KEY POINTS
Targeted cancer therapies are a group of oral medications directed at tumours harbouring specific driver mutations that occur in a subset of patients with cancer.
Around one-third to one-half of patients with advanced non–small cell lung carcinoma may harbour an actionable mutation, which can be identified from molecular analysis of a biopsy or surgical specimen.
Patients treated with targeted therapy generally have better symptom control, response rates (i.e., shrinking tumours), and overall survival than those treated with conventional chemotherapy.
Targeted therapy is typically well tolerated and does not carry the same risks of emesis, alopecia, immunosuppression, and febrile neutropenia as chemotherapy.
Lung cancer is the leading cause of cancer-related death in Canada, with non–small cell lung carcinoma (NSCLC) making up 85% of cases.1 Lung cancer has been associated with a poor prognosis, particularly for patients with metastatic disease. Since Health Canada’s initial approval of gefitinib in patients with advanced NSCLC in 2003, targeted therapies have emerged as an important treatment option for patients and are now widely used in clinical practice.
What is targeted therapy?
Targeted therapies are a group of personalized anti-cancer medications. While chemotherapy attacks all rapidly dividing cells, targeted therapies select for cancer cells that harbour specific genomic driver alterations. Driver alterations are the principal trigger of growth for cancer cells, and many of these driver alterations now have matched targeted therapies. About 30%–50% of patients with NSCLC harbour a tumour-driver alteration and about 50%–75% of these are targetable.2 The prevalence of specific driver alterations varies by patient age, degree of tobacco exposure, ethnicity, and histology.2
Small-molecule tyrosine kinase inhibitors are the most common type of targeted therapy used in the treatment of NSCLC, and they work by inhibiting tyrosine kinase enzymes. Tyrosine kinase enzymes are membrane-spanning proteins made up of an extracellular (ligand-binding domain) and an intracellular kinase domain. Under normal physiologic conditions, ligand binding of the extracellular domain results in phosphorylation of downstream proteins, leading to their activation in a highly regulated fashion. The presence of an activating oncogenic alteration causes the tyrosine kinase enzyme to become constitutively active (i.e., does not need ligand binding to be in the “on” position), which drives cancer cell growth and proliferation. Targeted therapies treat cancer by inhibiting these overactive tyrosine kinase enzymes. Practically, targeted therapies are oral medications that can be taken at home without the number of hospital attendances associated with intravenous therapies.
Who is eligible for targeted therapy?
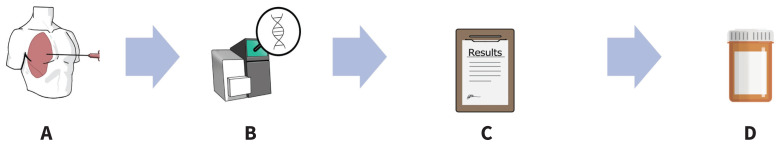
To assess whether targeted therapy is a suitable option, patients must undergo molecular profiling of their tumour. Molecular testing can be performed on most biopsy specimens, often on the same sample used to make the initial histopathologic cancer diagnosis. In some instances, especially when the procurement of a biopsy is difficult, analysis of circulating tumour DNA in the patient’s blood can also identify tumour-related gene alterations. Modern next-generation sequencing techniques can test for multiple alterations in parallel. Next-generation sequencing capacity has rapidly improved in Canada in recent years, although the turnaround time for molecular testing varies, with the ideal scenario being 1–2 weeks from biopsy to result. Figure 1 depicts the workflow in assessing candidacy for targeted therapy.
Figure 1:
A schematic depicting the workflow of assessing patients for and prescribing targeted therapy. (A) The patient undergoes a biopsy for histopathologic diagnosis of non–small cell lung carcinoma. (B) Tumour tissue is sent for molecular analysis. If a driver mutation is detected, the patient may be a candidate for a targeted agent. (C) The patient reviews the results with their oncologist and consents to targeted therapy. (D) The prescription can be filled at an outpatient pharmacy, and targeted therapy can be taken at home.
Molecular testing is performed reflexively (i.e., ordered by the pathologist) for newly diagnosed non-squamous lung cancers at most institutions, while others require a requisition from the oncologist. Many Canadian cancer centres offer in-house molecular testing, but others will send tissue samples externally for molecular analysis. All standard-of-care molecular testing can be performed in Canada.
