Abstract
The treatment of spinal pathologies has evolved significantly from the times of Hippocrates and Galen to the current era. This evolution has led to the development of cutting-edge technologies to improve surgical techniques and patient outcomes. The University of Missouri Health System is a high-volume, tertiary care academic medical center that serves a large catchment area in central Missouri and beyond. The Department of Neurosurgery has sought to integrate the best available technologies to serve their spine patients. These technological advancements include intra-operative image guidance, robotic spine surgery, minimally invasive techniques, motion preservation surgery, and interdisciplinary care of metastatic disease to the spine. These advances have resulted in safer surgeries with enhanced outcomes at the University of Missouri. This integration of innovation demonstrates our tireless commitment to ensuring excellence in the comprehensive care of a diverse range of patients with complex spinal pathologies.
Introduction
The study of spinal anatomy, pathology, and even management, dates back to the time of Hippocrates (460-370 BC). Correction of spinal deformity using the Hippocrates ladder and board marks the earliest interventions used to treat spine pathologies.1 Centuries later, the Greek physician Galen made groundbreaking contributions to the knowledge of spine anatomy, including the introduction of medical terms like kyphosis and scoliosis in addition to their treatment using devices such as chest binders and jackets. In the 19th and 20th centuries, further refinement in these non-surgical techniques and the development of surgical interventions birthed the field of spinal surgery.2 The 21st century marks the era of technological advancements in image guidance, robotics, and the introduction of minimally invasive techniques (MIS approaches). At the University of Missouri, we treat a wide spectrum of spinal pathologies from the occiput through the sacrum. We commonly treat pediatric and adult patients with degenerative diseases of the spine, inflammatory disorders, spinal trauma, infections, congenital disorders of the spine, spinal deformity, and malignancy.
Technological Advancements
Technological advancements in the past five decades have enabled spine surgeons to shift paradigms from open surgeries to minimally invasive spine surgery. Image guidance, new surgical techniques, and evolving instrumentation have laid the foundation for the current era of spine surgery. The following are a few of the fundamentally important innovations of the recent era.3,4
Intra-Operative Image Guidance
Image guidance in spine surgery has revolutionized both the safety and precision of instrumentation in spine surgery. Initially, the placement of screws and other hardware relied on anatomic landmarks that allowed for intraoperative assessment of known safe windows and trajectories. The advent of intraoperative fluoroscopy in 1955 all owed for real-time two-dimensional imaging to guide screw placement.5 While this marked a crucial step in the evolution of surgical technique, fluoroscopy guided pedicle screw placement still reported suboptimal screw positioning rates due to the inherent limitations of using two-dimensional imaging for guidance of three-dimensional techniques. The subsequent introduction of computed tomography (CT) in 1971 followed by the magnetic resonance imaging (MRI) in the early 1980s profoundly altered the pre-operative evaluation of surgical spine patients, but challenges persisted regarding intraoperative image guidance.6,7
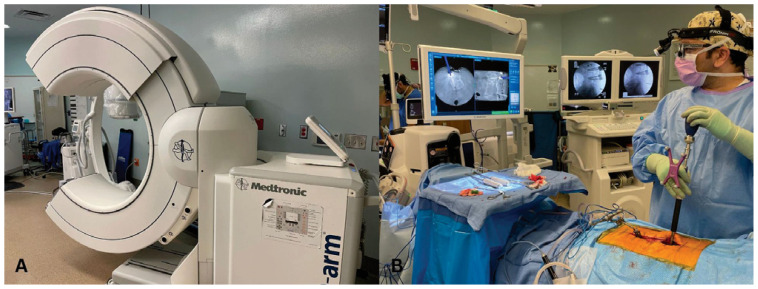
The foundation of contemporary image guidance in spine surgery consists of a navigation system that integrates patient-specific imaging data with real-time three-dimensional positioning of instruments and spinal implants. Patient-specific imaging is obtained via several mechanisms at the University of Missouri. The most basic technique comprises a pre-operative high-resolution CT image of the levels of interest which is then intraoperatively registered to the patient by selecting points on the patient’s spine for surface registration. An alternative workflow consists of obtaining a patient-specific intraoperative 2D/3D image with an intraoperative CT scanner (Figure 1). This workflow allows for immediate registration of the patient’s spine to the navigation system. Instruments and attached implants are outfitted with optical sensors which are tracked by a mobile optical sensing unit. Confirmation of expected implant placement is obtained with an intraoperative CT scanner. This significantly decreases the need for “take back” surgeries to revise implant placement.
