Abstract
Background
Exercise therapy (ET) is frequently an early treatment of choice when managing shoulder pain, yet evidence on its efficacy to expedite recovery is inconsistent. Moreover, the value of adding adjunct therapies (i.e. injections, manual therapy, electrotherapy) to ET is currently unclear. This study combined both direct and indirect evidence across studies on the effectiveness of ET with/without adjunct therapies compared to usual medical care for adults with chronic shoulder pain.
Methods and findings
Using a network meta-analysis, randomized control trials comparing ET along with adjunct therapies were identified in MEDLINE, Embase, CINAHL, Sportdiscus, CENTRAL, Conference Proceedings Citation Index-Science, clinicaltrials.gov, and association websites. Outcomes included pain, range of motion (ROM), and health-related quality of life (HRQL) measures in adult patients with chronic shoulder pain. Data analysis used a Frequentist hierarchical model. CINeMA tool assessed the confidence in the results and Cochrane Risk of Bias tool assessed quality of studies.
54 studies primarily from Europe (40.38%) included 3,893 participants who were followed up to 52 weeks. Shoulder-specific ET (Mean difference (MD) = -2.1; 95% confidence interval (CI) = -3.5 to -0.7) or in combination with electro-physical agents (MD = -2.5; 95% CI = -4.2 to -0.7), injections (MD = -2.4; 95% CI = -3.9 to—1.04) or manual therapy (MD = -2.3; 95% CI = -3.7 to -0.8) decreased pain compared to usual medical care. Trends with ROM and HRQL scores were seen; however, only Manual Therapy (MD = -12.7 and 95% CI = -24.4 to -1.0) achieved meaningfully important changes. Sensitivity analysis excluding studies with high risk of bias showed similar results, with exception of injections that did not reach significance (MD = -1.3; 95% CI = -4.3 to 1.7).
Conclusion(s)
Shoulder-specific ET provided pain relief up to 52 weeks. Adjunct therapies to shoulder-specific ET added little value in reducing pain. The quality of evidence varied between moderate and very low.
Introduction
Chronic shoulder pain is highly prevalent, with incidence rates ranging from 7.7 to 62 per 1000 persons per year and community prevalence ranging from 0.67 to 55.2% worldwide [1]. It significantly impacts patients’ quality of life including health-related quality of life (HRQL) and health care utilization [2]. In Canada, for example, treating chronic shoulder pain due to rotator cuff tears has an estimated cost between Can$43million and Can$101 million annually [3]. Evidence-based guidelines on effective management strategies for chronic shoulder pain are unclear due to high heterogeneity in treatment approaches, patient populations and study methodologies used [4]. Current clinical recommendations suggest a trial of conservative management (i.e., physiotherapy, medications) followed by surgery when conservative management is ineffective for chronic shoulder pain [5]. Rehabilitation of chronic shoulder pain through exercise therapy (ET) appear to be effective in pain relief and function gains, leading to increased participation in daily activities and better HRQL [6].
Following usual medical care (information, recommendations, and medical or pharmaceutical therapy as needed), exercise therapy (ET) is frequently a treatment choice when managing shoulder pain, yet evidence on its efficacy to expedite recovery is inconsistent [4,7]. Moreover, the value of adjunct therapies such as manual therapy, electro-physical agents, medications, and injections with ET is currently inconsistent. Although ET for shoulder pain is supported by 10 systematic reviews [7–16], only two [7,12] had strong recommendations for the use of ET. While findings from seven systematic reviews support using a combination of manual therapy (MT) and ET for pain relief and functional improvement, others state inconclusive evidence to support a combination of MT and ET. Inconclusive findings are also reported with the use of corticosteroid injections [15,17]. Shoulder diagnosis, ET definitions, follow-up time are highly variable among these systematic reviews and limit comparison.
Current systematic reviews on the benefits of conservative management for shoulder pain are mostly based on either descriptive analysis or limited meta-analysis, due to data heterogeneity with variability seen with outcomes, timelines, treatment length, follow-ups, and case definitions. A persuasive concern with many of these reviews is that ET was evaluated as one general approach, although ET consists of several different approaches including shoulder-specific strengthening and ROM exercises with/without scapular exercises to non-specific shoulder exercises such as postural and functional exercises. While the effectiveness of different types of ET has been evaluated with small clinical samples and systematic reviews, the different types of ET has yet to have head-to-head comparisons. Within a clinical context, ET is not always used alone but rather with adjunct therapy. Using a network meta-analysis, this study combined both direct and indirect evidence across studies on the effectiveness of ET with/without adjunct therapies compared to usual medical care for adults with chronic shoulder pain.
Material and methods
This network meta-analysis (NMA) is registered in the PROSPERO database (CRD 4201935093). Initially, the protocol published at PROSPERO stated that a meta-analysis was planned; however, we amended the protocol to include a network meta-analysis instead to enable the use of both direct and indirect evidence. We also amended the protocol to add the following inclusion criteria: “At least 6 weeks follow-up” and “More than 3 months of symptoms (chronic)”. Such criteria were important to better define the population being studied and the changes were made before the review started. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (S1 Appendix) extension statement for network meta- analyses was followed [18].
Eligibility criteria
This NMA included randomized or quasi-randomized control trials comparing ET with or without adjunct therapies in adult participants (aged 18 years or older) with shoulder pain for at least 3 months. At least one of the comparative groups needed to have ET as an intervention and follow-up time needed to be at least 6 weeks to detect true effect of ET. We excluded participants with previous surgery to the affected shoulder, history of shoulder dislocation, inflammatory disease, adhesive capsulitis (Frozen shoulder), scapular dyskinesis, major shoulder joint trauma, infection, avascular necrosis or neuropathy, or concomitant neck pathology. Studies not in English language, including fewer than 20 participants in the cohort, or examining holistic treatments were also excluded.
Information sources and search
A research health sciences librarian developed and conducted a systematic search of the following databases up to May/2022 and limited to English language: MEDLINE, Embase, CINAHL, Sportdiscus, CENTRAL, Conference Proceedings Citation Index- Science (CPCI-S), clinicaltrials.gov, and association websites (Canadian Academy of Sport and Exercise Medicine, Canadian Athletic Therapists Association, Canadian Physiotherapy Association, College of Family Physicians of Canada–Sport & Exercise Committee, Exercise is Medicine Canada, Ontario Athletic Therapist Association, Ontario Medical Association–Section on Sport & Exercise Medicine, Sport Physiotherapy Canada). Search strategy can be found in (S2 Appendix).
Study selection
Two independent reviewers (AS, CL) used CovidenceTM [19], for title, abstract, full text screening and data extraction. Disagreement of article inclusion was resolved through consensus between reviewers or through third party adjudication if the reviewers did not arrive at consensus. Study authors were contacted if further clarifications regarding study methods and/or results were needed.
Data extraction
Two independent reviewers (AS, CL) extracted the following data from eligible studies:
demographics (number participants, age, sex, year, country, and diagnosis), interventions (type, duration, retention, maximum follow-up time) and outcomes (Pain, ROM, HRQL). All outcomes were extracted for the following timelines: post-intervention (first follow-up once intervention was completed) and longest follow-up (last study follow-up). If outcome information was unclear in the manuscript, we contacted the authors for clarifications.
Quality and publication bias assessment
Two independent reviewers (AS, CM) used the Revised Cochrane risk-of-bias tool for randomized trials (RoB2) to assess the quality of each study [20]. Overall bias scores used the following criteria: low risk of bias (all domains were low), some concerns (at least one domain had some concerns, but none had high risk of bias) and high risk of bias (at least one domain was high or had some concerns in multiple domains that decreased the confidence in the result) [20].
Risk Of Bias due to Missing Evidence in Network meta-analysis (ROB-MEN) [21] assessed risk of bias due to missing evidence (publication bias) for all included pairwise comparisons. This assessment considered: 1. the contribution of direct comparisons to the network meta-analysis estimates, 2. the potential presence of small-study effects, and 3. any bias from unobserved comparisons. The automatized tool then assigned a level of low risk, some concerns, or high risk of bias [21].
Certainty of evidence
Reviewers used the Confidence in Network Meta-Analysis (CINeMA) [22,23] to assess the certainty of evidence for all outcomes considering the following domains: within-study bias, reporting/publication bias, indirectness, imprecision, heterogeneity, and incoherence. Final judgment summary across all domains were based on GRADE framework [22,23]. Reviewers took into consideration that domains may be interconnected and followed CINeMA guidelines for judgment to avoid downgrading the overall level of confidence more than once for related concerns. Indirectness and incoherence were considered correlated and heterogeneity, imprecision, within-study bias and reporting bias were considered correlated.
