Abstract
Background and Aims:
The largest cause of mortality in patients with inflammatory bowel disease (IBD) remains thrombo-embolic disease (TED). Recent reports have demonstrated that both monogenic and polygenic factors contribute to TED and 10% of ‘healthy’ subjects are genetically at high risk for TED. Our aim was to utilize whole exome sequencing (WES) and genome-wide genotyping to determine the proportion of IBD patients genetically at risk for TED and investigate the effect of genetic risk of TED in IBD.
Methods:
The TED polygenic risk score (PRS) was calculated from genome-wide genotyping. Thrombophilia pathogenic variants (TPV) were extracted from WES. In total, 792 IBD patients had both WES and genotyping data. We defined patients at genetically high risk for TED if they had a high TED PRS or carried at least one TPV.
Results:
We identified 122 out of 792 IBD patients (15.4%) as genetically high risk for TED. Among 715 out of 792 subjects whose documented TED status were available, 63 of the 715 patients (8.8 %) had TED events. Genetic TED risk was significantly associated with increased TED event (OR = 2.5, P = 0.0036).
Additionally, we confirmed an additive effect of monogenic and polygenic risk on TED (P = 0.0048). Patients with high TED genetic risk more frequently had thrombosis at multiple sites (78 % vs 42 %, OR = 3.96, P = 0.048).
Conclusions:
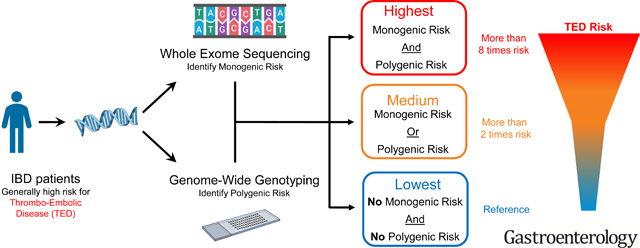
Genetic risk (both poly- and monogenic) was significantly associated with TED history. Our results suggest that genetic traits identify ~1 in 7 IBD patients who will experience 2.5-fold or greater risk for TED.
Keywords: Genetics, Inflammatory Bowel Diseases, Thrombosis
LAY SUMMARY
Our analyses demonstrated that approximately 1 in 7 inflammatory bowel disease patients are at around 2.5 times higher risk of developing Thrombo-embolic disease.
Graphical Abstract
Introduction:
The inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC), are chronic relapsing inflammatory conditions of the gastrointestinal tract1. The incidence of IBD is increasing with a prevalence of over 1.3% in the US2 and a global prevalence that surpasses 0.3%3. Patients with IBD are reported to have 3 – 4 fold increased risk of thrombo-embolic disease (TED) and the most significant cause of mortality in IBD remains TED4. This increased risk appears to be unique to IBD, as other chronic inflammatory diseases such as rheumatoid arthritis and celiac disease do not confer this risk5. More recently, TED also has been identified as a potential complication of JAK inhibition a mechanism of action recently approved for treating UC patients, which is now under FDA boxed warning for increased risk of thrombosis-associated morbidity and mortality. Due to the impact of TED on IBD prognosis and therapeutic management, developing methods to identify IBD patients at high risk for TED is an urgent clinical issue.
A number of factors may influence increased TED risk, including disease activity, hospitalization, age, pregnancy, medications, surgery, and genetics 6–10. Previous reports for genetic risks of TED in IBD patients have mainly focused on monogenic variants, such as Factor V Leiden deficiency10, 11. However, a recent study reported that risk of TED in the general population is influenced not only by monogenic risk, which classify individuals based on the presence or absence of discrete large effect variants, but also polygenic risk, which refers to the effect of numerous loci, often of small individual effect, across the genome. In a study containing two very large population cohorts, a combination of polygenic risk score (PRS, that aggregates multiple genetic TED risk variants) with two monogenic variants (Factor V Leiden deficiency and prothrombin G20210A mutation) delineated 10% of individuals with a ~2.5-fold increased likelihood of developing TED compared to non-high risk controls12. However, the prevalence and effects of polygenic and monogenic risk of TED in IBD patients has not previously been studied.
As the genetic etiology of TED becomes increasingly understood and the cost of genotyping/sequencing continues to decrease, the assessment of TED risk through genetics is becoming a viable clinical tool. If IBD patients with elevated risk of TED can be identified through genetic testing, there exists the potential to optimize drug therapy and the precise risks and benefits of anticoagulation prophylaxis can be considered on a personalized basis. To address this potential, the present study utilized whole exome sequencing (WES) to assess monogenic risk together with genome-wide genotyping to data to determine the proportion of IBD patients genetically at elevated risk for TED and investigate the effect of this risk on thrombotic events in IBD patients.