Figure 1.
A. Intraoperative CT navigation system. B. Placement of pedicle screws with image guidance.
Intraoperative spinal navigation was first developed to assist in the placement of C1-C2 screws (minimizing risk to the adjacent vertebral arteries) and thoracolumbar pedicle screws. However, intraoperative navigation has uses beyond the placement of pedicle screws. Intraoperative image guidance can assist in exact localization of spinal level in cases of abnormal anatomy, osteotomy planning, interbody placement, and decompression planning.8,9 In a study by Wang YC, the author describes navigated accuracy of cervical pedicle screw placement up to 94.1%.10 In the thoracolumbar region optimal hardware placement via intra-operative CT has accuracy reported up to 98%.11,12 Gelalis et al. assessed the accuracy of pedicle screw placement using freehand, fluoroscopy, and CT navigation.13 The results revealed free-hand technique accuracy to range from 69 to 94%, fluoroscopy-based navigation from 81 to 92%, and CT navigation from 89 to 100% accurate. Intraoperative CT navigation exhibits a higher accuracy in pedicle screw placement than free-hand technique and use of fluoroscopy.13
Robot-Assisted Spine Surgery
The University of Missouri is one of a select group of medical centers in the state of Missouri that also performs robot-assisted spine surgery. The advent of robotics in spine surgery represents a significant leap forward in the evolution of technology. This leap combines diverse advancements into a single platform to enhance both the precision of surgery and the subsequent patient outcomes.
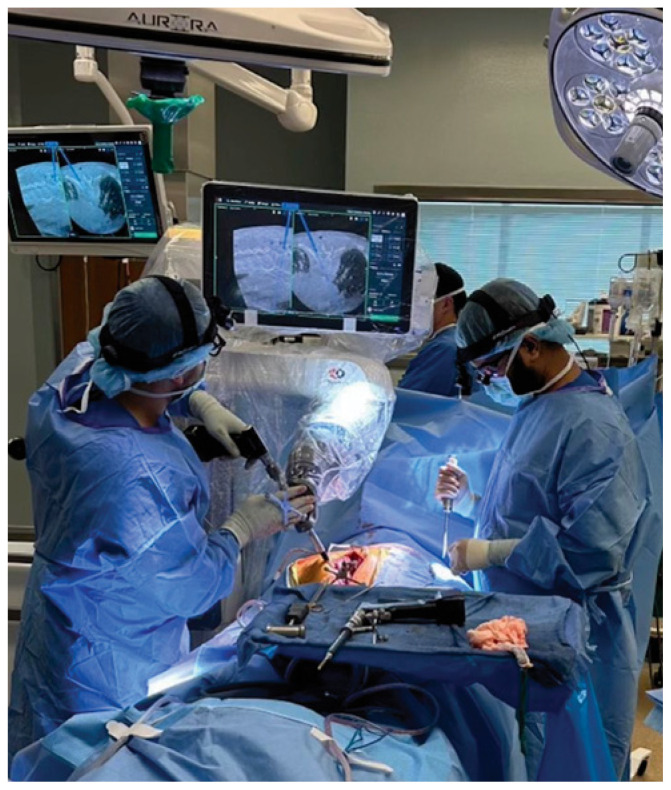
Robot-assisted spine surgery necessitates a complex workflow that requires extensive training for both spine surgeons and operating room nurses and technicians. The University of Missouri currently employs an advanced platform for robot-assisted spine surgery (Figure 2). This platform is integrated with both image acquisition and intraoperative image guidance to allow for seamless transitions between points in the workflow. After obtaining an intraoperative, three-dimensional image of the patient’s bony anatomy, the system automatically performs segmental registration (each vertebral body individually) to each spinal level. Segmental registration is a critical step as the spine is mobile and the bony anatomy may be altered significantly by patient positioning. Pedicle screws are then planned on the navigation station and saved into the robotic system. The robotic system then automatically positions itself for placement of each pedicle screw at the appropriate depth and trajectory. Surgeons are then able to perform both drilling, tapping to create threads in the bone, and placement of the pedicle screw through the robotically-positioned guide.
