in male rats.
Conclusion
In the present study, the MOEs of PAHs and HCAs in meat and fish-based products consumed by the adult Malaysian population were calculated by utilising the modelled BMDL10 and EDI. The MOE of BaP was higher than 10,000, which indicates that BaP can be considered a low concern. FA, MeIQx and PhIP had MOEs lower than 10,000, which show that these three compounds are of high concern and are a priority for risk management actions.
Acknowledgements
None.
Footnotes
Conflict of Interest: None.
Funds: The authors would like to thank the Ministry of Education of Malaysia for the financial support (Project no. FRGS/1/2020/STG04/UPM/02/16 and UPM/800-4/11/MRUN/2019/5539123).
Authors’ Contributions: Conception and design: NIAL, RA, MS
Analysis and interpretation of the data: NIAL, RA, MS
Drafting of the article: NIAL, MS
Critical revision of the article for important intellectual content: RA, SO, MS
Final approval of the article: RA, SO, MS
Provision of study materials or patients: NIAL, MS
Statistical expertise: NIAL, RA, MS
Obtaining funding: MS
References
- 1.Uran H, Gokoglu N. Effects of cooking methods and temperatures on nutritional and quality characteristics of anchovy (Engraulis encrasicholus) J Food Sci Technol. 2014;51(4):722–728. doi: 10.1007/s13197-011-0551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meurillon M, Engel E. Mitigation strategies to reduce the impact of heterocyclic aromatic amines in proteinaceous foods. Trends Food Sci Technol. 2016;50:70–84. doi: 10.1016/j.tifs.2016.01.007. [DOI] [Google Scholar]
- 3.Tamanna N, Mahmood N. Food processing and maillard reaction products: effect on human health and nutrition. Int J Food Sci. 2015;2015:526762. doi: 10.1155/2015/526762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oz F, Yuzer MO. The effects of different cooking methods on the formation of heterocyclic aromatic amines in turkey meat. J Food Process Preserv. 2017;41(5):e13196. doi: 10.1111/jfpp.13196. [DOI] [Google Scholar]
- 5.Dutta K, Shityakov S, Zhu W, Khalifa I. High-risk meat and fish cooking methods of polycyclic aromatic hydrocarbons formation and its avoidance strategies. Food Control. 2022;142:109253. doi: 10.1016/j.foodcont.2022.109253. [DOI] [Google Scholar]
- 6.Bulanda S, Janoszka B. Consumption of thermally processed meat containing carcinogenic compounds (polycyclic aromatic hydrocarbons and heterocyclic aromatic amines) versus a risk of some cancers in humans and the possibility of reducing their formation by natural food additives—a literature review. Int J Environ Res Public Health. 2022;19:4781. doi: 10.3390/ijerph19084781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nor Hasyimah AK, Jinap S, Sanny M, Ainaatul AI, Sukor R, Jambari NN, et al. Effects of honey-spices marination on polycyclic aromatic hydrocarbons and heterocyclic amines formation in gas-grilled beef satay. Polycyclic Aromatic Compounds. 2022;42(4):1620–1648. doi: 10.1080/10406638.2020.1802302. [DOI] [Google Scholar]
- 8.Alam Shah S, Selamat J, Haque Akanda MJ, Sanny M, Khatib A. Effects of different types of soy sauce on the formation of heterocyclic amines in roasted chicken. Food Addit Contam Part A Chem Anal Control Exposure Risk Assess. 2018;35(5):870–881. doi: 10.1080/19440049.2018.1440639. [DOI] [PubMed] [Google Scholar]
- 9.Jinap S, Hasnol NDS, Sanny M, Jahurul MHA. Effect of organic acid ingredients in marinades containing different types of sugar on the formation of heterocyclic amines in grilled chicken. Food Control. 2018;84:478–484. doi: 10.1016/j.foodcont.2017.08.025. [DOI] [Google Scholar]
- 10.Khan IA, Yiqun C, Zongshuai Z, Ijaz MU, Brohi SA, Ahmad MI, et al. Occurrence of heterocyclic amines in commercial fast-food meat products available on the chinese market and assessment of human exposure to these compounds. J Food Sci. 2019;84(1):192–200. doi: 10.1111/1750-3841.14418. [DOI] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer (IARC) IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC Publication; 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins; pp. 245–396. [Google Scholar]