Methods:
Study design and subjects:
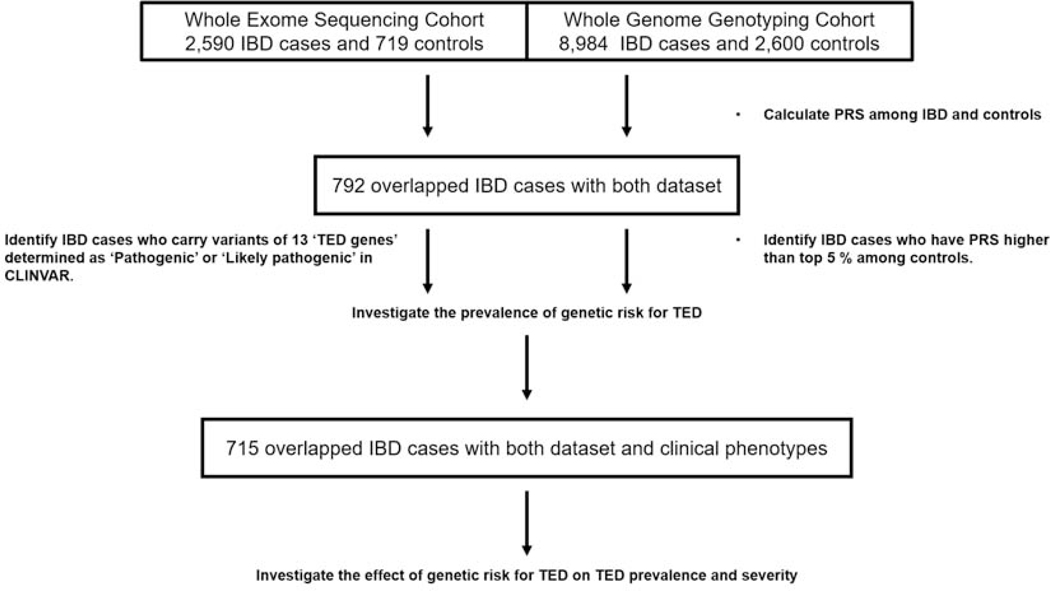
The study design is summarized in Figure 1. Samples were genotyped as part of the NIDDK IBDGC genotyping efforts. 11,584 samples were available following stringent quality control (details below), of which n=2,452 samples were recruited at Cedars-Sinai Medical Center (CSMC). The whole exome sequencing (WES) cohort consists of 3,198 subjects recruited at CSMC and 340 subjects provided to CSMC by the National Laboratory for the Genetics of Israeli Populations; all samples were sequenced at the BROAD Institute (see below). Admixture13 analysis was used to calculate ethnicity proportion estimations for all individuals. Only subjects identified by admixture as European ancestry [EUR proportion ≥ 0.70] were included in the further analyses.
Figure 1. Study design and cohorts.
‘Flow’ of study and outline of study cohorts. Detailed information is described in the methods section.
IBD, Inflammatory bowel disease; PRS, Polygenic Risk Score; TED, thrombo-embolic disease
792 Cedars-Sinai European ancestry IBD cases had both whole genome genotyping and WES data available. IBD was defined on the basis of clinical symptoms as well as standard endoscopic, radiographic, and histological findings. Detailed clinical data, including patient gender, age at diagnosis, disease location and behavior (according to the Montreal Classification), surgical history, family history of IBD and thrombo-embolic disease (TED) history were available in 715 cases and was collected from the medical records by two phenotypers (S.Y and L.A) who were blinded to the patients’ genotype information. The Institutional Review Board of Cedars-Sinai Medical Center approved the study, and all patients provided written informed consent.
GSA genotyping, QC and imputation:
Samples were genotyped on the Illumina Global Screening Array (GSA) at Feinstein Institute for Medical Research or at the Broad Institute in Massachusetts. Pre-imputation SNP QC metrics were applied for 700,078 SNPs including exclusion of non-autosomal markers and variants with MAF <1%, genotyping missingness >3% and deviation from Hardy-Weinberg equilibrium in controls P<1×10–6. A total of 11,584 samples (8,984 IBD and 2,600 control) passed sample quality control, which included exclusion of samples with genotyping call rate <95%, gender discrepancies, duplicated samples, EUR proportion < 0.70, ambiguous disease information and sample permission use restrictions. Genotypes were phased using Eagle v2.314 and imputation was performed using the Michigan Imputation Server15 per instructions and HRC r1.116 reference panel. Variants with estimated imputation accuracy (Rsq) <0.3 were excluded post-imputation prior to analyses. In total, 10,357,915 SNPs passed variant QC.
PRS calculation and normalization:
Among 297 variants reported in the TED PRS12, 265 variants were available in our imputed GSA cohort. Using these 265 variants we generated the TED PRS using PLINK v2.00a software17 for 2,600 controls and 8,984 IBD cases. We normalized PRS into Z-score and defined the top 5% normalized PRS among healthy control group as threshold.
Whole Exome-sequencing:
Paired-end WES was performed based on Illumina platform with 20X reading depth in 3,538 subjects. Reads alignment to the human reference genome GRCh37 were performed using BWA and variant calling were performed based on GATK best practices. Individual variants with Genotyping Quality (GQ) < 65, depth (DP) < 20, Strand Odds Ratio (SOR) > 3 or call rate < 95% were removed. For SNPs, variants with ReadPosRankSum < - 4 or Fisher Strand filter (FS) > 60 were also removed. For indels, variants with ReadPosRankSum < - 20 or FS > 200 were also removed. In total, 3,349,656 variants passed QC. Samples with a mean genotype quality (GQ) < 65, a depth < 25, a genotype rate < 96.5%, or a transition/transversion (Ti/Tv) ratio < 2.5 were removed from further analysis. Individuals of ambiguous disease information were removed. Individuals of ambiguous imputed sex or imputed sex inconsistent with reported sex were also removed. A total of 3,309 samples (2590 IBD and 719 controls) passed QC.
Thrombophilia pathogenic variants extraction:
Utilizing CLINVAR “Pathogenic” or “Likely Pathogenic” classification18 we extracted variants located within 15 blood clotting related genes yielding a total of 7 different thrombophilia pathogenic variants (TPV) (Supplementary Table 1 and Table 2). All QC and variant annotation was performed using Hail (Hail Team. Hail 0.2.36-ed011219dd93 https://github.com/hail-is/hail/releases/tag/0.2.36).