What is the evidence of benefit?
Metastatic NSCLC is associated with poor clinical outcomes. Efficacy of cancer treatments is generally measured by response rates (proportion of patients with substantial tumour shrinkage), quality of life, and overall survival. Before the introduction of targeted therapy in the management of metastatic lung cancer, patients treated with chemotherapy had a median survival of less than 9 months, with response rates in the range of 25%–35%; only 10% of patients survived to the 2-year mark.3
Targeted therapy has improved outcomes tremendously, whereby most patients receiving a targeted drug have major tumour shrinkage, symptom improvement, and longer survival. 4 In the context of metastatic cancer, targeted therapies are given with palliative intent, with the goal improving quality of life and overall survival rather than to cure cancer. However, 2 targeted therapies, alectinib and osimertinib, have been shown to significantly decrease rates of recurrence when given as adjuvant treatment after surgery among patients with non-metastatic disease.5,6
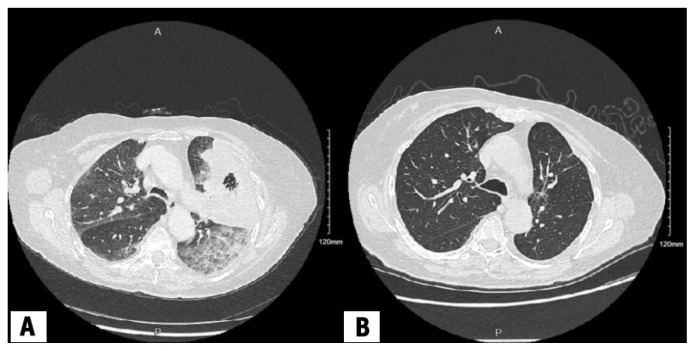
Ten different alterations have been identified, treated with 20 effective targeted therapies (Table 1).7 Mutations in the epidermal growth factor receptor (EGFR) gene were initially discovered in 2004, and are the most common mutations identified in NSCLC. Among patients harbouring a classic EGFR mutation, osimertinib, an EGFR kinase inhibitor, approved by Health Canada for the treatment of non-metastatic NSCLC in 2021, has proven to be the optimal agent, with a median overall survival of 38.6 months, compared with 31.8 months for people receiving older generations of EGFR kinase inhibitors.8 Patients with metastatic NSCLC involving anaplastic lymphoma kinase (ALK) translocations have a 5-year survival rate of more than 60% when treated with alectinib, compared with 45.5% among those treated with crizotinib, the first ALK inhibitor approved.9 For patients with RET translocations treated with selpercatinib, 84% of patients had a major tumour shrinkage, with the response being durable for close to 2 years, although no comparator arm was used in this single-arm, phase 2 study.10 Figure 2 shows the radiographic response of a patient harbouring a RET mutation treated with selpercatinib. Targeted agents directed at KRAS, BRAF, MET, NTRK, ROS1, and HER2 have also shown meaningful clinical benefit, although in some cases, the data are too recent to report 5-year survival rates.2,4 Patients can receive targeted therapy at home with remarkable response rates and the ability to maintain quality of life and symptom control, in addition to better odds at long-term survival.11–13
Table 1:
List of Health Canada–approved targeted agents by the genes harbouring the sensitizing alteration7
| Affected gene | Estimated prevalence in NSCLC tumours,* % | Approved targeted therapies |
|---|---|---|
| KRAS (G12C) | 12–15 | Sotorasib |
| EGFR | 12–15 | Afatinib, amivantamab, dacomitinib, erlotinib, gefinitib, osimertinib† |
| ALK | 2–7 | Alectinib, brigatinib, ceritinib, crizotinib, lorlatinib |
| BRAF | 2–4 | Dabrafenib–tramentinib |
| MET | 3 | Capmatinib, tepotinib |
| ROS1 | 1–2 | Crizotinib, entrectinib |
| RET | 1–2 | Pralsetinib, selpercatinib |
| NTRK | < 1 | Entrectinib, larotrectinib |
Note: NSCLC = non–small cell lung carcinoma.
Prevalence varies significantly by ethnicity.
Osimertinib is approved as adjuvant therapy after surgical resection.