This NMA included the assumptions of consistency and transitivity. CINeMA assessed consistency though the design-by-treatment test and by separating indirect from direct evidence (SIDE test) using the R netmeta packageTM. For the transitivity assumption, CINeMA considers indirectness through the distribution of potential effect modifiers, and statistical incoherence through the SIDE test [22,23].
Outcomes
Based on the literature, we anticipated ET with or without adjunct therapies to have an impact on shoulder pain, ROM, strength, and HRQL. Such domains are clinically important to both the patients and the clinicians to access effectiveness of therapies [24].
Because shoulder-specific pain was measured through several pain scales, all scores were transformed to a scale from 0 (no pain) to 10 (worst pain) for comparison. We considered a difference of 20% to be clinically important [25].
Shoulder abduction and external rotation are the most restricted ROM in patients with chronic shoulder pain and dysfunction. Even though a minimal clinically important difference has yet to be established for chronic shoulder pain population, based on the current literature a difference of 10 degrees was considered clinically important for this NMA [26].
Disease-specific HRQL measures such as Shoulder Pain and Disability Index (SPADI), The Disabilities of the Arm, Shoulder and Hand (DASH), and Quick-DASH were included. A 10 points difference was considered clinically important [27–30].
Data synthesis and analysis
Data synthesis pooled data for the outcomes of interest in the pre-specified groups, including mean or mean differences, standard deviations (SD) and/or 95% confidence intervals (Cis), follow up time, number of included participants per group, demographics (age, gender), and exercises program characteristics (total duration, post-intervention and retention). Groups were classified as the following (S3 Appendix).
Rotator Cuff and Scapula Exercise (RC+SCAP): Participants allocated to this group were treated with an exercise program that targeted both rotator cuff and scapular muscles.
Rotator Cuff Exercise (RC): Participants allocated to this group were treated with an exercise program that targeted mostly rotator cuff muscles without focusing on scapula muscles.
Non-Specific RC Exercises: Participants allocated to this group were treated with an exercise program that did not target specifically RC muscles.
Electro-physical agent (EPA) + Exercise Therapy (ET): Participants allocated to this group used electro-physical modalities in addition to their exercise program. Modalities included electrotherapy (i.e. TENS, ultrasound, laser, IFC, microwave diathermy, and/or radial extracorporeal shock-wave), thermotherapy (cold and/or heat), and dry needling.
Manual Therapy (MT) + ET: Participants allocated to this group used manual therapy in addition to their exercise program. Manual therapy techniques could include any of the following: soft tissue massage, joint mobilization (i.e. Glenohumeral, scapula, acromioclavicular, sternoclavicular, cervical and/ or thoracic), and/or manual compression of trigger points.
Injections + ET: Participants allocated to this group used injections (i.e. corticosteroid, prolotherapy, platelet-rich plasma) in addition to their exercise program.
Usual Care: Participants allocated to this group saw their family physician who gave them information, recommendations, and medical or pharmaceutical therapy as needed. Patients followed a wait-and-see approach and re-consulted with their family physician if symptoms persisted for further evaluation. We also included participants that received no treatment during the study in this group.
Statistical analysis
Data analysis combined direct and indirect comparisons in a Frequentist hierarchical model. Data was combined using a random-effects model and included information from all studies. Relative effects (Mean differences) and a common heterogeneity parameter (τ2) using R Net-Meta packageTM were estimated using CINeMATM for all outcomes. Assessment of the agreement of the various sources of evidence was calculated using the design-by-treatment test and by separating indirect from direct evidence (SIDE test) using the R netmeta packageTM in CINeMATM [22,23].
CINeMA used the flow decomposition method [22,23] to calculate the contribution matrix. Contribution matrix included the percentage contribution of information from each study and each direct comparison to the estimation of each relative effect. Contribution matrix was used in the evaluation of contribution of within-study bias and indirectness to the confidence in the results.
NMA plots visually showed direct comparisons through edges. Nodes size represented the number of participants assigned to each intervention and node color represented ROB as described above. We imputed baseline standard deviation (SD) values when presented with mean differences from the baseline, but without a correspondent SD. Publication Bias used ROBMENTM [21] to assess the risk of bias due to missing evidence for all possible pairwise comparisons in the network. Sensitivity analyses (excluding studies at overall high risk of bias) controlled for residual bias. The strength of evidence was measured by a synthesis of each outcome using the framework described by Salanti and colleagues [31] and implemented using the CINeMATM [22,23] which allowed confidence in the results to be graded as high, moderate, low, and very low.
Results
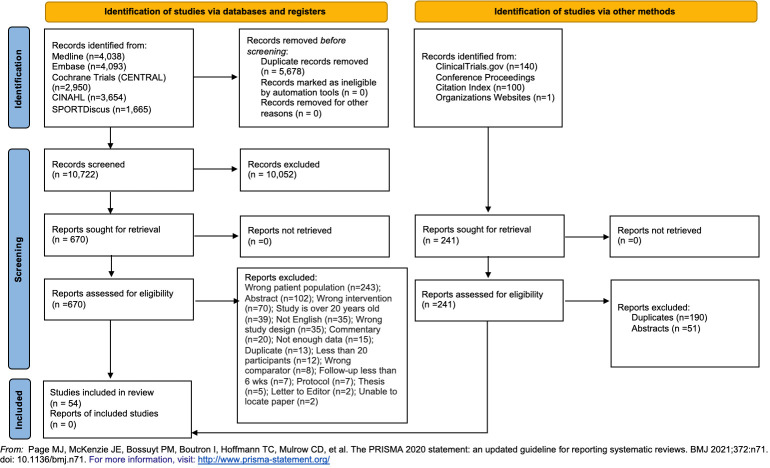
Literature search identified 16,641 citations, of which 5,678 duplicates and 10,052 were excluded. Of the 911 full-text studies reviewed, 54 studies were included [32–86] in the Network Meta-analysis (Fig 1). 4 articles [60,61,73,74] were from 2 studies and, in the analysis, we accounted as 2 studies instead of 4. 22 (43.31%) studies received research grant funds, 3 (5.77%) received industry funds, 5 (9.62%) stated no funds and 22 (42.31%) had no information regarding funds. The majority of included studies were from Europe (21.40.38%) and the remaining from Asia, (18, 34.6%), South America (5 (9.62%), North America (4,7.69%), and Australia (4 (7.69%). 22 out 52 studies had published protocols available [32,33,36,37,40,43,44,47,53,58,61,65,68,69,71,72,74–76,78,80,82].
Fig 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources.
Characteristics of the included studies
Of the 3,893 participants, the mean age was 51.26 years (SD: 7.55) with slightly over half being female (2,053 (52.7%)). The primary diagnosis was rotator cuff related shoulder pain (79%) with the remaining diagnosed as unspecified shoulder pain (21%). 6 interventions were compared to usual care. The mean intervention duration was 7.09 weeks (SD = 3.67).
Quality and publication bias assessment
Overall risk of bias assessment (S4 Appendix) for pain found 19 studies at high risk [34,38,39,41,43,45–48,57–59,64,66,70,78,79,81,84], 21 with some concerns [33,35,37,40,42,44,51,52,54,56,60–62,65,68,77,80,82,85] and 4 at low risk [53,71,73,74]. For ABD ROM, 9 studies had high risk [34,41,57,59,62,70,75,81,84] and 6 had some concerns [35,40,49,56,77,85]. ER ROM had 12 studies with high risk [34,38,39,41,48,57,59,62,70,75,81,84] and 5 with some concerns [35,40,56,63,77]. SPADI consisted of 8 studies at high risk [32,38,43,59,75,76,83,84], 13 with some concerns [35,42,51,52,60,61,65,67,72,80,82,85,86] and 3 with low risk of bias [69,73,74]. Finally, DASH had 8 studies at high risk [32,34,47,50,66,70,79,81], 7 with some concerns [33,36,37,44,51,52,68,80] and 2 at low risk [53,71].
ROB-MEN risk of bias due to missing evidence showed some concerns for EPA+ET, RC+SCAP and Non-specific RC exercises compared to usual care for pain. For ROM, some concerns were seen for RC and EPA+ET. Finally, SPADI had some concerns with RC+SCAP. (S5 Appendix)
Outcomes
Pain
All ET approaches showed large significant pain relief when compared to usual medical care post-intervention: EPA+ET (MD = -2.5; 95% CI = -4.2 to -0.7), Injections+ET (MD = -2.4; 95% CI = -3.9 to -1.04), MT+ET (MD = -2.3; 95% CI = -3.7 to -0.8), and RC+SCAP (MD = -2.1; 95% confidence interval (CI) = -3.5 to -0.7) (Table 1). When studies with high RoB were removed, the sensitivity analysis (S6 Appendix), however, showed that injections lost both statistical and clinical significance (MD = -1.28; 95% CI = -4.28 to 1.73). SIDE test showed no major concerns with inconsistency (P>0.05; S6 Appendix).