Figure 2.
Robot-assisted placement of pedicle screws.
The use of robotics in spine surgery has revealed promising clinical results with minimally invasive techniques. Multiple studies have shown robotic assisted pedicle screw placement to be more accurate than free-hand surgeries.14–16 Additionally, the use of robotic guidance was shown to reduce radiation exposure and length of hospital stay.14 However, there are still many limitations to the use of robotics in spine surgery including longer surgery times compared to free-hand techniques.16 Additionally, the number of patients robotic surgery in general can serve is limited based on the small number of centers that can afford such technology. The current pricing on a single da Vinci robot (non-spinal robot) is approximately $1.5 to 2 million, resulting in higher initial capital investments for the hospitals and higher surgical costs for the patients.17,18 Yet, an overall cost-analysis of the use of robotics in spine surgery revealed an overall savings estimate of $608,546 during a one-year period because of reduced infections and shorter length of hospital stay with the use of robotics.17
Motion Preservation Surgery in the Cervical Spine
Degenerative disc disease affects 80–90% of the population by age 50.19 Anterior cervical discectomy and fusion (ACDF) is a well-established, clinically effective treatment option for advanced degenerative disc disease of the cervical spine. However, there are certain drawbacks including restricted mobility at the treated level, adjacent level disease, and hardware failure.20–22 Artificial disc replacement (ADR) is an established procedure used to treat cervical disc disease that has particular benefits such as preservation of motion and reduction in adjacent level degeneration in comparison to traditional ACDF.
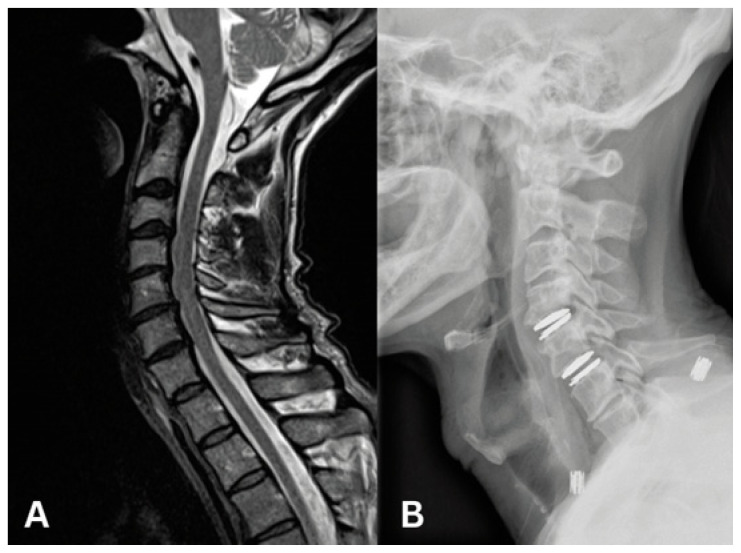
Results from a number of randomized-controlled trials have demonstrated that ADR may prove to be an excellent surgical option when careful patient selection and well-trained, meticulous technique are employed.23–26 Complications of ADR include heterotopic ossification (bony overgrowth) and implant migration or subsidence.27,28 Careful patient selection in each treatment is key to a successful outcome. Recent series have also demonstrated that ADR may be associated with shorter length of stay and enhanced recovery times.29–31 Spine surgeons at the University of Missouri are highly experienced in motion preservation surgery in the cervical spine via ADR (Figure 3). Our surgeons use a diverse number of available ADR devices to perform successful motion preservation surgery.
Figure 3.