Table 2.
Characteristics of IBD patients with TED events.
| Low risk | High risk | P value | |
|---|---|---|---|
| N | 45 | 18 | |
| Female | 21 (46.67%) | 10 (55.56%) | 0.5850 |
| Male | 24 (53.33%) | 8 (44.44%) | |
| Interval from IBD onset to TED (year; mean +− SD) | 18.91 +− 13.97 | 11.62 +− 14.21 | 0.0863 |
| Age at TED (year; mean +− SD) | 45.45 +− 17.24 | 50.58 +− 18.84 | 0.3441 |
| Active disease at TED | 25 (71.43%) | 10 (83.33%) | 0.7027 |
| Subjects missing disease activity data | 10 | 6 | |
| Hospitalization within 3 months before TED | 14 (41.18%) | 8 (61.54%) | 0.3279 |
| Subjects missing hospitalizations data | 11 | 5 | |
| Current smoking | 13 (30.95%) | 3 (16.67%) | 0.5224 |
| Past smoking | 4 (9.52%) | 1 (5.56%) | 1.0000 |
| Never smoking | 25 (59.52%) | 14 (77.78%) | 0.1516 |
| Subjects missing smoking data | 3 | 0 | |
| IBD-related surgery within 6 months and prior to TED | 2 (5.41%) | 0 (0%) | 1.0000 |
| Subjects missing surgery data | 8 | 3 | |
| Women taking OCP at time of TED | 4 (22.22%) | 0 (0%) | 0.2800 |
| Subjects missing OCP data | 3 | 1 | |
| Biologic use at the time of TED | 16 (37.21%) | 12 (70.59%) | 0.0244 |
| Subjects missing biologic use data | 2 | 1 | |
| Multiple sites of TED | 19 (45.24%) | 14 (77.78%) | 0.0447 |
| Subjects missing multiple sites data | 3 | 0 | |
| Number of sites | 2.15 +− 2.23 | 3.33 +− 3.44 | 0.2343 |
Data are presented as means ± standard deviation (range), or n (%).
Percentage of OCP represents percentage within females.
TED, thrombo-embolic disease; High risk, genetically high risk for TED; Low risk, genetically low risk for TED; IBD, inflammatory bowel disease; OCP, oral contraceptive pills; Biologics, Infliximab, Adalimumab or Certolizumab.
Definitions:
We defined patients at high genetic risk for TED if they had a TED PRS more than the top 5% of the control population distribution or carried at least one TPV. This aligns with the report that individuals within the top 5% of the TED PRS have a ~2.5-fold increased risk of VTE relative to the rest of the population which is similar to the risk attributed to the presence of monogenic variants 12. Disease activity at the time of TED for Crohn’s Disease was measured by the Harvey-Bradshaw Index (HBI) and colonoscopy report at the time of clotting event (when available). Patients were considered to have active disease if they had HBI scores greater than or equal to 5 and/or endoscopy showed active disease, Disease activity at the time of TED for Ulcerative Colitis was evaluated by the full Mayo score. A full Mayo score above 2 was considered as active disease. Smoking status was defined as either currently smoking, past smoker, or never smoker as assessed at the most recent clinical visit. Patients who had history of IBD surgery were defined as having history of IBD related surgery (colectomy for UC and any bowel resection for CD) from their disease onset to last time of follow up. Hospitalization for any reason within 3 months before TED event and use of oral contraceptive pills (OCP) at the time of TED event was investigated. The use of any biologic therapy at the time of TED event were also investigated. Patients with TED were defined as patients who had a history of venous thrombosis at any site identified by ultrasonography or computed tomographic scanning. If patients had multiple episodes of TED events in their disease course, we considered the 1st time of TED in terms of time of TED event.
Statistical Analysis:
Fisher’s exact test (two-sided) was used to explore associations of categorical data in Table 1 and Table 2. Unpaired t test was used to explore quantitative data between two groups in Table 1 and Table 2. For the multivariate model in Table 1 and Supplementary Table 4, logistic regression with all univariate risk factors with a p value < 0.05 were included together in the multivariable logistic regression model along with the first two principle components. Linear trend test in logistic regression with age at last visit, history of IBD surgery, and the first two principle components was performed for Figure 2. Logistic regression with age at last visit, history of IBD surgery, and the first two principle components was performed in Figure 3. Logistic regression was performed with the first two principle components in Supplementary Table 5 and Supplementary Table 6. A p value of <0.05 was considered statistically significant. All statistical analyses were performed with R software (version 3.6.1) [http://www.rproject.org/].
Table 1.