Table 1. Mean differences (95%CI) for pain relief post-intervention.
Statistically significant differences are in red.
| EPA | -0.042 (-1.275, 1.191) | -0.200 (-1.186, 0.785) | -1.174 (-2.810, 0.463) | -0.955 (-2.189, 0.278) | -0.363 (-1.280, 0.554) | -2.459 (-3.875, -1.044) |
| 0.042 (-1.191, 1.275) | Injections | -0.159 (-1.558, 1.241) | -1.132 (-3.010, 0.747) | -0.914 (-2.446, 0.620) | -0.321 (-1.606, 0.964) | -2.418 (-4.152, -0.684) |
| 0.200 (-0.785, 1.186) | 0.159 (-1.241, 1.558) | Manual Therapy | -0.973 (-2.675, 0.729) | -0.755 (-2.183, 0.673) | -0.162 (-1.222, 0.897) | -2.259 (-3.674, -0.844) |
| 1.174 (-0.463, 2.810) | 1.132 (-0.747, 3.010) | 0.973 (-0.729, 2.675) | Non-specific RC exercises | 0.218 (-1.607, 2.043) | 0.811 (-0.606, 2.227) | -1.286 (-3.050, 0.478) |
| 0.955 (-0.278, 2.189) | 0.914 (-0.620, 2.446) | 0.755 (-0.673, 2.183) | -0.218 (-2.043, 1.607) | RC | 0.592 (-0.634, 1.819) | -1.504 (-3.123, 0.114) |
| 0.363 (-0.554, 1.280) | 0.321 (-0.964, 1.606) | 0.162 (-0.897, 1.222) | -0.811 (-2.227, 0.606) | -0.592 (-1.819, 0.634) | RC+SCAP | -2.097 (-3.491, -0.702) |
| 2.459 (1.044, 3.875) | 2.418 (0.684, 4.152) | 2.259 (0.844, 3.674) | 1.286 (-0.478, 3.050) | 1.504 (-0.114, 3.123) | 2.097 (0.702, 3.491) | Usual Medical Care |
Up to 52 weeks post-intervention (longest follow-up), pain relief was retained for EPA+ET (MD = -2.6 and 95% CI = -4.0 to -1.2), Injections+ET (MD = -2.9 and 95% CI = -4.6 to -1.2), MT+ET (MD = -2.3 and 95% CI = -3.6 to -0.9), RC (MD = -1.7 and 95% CI = -3.3 to -0.1) and RC+SCAP (MD = -2.1 and 95% CI = -3.5 to -0.8). However, once again adding injections to ET did not show significant or clinically important pain relief when excluding high RoB studies. Confidence in the results varied between moderate to very low (Table 2). SIDE test showed no major concerns with inconsistency (P>0.05; S6 Appendix).
Table 2. Pain Confidence in the results.
| Compari-son | Number of studi-es | Within-study bias | Report-ing bias | Indirectness | Impreci-sion | Heteroge-neity | Incohe-rence | Confi-dence rating | Reason(s) for downgrading |
|---|---|---|---|---|---|---|---|---|---|
| MIXED EVIDENCE | |||||||||
| EPA+ET:Injections+ET | 3 | Major concerns | Low risk | Some concerns | No concerns | Major concerns | No concerns | Very low | 2 levels for major concerns with heterogeneity and within-study bias. 1 level for some concerns with indirectness. |
| EPA+ET:MT | 6 | Some concerns | Low risk | Some concerns | No concerns | Major concerns | No concerns | Very low | 2 levels for major concerns with heterogeneity and some concerns within-study bias. 1 level for some concerns with indirectness. |
| EPA+ET:RC | 3 | Some concerns | Low risk | Some concerns | Some concerns | Some concerns | No concerns | Low | 1 level for some concerns with heterogeneity, imprecision and within-study bias. 1 level for some concerns with indirectness. |
| EPA+ET:RC+SCAP | 6 | Some concerns | Low risk | Some concerns | No concerns | Major concerns | No concerns | Very low | 2 levels for major concerns with heterogeneity and some concerns within-study bias. 1 level for some concerns with indirectness. |
| EPA+ET:Usual Medical Care | 1 | Some concerns | Some concerns | Some concerns | No concerns | Some concerns | No concerns | Low | 1 level for some concerns with heterogeneity, reporting bias and within-study bias. 1 level for some concerns with indirectness. |
|
Injections+ET:
MT |
1 | Major concerns | Low risk | Some concerns | No concerns | Major concerns | No concerns | Very low | 2 levels for major concerns with heterogeneity and within-study bias. 1 level for some concerns with indirectness. |
| Injections+ET:RC | 1 | Major concerns | Some concerns | Some concerns | Some concerns | Some concerns | No concerns | Very low | 2 levels for major concerns with within-study bias and some concerns with heterogeneity and reporting bias. 1 level for some concerns with indirectness. |
|
Injections+ET:
RC+SCAP |
2 | Major concerns | Low risk | Some concerns | No concerns | Major concerns | No concerns | Very low | 2 levels for major concerns with heterogeneity and within-study bias. 1 level for some concerns with indirectness. |
|
MT:
RC+SCAP |
4 | Some concerns | Low risk | Some concerns | No concerns | Major concerns | No concerns | Very low | 2 levels for major concerns with heterogeneity and some concerns within-study bias. 1 level for some concerns with indirectness. |
| MT:Usual Medical Care | 2 | Some concerns | Low risk | Some concerns | No concerns | Some concerns | No concerns | Low | 1 level for some concerns with heterogeneity and within-study bias. 1 level for some concerns with indirectness. |
|
Non-specific RC exercises:
RC+SCAP |
4 | Some concerns | Low risk | No concerns | Some concerns | Some concerns | No concerns | Moderate | 1 level for some concerns with heterogeneity, imprecision and within-study bias. |
| Non-specific RC exercises:Usual Medical Care | 1 | Major concerns | Some concerns | No concerns | Some concerns | Some concerns | No concerns | Low | 2 levels for major concerns with within-study bias and some concerns with heterogeneity, imprecision and reporting bias. |
| RC:RC+SCAP | 3 | Major concerns | Low risk | Some concerns | No concerns | Major concerns | No concerns | Very low | 2 levels for major concerns with within-study bias and heterogeneity. 1 level for some concerns with indirectness. |
| RC:Usual Medical Care | 1 | Some concerns | Low risk | No concerns | Some concerns | No concerns | No concerns | Moderate | 1 level some concerns with imprecision and within-study bias. |
| RC+SCAP:Usual Medical Care | 1 | Some concerns | Some concerns | No concerns | No concerns | Some concerns | Some concerns | Moderate | 1 level some concerns with imprecision, reporting bias, heterogeneity, incoherence and within-study bias. |
| INDIRECT EVIDENCE | |||||||||
| EPA+ET:Non-specific RC exercises | 0 | Some concerns | Low risk | No concerns | Some concerns | Some concerns | No concerns | Moderate | 1 level for some concerns with imprecision, heterogeneity, and within-study bias. |
| Injections+ET:Non-specific RC exercises | 0 | Major concerns | Low risk | No concerns | Some concerns | Some concerns | No concerns | Low | 2 levels for major concerns with within-study bias and some concerns with imprecision and heterogeneity. |
| Injections+ET:Usual Medical Care | 0 | Major concerns | Low risk | Some concerns | No concerns | Some concerns | No concerns | Very low | 2 levels for major concerns with within-study bias and some concerns with heterogeneity. 1 level for some concerns with indirectness. |
| MT:Non-specific RC exercises | 0 | Some concerns | Low risk | No concerns | Some concerns | Some concerns | No concerns | Moderate | 1 level for some concerns with imprecision, heterogeneity, and within-study bias. |
| MT:RC | 0 | Some concerns | Low risk | Some concerns | Some concerns | Some concerns | No concerns | Low | 1 level for some concerns with heterogeneity, imprecision and within-study bias. 1 level for some concerns with indirectness. |
| Non-specific RC exercises:RC | 0 | Some concerns | Low risk | No concerns | Some concerns | Some concerns | No concerns | Moderate | 1 level for some concerns with imprecision, heterogeneity, and within-study bias. |
Shoulder ROM and HRQL
ROM (ER; ABD) included 917 and 894 participants post-intervention. The average ER for the shoulder was 67.38 degrees (Min 36.5 to Max 95) [34,35,38–41,46,56,57,59,62,70,75,77,81,84] and for abduction was 135.8 degrees (Min 9.33 to Max 179.5). [34,35,40,41,49,57,59,62,70,75,77,81,84,85] Shoulder-specific HRQL (SPADI, DASH) included 2,375 and 1,154 participants respectively post-intervention. SPADI [32,35,38,42,43,49–52,59–61,65,67,69,72–74,76,80,82–86] average was 30.22 points (Min 10.1 to Max 61.4) and for DASH [32,34,36,37,44,47,50–53,66,68,70,71,77,80,81] was 26.46 points (Min 9.3 to Max 51.35). There was a trend in improving ROM (ER; ABD) and HRQL (SPADI, DASH) when compared to usual medical care; however, none achieved statistical and clinically important significant improvements (Tables 3–6). When high RoB studies were excluded, the improving trend was not seen with Injections+ET (SPADI; DASH) and non-specific RC exercises (DASH) (S6 Appendix). Post-intervention trends were retained up to 52 weeks with exception of MT+ET that showed a significant and clinically important improvement in DASH scores (MD = -12.7 and 95% CI = -24.4 to -1.02); however, such improvement disappeared when excluding high RoB studies (MD = -7.7 and 95% CI = -21.1 to 5.7). Confidence in the results varied between moderate to very low (S7 Appendix). SIDE test showed some to major concerns with inconsistency for DASH retention, mainly in the indirect comparisons (P = 0.047); S6 Appendix). For all other outcomes, SIDE test showed no major concerns with inconsistency (P>0.05; S6 Appendix).