A. 56-year old male presenting with neck pain and bilateral arm pain.
B. Post-operative lateral XR C4/5 and C5/6 artificial disc replacement.
Minimally-Invasive Spine (MIS) Surgery
Degenerative disease of the lumbar spine diseases is estimated to impact around 2.06 million people annually in the U.S.32 Historically, the approach for the treatment of most pathologies in the lumbar has been with a posterior midline incision. Such interventions can include decompression surgeries with laminectomy, discectomy, and fusion with a wide array of instrumentation. In the last several decades, several minimally invasive surgical techniques have been developed. These techniques are associated with decreased blood loss, decreased tissue injury, and in many cases, decreased risk of complications such as infection.
Percutaneous Pedicle Screw Placement
Magerl first described fixation of the lumbar spine using external fixators.33 Later Mathews and Long used percutaneous pedicle fixation using plates.34 This subsequently evolved into the use of rods to connect the percutaneously placed pedicle screws. In such techniques, real time neuronavigational guidance is used to identify the trajectory of pedicle screw placement. The incisions are planned based on the trajectory of each pedicle screw with minimal need for muscle retraction or dissection. Percutaneous pedicle screw placement can be utilized as a standalone treatment of vertebral fractures acting as an internal brace or supplement to anterior spinal procedures.31, 32
Minimally Invasive Transforaminal Lumbar Interbody Fusion (MIS-TLIF)
Interbody fusion remains an effective treatment option for patients with degenerative disc and facet joint disease. Such patients often present with mechanical back pain, radiculopathy, and claudication. In 1982, Harms and Rominger described the transforaminal approach to lumbar interbody fusion (TLIF).35 In 2002, with the availability of more sophisticated instrumentation, minimally invasive interbody fusion was introduced by Foley and Lefkowitz.36
A paramedian incision is usually planned on the side of symptoms to allow for ipsilateral facetectomy and laminectomy as a mean of direct nerve root decompression. The muscle dissection is performed using tubular retractors with increasing diameters centered at the disc level of interest. The tubular retractor is placed under image guidance. Once the disc space is identified and confirmed, a microscope is brought to the field to perform a discectomy and end plate preparation for interbody cage placement. The interbody cage can be packed with graft material to facilitate fusion. Stab incisions are used to place pedicle screws at the vertebral levels above and below the disc space utilizing neuronavigational guidance. Rods are then passed through break-way guides connected to the screw heads. Once the rods are secured to the screws, the guides are broken off. The MIS-TLIF technique is associated with less blood loss, less muscle retraction, reduced postoperative pain, and shorter length of hospital stay when compared with open TLIF. However, patient reported pain scores and fusion rates are comparable for the two surgical techniques.36,37
Lateral Lumbar Interbody Fusion
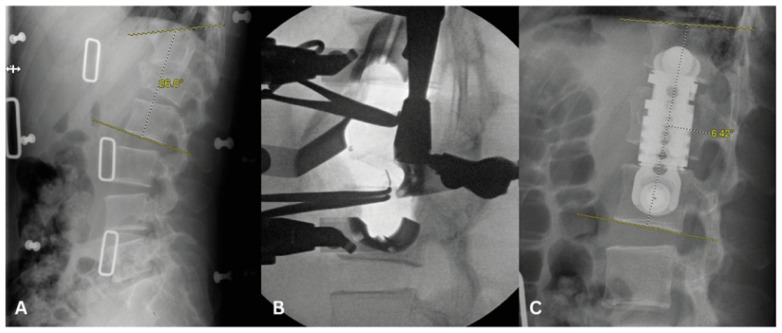
In recent years, anterolateral approaches to the spine have gained popularity and have been refined to the point of becoming commonly used approaches for spine surgery, particularly the lumbar spine.38 Through a small (~6cm) abdominal incision, the surgeon is able to access most of the lumbar spine. Once the skin is cut and the external fascia of the abdominal wall muscles is open, the rest of the dissection is performed bluntly and there is no additional injury to the tissues. This can obviate the need for the large midline lumbar incisions and extensive paraspinal muscle dissections that may be necessary in certain posterior approaches to the spine. This allows for more rigorous disc removal and the ability to place larger interbody grafts than are possible through a posterior approach.39 This, in turn, results in increased ability to restore disc height, indirectly decompress the neural elements, correct spinal deformities and reduce certain fractures, making it a useful tool in cases of degenerative spinal disease, trauma, infections and even spinal tumors (Figure 4). However, these approaches are not suitable for all patients, particularly those with prior retroperitoneal surgeries.40,41 Some of the possible complications of these approaches include hip flexion weakness related to manipulation of the psoas muscle during dissection of the lateral aspect of the spine, abdominal pseudohernias related to disruption of the abdominal wall innervation, or injury to the greater vessels, among others.40,41 Consequently, these approaches require the spine surgeon have additional training on the retroperitoneal exposure or the help of a general surgeon for access to the spine. The use of stereotactic navigation and/or neuromonitoring can also enhance the safety of these procedures.42
Figure 4.