Figure 2:
Axial computed tomography (CT) chest scans of a female patient in her late 80s, diagnosed with metastatic lung adenocarcinoma harbouring a RET rearrangement (ERC1 exon 17 to RET exon 12) treated with selpercatinib (A) at the time of diagnosis and (B) 9 months after starting therapy, showing radiographic response below the aortic arch.
The efficacy of targeted therapies is heterogeneous and varies depending on the specific drug and target protein. This results in variable magnitudes of benefit with regard to response rate, progression-free survival, and overall survival. Given the rarity of certain actionable mutations, many targeted agents have been studied only in single-arm, phase 2 clinical trials.
What are the harms?
Targeted therapies have more favourable adverse effect profiles than chemotherapy. Adverse effects are specific to the agent used and the protein being inhibited. They are usually mild and can typically be managed supportively. Table 2 provides an overview of adverse effects related to targeted therapy. If symptoms persist or affect a patient’s quality of life or functionality, dose reductions are usually considered as the next step in management. Severe or life-threatening adverse effects are uncommon; thus, the risk–benefit balance for targeted therapies almost always favours treatment, unlike traditional cytotoxic chemotherapy. Unlike chemotherapy, targeted therapies do not cause notable immunosuppression and most patients presenting with fever can be managed as per routine clinical practice. Most targeted agents require regular blood work to monitor blood counts and electrolytes, as well as kidney and liver function. Some agents may require cardiac testing (e.g., electrocardiography, echocardiography). This monitoring is typically organized by the patient’s oncologist.
Table 2:
Adverse effects related to targeted therapy classes, grouped by their target gene*
| Target gene | Common adverse effects | Serious adverse effects |
|---|---|---|
| KRAS (G12C) | Fatigue, nausea, diarrhea, elevated liver enzymes, arthralgias | Pneumonitis |
| EGFR | Rash, nail changes, diarrhea | Pneumonitis, cardiomyopathy |
| ALK | Laboratory abnormalities (elevation in cholesterol or triglycerides, creatine kinase, and glucose), peripheral edema, diarrhea, cognitive changes‡ | Bradycardia, pneumonitis |
| BRAF | Pyrexia, nausea, diarrhea, hypertension | Cardiomyopathy |
| MET | Peripheral edema, nausea, dyspnea, elevated creatinine, elevated amylase without pancreatitis§ | Pneumonitis, pleural effusions |
| ROS1 † | Diarrhea, nausea, visual changes, elevated liver enzymes, hypophosphatemia | – |
| RET | Diarrhea, dry mouth, hypertension, nausea, peripheral edema, hyponatremia | – |
| NTRK | Fatigue, constipation, dysgeusia, dizziness, dysesthesia, mood changes, peripheral edema | Mood disorder, increased risk of fractures (falls) |
List of adverse effects is not exhaustive; adverse effects may vary depending on the exact drug used.
In some instances, neurotrophic tyrosine receptor kinase inhibitors (e.g., entrectinib) are also used for patients with mutations in ROS1 fusion genes; shared adverse effects are listed under NTRK.
Adverse effects vary substantially from drug to drug.
Peripheral edema may be substantial.
What are the resource implications and how is targeted therapy accessed?
Targeted therapies are usually more costly than chemotherapy and sometimes more than immunotherapy. Using estimates from wholesale prices in the United States, the average cost of targeted therapy is US$5000–US$10 000 monthly. Treatments are usually continuous until signs of cancer progression in the metastatic setting. 14,15 However, costs vary considerably between agents, and are much lower in Canada than the list price as the prices paid by provinces are heavily discounted based on negotiation by the pan-Canadian Pharmaceutical Alliance, although these negotiated prices are not publicly available. In general, the costs of targeted therapies are likely to decrease as more agents expire from patent and become available as generic medications.
Interprovincial differences to access these drugs exist, leading to disparities across Canada. For instance, oral cancer drugs are funded the same way as intravenous drugs in some provinces (e.g., British Columbia), but through different pathways in other provinces (e.g., Ontario). Accessing therapies when they are not provincially funded is challenging. Options include public or private insurance, private pay, and compassionate access or co-pay programs through pharmaceutical companies.
What can be expected in the future?