Table 3. Mean differences for the post-intervention ROM_ER.
| EPA | 2.205 (-7.421, 11.830) | -0.441 (-10.981, 10.099) | 1.798 (-13.672, 17.268) | 1.678 (-6.449, 9.805) | 13.998 (-13.972, 41.968) |
| -2.205 (-11.830, 7.421) | Injections | -2.646 (-16.221, 10.930) | -0.406 (-17.760, 16.948) | -0.526 (-10.658, 9.605) | 11.793 (-17.261, 40.848) |
| 0.441 (-10.099, 10.981) | 2.646 (-10.930, 16.221) | Manual Therapy | 2.239 (-15.795, 20.273) | 2.119 (-9.340, 13.579) | 14.439 (-15.026, 43.905) |
| -1.798 (-17.268, 13.672) | 0.406 (-16.948, 17.760) | -2.239 (-20.273, 15.795) | RC | -0.120 (-15.376, 15.136) | 12.200 (-11.102, 35.502) |
| -1.678 (-9.805, 6.449) | 0.526 (-9.605, 10.658) | -2.119 (-13.579, 9.340) | 0.120 (-15.136, 15.376) | RC+SCAP | 12.320 (-15.532, 40.172) |
| -13.998 (-41.968, 13.972) | -11.793 (-40.848, 17.261) | -14.439 (-43.905, 15.026) | -12.200 (-35.502, 11.102) | -12.320 (-40.172, 15.532) | Usual Medical Care |
Table 6. Mean differences for the post-intervention DASH.
| EPA | -7.281 (-17.034, 2.472) | -1.028 (-10.480, 8.423) | -10.511 (-24.302, 3.280) | -8.358 (-20.422, 3.705) | -2.159 (-12.180, 7.861) | -13.057 (-27.963, 1.850) |
| 7.281 (-2.472, 17.034) | Injections | 6.253 (-2.234, 14.739) | -3.230 (-16.598, 10.138) | -1.078 (-12.894, 10.738) | 5.122 (-4.308, 14.551) | -5.776 (-20.264, 8.712) |
| 1.028 (-8.423, 10.480) | -6.253 (-14.739, 2.234) | Manual Therapy | -9.482 (-21.951, 2.986) | -7.330 (-19.702, 5.041) | -1.131 (-9.235, 6.974) | -12.028 (-25.276, 1.220) |
| 10.511 (-3.280, 24.302) | 3.230 (-10.138, 16.598) | 9.482 (-2.986, 21.951) | Non-specific RC exercises | 2.152 (-14.191, 18.495) | 8.352 (-1.124, 17.827) | -2.546 (-20.230, 15.139) |
| 8.358 (-3.705, 20.422) | 1.078 (-10.738, 12.894) | 7.330 (-5.041, 19.702) | -2.152 (-18.495, 14.191) | RC | 6.199 (-7.116, 19.515) | -4.698 (-18.619, 9.222) |
| 2.159 (-7.861, 12.180) | -5.122 (-14.551, 4.308) | 1.131 (-6.974, 9.235) | -8.352 (-17.827, 1.124) | -6.199 (-19.515, 7.116) | RC+SCAP | -10.898 (-25.829, 4.034) |
| 13.057 (-1.850, 27.963) | 5.776 (-8.712, 20.264) | 12.028 (-1.220, 25.276) | 2.546 (-15.139, 20.230) | 4.698 (-9.222, 18.619) | 10.898 (-4.034, 25.829) | Usual Medical Care |
Table 4. Mean differences for the post-intervention ROM_ABD.
| EPA | 17.920 (-5.373, 41.213) | -0.272 (-26.125, 25.581) | 6.153 (-21.782, 34.089) | 3.989 (-14.827, 22.805) | 15.853 (-35.645, 67.352) |
| -17.920 (-41.213, 5.373) | Injections | -18.192 (-49.442, 13.058) | -11.767 (-45.119, 21.585) | -13.932 (-36.812, 8.949) | -2.067 (-56.694, 52.560) |
| 0.272 (-25.581, 26.125) | 18.192 (-13.058, 49.442) | Manual Therapy | 6.425 (-22.798, 35.649) | 4.261 (-19.772, 28.293) | 16.125 (-36.083, 68.334) |
| -6.153 (-34.089, 21.782) | 11.767 (-21.585, 45.119) | -6.425 (-35.649, 22.798) | RC | -2.165 (-29.271, 24.941) | 9.700 (-33.564, 52.964) |
| -3.989 (-22.805, 14.827) | 13.932 (-8.949, 36.812) | -4.261 (-28.293, 19.772) | 2.165 (-24.941, 29.271) | RC+SCAP | 11.865 (-39.189, 62.919) |
| -15.853 (-67.352, 35.645) | 2.067 (-52.560, 56.694) | -16.125 (-68.334, 36.083) | -9.700 (-52.964, 33.564) | -11.865 (-62.919, 39.189) | Usual Medical Care |
Table 5. Mean differences for the post-intervention SPADI.
| EPA | -0.205 (-15.169, 14.759) | -5.132 (-15.930, 5.666) | 7.539 (-13.711, 28.790) | -6.452 (-22.131, 9.227) | -4.491 (-14.528, 5.545) | -14.390 (-31.776, 2.995) |
| 0.205 (-14.759, 15.169) | Injections | -4.927 (-19.093, 9.239) | 7.744 (-17.382, 32.871) | -6.247 (-25.779, 13.285) | -4.286 (-19.228, 10.655) | -14.185 (-34.711, 6.341) |
| 5.132 (-5.666, 15.930) | 4.927 (-9.239, 19.093) | Manual Therapy | 12.671 (-10.192, 35.534) | -1.320 (-16.804, 14.164) | 0.640 (-9.987, 11.268) | -9.258 (-25.721, 7.204) |
| -7.539 (-28.790, 13.711) | -7.744 (-32.871, 17.382) | -12.671 (-35.534, 10.192) | Non-specific RC exercises | -13.992 (-39.447, 11.464) | -12.031 (-33.584, 9.523) | -21.930 (-48.328, 4.468) |
| 6.452 (-9.227, 22.131) | 6.247 (-13.285, 25.779) | 1.320 (-14.164, 16.804) | 13.992 (-11.464, 39.447) | RC | 1.961 (-13.307, 17.229) | -7.938 (-23.403, 7.527) |
| 4.491 (-5.545, 14.528) | 4.286 (-10.655, 19.228) | -0.640 (-11.268, 9.987) | 12.031 (-9.523, 33.584) | -1.961 (-17.229, 13.307) | RC+SCAP | -9.899 (-26.396, 6.598) |
| 14.390 (-2.995, 31.776) | 14.185 (-6.341, 34.711) | 9.258 (-7.204, 25.721) | 21.930 (-4.468, 48.328) | 7.938 (-7.527, 23.403) | 9.899 (-6.598, 26.396) | Usual Medical Care |
Discussion
Findings from this NMA were that shoulder-specific strengthening along with scapular exercises and ROM exercises are more effective in providing pain relief for chronic shoulder pain than usual medical care. Pain relief can last up to 52 weeks following an average of 7.09 weeks ET program. Evidence shows that targeting specifically shoulder muscles improves shoulder biomechanics, leading to better movement patterns that decreases shoulder impingement and allows shoulder healing [87]. A recent RCT [88] showed that a 12-weeks supervised rehabilitation program using shoulder-specific exercises with the addition of scapular retraction exercises was effective in decreasing patients’ pain and improving HRQL. However, another RCT [89] stated that adding 12-week ET (shoulder-specific or functional exercises) to formal shoulder pain education did not result in further benefits to the patients. Dube’s (2023) [89] study had a well-defined education group including information on shoulder (anatomy and function), pain mechanism, pain management and activity modification. Moreover, participants watched educational videos on shoulder pain/function, chronic pain, stress, and the importance of healthy habits (sleep, eating and physical exercise). Usual care in this NMA may or may not have included an education component as part of their intervention and the content of education intervention varied among studies. Furthermore, it is important to take into account that education interventions are highly correlated with patients’ levels of education and their ability to understand and implement the recommendations [90,91]. Exercise recommendations were also part of the education component in Dube (2023) [89] study and may also have contributed to their findings.