Univariate and multivariate models of associations of Thrombo-embolic Disease (TED) history.
| TED = No | TED = Yes | Univariate P value | Multivariate P value | OR | 95%-CI | |
|---|---|---|---|---|---|---|
| N | 652 | 63 | ||||
| Disease type | ||||||
| CD | 511 (78.37%) | 46 (73.02%) | 0.3408 | |||
| UC | 129 (19.79%) | 16 (25.4%) | 0.3240 | |||
| IBDU | 12 (1.84%) | 1 (1.59%) | 1.0000 | |||
| Gender | ||||||
| Male | 349 (53.53%) | 32 (50.79%) | 0.6935 | |||
| Female | 303 (46.47%) | 31 (49.21%) | ||||
| Age at IBD onset (year) | 23.77 +- 12.74 | 30.11 +- 17.83 | 0.0075 | 0.0001 | 1.04 | 1.02–1.06 |
| Disease duration (year) | 19.83+−11.65 | 23.74+−13.87 | 0.0340 | 0.0083 | 1.03 | 1.01–1.05 |
| A1 | 173 (34.06%) | 12 (26.09%) | 0.1515 | |||
| A2 | 287 (56.5%) | 27 (58.7%) | 0.6424 | |||
| A3 | 48 (9.45%) | 7 (15.22%) | 0.3119 | |||
| Subjects missing age at onset data | 3 | 0 | ||||
| Disease location | ||||||
| L1 | 66 (13.15%) | 2 (4.35%) | 0.1015 | |||
| L2 | 61 (12.15%) | 5 (10.87%) | 1.0000 | |||
| L3 | 375 (74.7%) | 39 (84.78%) | 0.1121 | |||
| L4 | 85 (16.63%) | 7 (15.22%) | 1.0000 | |||
| Subjects missing disease location data | 9 | 0 | ||||
| Disease behavior | ||||||
| B1 | 183 (36.31%) | 12 (26.09%) | 0.2008 | |||
| B2 | 253 (50.2%) | 27 (58.7%) | 0.2340 | |||
| B3 | 192 (38.1%) | 25 (54.35%) | 0.0600 | |||
| Subjects missing disease behavior data | 7 | 0 | ||||
| Perianal disease | 296 (57.93%) | 28 (60.87%) | 0.7565 | |||
| Disease extent | ||||||
| E1 | 12 (9.68%) | 0 (0%) | 0.3620 | |||
| E2 | 41 (33.06%) | 4 (25%) | 0.7762 | |||
| E3 | 71 (57.26%) | 12 (75%) | 0.1811 | |||
| Subjects missing disease extent data | 5 | 0 | ||||
| Extensive disease (E3 or L3) | 446 (69.91%) | 51 (80.95%) | 0.1296 | |||
| Smoking status | ||||||
| Current smoking | 141 (22.63%) | 16 (26.67%) | 0.5203 | |||
| Past smoking | 38 (6.1%) | 5 (8.33%) | 0.4148 | |||
| Never smoking | 444 (71.27%) | 39 (65%) | 0.3024 | |||
| Subjects missing smoking data | 29 | 3 | ||||
| IBD family history | 200 (31.6%) | 13 (21.67%) | 0.1425 | |||
| Subjects missing IBD family history data | 19 | 3 | ||||
| Surgery history | ||||||
| IBD-related bowel surgery | 375 (57.52%) | 48 (76.19%) | 0.0045 | 0.0120 | 2.24 | 1.19–4.20 |
| CD-related bowel surgery | 333 (65.17%) | 38 (82.61%) | 0.0147 | |||
| Colectomy in UC | 38 (29.46%) | 10 (62.5%) | 0.0116 | |||
| Genetic risk (high) | 91 (13.96%) | 18 (28.57%) | 0.0050 | 0.0036 | 2.5 | 1.35–4.64 |
Data are presented as means ± standard deviation (range), or n (%).
UC, ulcerative colitis; CD, Crohn’s disease; IBDU, Inflammatory bowel disease undetermined;
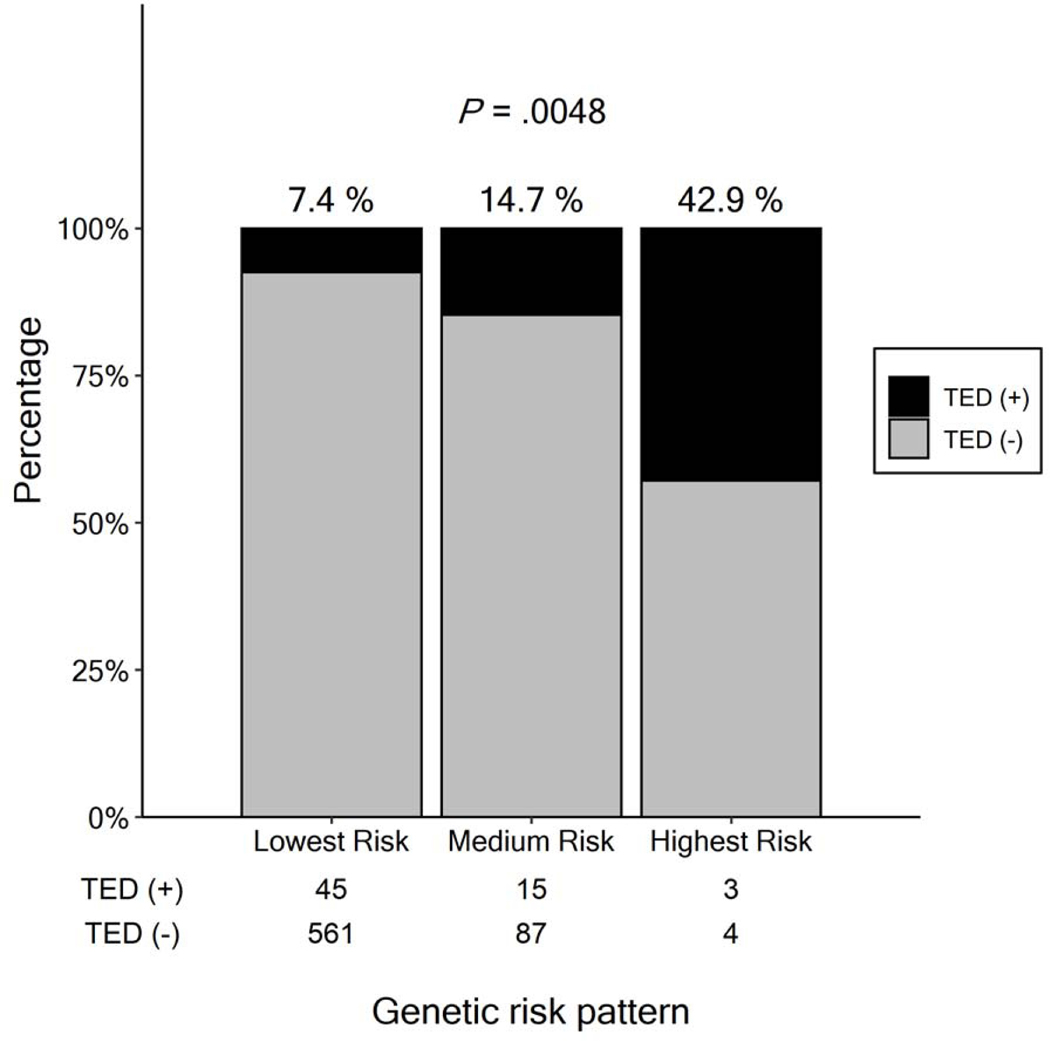
Figure 2. Percentage of TED events in each Genetic Risk Group.
