Abstract
The surface chemical composition and physicochemical properties (hydrophobicity and zeta potential) of two lactic acid bacteria, Lactococcus lactis subsp. lactis bv. diacetilactis and Lactobacillus helveticus, have been investigated using cells harvested in exponential or stationary growth phase. The surface composition determined by X-ray photoelectron spectroscopy (XPS) was converted into a molecular composition in terms of proteins, polysaccharides, and hydrocarbonlike compounds. The concentration of the last was always below 15% (wt/wt), which is related to the hydrophilic character revealed by water contact angles of less than 30°. The surfaces of L. lactis cells had a polysaccharide concentration about twice that of proteins. The S-layer of L. helveticus was either interrupted or crossed by polysaccharide-rich compounds; the concentration of the latter was higher in the stationary growth phase than in the exponential growth phase. Further progress was made in the interpretation of XPS data in terms of chemical functions by showing that the oxygen component at 531.2 eV contains a contribution of phosphate in addition to the main contribution of the peptide link. The isoelectric points were around 2 and 3, and the electrophoretic mobilities above pH 5 (ionic strength, 1 mM) were about −3.0 × 10−8 and −0.6 × 10−8 m2 s−1 V−1 for L. lactis and L. helveticus, respectively. The electrokinetic properties of the latter reveal the influence of carboxyl groups, while the difference between the two strains is related to a difference between N/P surface concentration ratios, reflecting the relative exposure of proteins and phosphate groups at the surface.
In many instances, the behavior of lactic acid bacteria is dependent on interfacial processes and thus on cell surface physicochemical properties and chemical composition. A better knowledge of these aspects would allow a deeper understanding of the autolysis of lactic acid bacteria (29, 36) and the production of texturing exopolysaccharides (4). It would help in controlling the sedimentation of starters for commercial production (7) and in understanding the roles of specific and nonspecific interactions in phage attachment (7, 46, 56).
In dairy product manufacturing, adhesion of lactic bacteria to a material may be the first step leading to biofilm formation, which can be either deleterious (contamination, taste alteration, and biofouling on heat exchangers) (17, 25) or beneficial (continuous inoculation in yogurt or cheese making) (5). The adhesion behavior of microbial cells has been shown to depend on the balance of electrostatic and van der Waals interactions and on the hydrophobic character of the surfaces involved (38, 42, 53, 54), pointing to the possible influence of the respective zeta potentials and surface hydrophobicities. Moreover, the production of extracellular substances either at the cell surface or in the surrounding medium has been shown to influence adhesion (14, 55).
The surface hydrophobicity and composition of lactic acid bacteria have been studied primarily by microbial adhesion to hydrocarbon (7, 18, 40, 46, 56) and by biochemical analysis (7, 18, 22, 35, 45, 49, 50, 56), respectively. However, the relevance of these methods is questionable for interfacial phenomena. Electrostatic interactions may play a major role in the adhesion of microorganisms to hydrocarbons (2, 51); in contrast, water contact angle measures the surface hydrophobicity without the interference of electrostatic interactions. Biochemical analysis refers to constituents of the whole cell wall and not to the cell surface; in contrast (16), X-ray photoelectron spectroscopy (XPS) provides information on the element and chemical function compositions of the outermost cell surface (2 to 10 nm). XPS is based on irradiation of the freeze-dried sample by an X-ray beam and analysis of the kinetic energy of the emitted photoelectrons (43, 44). The relevance of XPS for probing microbial surfaces is supported by correlations between the XPS results and cell surface properties and by relationships with the cell behavior at interfaces (13, 14, 15, 16, 21, 44).
Lactococcus lactis and Lactobacillus helveticus are two lactic acid bacteria important in cheese ripening. The former is present in Cheddar and Saint-Paulin cheeses, while the latter is found in Swiss-type and Italian cheeses (6, 8, 29, 48). L. helveticus is also involved in other applications: production of fermented milk with antihypertensive effect and production of lactic acid from whey permeate (33). Moreover, L. lactis and Lactobacillus spp. could be considered probiotic agents (23, 41) and used as live delivery vehicles for the administration of oral vaccines (19, 32).