A. 38 year-old male presenting with disabling pain after MVC found to have L1 burst fracture with retropulsion and kyphosis.
B. Intra-operative lateral XR with retractors in place for lateral corpectomy.
C. L1 lateral corpectomy with restoration of the anterior column and resolution of kyphosis.
Treatment of Metastatic Spinal Disease
Spinal metastasis is the most common bony spine tumor and the thoracic spine is the most frequently involved segment of the spine.43 Breast (21%), lung (19%), prostate (7.5%), renal (5%), gastrointestinal (5.5%), and thyroid (2.5%) are the most common tumors that metastasize to the spine. The treatment algorithm for patients with metastatic spine disease is based on neurological dysfunction, histopathology, mechanical instability, and systemic burden of the disease. Patients who have cord compression, acute onset paralysis/paresis, and mechanical instability are often candidates for decompression and fusion. Patients with oligometastatic disease and favorable survival are also candidates for surgery. In patients with advanced disease, subtotal tumor resection with internal fixation can be used as part of a palliative approach to cancer treatment. Surgical intervention has been shown to improve morbidity and mortality in patients with acute paralysis secondary to metastatic disease to the spind.44,45 The recent treatment algorithm is based on Patchel R. et al,44 landmark randomized control trial where he compared the clinical outcomes between patients with metastatic spinal cord compression treated with surgery followed by radiation and radiation alone. Patients who underwent surgical intervention followed by radiation demonstrated improved outcomes with respect to: ambulation, continence, Frankel score, and survival.
Advances in image guidance, intraoperative navigation, and percutaneous instrumentation allow spinal surgeons to deliver care to an even larger number of patients with spinal tumors of all types. By minimizing injury to adjacent tissues and shortening incisions, these tools allow patients undergoing surgery for their cancers to progress to radiation and systemic therapy sooner than when undergoing traditional surgery. “Separation surgery” involves decreasing the tumor burden around radiosensitive tissues. Stereotactic Body Radiation Therapy (SBRT) allows a team of radiation oncologists and spinal surgeons to deliver high doses of radiation to very small volumes of tissue with less injury to surrounding tissues than conventional radiotherapy. The combination of “separation surgery” and SBRT vs conventional radiotherapy allows for more effective treatment of spinal tumor burden, particularly in cases of recurrent disease.
Patients with spinal malignancies are treated by a large interdisciplinary team. This interdisciplinary team involves spine surgeons, medical oncologists, radiation oncologists, neuropathologists, and neuroradiologists. These cases are typically discussed in tumor board meetings where recommendations from all services are considered, and a unified care plan is formulated to care for these complex patients. This combined effort represents a significant advancement in the care of patients with spinal cancer.
Conclusion
The Division of Spinal Neurosurgery at the University of Missouri has sought to integrate currently developing technological advancements such as intra-operative image guidance, robotic spine surgery, minimally invasive techniques, motion preservation surgery, and the interdisciplinary care of spinal malignancy. This integration allows us to serve the needs of patients with straightforward and challenging spinal pathologies both now and in the future.