The percentage of patients with thrombo-embolic disease (TED) in each group. X-axis shows classification of genetic risk for TED (“Lowest Risk”: patients without genetic risk; “Medium Risk”: patients who have one risk, either high PRS OR carriage of a TPV; “Highest Risk”: patients who both high PRS and a TPV). The numbers below each bar represents number of TED positive and negative cases in each group. P value was calculated by trend test.
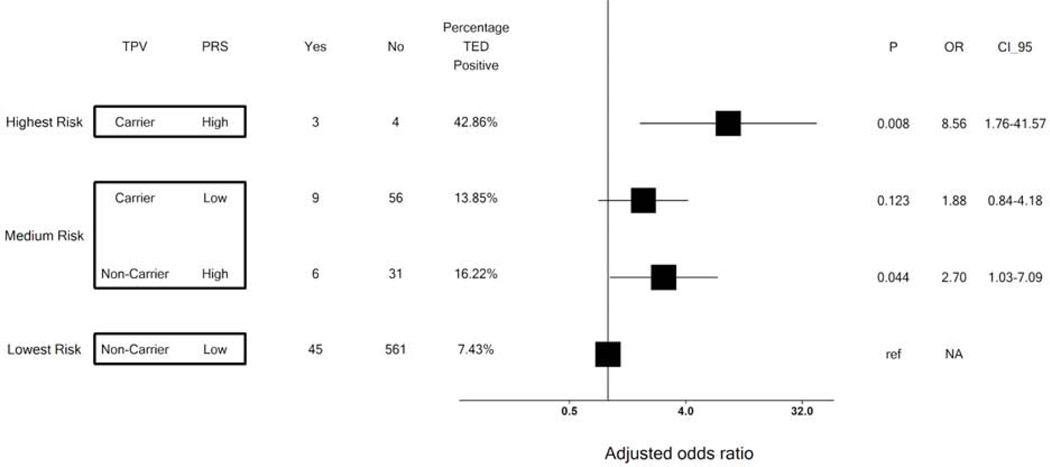
Figure 3. Risk of TED by monogenic and polygenic risk status.
Patients were stratified into two groups according to their polygenic risk score (PRS) — high or low defined as more than the top 5% of the control population distribution or the other 95%, respectively. For carriers and non-carriers of thrombophilia pathogenic variant (TPV) in each PRS group, the p value and odds ratio (OR) for thrombo-embolic disease (TED) were calculated in a logistic regression model with age at last visit and the first two principal components as covariates. Non-carriers with low PRS served as the reference group. Each black square represents mean of OR in each group and horizontal line around each square represents the 95% confidence.
Results:
Prevalence of IBD patients who are genetically high risk at TED;
Among the 792 IBD subjects with both PRS and WES data, 49 subjects had a high PRS and 82 subjects carried at least one variant among the 7 identified TPVs, including Factor V Leiden, and the prothrombin G20210A mutation (Supplementary Table 2 for full list of TPVs). In total, 122 out of 792 IBD patients (15.4%) were identified as genetically ‘high risk’ for TED.
Difference of TED genetic risk among IBD and controls;
There was no difference in: TED PRS distribution; frequency of Factor V Leiden mutation; and frequency of prothrombin G20210A mutation between 8,984 IBD cases and 2,600 controls (P = 0.84, 0.26, and 0. 94 respectively; Supplementary Table 3).
Effect of genetic risk on TED event in multivariate model;
Among the 792 subjects with WES and genotyping data, detailed longitudinal clinical information on TED events was available for 715 subjects (109 high risk and 606 non risk patients). In total, 63 of the 715 patients (8.8 %) had a documented TED event. TED patients had significantly longer disease duration (23.7 years vs 19.8 years, P = 0.034), were older at IBD onset (30.1 years vs 23.7 years, P = 0.0075) and were more likely to have had IBD surgery (OR = 2.36, P = 0.0045). No other demographic or clinical factors were statistically associated with TED (Table 1). After adjustment for age, disease duration and history of IBD surgery, genetic TED risk was significantly associated with increased TED event (OR = 2.5, P = 0.0036, Table 1). Additionally, after adjusting multicollinearity between disease duration and age at disease onset, both high PRS and carriage of TPV were independently associated with TED, respectively (OR = 3.13, P = 0.0070 and OR = 2.11, P = 0.042, Supplementary Table 5B).