In the present paper, the relationships between surface chemical composition (XPS), hydrophobicity (water contact angle), and electrical properties (microelectrophoresis) are examined for L. helveticus and L. lactis. These are taken as models of lactic acid bacteria, rodlike and cocci, respectively; moreover, the L. helveticus strain possesses an S-layer (31).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. helveticus ATCC 12046 (Institut Pasteur, Paris, France) and L. lactis subsp. lactis bv. diacetilactis LMG 9452 (LMG Culture Collection, Universiteit Gent, Ghent, Belgium) were stored at −20°C in MRS (9) and M17 (47) media (Merck, Darmstadt, Germany), respectively, containing 15% (vol/vol) glycerol (Vel, Leuven, Belgium). L. helveticus and L. lactis were incubated at 37 and 30°C, respectively, without agitation under aerobic conditions. The volumes for the preculture and the culture were 10 (test tube) and 100 (Erlenmeyer flask) ml, respectively. Precultures were carried out by inoculation with a frozen sample, and the cultures were inoculated at 1% (vol/vol) with an overnight preculture. The cells were harvested (13,873 × g; 4°C; 10 min) either in the exponential (after 4 and 8 to 9 h for L. lactis and L. helveticus, respectively) or the stationary (after 24 h for both strains) growth phase.
Surface characterization.
The electrical properties of the cell surfaces were assessed by microelectrophoresis (24) with a Pen Kem Laser Zee Meter (model 500). For this purpose, cells were resuspended in 1 mM KNO3 (UCB, Leuven, Belgium) at concentrations of about 2 × 108 and 5 × 108 per ml for L. helveticus and L. lactis, respectively. The pH was adjusted with KOH (Janssen Chimica, Beerse, Belgium) or HNO3 (Merck), with a new suspension made for each determination.
Cell surface hydrophobicity was estimated by water contact angle (θw) measurements on a cell lawn using the sessile drop method (52). The cell lawns (30 to 100 layers) were obtained by filtration on a filter (4.5-cm diameter) with a pore diameter of 0.45 μm (HAWP filter; Millipore Co., Bedford, Mass.). To standardize the moisture content, the filters were then transferred onto agar for 30 min (1% [wt/vol] agar in 10% [vol/vol] glycerol). Finally, the filters were mounted on a microscope slide, and θw (drop volume, 0.3 μl) was measured as a function of drying time, using water of high-performance liquid chromatography grade produced by a MilliQ plus system (Millipore). For each filter, 10 measurements were made at different places (standard deviations were less than 10% of the mean value).
The sample preparation for XPS analysis was as follows. The cell pellet was resuspended in MilliQ water (Millipore) at a final concentration of 1011 cells per ml. Two milliliters of this suspension was transferred into a glass flask (diameter, 3 cm; height, 2.5 cm) precooled in liquid nitrogen. After 15 min, the flasks were either freeze-dried immediately or stored at −80°C until they were freeze-dried. The freeze-drying was carried out in an apparatus specially designed by Leybold (Brussels, Belgium). The temperature of the freeze-dryer shelf was maintained at −50°C for 3 h; then it was increased to −5°C over 15 h and maintained at −5°C for 6 to 12 h; finally, it was increased to 23°C over 3 h. The dehydrated cell powder was homogenized with a spatula and pressed with a polyacetal cylinder cleaned with isopropanol. More details of the procedure can be found in a previous paper (10).
The XPS analysis was performed with an SSI X-Probe (SSX-100/206; Surface Science Instruments, Mountain View, Calif.) equipped with an aluminum anode and a quartz monochromator and interfaced with a Hewlett-Packard 9000/310 computer, allowing analysis control, data accumulation, and data treatment. Pressure during analysis varied between 4 × 10−7 and 2.7 × 10−6 Pa. The analyzed area was an elliptical spot with a minor axis of 1,000 μm. Sample charging was stabilized with a flood gun (6 or 8 eV) and a nickel grid placed 2 mm above the surface sample. The peaks were recorded in the following sequence: survey spectrum, C1s, O1s, N1s, P2p, K2p, Na1s, and C1s again to check for the absence of sample degradation and charging stability during analysis (12). The constant pass energy was 150 and 50 eV for the survey spectrum and individual spectra, respectively. Under these conditions, the resolutions determined by the full width at half maximum (FWHM) of the Au4f7/2 peak of a standard gold sample were about 1.6 and 1.1 eV, respectively. To assess the eventual hydrocarbon contamination of the sample during freeze-drying, a sorbitol specimen was included in each set of samples, starting with the freezing step. Data treatment was performed as described by Dufrêne and Rouxhet (13).
RESULTS
The surface properties, i.e., hydrophobicity (Table 1), and electrical properties (Fig. 1) of L. lactis and L. helveticus were not significantly affected by the growth phase of the cells. The surfaces were hydrophilic whatever the strain, as indicated by the water contact angle in the range of 21 to 29°. The isoelectric points were around pH 2 and 3 for L. lactis and L. helveticus, respectively. Between pH 2 and 5, the electrophoretic mobility decreased continuously. Above pH 5, the electrophoretic mobilities were about −3.0 × 10−8 and −0.6 × 10−8 m2 s−1 V−1 for L. lactis and L. helveticus, respectively.