Footnotes
by Ravi Nunna, MD, Farzana Tariq, MD, Michael Ortiz, MD, Inamullah Khan, MD, Paul Santiago, MD, (pictured), are all in the Department of Neurosurgery, University of Missouri - Columbia, Columbia, Missouri. Sabrina Genovese, BS, is at the School of Medicine, University of Missouri - Columbia, Columbia, Missouri.
Disclosure: No financial disclosures reported. Artificial intelligence was not used in the study, research, preparation, or writing of this manuscript.
References
- 1.Vasiliadis ES, Grivas TB, Kaspiris A. Historical overview of spinal deformities in ancient. Greece Scoliosis. 2009;4(1):6. doi: 10.1186/1748-7161-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarpada SP, Morris MT, Burton DA. Spinal fusion surgery: A historical perspective. Journal of Orthopaedics. 2017;14(1):134–136. doi: 10.1016/j.jor.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momin AA, Steinmetz MP. Evolution of Minimally Invasive Lumbar Spine Surgery. World Neurosurgery. 2020;140:622–626. doi: 10.1016/j.wneu.2020.05.071. [DOI] [PubMed] [Google Scholar]
- 4.Moses ZB, Mayer RR, Strickland BA, et al. Neuronavigation in minimally invasive spine surgery. FOC. 2013;35(2):E12. doi: 10.3171/2013.5.FOCUS13150. [DOI] [PubMed] [Google Scholar]
- 5.Nair S, Nambiar M, Pope A, Parkes M, De Jong K, Hau R. Intraoperative fluoroscopy alone versus routine post-operative X-RAYS in identifying return to theatre after fracture fixation. ANZ Journal of Surgery. 2021;91(3):392–397. doi: 10.1111/ans.16610. [DOI] [PubMed] [Google Scholar]
- 6.Schulz RA, Stein JA, Pelc NJ. How CT happened: the early development of medical computed tomography. J Med Imaging (Bellingham) 2021;8(5):052110. doi: 10.1117/1.JMI.8.5.052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabasawa H. MR Imaging in the 21st Century: Technical Innovation over the First Two Decades. Magn Reson Med Sci. 2022;21(1):71–82. doi: 10.2463/mrms.rev.2021-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alqurashi A, Alomar SA, Bakhaidar M, Alfiky M, Baeesa SS. Accuracy of Pedicle Screw Placement Using Intraoperative CT-Guided Navigation and Conventional Fluoroscopy for Lumbar Spondylosis. Cureus. 2021;13(8):e17431. doi: 10.7759/cureus.17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin KD, Kadiyala M, Talwar D, Sankar WN, Flynn JM, Anari JB. Does intraoperative CT navigation increase the accuracy of pedicle screw placement in pediatric spinal deformity surgery? A systematic review and meta-analysis. Spine Deform. 2022;10(1):19–29. doi: 10.1007/s43390-021-00385-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Zhou Z, Wang B, et al. Occipitocervical Fusion via Cervical Pedicle Fixation Assisted with O-arm Navigation. Orthopaedic Surgery. 2020;12(4):1100–1107. doi: 10.1111/os.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tehli O, Harman F, Temiz C, et al. The use of neuronavigation and intraoperative imaging in spinal stabilization surgery. Turkish Neurosurgery. doi: 10.5137/1019-5149.JTN.13226-14.1. Published online 2015. [DOI] [PubMed] [Google Scholar]
- 12.Sundaram PPM, Oh JYL, Tan M, Nolan CP, Yu CS, Ling JM. Accuracy of Thoracolumbar Pedicle Screw Insertion Based on Routine Use of Intraoperative Imaging and Navigation. Asian Spine J. 2021;15(4):491–497. doi: 10.31616/asj.2020.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelalis ID, Paschos NK, Pakos EE, et al. Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J. 2012;21(2):247–255. doi: 10.1007/s00586-011-2011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Minimally Invasive Robotic Versus Open Fluoroscopic-guided Spinal Instrumented Fusions: A Randomized Controlled Trial. Spine. 2017;42(6):353–358. doi: 10.1097/BRS.0000000000001778. [DOI] [PubMed] [Google Scholar]