Usual medical care frequently relies on the use of pharmaceutical management including NSAIDS and corticosteroid injections to reduce pain by decreasing the inflammatory process commonly seen in patients with chronic shoulder pain; however, the evidence is of low quality [15]. Even though a systematic review showed that both NSAIDS (SMD of −0.29, 95% CI −0.53 to −0.05) and corticosteroid injections (SMD −0.65, 95% CI −1.04 to −0.26) were more effective than no treatment, included studies were of low quality and it remained unclear how pharmaceutical management compared to ET [15]. This NMA adds value to the current literature since it shows that shoulder-specific strengthening and ROM exercises including scapular exercises provides long-lasting pain relief for chronic shoulder pain compared to usual medical care. It also included studies that had at least 6 weeks follow-up, an important factor to detect true effect of ET. Finally, this NMA was not restricted to a specific shoulder diagnosis, rather it englobed the most common diagnosis of shoulder pain under the umbrella of rotator cuff related shoulder pain as well as unspecified shoulder pain that better reflects the population seen under current primary care.
Shoulder pain is the primary reason people seek medical treatment, since pain impacts patients both physically and emotionally [24]. Adding adjunct therapies to ET added little value when compared to shoulder-specific ET in reducing pain. We found that the addition of injections to ET lost both statistical and clinical importance compared to usual care which typically included medication. A systematic review [16] however showed that injections (SMD −0.65, 95% CI −1.04 to −0.26) were more effective than no treatment. Injections may be effective in reducing pain by decreasing the inflammatory process commonly seen in patients with chronic shoulder pain; however, the evidence is of low quality [16].
Strengths and limitations
This NMA has several strengths. Inclusion of RCTs studies ensured conclusions were based on best available evidence. Exclusion of studies with less than 6 weeks follow-ups enabled reliable assessment of the effect of ET with or without the addition of adjunct therapies. To the best of our knowledge, this is the first NMA that classified ET interventions taking into consideration targeted muscles (RC muscles, RC and scapula muscles or non-specific shoulder muscles) as well as did not focus the interventions to a specific diagnosis. The large sample size (3,893) increased the power of the results. We also considered the effects of the interventions immediately post intervention and at the longest follow-up, enabling conclusions regarding intervention effect and retention.
This NMA is limited by the quality of included studies, since most studies were considered as moderate to high risk of bias. Definition of ET depended on exercises strategies; however, authors are physical therapists with specialty training in shoulder treatments increasing the reliability of definitions [4]. Adjunct therapies were considered in combination with ET and not as stand-alone interventions, limiting the conclusions regarding their effectiveness on their own. Usual care group included variable approaches, including advice, wait-and-see and potential use of pain medications; however, in current practice this is a very common pattern [92,93]. The diagnosis criteria were variable among studies, but we used rotator cuff related shoulder pain or undefined shoulder pain terms to address this concern. We were unable to compile strength data, an important outcome to reflect ET effectiveness, due to inconsistency in measurement methods. The classification of groups in this NMA limits the ability to effectively assess targeted treatment effects of individual interventions that may have different mechanisms and effects if considered separately. In some cases, the small number of studies prevented the analysis of specific interventions such as injections and dry needling. The authors considered the purpose of each intervention to group interventions with similar approaches so there was less variability within groups.
Implications for clinical practice
Shoulder pain has deleterious impact on functional activities, overall health status and is associated with increased health care utilization and associated costs [3]. Health care providers need to take into consideration not only the best treatments available to treat shoulder pain but also the costs associated with each treatment. In this NMA, ET targeting shoulder muscles decreased meaningfully shoulder pain and the addition of adjunct therapies had questionable value. On the other hand, the effect of ET and adjunct therapies on shoulder ROM and HRQL did not show significant differences. Since pain is the major reason patients seek treatment [93], we advocate that ET be considered as the first line of treatment when dealing with chronic shoulder pain.
Implications for future research
Pulling data for this NMA highlighted important barriers that need to be addressed in future trials. First, most included studies lacked published protocols, limiting the ability to judge their findings and increasing the risk of bias of included trials. Secondly, replicability and quality of studies requires detailed information on study methodology [94]. Most studies included in this NMA included general descriptions of interventions limiting the ability to properly combine information into groups and to replicate interventions in real-life clinical settings. Thirdly, the length of interventions varied between 2 to 16 weeks, yet ET requires at least 12-weeks to decrease pain and increase function [94]. Finally, even though strength is an important outcome when assessing the effectiveness of ET [95,96], it has been poorly reported and not feasible to synthesize the results in this NMA. Future studies need to address these barriers to increase confidence in the results and facilitate the implementation of effective interventions in clinical settings. These findings need be interpreted with caution, given the quality of evidence.
Conclusion
Compared to usual care, shoulder-specific ET including scapular exercises are more effective in decreasing pain and maintaining pain relief. Adding adjunct therapies to ET resulted in little pain relief when compared to shoulder-specific ET and usual care. Although augmenting ET with MT had clinically important effects on health status, such effects were not seen when low quality studies were removed. Future studies need to consider important barriers such as having published protocols, including detailed information on study methodology and considering intervention lengths and responsive outcomes.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Liza Bialy, MSc and Alberta Strategy for Patient-Oriented Research (SPOR) SUPPORT Unit Knowledge Translation Platform at University of Alberta for support with methodological design and data collection. We also thank Ben Vandermeer, MSc for support with data analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Lucas J, van Doorn P, Hegedus E, Lewis J, van der Windt D. A systematic review of the global prevalence and incidence of shoulder pain. BMC Musculoskelet Disord. 2022;23(1):1073. doi: 10.1186/s12891-022-05973-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shigley C, Green A. Shoulder conditions and health related quality of life and utility: a current concepts review. JSES Int. 2022;6(1):167–74. doi: 10.1016/j.jseint.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eubank BH, Emery JCH, Lafave MR, Wiley JP, Sheps DM, Mohtadi NG. Exploring the Business Case for Improving Quality of Care for Patients with Chronic Rotator Cuff Tears. Quality management in health care. 2019;28(4):209. doi: 10.1097/QMH.0000000000000231 [DOI] [PubMed] [Google Scholar]
- 4.Eubank BH, Mohtadi NG, Lafave MR, Wiley JP, Bois AJ, Boorman RS, et al. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med Res Methodol. 2016;16:56. doi: 10.1186/s12874-016-0165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler M, Forte M, Braman J, Swiontkowski M, Kane RL. Nonoperative and Operative Treatments for Rotator Cuff Tears: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 22. 2013. [PubMed] [Google Scholar]