Additive effect of genetic risk on TED event in IBD;
We subsequently confirmed an additive effect of genetic risk on TED: patients with both a high PRS and carrying pathogenic variants are at the highest risk of TED; patients who have one risk factor (either high PRS OR carriage of a TPV) were medium risk: and patients without either of these genetic risk factors were at lowest risk (43 %, 15 % and 7 % respectively, P = 0.0048; trend test) (Figure 2). Among patients with medium risk, patients with PRS had slightly higher risk than patients with TPV only (OR = 2.8 vs 1.8, respectively, Figure 3).
Characteristic of patients with genetic risk within TED cases;
Patients with high TED genetic risk tended to have shorter time from IBD onset to TED event (11.62 years vs 18.91 years, P= 0.086) and were more likely to have thrombosis documented at multiple sites (78 % vs 42 %,, OR = 3.96, P = 0.048) (Table 2). Among these 2 groups, disease activity at the time of TED, hospitalization within 3 months before TED and surgery before TED were not significantly different between the two 2 groups. Patients with high TED genetic risk were more likely to have received biologics (71% vs 37%, OR = 3.95, P = 0.024). Biologics have previously been reported to have protective effect19, 20 on TED.
Discussion:
By aggregating whole genome genotyping and WES data, our analyses demonstrate that ~1 in 7 IBD patients have odds 2.5 times higher than non-genetically high risk IBD patients for experiencing TED. Higher genetic ‘risk’ was associated with TED events suggesting that these IBD patients may warrant more aggressive prophylaxis against TED and also might be subjects in whom JAK inhibitors may need to be used judiciously. TED PRS and TPV were independently associated with TED and also have additive effects on TED events. Furthermore, our analysis within TED cases suggests genetic risk also affects disease severity of TED. Although the number of patients who ‘carry’ both PRS and TPV risks is small (7 patients), three developed TED at multiple sites and at a young age (mean 27.5 years).
The prevalence of TED in our IBD cohort was 8.8 %, consistent with previous findings21. The risk of TED in IBD patients is reported to be 3 to 4-fold higher compared to the general population6, which is attributed to risk factors including disease flare, extended disease location, and steroid use22. Our study confirmed long disease duration, older age at IBD onset and history of IBD-related surgery are risk factors for TED. Disease duration and age at onset are strongly associated with age at last visit to hospital (Supplementary Figure 1A and 1B). If we include all these 3 parameters into multivariate model, all of them became nonsignificant because of multicollinearity. Variance inflation factors (VIF) of age at last visit, disease duration and age at diagnosis in the model are 26.50, 13.65 and 17.11, respectively, which indicates strong multicollinearity among these variables (Supplementary Table 4A). If we combined disease duration or age at IBD onset with age at last visit, only age at last visit remains significant (Supplementary Table 4B and 4C). Age at last visit approximates current age, therefore, our study suggests older age, a well-established risk factor for TED, is the significant demographic risk factor for TED history 23, 24. We also confirmed that history of IBD-related surgery was significantly associated with TED history (OR = 3.95 and 2.54 for colectomy history in UC and bowel resection for CD respectively). Importantly, this effect was independent from time-dependent parameters. IBD-related surgery has previously been reported to be one of the established risk factors of TED in multiple studies with a higher risk observed in UC cases requiring colectomy than CD-related surgeries25, 26,27, 28. The elevated risk of UC-related surgeries probably reflects, in part, the higher inflammatory burden and perhaps this is a population in whom an increased understanding of genetic risk might have the largest impact. Importantly, genetic factors remained significant even after adjusting for age at last visit and history of IBD-related surgery, (Supplementary Table 5A and 5B).
In the within TED patients analyses (Table 2), we found that a high proportion of patients (74 %) had active disease at the time of TED event which is consistent with previous reports including in a single-center retrospective study, where 71% of IBD patients were found to have active disease at the time of the TED event29. Additionally, nearly half (47 %) of patients had experience of hospitalization within 3 months before TED event. These factors were not different between patients with or without genetic TED risk. Our results support prior evidence that active disease and hospitalization are risk factors of TED4, 6, 30, and demonstrate that these are independent from genetic risk. In our cohort, very few patients had undergone IBD-related surgery within 6 months before TED event, were taking OCPs at the time of TED (table 2) and no one was receiving prophylactic warfarin nor JAK inhibitor at the time of TED. Interestingly, patients with genetic risk of TED were receiving biologics more frequently than those without genetic risk at time of TED, whereas disease activity was not different between the 2 groups. Generally, biologics have been reported to have protective effect on TED19, 20 suggesting that our findings may have underestimated the effect of genetic variation on TED risk. Finally, we found patients with genetic risk had multiple site TED more frequently than those without genetic risk, which may indicate a more aggressive course of TED.
We did not find an elevated PRS TED distribution in IBD compared to controls. Additionally, the frequency of the two major TPVs (factor V Leiden and prothrombin G20210A mutation) were not elevated in IBD, consistent with previous studies21 10. Thus, elevated genetic burden of TED risk does not alone explain the increased risk of TED in IBD patients (Supplementary Table 3).