TABLE 1.
Water contact angle (θw) determined on cell lawns of L. helveticus ATCC 12046 and L. lactis subsp. lactis bv. diacetilactis LMG 9452
| Strain | Growth phase | θw (°) |
|---|---|---|
| L. helveticusa,c | Exponential | 21 (2) |
| Stationary | 26 (3) | |
| L. lactisb,c | Exponential | 27 (3) |
| Stationary | 29 (3) |
Mean values after three different drying times of less than 1 h.
Mean values after five different drying times of less than 1 h.
Mean values of two sets of independent data with standard deviations in parentheses.
FIG. 1.
Variation of electrophoretic mobility as a function of pH for L. helveticus ATCC 12046 (circles) and L. lactis subsp. lactis bv. diacetilactis LMG 9452 (squares) cells harvested in the exponential (open symbols) and stationary (closed symbols) growth phases. Measurements were made in 1 mM KNO3 solution on two independent sets of data; the bars represent 95% confidence intervals.
Table 2 presents the surface elemental composition, determined by XPS, for L. helveticus and L. lactis cells harvested in the exponential and stationary growth phases. No significant difference was observed between the two growth phases for L. lactis, while L. helveticus cells were slightly richer in oxygen and poorer in nitrogen in the stationary growth phase. Except for the N/C ratio, which was slightly inferior, the outermost surface chemical composition of L. lactis was similar to that of L. helveticus in the stationary growth phase. The O/C, N/C, and P/C ratios determined on L. helveticus in the exponential growth phase were similar to those reported by Mozes and Lortal (36). The concentration of phosphorus detected at the extreme cell surface was not affected significantly by the strain or growth phase of the cells. Potassium was also detected in a concentration similar to that of phosphorus.
TABLE 2.
XPS peak assignments and surface chemical compositions for L. helveticus ATCC 12046 and L. lactis subsp. lactis bv. diacetilactis LMG 9452 cells harvested in exponential or stationary growth phase
| Species | Growth phase | Molar ratio vs total carbona
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 284.8bC—(C,H) | 286.3 C—(O,N) | 287.9 C=O, O—C—O | 532.6 O—C, P—O—C, P—OH | 531.2 O=C, P=O, P—O− | 399.8 Nnonproton | 401.3 Nproton | O | N | P | K | ||
| L. helveticus | Exponential | 0.38 (0.04) | 0.42 (0.03) | 0.20 (0.02) | 0.29 (0.03) | 0.18 (0.03) | 0.14 (0.03) | 0.013 (0.002) | 0.47 (0.01) | 0.15 (0.03) | 0.010 (0.004) | —c |
| Stationary | 0.28 (0.04) | 0.51 (0.04) | 0.21 (0.02) | 0.40 (0.05) | 0.16 (0.04) | 0.11 (0.03) | 0.010 (0.006) | 0.56 (0.05) | 0.12 (0.03) | 0.007 (0.001) | —d | |
| L. lactis | Exponential | 0.29 (0.02) | 0.51 (0.01) | 0.19 (0.01) | 0.42 (0.04) | 0.15 (0.03) | 0.09 (0.02) | 0.008 (0.002) | 0.57 (0.06) | 0.09 (0.01) | 0.012 (0.002) | 0.019 (0.006) |
| Stationary | 0.31 (0.05) | 0.50 (0.04) | 0.19 (0.02) | 0.40 (0.04) | 0.15 (0.01) | 0.08 (0.02) | 0.011 (0.004) | 0.55 (0.03) | 0.09 (0.02) | 0.012 (0.002) | 0.012 (0.005) | |
Mean values of four sets of independent data; 95% confidence interval is in parentheses.
Binding energy (electron volts) and XPS peak assignment.
Detected on only three samples (K/C = 0.007, 0.003, and 0.003).
Detected on only two samples (K/C = 0.007 and 0.005).