- 6.Kuhn JE, Dunn WR, Sanders R, An Q, Baumgarten KM, Bishop JY, et al. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. Journal Of Shoulder And Elbow Surgery. 2013;22(10):1371. doi: 10.1016/j.jse.2013.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pieters L, Lewis J, Kuppens K, Jochems J, Bruijstens T, Joossens L, et al. An Update of Systematic Reviews Examining the Effectiveness of Conservative Physical Therapy Interventions for Subacromial Shoulder Pain. J Orthop Sports Phys Ther. 2020;50(3):131–41. doi: 10.2519/jospt.2020.8498 [DOI] [PubMed] [Google Scholar]
- 8.Littlewood C, Ashton J, Chance-Larsen K, May S, Sturrock B. Exercise for rotator cuff tendinopathy: a systematic review. Physiotherapy. 2012;98(2):101. doi: 10.1016/j.physio.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Littlewood C, Malliaras P, Chance-Larsen K. Therapeutic exercise for rotator cuff tendinopathy: a systematic review of contextual factors and prescription parameters. International Journal of Rehabilitation Research. 2015;38(2):95. doi: 10.1097/MRR.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 10.Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. Journal Of Shoulder and Elbow Surgery. 2009;18(1):138. doi: 10.1016/j.jse.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Kelly SM, Wrightson PA, Meads CA. Clinical outcomes of exercise in the management of subacromial impingement syndrome: a systematic review. Clin Rehabil. 2010;24(2):99–109. doi: 10.1177/0269215509342336 [DOI] [PubMed] [Google Scholar]
- 12.Haik MN, Alburquerque-Sendin F, Moreira RF, Pires ED, Camargo PR. Effectiveness of physical therapy treatment of clearly defined subacromial pain: a systematic review of randomised controlled trials. Br J Sports Med. 2016;50(18):1124–34. doi: 10.1136/bjsports-2015-095771 [DOI] [PubMed] [Google Scholar]
- 13.Desmeules F, Boudreault J, Dionne CE, Frémont P, Lowry V, MacDermid JC, et al. Efficacy of exercise therapy in workers with rotator cuff tendinopathy: a systematic review. Journal Of Occupational Health. 2016;58(5):389. doi: 10.1539/joh.15-0103-RA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong W, Goost H, Lin XB, Burger C, Paul C, Wang ZL, et al. Treatments for shoulder impingement syndrome a prisma systematic review and network meta-analysis. Medicine (United States). 2015;94(10). doi: 10.1097/MD.0000000000000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steuri R, Sattelmayer M, Elsig S, Kolly C, Tal A, Taeymans J, et al. Effectiveness of conservative interventions including exercise, manual therapy and medical management in adults with shoulder impingement: a systematic review and meta-analysis of RCTs. British journal of sports medicine. 2017;51(18):1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, Green S, McBain B, Surace SJ, Deitch J, Lyttle N, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;2016(6):CD012224. doi: 10.1002/14651858.CD012224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdulla SY, Southerst D, Côté P, Shearer HM, Sutton D, Randhawa K, et al. Is exercise effective for the management of subacromial impingement syndrome and other soft tissue injuries of the shoulder? A systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Manual therapy. 2015;20(5):646. doi: 10.1016/j.math.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. [The PRISMA 2020 statement: an updated guideline for reporting systematic reviewsDeclaracion PRISMA 2020: una guia actualizada para la publicacion de revisiones sistematicas]. Rev Panam Salud Publica. 2022;46: e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
- 20.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 21.Chiocchia V, Nikolakopoulou A, Higgins JPT, Page MJ, Papakonstantinou T, Cipriani A, et al. ROB-MEN: a tool to assess risk of bias due to missing evidence in network meta-analysis. BMC Medicine. 2021;19(1):304. doi: 10.1186/s12916-021-02166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: An approach for assessing confidence in the results of a network metaanalysis. PLoS Med. 2020;17(4):e1003082. doi: 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: Software for semiautomated assessment of the confidence in the results of network metaanalysis. Campbell Systematic Reviews. 2020;16(1):e1080. doi: 10.1002/cl2.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maxwell C, Robinson K, McCreesh K. Understanding Shoulder Pain: A Qualitative Evidence Synthesis Exploring the Patient Experience. Phys Ther. 2021;101(3). doi: 10.1093/ptj/pzaa229 [DOI] [PubMed] [Google Scholar]
- 25.Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18(6):927–32. doi: 10.1016/j.jse.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 26.Simovitch R, Flurin PH, Wright T, Zuckerman JD, Roche CP. Quantifying success after total shoulder arthroplasty: the minimal clinically important difference. J Shoulder Elbow Surg. 2018;27(2):298–305. doi: 10.1016/j.jse.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 27.Hao Q, Devji T, Zeraatkar D, Wang Y, Qasim A, Siemieniuk RAC, et al. Minimal important differences for improvement in shoulder condition patient-reported outcomes: a systematic review to inform a BMJ Rapid Recommendation. BMJ Open. 2019;9(2):e028777. doi: 10.1136/bmjopen-2018-028777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Grant JA, Miller BS, Mirza FM, Gagnier JJ. A Systematic Review of the Psychometric Properties of Patient-Reported Outcome Instruments for Use in Patients With Rotator Cuff Disease. Am J Sports Med. 2015;43(10):2572–82. doi: 10.1177/0363546514565096 [DOI] [PubMed] [Google Scholar]
- 29.Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther. 2014;44(1):30–9. doi: 10.2519/jospt.2014.4893 [DOI] [PubMed] [Google Scholar]
- 30.Paul A, Lewis M, Shadforth MF, Croft PR, Van Der Windt DA, Hay EM. A comparison of four shoulder-specific questionnaires in primary care. Ann Rheum Dis. 2004;63(10):1293–9. doi: 10.1136/ard.2003.012088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. doi: 10.1371/journal.pone.0099682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aceituno-Gomez J, Avendano-Coy J, Gomez-Soriano J, Garcia-Madero VM, AvilaMartin G, Serrano-Munoz D, et al. Efficacy of high-intensity laser therapy in subacromial impingement syndrome: a three-month follow-up controlled clinical trial. Clinical rehabilitation. 2019;33(5):894–903. \ doi: 10.1177/0269215518824691 [DOI] [PubMed] [Google Scholar]
- 33.Ager AL, Roy J-S, Gamache F, Hebert LJ. The Effectiveness of an Upper Extremity Neuromuscular Training Program on the Shoulder Function of Military Members With a Rotator Cuff Tendinopathy: A Pilot Randomized Controlled Trial. Military medicine. 2019;184(56):e385–e93. doi: 10.1093/milmed/usy294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbaba YA, Mutlu EK, Altun S, Turkmen E, Birinci T, Celik D. The effectiveness of trigger point treatment in rotator cuff pathology: A randomized controlled double-blind study. Journal of back and musculoskeletal rehabilitation. 2019;32(3):519–27. doi: 10.3233/BMR-181306 [DOI] [PubMed] [Google Scholar]
- 35.Akyol Y, Ulus Y, Durmus D, Canturk F, Bilgici A, Kuru O, et al. Effectiveness of microwave diathermy on pain, functional capacity, muscle strength, quality of life, and depression in patients with subacromial impingement syndrome: a randomized placebocontrolled clinical study. Rheumatology international. 2012;32(10):3007–16. doi: 10.1007/s00296-011-2097-2 [DOI] [PubMed] [Google Scholar]
- 36.Arias-Buría JL, Fernández-de-las-Peñas C, Palacios-Ceña M, Koppenhaver SL, SalomMoreno J. Exercises and Dry Needling for Subacromial Pain Syndrome: A Randomized ParallelGroup Trial. Journal of Pain. 2017;18(1):11. doi: 10.1016/j.jpain.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 37.Arias-Buría JL, Truyols-Domínguez S, Valero-Alcaide R, Salom-Moreno J, AtínArratibel MA, Fernández-de-las-Peñas C. Ultrasound-Guided Percutaneous Electrolysis and Eccentric Exercises for Subacromial Pain Syndrome: A Randomized Clinical Trial. Evidencebased Complementary & Alternative Medicine (eCAM). 2015;2015:1. doi: 10.1155/2015/315219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atici E, Aydin G, Gulsen M, Surenkok O. Effects of subscapularis muscle soft tissue mobilization on pain and functionality in shoulder dysfunction. Turkish Journal of Physiotherapy and Rehabilitation. 2021;32(2):148–54. [Google Scholar]
- 39.Aytar A, Baltaci G, Uhl TL, Tuzun H, Oztop P, Karatas M. The effects of scapular mobilization in patients with subacromial impingement syndrome: a randomized, double-blind, placebo-controlled clinical trial. Journal of Sport Rehabilitation. 2015;24(2):116–29. doi: 10.1123/jsr.2013-0120 [DOI] [PubMed] [Google Scholar]