The relationship between TPVs and disease behavior or extent in IBD has not previously been studied and any relationship is important for interpreting our findings. We observed no association between elevated genetic burden (carriage of TPV and high PRS) and disease behavior and extent of disease (Supplementary Table 6 A and B).
In our analyses, access to WES enabled us to also include ‘rare’ TPVs beyond factor V Leiden and prothrombin G20210A mutation. As shown in Supplementary Table2, we identified 5 out of 13 (38%) TED patients with ‘other’ TPVs suggesting that including only factor V Leiden and Prothrombin variants would miss over one third of ‘monogenic’ TPVs. Thus, looking at both common and rare TPVs is necessary for a more comprehensive estimate of monogenic TED risk. Furthermore, the additive effect we have shown here suggests that an accurate estimation of genetic risk requires both TPV and PRS assessment and that PRS may define a higher TED risk than TPVs alone.
With decreasing costs of sequencing and moves towards genomic medicine, it is likely that increasing numbers of people will have their genome sequenced making ‘routine’ integration of genetic risk assessment for various traits including TED possible. Our data suggest that ~1 in 7 IBD patients is at higher risk of TED and those IBD patients are at around 2.5 times increased risk of TED. Considering the relatively high prevalence of genetic risk and the fact that TED can lead to significant morbidity and even mortality, ‘routine’ screening of TED genetic risk for IBD patients may beneficially impact these poor outcomes. Further studies are warranted including, perhaps, randomized controlled trials of prophylactic anticoagulation in IBD patients at high risk of TED stratified by ‘genetic risk.’ With likely increasing availability of these types of data the IBD community requires the development of guidelines and strategies to determine whether these patients should be counseled about long term TED prophylaxis and also whether drugs such as JAK inhibitors should be used cautiously or avoided in this setting.
Our study has limitations. First, since our study is a retrospective study and there are some missing clinical information such as corticosteroid use, indwelling catheters, and family history of TED. Thus, it is difficult to estimate accurate effects of some clinical factors on TED. Second, since not all variants of TED PRS were available in our study, the result might be slightly different if all variants were included although if this has had an effect it is likely to have underestimated the true genetic contribution to TED. Third, since our study is a single centered analysis, the number of subjects is relatively small and additional cohorts will need to be studied. In addition, as is the case with the majority of genetic studies currently, our study is limited to European ancestry subjects and future studies will be needed in other populations so that the benefits of Precision Medicine approaches such as the one described are available to all parts of society.
In conclusion, we have demonstrated that ~1 in 7 IBD patients are genetically at a higher risk for TED, and genetic risk is independently associated with TED events when adjusting for time-dependent parameters and IBD-related surgery. For comprehensive ‘prediction’ of genetic risk of TED both monogenic and polygenic approaches are needed. To our knowledge, this is the first report suggesting benefits for clinical decision making in IBD through combining both WES and whole genome genotyping. With increased interest in genomic sequencing/genotyping for clinical utility our findings suggest that strategies for managing patients at high risk of TED identified through genetic approaches should be developed.
Supplementary Material
Supplementary Figure 1. Association of age at last visit with disease duration and age at diagnosis.
Figure 1A shows association between age at last visit and disease duration. X axis represents age at last visit and Y axis represents disease duration. P value for association was calculated by linear regression without any covariates. Blue line represents regression line and shaded area represents 95 % confidence.
Figure 1B shows association between age at last visit and age at diagnosis. The form of figure is the same as described for Figure 1A.
WHAT YOU NEED TO KNOW.
Background and Context:
Patients with inflammatory bowel disease (IBD) are at high risk of Thrombo-embolic disease (TED); however, prevalence and effect of genetic risk of TED in IBD remains unknown.
New Findings:
By aggregating whole exome sequencing (WES) and whole-genome genotyping data, we identified that ~1 in 7 IBD patients are genetically at around 2.5 times higher risk for TED. Genetic risk was significantly related with increased risk of TED events and there was an additive effect of monogenic and polygenic risks.
Limitations:
Number of cases in our study was limited (N = 792). Thrombo-embolic events were determined retrospectively by chart review blinded to genetic status.
Impact:
We found that more than 15 % of IBD patients are at higher risk of TED. Genetic test by combining monogenic and polygenic risk can identify IBD patients with higher risk of TED. WES provides a more comprehensive evaluation of genetic risk in IBD.
Acknowledgement;
DNA samples for a subset of Jewish controls from Cedars-Sinai Medical Center were obtained from The National Laboratory for the Genetics of Israeli Populations at Tel-Aviv University http://yoran.tau.ac.il/nlgip/. This study was supported by the Cedars-Sinai MIRIAD IBD Biobank. The MIRIAD IBD Biobank is supported by the Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, National Institute of Diabetes and Digestive and Kidney Disease Grants P01DK046763 and U01DK062413, and The Leona M. and Harry B. Helmsley Charitable Trust.
Funding:
This work was supported by internal funds from the F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute. The Cedars-Sinai MIRIAD IBD Biobank is supported by the F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases [NIH/NIDDK] [grants P01 DK046763 and U01 DK062413], and The Leona M and Harry B Helmsley Charitable Trust.
Footnotes
Disclosure;
DPBM, TH, DL, and JB are faculty members at Cedars-Sinai Medical Center. TN, MK, GB, SH, LA, EM are employees at Cedars-Sinai. Cedars-Sinai has financial interests in Prometheus Biosciences, Inc., a company which has access to the data and specimens in Cedars-Sinai’s MIRIAD Biobank (including the data and specimens used in this study). Prometheus Biosciences, Inc. seeks to develop commercial products. DPBM and DL are paid consultants and shareholders of Prometheus Biosciences, Inc. DPBM: Consultant (Gilead Sciences, Boehringer-Ingelheim, Pfizer, Bridge Biotherapeutics, Qu Biologics, Prometheus Biosciences, Takeda, Palatin Technologies). Grant support (Janssen).