The sorbitol control did not reveal any abnormal surface contamination due to freeze-drying. The carbon peaks were decomposed in three components (FWHM = 1.45 eV), attributed to carbon singly bound to carbon and hydrogen [C—(C,H) at 284.8 eV], carbon singly bound to oxygen or nitrogen [C—(O,N) at 286.2 eV, including ether, alcohol, amine, or amide], and carbon making a double bond or two single bonds with oxygen (at 287.9 eV, denoted C=O, including amide, carbonyl, carboxylate, acetal, or hemiacetal) (20, 44). The oxygen peaks were decomposed in two components (FWHM = 1.63 eV), attributed mainly to oxygen making a double bond with carbon (at 531.2 eV, denoted O531.2, including carboxylic acid, carboxylate, ester, carbonyl, or amide), and to oxygen involved in hydroxide or ether functions (O—C at 532.6 eV). The nitrogen peaks were decomposed in two components (FWHM = 1.53 eV), attributed to nonprotonated nitrogen (Nnonproton at 399.8 eV, involved in amine or amide) and to protonated nitrogen (Nproton at 401.3 eV, involved in ammonium or protonated amine). The phosphorus peak appearing at 133.4 eV was attributed to phosphate groups.
Table 2 presents the results of these decompositions. L. helveticus stationary cells and L. lactis cells in both growth phases presented high C—(O,N)/C and O—C/C ratios (around 0.5 and 0.4, respectively). For L. helveticus cells, C—(O,N)/C and O—C/C ratios increased 25% during culture, while the C—(C,H)/C ratio decreased. The C=O/C and O531.2/C ratios were not affected significantly by the strain or growth phase of the cells. Finally, most of the nitrogen was involved in nonprotonated amine or amide functions.
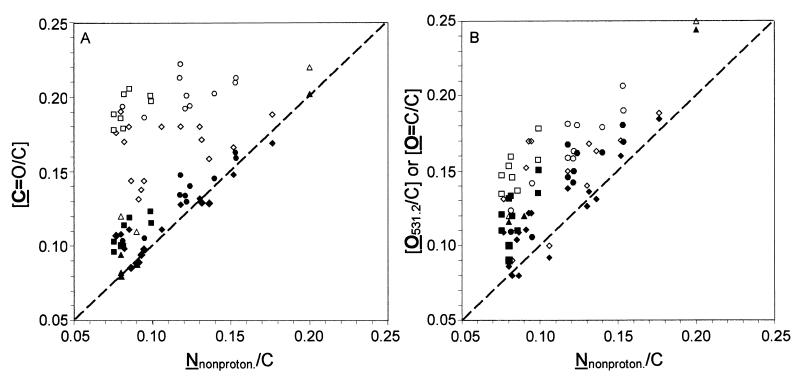
Figure 2 presents the plot of the molar concentration ratios C=O/C and O531.2/C as a function of Nnonproton/C. If these three ratios were exclusively due to amide functions, O=C—N, the graphs should give a straight line with unit slope. The higher C=O/C ratio may be due to the presence of acetal functions, present in polysaccharides. The part of C=O/C due to acetal (subscript Ac) may be evaluated as previously described (13, 44). Figure 2A shows that [C=O/C − (C=O)AcC] is very close to Nnonproton/C for L. helveticus and L. lactis; the same is found for other gram-positive and gram-negative bacteria analyzed with a spectral resolution similar to that used here. This indicates that most of the nonprotonated nitrogen may be attributed to the peptidic link of proteins. The higher value of O531.2/C with respect to Nnonproton/C (Fig. 2B) will be discussed below.
FIG. 2.
Molar concentration ratio with respect to total carbon, as a function of the molar ratio of nonprotonated nitrogen to total carbon (Nnonproton/C), of carbon making one double or two single bonds with oxygen (C=O/C) (open symbols) and after deduction of the acetal contribution (closed symbols) (A) and of oxygen responsible for the peak at 531.2 eV (O531.2/C, mainly due to O=C/C) (open symbols) and after deduction of 2 P/C (closed symbols) (B). Circles, L. helveticus ATCC 12046, including data from Mozes and Lortal (36); squares, L. lactis subsp. lactis bv. diacetilactis LMG 9452; triangles and diamonds, other gram-positive (triangles) (16) and gram-negative (diamonds) (13, 27, 39) bacteria analyzed earlier.