- 40.Barra Lopez ME, Lopez dC, Fernandez Jentsch G, Raya dC, Lucha Lopez MO, Tricas Moreno JM. Effectiveness of Diacutaneous Fibrolysis for the treatment of subacromial impingement syndrome: a randomised controlled trial. Manual therapy. 2013;18(5):418–24. doi: 10.1016/j.math.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 41.Baskurt Z, Baskurt F, Gelecek N, Ozkan MH. The effectiveness of scapular stabilization exercise in the patients with subacromial impingement syndrome. Journal of Back & Musculoskeletal Rehabilitation. 2011;24(3):173–9. [DOI] [PubMed] [Google Scholar]
- 42.Bennell K, Wee E, Coburn S, Green S, Harris A, Staples M, et al. Efficacy of standardised manual therapy and home exercise programme for chronic rotator cuff disease: Randomised placebo-controlled trial. BMJ (Online). 2010;341(7763):c2756. doi: 10.1136/bmj.c2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berg OK, Paulsberg F, Brabant C, Arabsolghar K, Ronglan S, Bjornsen N, et al. HighIntensity Shoulder Abduction Exercise in Subacromial Pain Syndrome. Medicine and science in sports and exercise. 2021;53(1):1–9. doi: 10.1249/MSS.0000000000002436 [DOI] [PubMed] [Google Scholar]
- 44.Bron C, de Gast A, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. Treatment of myofascial trigger points in patients with chronic shoulder pain: a randomized, controlled trial. BMC Med. 2011; 9:8. doi: 10.1186/1741-7015-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celik D, Akyuz G, Yeldan I. [Comparison of the effects of two different exercise programs on pain in subacromial impingement syndrome]. Acta Orthop Traumatol Turc. 2009;43(6):504–9. [DOI] [PubMed] [Google Scholar]
- 46.Celik D, Atalar AC, Guclu A, Demirhan M. [The contribution of subacromial injection to the conservative treatment of impingement syndrome]. Acta Orthop Traumatol Turc. 2009;43(4):331–5. [DOI] [PubMed] [Google Scholar]
- 47.Centeno C, Fausel Z, Stemper I, Azuike U, Dodson E. A Randomized Controlled Trial of the Treatment of Rotator Cuff Tears with Bone Marrow Concentrate and Platelet Products Compared to Exercise Therapy: A Midterm Analysis. Stem cells international. 2020;2020(101535822):5962354. doi: 10.1155/2020/5962354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cha JY, Kim JH, Hong J, Choi YT, Kim MH, Cho JH, et al. A 12-week rehabilitation program improves body composition, pain sensation, and internal/external torques of baseball pitchers with shoulder impingement symptom. J Exerc Rehabil. 2014;10(1):35–44. doi: 10.12965/jer.140087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen JF, Ginn KA, Herbert RD. Passive mobilisation of shoulder region joints plus advice and exercise does not reduce pain and disability more than advice and exercise alone: a randomised trial. Aust J Physiother. 2009;55(1):17–23. doi: 10.1016/s0004-9514(09)70056-x [DOI] [PubMed] [Google Scholar]
- 50.Crawshaw DP, Helliwell PS, Hensor EM, Hay EMA, Aldous SJ, Conaghan PG. Exercise therapy after corticosteroid injection for moderate to severe shoulder pain: large pragmatic randomised trial. BMJ: British Medical Journal (Overseas & Retired Doctors Edition). 2010;341(7762):c3037–c. doi: 10.1136/bmj.c3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daghiani M, Negahban H, Ebrahimzadeh MH, Moradi A, Kachooei AR, Raeesi J, et al. The effectiveness of comprehensive physiotherapy compared with corticosteroid injection on pain, disability, treatment effectiveness, and quality of life in patients with subacromial pain syndrome: a parallel, single-blind, randomized controlled trial. Physiotherapy theory and practice. 2022(9015520):1–15. doi: 10.1080/09593985.2022.2044421 [DOI] [PubMed] [Google Scholar]
- 52.de Miguel Valtierra L, Salom Moreno J, Fernandez-de-Las-Penas C, Cleland JA, AriasBuria JL. Ultrasound-Guided Application of Percutaneous Electrolysis as an Adjunct to Exercise and Manual Therapy for Subacromial Pain Syndrome: A Randomized Clinical Trial. The journal of pain. 2018;19(10):1201–10. doi: 10.1016/j.jpain.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 53.de Oliveira FCL, Pairot de Fontenay B, Bouyer LJ, Desmeules F, Roy J-S. Kinesiotaping for the Rehabilitation of Rotator Cuff-Related Shoulder Pain: A Randomized Clinical Trial. Sports health. 2021;13(2):161–72. doi: 10.1177/1941738120944254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dejaco B, Habets B, van Loon C, van Grinsven S, van Cingel R. Eccentric versus conventional exercise therapy in patients with rotator cuff tendinopathy: a randomized, single blinded, clinical trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2051–9. doi: 10.1007/s00167-016-4223-x [DOI] [PubMed] [Google Scholar]
- 55.Dickens VA, Williams JL, Bhamra MS. Role of physiotherapy in the treatment of subacromial impingement syndrome: a prospective study. Physiotherapy. 2005;91(3):159–64. [Google Scholar]
- 56.Dilek B, Gulbahar S, Gundogdu M, Ergin B, Manisali M, Ozkan M, et al. Efficacy of Proprioceptive Exercises in Patients with Subacromial Impingement Syndrome. American Journal of Physical Medicine & Rehabilitation. 2016;95(3):169–82. [DOI] [PubMed] [Google Scholar]
- 57.Dogan C, Ketenci S, Uzuner B, Sen HE, Bilgici A, Alayli G, et al. Comparison of subacromial corticosteroid injection and physical therapy in patients with subacromial impingement syndrome: A prospective, randomized trial. Journal of Experimental and Clinical Medicine (Turkey). 2021;38(4):511–5. [Google Scholar]
- 58.Ekici G, Özcan Ş, Öztürk BY, Öztürk B, Ekici B. Effects of deep friction massage and dry needling therapy on night pain and shoulder internal rotation in subacromial pain syndrome: 1-year follow up of a randomised controlled trial. International Journal of Therapy & Rehabilitation. 2021;28(2):1–12. [Google Scholar]
- 59.Elsodany AM, Alayat MSM, Ali MME, Khaprani HM. Long-Term Effect of Pulsed Nd:YAG Laser in the Treatment of Patients with Rotator Cuff Tendinopathy: A Randomized Controlled Trial. Photomedicine and laser surgery. 2018;36(9):506–13. doi: 10.1089/pho.2018.4476 [DOI] [PubMed] [Google Scholar]
- 60.Engebretsen K, Grotle M, Bautz-Holter E, Ekeberg OM, Juel NG, Brox JI. Supervised Exercises Compared With Radial Extracorporeal Shock-Wave Therapy for Subacromial Shoulder Pain: 1-Year Results of a Single-Blind Randomized Controlled Trial. Physical Therapy. 2011;91(1):37–47. doi: 10.2522/ptj.20090338 [DOI] [PubMed] [Google Scholar]
- 61.Engebretsen K, Grotle M, Bautz-Holter E, Sandvik L, Juel NG, Ekeberg OM, et al. Radial extracorporeal shockwave treatment compared with supervised exercises in patients with subacromial pain syndrome: single blind randomised study. Bmj. 2009;339. doi: 10.1136/bmj.b3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eslamian F, Shakouri SK, Ghojazadeh M, Nobari OE, Eftekharsadat B. Effects of lowlevel laser therapy in combination with physiotherapy in the management of rotator cuff tendinitis. Lasers in medical science. 2012;27:951–8. doi: 10.1007/s10103-011-1001-3 [DOI] [PubMed] [Google Scholar]
- 63.Galace de Freitas D, Marcondes FB, Monteiro RL, Rosa SG, Maria de Moraes Barros Fucs P, Fukuda TY. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: a randomized, double-blind, placebo-controlled clinical trial. Arch Phys Med Rehabil. 2014;95(2):345–52. doi: 10.1016/j.apmr.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 64.Geraets JJ, Goossens ME, de Groot IJ, de Bruijn CP, de Bie RA, Dinant GJ, et al. Effectiveness of a graded exercise therapy program for patients with chronic shoulder complaints. Aust J Physiother. 2005;51(2):87–94. doi: 10.1016/s0004-9514(05)70037-4 [DOI] [PubMed] [Google Scholar]
- 65.Gomes C, Dibai-Filho AV, Moreira WA, Rivas SQ, Silva EDS, Garrido ACB. Effect of Adding Interferential Current in an Exercise and Manual Therapy Program for Patients with Unilateral Shoulder Impingement Syndrome: A Randomized Clinical Trial. J Manipulative Physiol Ther. 2018;41(3):218–26. doi: 10.1016/j.jmpt.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 66.Hallgren HC, Holmgren T, Oberg B, Johansson K, Adolfsson LE. A specific exercise strategy reduced the need for surgery in subacromial pain patients. Br J Sports Med. 2014;48(19):1431–6. doi: 10.1136/bjsports-2013-093233 [DOI] [PubMed] [Google Scholar]