- 12.Alomirah H, Al-Zenki S, Al-Hooti S, Zaghloul S, Sawaya W, Ahmed N, et al. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control. 2011;22(12):2028–2035. doi: 10.1016/j.foodcont.2011.05.024. [DOI] [Google Scholar]
- 13.Khalili F, Shariatifar N, Dehghani MH, Yaghmaeian K, Nodehi RN, Yaseri M, et al. Polycyclic aromatic hydrocarbons (PAHs) in meat, poultry, fish and related product samples of Iran: a risk assessment study. J Environ Health Sci Eng. 2023;21(1):215–224. doi: 10.1007/s40201-023-00854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamidi EN, Hajeb P, Selamat J, Razis AFA. Polycyclic aromatic hydrocarbons (PAHs) and their bioaccessibility in meat: a tool for assessing human cancer risk. Asian Pac J Cancer Prev. 2016;17(1):15–23. doi: 10.7314/APJCP.2016.17.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Penning TM. Polyaromatic hydrocarbons. In: Stadler RH, Lineback DR, editors. Process-induced food toxicants: occurence, formation, mitigation, and health risks. New Jersey: John Wiley & Sons, Inc; 2009. [DOI] [Google Scholar]
- 16.Aaslyng MD, Duedahl-Olesen L, Jensen K, Meinert L. Content of heterocyclic amines and polycyclic aromatic hydrocarbons in pork, beef and chicken barbecued at home by Danish consumers. Meat Sci. 2013;93(1):85–91. doi: 10.1016/j.meatsci.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Food Standards Australia New Zealand. Risk assessment. 2014. Available at: http://www.foodstandards.gov.au/science/riskanalysis/riskassessment/Pages/Risk-assessment.aspx.
- 18.Singh L, Varshney JG, Agarwal T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016;199:768–781. doi: 10.1016/j.foodchem.2015.12.074. [DOI] [PubMed] [Google Scholar]
- 19.Diggs DL, Huderson AC, Harris KL, Myers JN, Banks LD, Rekhadevi PV, et al. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J Environ Sci Health C. 2011;29(4):324–357. doi: 10.1080/10590501.2011.629974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahurul MHA, Jinap S, Zaidul ISM, Sahena F, Farhadian A, Hajeb P. Determination of fluoranthene, benzo[b]fluoranthene and benzo[a]pyrene in meat and fish products and their intake by Malaysian. Food Biosci. 2013;1:73–80. doi: 10.1016/j.fbio.2013.03.006. [DOI] [Google Scholar]
- 21.Jahurul MHA, Jinap S, Ang SJ, Abdul-Hamid A, Hajeb P, Lioe HN, et al. Dietary exposure to heterocyclic amines in high-temperature cooked meat and fish in Malaysia. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27(8):1060–1071. doi: 10.1080/19440041003801190. [DOI] [PubMed] [Google Scholar]
- 22.Kirkland D, Kasper P, Martus HJ, Müller L, van Benthem J, Madia F, et al. Updated recommended lists of genotoxic and non-genotoxic chemicals for assessment of the performance of new or improved genotoxicity tests. Mutat Res Genet Toxicol Environ Mutagen. 2016;795:7–30. doi: 10.1016/j.mrgentox.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Benford D, Bolger PM, Carthew P, Coulet M, DiNovi M, Leblanc JC, et al. Application of the margin of exposure (MOE) approach to substances in food that are genotoxic and carcinogenic. Food Chem Toxicol. 2010;48(Suppl 1):S2–S24. doi: 10.1016/j.fct.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Barlow S, Renwick AG, Kleiner J, Bridges J, Busk L, Dybing E, et al. Risk assessment of substances that are both genotoxic and carcinogenic. Report of an international conference organized by EFSA and WHO with support of ILSI Europe. Food Chem Toxicol. 2006;44:1636–1650. doi: 10.1016/j.fct.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Committee ES. Statement on the applicability of the margin of exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA J. 2012;10(3):2578. doi: 10.2903/j.efsa.2012.2578. [DOI] [Google Scholar]
- 26.Haber LT, Dourson ML, Allen BC, Hertzberg RC, Parker A, Vincent MJ, et al. Benchmark dose (BMD) modeling: current practice, issues, and challenges. Crit Rev Toxicol. 2018;48(5):387–415. doi: 10.1080/10408444.2018.1430121. [DOI] [PubMed] [Google Scholar]