- 15.Devito DP, Kaplan L, Dietl R, et al. Clinical Acceptance and Accuracy Assessment of Spinal Implants Guided With SpineAssist Surgical Robot: Retrospective Study. Spine. 2010;35(24):2109–2115. doi: 10.1097/BRS.0b013e3181d323ab. [DOI] [PubMed] [Google Scholar]
- 16.Fatima N, Massaad E, Hadzipasic M, Shankar GM, Shin JH. Safety and accuracy of robot-assisted placement of pedicle screws compared to conventional free-hand technique: a systematic review and meta-analysis. Spine J. 2021;21(2):181–192. doi: 10.1016/j.spinee.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Menger RP, Savardekar AR, Farokhi F, Sin A. A Cost-Effectiveness Analysis of the Integration of Robotic Spine Technology in Spine Surgery. Neurospine. 2018;15(3):216–224. doi: 10.14245/ns.1836082.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezeokoli EU, Pfennig M, John J, Gupta R, Khalil JG, Park DK. Index Surgery Cost of Fluoroscopic Freehand Versus Robotic-Assisted Pedicle Screw Placement in Lumbar Instrumentation: An Age, Sex, and Approach-Matched Cohort Comparison. JAAOS Glob Res Rev. 2022;6(12) doi: 10.5435/JAAOSGlobal-D-22-00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teraguchi M, Yoshimura N, Hashizume H, et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthritis and Cartilage. 2014;22(1):104–110. doi: 10.1016/j.joca.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta neurochir. 1989;99(1–2):41–50. doi: 10.1007/BF01407775. [DOI] [PubMed] [Google Scholar]
- 21.Fielding JW. Complications of anterior cervical disk removal and fusion. Clin Orthop Relat Res. 1992;(284):10–13. [PubMed] [Google Scholar]
- 22.Flynn TB. Neurologic Complications of Anterior Cervical Interbody Fusion. Spine. 1982;7(6):536–539. doi: 10.1097/00007632-198211000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Davis RJ, Nunley PD, Kim KD, et al. Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: a prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. SPI. 2015;22(1):15–25. doi: 10.3171/2014.7.SPINE13953. [DOI] [PubMed] [Google Scholar]
- 24.Delamarter RB, Zigler J. Five-Year Reoperation Rates, Cervical Total Disc Replacement Versus Fusion, Results of a Prospective Randomized Clinical Trial. Spine. 2013;38(9):711–717. doi: 10.1097/BRS.0b013e3182797592. [DOI] [PubMed] [Google Scholar]
- 25.Luo J, Huang S, Gong M, et al. Comparison of artificial cervical arthroplasty versus anterior cervical discectomy and fusion for one-level cervical degenerative disc disease: a meta-analysis of randomized controlled trials. Eur J Orthop Surg Traumatol. 2015;25(S1):115–125. doi: 10.1007/s00590-014-1510-4. [DOI] [PubMed] [Google Scholar]
- 26.Radcliff K, Coric D, Albert T. Five-year clinical results of cervical total disc replacement compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled, multicenter investigational device exemption clinical trial. SPI. 2016;25(2):213–224. doi: 10.3171/2015.12.SPINE15824. [DOI] [PubMed] [Google Scholar]
- 27.Chutkan NB, editor. Results of Cervical Arthroplasty Compared With Anterior Discectomy and Fusion: Four-year Clinical Outcomes in a Prospective, Randomized Controlled Trial. Orthopedics. 2011;34(11):889–889. doi: 10.3928/01477447-20110922-24. [DOI] [PubMed] [Google Scholar]
- 28.Peng Z, Hong Y, Meng Y, Liu H. A meta-analysis comparing the short- and mid- to long-term outcomes of artificial cervical disc replacement(ACDR) with anterior cervical discectomy and fusion (ACDF) for the treatment of cervical degenerative disc disease. International Orthopaedics (SICOT) 2022;46(7):1609–1625. doi: 10.1007/s00264-022-05318-z. [DOI] [PubMed] [Google Scholar]