- 67.Heron SR, Woby SR, Thompson DP. Comparison of three types of exercise in the treatment of rotator cuff tendinopathy/shoulder impingement syndrome: A randomized controlled trial. Physiotherapy. 2017;103(2):167. doi: 10.1016/j.physio.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 68.Holmgren T, Bjornsson Hallgren H, Oberg B, Adolfsson L, Johansson K. Effect of specific exercise strategy on need for surgery in patients with subacromial impingement syndrome: randomised controlled study. BMJ (Clinical research ed). 2012;344(8900488, bmj, 101090866):e787. doi: 10.1136/bmj.e787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hopewell S, Keene DJ, Heine P, Marian IR, Dritsaki M, Cureton L, et al. Progressive exercise compared with best-practice advice, with or without corticosteroid injection, for rotator cuff disorders: the GRASP factorial RCT. Health technology assessment (Winchester, England). 2021;25(48):1–158. doi: 10.3310/hta25480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ilhanli I, Guder N, Gul M. Platelet-Rich Plasma Treatment with Physical Therapy in Chronic Partial Supraspinatus Tears. Iran Red Crescent Med J. 2015;17(9): e23732. doi: 10.5812/ircmj.23732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ingwersen KG, Vobbe JW, Pedersen LL, Sorensen L, Wedderkopp N. Effect of Psychomotricity in Combination With 3 Months of Active Shoulder Exercises in Individuals With Chronic Shoulder Pain: Primary Results From an Investigator-Blinded, Randomized, Controlled Trial. Archives of physical medicine and rehabilitation. 2019;100(11):2136–43. doi: 10.1016/j.apmr.2019.05.032 [DOI] [PubMed] [Google Scholar]
- 72.Kazempour Mofrad M, Rezasoltani Z, Dadarkhah A, Kazempour Mofrad R, Abdorrazaghi F, Azizi S. Periarticular Neurofascial Dextrose Prolotherapy Versus Physiotherapy for the Treatment of Chronic Rotator Cuff Tendinopathy: Randomized Clinical Trial. Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases. 2021;27(4):136–42. doi: 10.1097/RHU.0000000000001218 [DOI] [PubMed] [Google Scholar]
- 73.Kvalvaag E, Brox JI, Engebretsen KB, Soberg HL, Juel NG, Bautz-Holter E, et al. Effectiveness of radial extracorporeal shock wave therapy (rESWT) when combined with supervised exercises in patients with subacromial shoulder pain: a double-masked, randomized, sham-controlled trial. The American journal of sports medicine. 2017;45(11):2547–54. doi: 10.1177/0363546517707505 [DOI] [PubMed] [Google Scholar]
- 74.Kvalvaag E, Roe C, Engebretsen KB, Soberg HL, Juel NG, Bautz-Holter E, et al. One year results of a randomized controlled trial on radial Extracorporeal Shock Wave Treatment, with predictors of pain, disability and return to work in patients with subacromial pain syndrome. Eur J Phys Rehabil Med. 2018;54(3):341–50. doi: 10.23736/S1973-9087.17.04748-7 [DOI] [PubMed] [Google Scholar]
- 75.Lewis J, Sim J, Barlas P. Acupuncture and electro‐acupuncture for people diagnosed with subacromial pain syndrome: A multicentre randomized trial. European Journal of Pain. 2017;21(6):1007–19. doi: 10.1002/ejp.1001 [DOI] [PubMed] [Google Scholar]
- 76.Littlewood C, Bateman M, Brown K, Bury J, Mawson S, May S, et al. A self-managed single exercise programme versus usual physiotherapy treatment for rotator cuff tendinopathy: a randomised controlled trial (the SELF study). Clin Rehabil. 2016;30(7):686–96. doi: 10.1177/0269215515593784 [DOI] [PubMed] [Google Scholar]
- 77.Lombardi I Jr., Magri AG, Fleury AM, Da Silva AC, Natour J. Progressive resistance training in patients with shoulder impingement syndrome: a randomized controlled trial. Arthritis Rheum. 2008;59(5):615–22. doi: 10.1002/art.23576 [DOI] [PubMed] [Google Scholar]
- 78.Martins da Silva L, Maciel Bello G, Chuaste Flores B, Silva Dias L, Camargo P, Mengue LF, et al. Kinesio Tape In Shoulder Rotator Cuff Tendinopathy: A Randomized, Blind Clinical Trial. Muscles, Ligaments & Tendons Journal (MLTJ). 2020;10(3):364–75. [Google Scholar]
- 79.Marzetti E, Rabini A, Piccinini G, Piazzini DB, Vulpiani MC, Vetrano M, et al. Neurocognitive therapeutic exercise improves pain and function in patients with shoulder impingement syndrome: a single-blind randomized controlled clinical trial. Eur J Phys Rehabil Med. 2014;50(3):255–64. [PubMed] [Google Scholar]
- 80.Mintken PE, McDevitt AW, Cleland JA, Boyles RE, Beardslee AR, Burns SA, et al. Cervicothoracic Manual Therapy Plus Exercise Therapy Versus Exercise Therapy Alone in the Management of Individuals With Shoulder Pain: A Multicenter Randomized Controlled Trial. Journal of Orthopaedic & Sports Physical Therapy. 2016;46(8):617–28. doi: 10.2519/jospt.2016.6319 [DOI] [PubMed] [Google Scholar]
- 81.Nejati P, Ghahremaninia A, Naderi F, Gharibzadeh S, Mazaherinezhad A. Treatment of Subacromial Impingement Syndrome: Platelet-Rich Plasma or Exercise Therapy? A Randomized Controlled Trial. Orthop J Sports Med. 2017;5(5):2325967117702366. doi: 10.1177/2325967117702366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santello G, Rossi DM, Martins J, Libardoni TdC, de Oliveira AS. Effects on shoulder pain and disability of teaching patients with shoulder pain a home-based exercise program: a randomized controlled trial. Clinical rehabilitation. 2020;34(10):1245–55. doi: 10.1177/0269215520930790 [DOI] [PubMed] [Google Scholar]
- 83.Schedler S, Brueckner D, Hagen M, Muehlbauer T. Effects of a Traditional versus an Alternative Strengthening Exercise Program on Shoulder Pain, Function and Physical Performance in Individuals with Subacromial Shoulder Pain: A Randomized Controlled Trial. Sports (Basel, Switzerland). 2020;8(4). doi: 10.3390/sports8040048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seven MM, Ersen O, Akpancar S, Ozkan H, Turkkan S, Yildiz Y, et al. Effectiveness of prolotherapy in the treatment of chronic rotator cuff lesions. Orthop Traumatol Surg Res. 2017;103(3):427–33. doi: 10.1016/j.otsr.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 85.van den Dolder PA, Roberts DL. A trial into the effectiveness of soft tissue massage in the treatment of shoulder pain. Australian Journal of Physiotherapy. 2003;49(3):183–8. doi: 10.1016/s0004-9514(14)60238-5 [DOI] [PubMed] [Google Scholar]
- 86.Yiasemides R, Halaki M, Cathers I, Ginn KA. Does Passive Mobilization of Shoulder Region Joints Provide Additional Benefit Over Advice and Exercise Alone for People Who Have Shoulder Pain and Minimal Movement Restriction? A Randomized Controlled Trial. Physical Therapy. 2011;91(2):178–89. doi: 10.2522/ptj.20100111 [DOI] [PubMed] [Google Scholar]
- 87.Ludewig PM, Braman JP. Shoulder impingement: biomechanical considerations in rehabilitation. Man Ther. 2011;16(1):33–9. doi: 10.1016/j.math.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eraslan L, Yar O, Ergen F, Huri G, Duzgun I. Utilizing Scapula Retraction Exercises With or Without Glenohumeral Rotational Exercises With a Gradual Progression for Subacromial Pain Syndrome. Sports health. 2023:19417381231155190. doi: 10.1177/19417381231155190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dubé M-O, Desmeules F, Lewis JS, Roy J-S. Does the addition of motor control or strengthening exercises to education result in better outcomes for rotator cuff-related shoulder pain? A multiarm randomised controlled trial. British Journal of Sports Medicine. 2023:bjsports2021–105027. doi: 10.1136/bjsports-2021-105027 [DOI] [PubMed] [Google Scholar]
- 90.Silles M. The causal effect of education on health: Evidence from the United Kingdom. Economics of Education Review. 2009;28(1):122–8. [Google Scholar]
- 91.Cusack L, Del Mar CB, Chalmers I, Gibson E, Hoffmann TC. Educational interventions to improve people’s understanding of key concepts in assessing the effects of health interventions: a systematic review. Systematic Reviews. 2018;7(1):68. doi: 10.1186/s13643-018-0719-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lowry V, Lavigne P, Zidarov D, Perreault K, Roy JS, Desmeules F. Knowledge and appropriateness of care of family physicians and physiotherapists in the management of shoulder pain: a survey study in the province of Quebec, Canada. BMC Prim Care. 2023;24(1):49. doi: 10.1186/s12875-023-01999-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walker-Bone K, van der Windt DAWM. Shoulder Pain—Where Are We Now? Current Treatment Options in Rheumatology. 2021;7(4):285–306. [Google Scholar]
- 94.Page P, Hoogenboom B, Voight M. Improving the Reporting of Therapeutic Exercise Interventions in Rehabilitation Research. Int J Sports Phys Ther. 2017;12(2):297–304. [PMC free article] [PubMed] [Google Scholar]
- 95.Edwards P, Ebert J, Joss B, Bhabra G, Ackland T, Wang A. Exercise Rehabilitation in the Non-Operative Management of Rotator Cuff Tears: A Review of the Literature. Int J Sports Phys Ther. 2016;11(2):279–301. [PMC free article] [PubMed] [Google Scholar]
- 96.Verma B, Multani N, Singh Z. Predictors of shoulder pain among adults. International Journal of Advanced Science and Technology. 2020; 29: 1813–25. [Google Scholar]