- 27.Hardy A, Benford D, Halldorsson T, Jeger M, Knutsen K, More S, et al. Update: use of the benchmark dose approach in risk assessment. EFSA J. 2017;15(1):e04658. doi: 10.2903/j.efsa.2017.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carthew P, Dinovi M, Setzer R. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic example: Furan (CAS No. 110-00-9) Food Chem Toxicol. 2010;48(Suppl 1):S69–S74. doi: 10.1016/j.fct.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Kushida H, Wakabayashi K, Sato H, Katami M, Kurosaka R, Nagao M. Dose-response study of MeIQx carcinogenicity in F344 male rats. Cancer Lett. 1994;83(1–2):31–35. doi: 10.1016/0304-3835(94)90295-X. [DOI] [PubMed] [Google Scholar]
- 30.Wang JS, Busby WF. Induction of lung and liver tumors by flouranthene in a preweanling CD-1 mouse bioassay. Carcinogenesis. 1993;14(9):1871–1874. doi: 10.1093/carcin/14.9.1871. [DOI] [PubMed] [Google Scholar]
- 31.Wester PW, Muller JJ, Slob W, Mohn GR, Dortant PM, Kroese ED. Carcinogenic activity of benzo[a]pyrene in a 2-year oral study in Wistar rats. Food Chem Toxicol. 2012;50(3–4):927–935. doi: 10.1016/j.fct.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Haber LT, Dourson ML, Allen BC, Hertzberg RC, Parker A, Vincent MJ, et al. Benchmark dose (BMD) modeling: current practice, issues, and challenges. Crit Rev Toxicol. 2018;48(5):387–415. doi: 10.1080/10408444.2018.1430121. [DOI] [PubMed] [Google Scholar]
- 33.Majek O, Gondos A, Jansen L, Emrich K, Holleczek B, Katalinic A, et al. Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS ONE. 2013;8(7):e68077. doi: 10.1371/journal.pone.0068077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White A, Ironmonger L, Steele RJC, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(1):906. doi: 10.1186/s12885-018-4786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oz F, Kızıl M, Çelık T. Effects of different cooking methods on the formation of heterocyclic aromatic amines in goose meat. J Food Process Preserv. 2016;40(5):1047–1053. doi: 10.1111/jfpp.12685. [DOI] [Google Scholar]
- 36.Zheng Z, Park JK, Kwon OW, Ahn SH, Kwon YJ, Jiang L, et al. The risk of gastrointestinal cancer on daily intake of low-dose BaP in C57BL/6 for 60 days. J Korean Med Sci. 2022;37(30):e235. doi: 10.3346/jkms.2022.37.e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahin S, Ulusoy HI, Alemdar S, Erdogan S, Agaoglu S. The presence of polycyclic aromatic hydrocarbons (PAHs) in grilled beef, chicken and fish by considering dietary exposure and risk assessment. Food Sci Anim Resour. 2020;40(5):675–688. doi: 10.5851/kosfa.2020.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rozentale I, Zacs D, Bartkiene E, Bartkevics V. Polycyclic aromatic hydrocarbons in traditionally smoked meat products from the Baltic states. Food Addit Contam Part B. 2018;11(2):138–145. doi: 10.1080/19393210.2018.1440637. [DOI] [PubMed] [Google Scholar]
- 39.Duedahl-Olesen L, Aaslyng M, Meinert L, Christensen T, Jensen AH, Binderup ML. Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control. 2015;57:169–176. doi: 10.1016/j.foodcont.2015.04.012. [DOI] [Google Scholar]
- 40.Lee Y, Lee KS, Kim CI, Lee JY, Kwon SO, Park HM. Assessment of dietary exposure to heterocyclic amines based on the Korean total diet study. Food Addit Contam Part A. 2022;39(3):429–439. doi: 10.1080/19440049.2021.2012601. [DOI] [PubMed] [Google Scholar]
- 41.Manan WA, Firdaus N, Safiah M, Haslinda S, Poh B, Norimah A, et al. Meal patterns of Malaysian adults: findings from the Malaysian adults nutrition survey (MANS) Malays J Nutr. 2012;18(2):221–230. [PubMed] [Google Scholar]
- 42.Pouzou JG, Costard S, Zagmutt FJ. Probabilistic assessment of dietary exposure to heterocyclic amines and polycyclic aromatic hydrocarbons from consumption of meats and breads in the United States. Food and Chemical Toxicology. 2018;114:361–374. doi: 10.1016/j.fct.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerli B, Rhyn P, Zoller O, Schlatter J. Occurrence of heterocyclic aromatic amines in the Swiss diet: analytical method, exposure estimation and risk assessment. Food Addit Contam. 2001;18(6):533–551. doi: 10.1080/02652030119545. [DOI] [PubMed] [Google Scholar]