Targeted therapy is the standard of care for patients with metastatic NSCLC driven by alterations in select oncogenes. In the future, these agents are likely to be used in earlier stages of disease with the aim of improving cure rates. Further expansion of targeted therapies will be observed as molecular testing becomes part of routine practice, as more patients with targetable mutations will be identified. More targetable alterations are being discovered. From the first mutation (EGFR) in 2004, to the second (ALK fusion) in 2009, 10 alterations have been identified, with other candidates in discovery. In addition, as mechanisms of resistance to targeted therapies are better understood, new agents are being developed that target resistant cancer cell clones.
Acknowledgement
The authors acknowledge Joshua Belair for assistance in preparing Figure 1.
Footnotes
Competing interests: Natasha Leighl reports research funding or materials from Amgen, AstraZeneca, Eli Lilly, EMD Serono, Guardant Health, Inivata, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda; honoraria from BeiGene, Bristol Myers Squibb, Janssen, Merck, Novartis, and Takeda; travel support from AstraZeneca, Merck Sharp & Dohme, Roche, Janssen, Sanofi, and Guardant Health; and participation on data safety monitoring boards for Mirati and Daichii Sankyo. Normand Blais reports honoraria from Amgen, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb, Eli Lilly, EMD Serono, Ipsen, Janssen, Merck, Novartis, Pfizer, Roche, Sanofi, Servier, and Takeda. Paul Wheatley-Price reports consulting fees or honoraria from Merck, AstraZeneca, Roche, Bristol Myers Squibb, Amgen, Lilly, Novartis, Sanofi, Pfizer, GSK, Janssen, SteriMax, Bayer, and Daiichi Sankyo. He participated on data safety monitoring board for the REaCT-HER TIME and POISE trials, and is a past president and current board member with Lung Cancer Canada. No other competing interests were declared.
This article has been peer reviewed.
For a patient perspective on targeted cancer therapy, see www.cmaj.ca/lookup/doi/10.1503/cmaj.240465
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
References
- 1.Brenner DR, Poirier A, Woods RR, et al. Projected estimates of cancer in Canada in 2022. CMAJ 2022;194:E601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chevallier M, Borgeaud M, Addeo A, et al. Oncogenic driver mutations in non-small cell lung cancer: past, present and future. World J Clin Oncol 2021;12:217–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8. [DOI] [PubMed] [Google Scholar]
- 4.Xiao Y, Liu P, Wei J, et al. Recent progress in targeted therapy for non-small cell lung cancer. Front Pharmacol 2023;14:1125547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y-L, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020;383:1711–23. [DOI] [PubMed] [Google Scholar]
- 6.Solomon BJ, Ahn JS, Dziadziuszko R, et al. LBA2 ALINA: efficacy and safety of adjuvant alectinib versus chemotherapy in patients with early-stage ALK+ nonsmall cell lung cancer (NSCLC). Ann Oncol 2023;34:S1295–6. [Google Scholar]
- 7.Tsao AS, Scagliotti GV, Bunn PA, et al. Scientific advances in lung cancer 2015. J Thorac Oncol 2016;11:613–38. [DOI] [PubMed] [Google Scholar]
- 8.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 9.Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056–64. [DOI] [PubMed] [Google Scholar]
- 10.Drilon A, Subbiah V, Gautschi O, et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol 2023;41:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majem M, Goldman JW, John T, et al. Health-related quality of life outcomes in patients with resected epidermal growth factor receptor–mutated non–small cell lung cancer who received adjuvant osimertinib in the phase III ADAURA trial. Clin Cancer Res 2022;28:2286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon BJ, Mok T, Kim D-W, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77. [DOI] [PubMed] [Google Scholar]
- 13.Petrillo LA, El-Jawahri A, Gallagher ER, et al. Patient-reported and end-of-life outcomes among adults with lung cancer receiving targeted therapy in a clinical trial of early integrated palliative care: a secondary analysis. J Pain Symptom Manage 2021;62:e65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih Y-CT, Smieliauskas F, Geynisman DM, et al. Trends in the cost and use of targeted cancer therapies for the privately insured nonelderly: 2001 to 2011. J Clin Oncol 2015;33:2190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skinner KE, Fernandes AW, Walker MS, et al. Healthcare costs in patients with advanced non-small cell lung cancer and disease progression during targeted therapy: a real-world observational study. J Med Econ 2018;21:192–200. [DOI] [PubMed] [Google Scholar]