Date repository;
Our original data including raw genetic data and metadata is available at github (https://github.com/mcgovernlab).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
NIDDK IBD Genetics Consortium:
Lisa Abbou, Emebet Mengesha, Christine Stevens, Atsushi Masamune, Mark Daly, and Dermot P.B. McGovern
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:1166–1169. [DOI] [PubMed] [Google Scholar]
- 3.Ng SC, Shi HY, Kaplan GG et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol 2008;103:2272–80. [DOI] [PubMed] [Google Scholar]
- 5.Miehsler W, Reinisch W, Valic E, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut 2004;53:542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63. [DOI] [PubMed] [Google Scholar]
- 7.Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697–706. [DOI] [PubMed] [Google Scholar]
- 8.Kappelman MD, Horvath-Puho E, Sandler RS, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut 2011;60:937–43. [DOI] [PubMed] [Google Scholar]
- 9.Sarlos P, Szemes K, Hegyi P, et al. Steroid but not Biological Therapy Elevates the risk of Venous Thromboembolic Events in Inflammatory Bowel Disease: A Meta-Analysis. J Crohns Colitis 2018;12:489–498. [DOI] [PubMed] [Google Scholar]
- 10.Papa A, Danese S, Grillo A, et al. Review article: inherited thrombophilia in inflammatory bowel disease. Am J Gastroenterol 2003;98:1247–51. [DOI] [PubMed] [Google Scholar]
- 11.Liang J, Wu S, Feng B, et al. Factor V Leiden and inflammatory bowel disease: a systematic review and meta-analysis. J Gastroenterol 2011;46:1158–66. [DOI] [PubMed] [Google Scholar]
- 12.Klarin D, Busenkell E, Judy R, et al. Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease. Nature Genetics 2019;51:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res 2009;19:1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh PR, Danecek P, Palamara PF, et al. Reference-based phasing using the Haplotype Reference Consortium panel. 2016;48:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das S, Forer L, Schönherr S, et al. Next-generation genotype imputation service and methods. Nat Genet 2016;48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy S, Das S. A reference panel of 64,976 haplotypes for genotype imputation. 2016;48:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CC, Chow CC, Tellier LC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landrum MJ, Lee JM, Benson M, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res 2018;46:D1062–d1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.deFonseka AM, Tuskey A, Conaway MR, et al. Antitumor Necrosis Factor-α Therapy Is Associated With Reduced Risk of Thromboembolic Events in Hospitalized Patients With Inflammatory Bowel Disease. J Clin Gastroenterol 2016;50:578–83. [DOI] [PubMed] [Google Scholar]
- 20.Higgins PD, Skup M, Mulani PM, et al. Increased risk of venous thromboembolic events with corticosteroid vs biologic therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 2015;13:316–21. [DOI] [PubMed] [Google Scholar]
- 21.Giannotta M, Tapete G, Emmi G, et al. Thrombosis in inflammatory bowel diseases: what’s the link? Thromb J 2015;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng K, Faye AS. Venous thromboembolism in inflammatory bowel disease. World J Gastroenterol 2020;26:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faye AS, Wen T, Ananthakrishnan AN, et al. Acute Venous Thromboembolism Risk Highest Within 60 Days After Discharge From the Hospital in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2020;18:1133–1141.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCurdy JD, Israel A, Hasan M, et al. A clinical predictive model for post-hospitalisation venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2019;49:1493–1501. [DOI] [PubMed] [Google Scholar]
- 25.Alhassan N, Trepanier M, Sabapathy C, et al. Risk factors for post-discharge venous thromboembolism in patients undergoing colorectal resection: a NSQIP analysis. Tech Coloproctol 2018;22:955–964. [DOI] [PubMed] [Google Scholar]
- 26.McKechnie T, Wang J, Springer JE, et al. Extended thromboprophylaxis following colorectal surgery in patients with inflammatory bowel disease: a comprehensive systematic clinical review. Colorectal Dis 2020;22:663–678. [DOI] [PubMed] [Google Scholar]
- 27.Wallaert JB, De Martino RR, Marsicovetere PS, et al. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum 2012;55:1138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alatri A, Schoepfer A, Fournier N, et al. Prevalence and risk factors for venous thromboembolic complications in the Swiss Inflammatory Bowel Disease Cohort. Scand J Gastroenterol 2016;51:1200–5. [DOI] [PubMed] [Google Scholar]
- 29.Bollen L, Vande Casteele N, Ballet V, et al. Thromboembolism as an important complication of inflammatory bowel disease. Eur J Gastroenterol Hepatol 2016;28:1–7. [DOI] [PubMed] [Google Scholar]
- 30.Jackson E, Curtis KM, Gaffield ME. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol 2011;117:691–703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Association of age at last visit with disease duration and age at diagnosis.
Figure 1A shows association between age at last visit and disease duration. X axis represents age at last visit and Y axis represents disease duration. P value for association was calculated by linear regression without any covariates. Blue line represents regression line and shaded area represents 95 % confidence.
Figure 1B shows association between age at last visit and age at diagnosis. The form of figure is the same as described for Figure 1A.