- 29.Wei J, Song Y, Sun L, Lv C. Comparison of artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. International Orthopaedics (SICOT) 2013;37(7):1315–1325. doi: 10.1007/s00264-013-1883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delamarter R, Zigler JE, Balderston RA, Cammisa FP, Goldstein JA, Spivak JM. Prospective, Randomized, Multicenter Food and Drug Administration Investigational Device Exemption Study of the ProDisc-L Total Disc Replacement Compared with Circumferential Arthrodesis for the Treatment of Two-Level Lumbar Degenerative Disc Disease: Results at Twenty-four Months. The Journal of Bone & Joint Surgery. 2011;93(8):705–715. doi: 10.2106/JBJS.I.00680. [DOI] [PubMed] [Google Scholar]
- 31.Lee WT, Liu G, Thambiah J, Wong HK. Clinical outcomes of single-level lumbar artificial disc replacement compared with transforaminal lumbar interbody fusion in an Asian population. Singapore Med J. 2015;56(4):208–211. doi: 10.11622/smedj.2015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterman BR, Belmont PJ, Schoenfeld AJ. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. The Spine Journal. 2012;12(1):63–70. doi: 10.1016/j.spinee.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Magerl F. The External Fixator. Springer; Berlin Heidelberg: 1985. External Spinal Skeletal Fixation; pp. 289–365. [DOI] [Google Scholar]
- 34.Mathews H, Long B. Endoscopy assisted percutaneous anterior interbody fusion with subcutaneous suprafascial internal fixation: evolution of technique and surgical considerations. Orthop Int Ed. 1995;3:496–500. [Google Scholar]
- 35.Harms J, Rolinger H. Die operative Behandlung der Spondylolisthese durch dorsale Aufrichtung und ventrale Verblockung. Z Orthop Ihre Grenzgeb. 2008;120(03):343–347. doi: 10.1055/s-2008-1051624. [DOI] [PubMed] [Google Scholar]
- 36.Wiltse LL, Bateman JG, Hutchinson RH, Nelson WE. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Joint Surg Am. 1968;50(5):919–926. [PubMed] [Google Scholar]
- 37.Lener S, Wipplinger C, Hernandez RN, et al. Defining the MIS-TLIF: A Systematic Review of Techniques and Technologies Used by Surgeons Worldwide. Global Spine Journal. 2020;10(2_suppl):151S–167S. doi: 10.1177/2192568219882346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawar A, Hughes A, Girardi F, Sama A, Lebl D, Cammisa F. Lateral Lumbar Interbody Fusion Asian. Spine J. 2015;9(6):978. doi: 10.4184/asj.2015.9.6.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wangaryattawanich P, Kale HA, Kanter AS, Agarwal V. Lateral Lumbar Interbody Fusion: Review of Surgical Technique and Postoperative Multimodality Imaging Findings. American Journal of Roentgenology. 2021;217(2):480–494. doi: 10.2214/AJR.20.24074. [DOI] [PubMed] [Google Scholar]
- 40.Salzmann SN, Shue J, Hughes AP. Lateral Lumbar Interbody Fusion—Outcomes and Complications. Curr Rev Musculoskelet Med. 2017;10(4):539–546. doi: 10.1007/s12178-017-9444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon B, Kim DH. Lateral Lumbar Interbody Fusion: Indications, Outcomes, and Complications. Journal of the American Academy of Orthopaedic Surgeons. 2016;24(2):96–105. doi: 10.5435/JAAOS-D-14-00208. [DOI] [PubMed] [Google Scholar]
- 42.DiGiorgio AM, Edwards CS, Virk MS, Mummaneni PV, Chou D. Stereotactic navigation for the prepsoas oblique lateral lumbar interbody fusion: technical note and case series. Neurosurgical Focus. 2017;43(2):E14. doi: 10.3171/2017.5.FOCUS17168. [DOI] [PubMed] [Google Scholar]
- 43.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 44.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. The Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 45.Van Den Bent MJ. Surgical resection improves outcome in metastatic epidural spinal cord compression. The Lancet. 2005;366(9486):609–610. doi: 10.1016/S0140-6736(05)66955-3. [DOI] [PubMed] [Google Scholar]