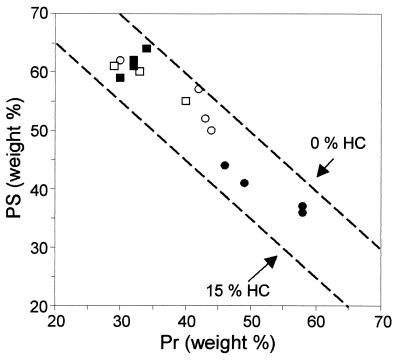
The XPS data were converted into concentrations of model compounds (i.e., proteins, polysaccharides, and hydrocarbonlike compounds) as previously described (13, 16). Table 3 presents the elemental compositions and carbon concentrations of the three model constituents considered. In this method of modeling the molecular composition, peptidoglycans are considered a mixture of proteins and polysaccharides, and (lipo)teichoic acids are considered a combination of hydrocarbonlike compounds and polysaccharides. Figure 3 presents the results obtained for different samples of L. helveticus and L. lactis harvested in the exponential and stationary growth phases. The cell surface was very poor in hydrocarbonlike compounds (less than 15% [wt/wt]); it was essentially made of proteins and polysaccharides. For L. helveticus, the polysaccharide/protein ratio increased between the exponential and stationary growth phases and showed an appreciable variation from one culture to another. This ratio was higher for L. lactis; it did not change during culture and showed a weak variability from one culture to another.
TABLE 3.
Chemical compositions of model constituents (molar ratio with respect to total carbon) considered for deduction of the molecular composition of cell surfaces
| Model constituent | O/C | N/C | Carbon concn (mmol/g) |
|---|---|---|---|
| Proteina | 0.347 | 0.286 | 43.1 |
| Hydrocarbonlike compoundb | 0.000 | 0.000 | 71.4 |
| Polysaccharidec | 0.833 | 0.000 | 37.0 |
S-layer protein of L. helveticus ATCC 12046 calculated from data of Lortal et al. (31).
Hydrocarbonlike compound, (CH2)n.
Polysaccharide, (C6H10O5)n.
FIG. 3.
Diagram showing the surface composition of L. helveticus ATCC 12046 (circles) and L. lactis subsp. lactis bv. diacetilactis LMG 9452 (squares) in terms of model compounds, as deduced from XPS. Pr, protein; PS, polysaccharide; HC, hydrocarbonlike compounds. Cells harvested in the exponential (closed symbols) and stationary (open symbols) growth phases are represented.
DISCUSSION
Relationships among surface functional groups.
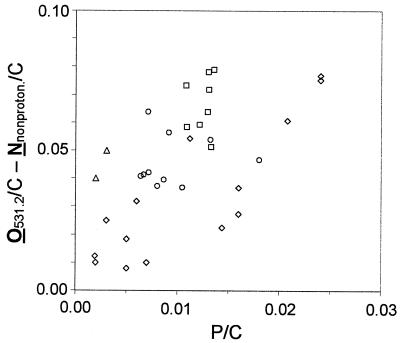
Figure 2A confirms that the higher values of the C=O/C ratio compared to the Nnonproton/C ratio may be attributed to the acetal of polysaccharides (Fig. 2A). The difference between the O531.2/C and Nnonproton/C (Fig. 2B) ratios did not receive attention before. When this difference is plotted as a function of the phosphate concentration (Fig. 4), a correlation is found (correlation coefficient, 0.61); the trend is preserved if other bacteria are included in the comparison. This difference may therefore be interpreted in the light of previous data obtained on phosphate-containing compounds. The O1s peaks of AlPO4, CaHPO4, and Ca2P2O7 were reported to appear at 531.6, 531.6, and 531.4 eV, respectively (26, 28). On the other hand, spectra of KH2PO4, CaHPO4, and FePO4 (G. Vereecke, personal communication) showed that the O1s peak of phosphate has two components appearing in the ranges of 530.9 to 531.5 and 532.4 to 532.9 eV, respectively, with the relative intensities given in Table 4. Table 4 also gives the relative intensities expected if the low binding energy peak is attributed to P=O or P—O− and the high binding energy peak is attributed to P—OH. A comparison of experimental and expected data supports this assignment. In addition, Table 4 gives the data obtained for glucose-6-phosphate (20) and shows that they are in agreement with the assignment of the low binding energy component to P=O and P—O− and the assignment of the high binding energy component to P—O—C and P—OH.
FIG. 4.
Difference between the molar concentration ratios of oxygen responsible for the peak at 531.2 eV and nonprotonated nitrogen with respect to total carbon, [O531.2/C − Nnonproton/C], as a function of the molar ratio of phosphate to total carbon (P/C). L. helveticus ATCC 12046, including data from Mozes and Lortal (36) (circles), L. lactis subsp. lactis bv. diacetilactis LMG 9452 (squares), and other gram-positive (triangles) (16) and gram-negative (diamonds) (13, 27, 39) bacteria analyzed previously are represented.
TABLE 4.
Proportions of the components in the O1s XPS peak for phosphate salts (G. Vereecke, personal communication) and for glucose-6-phosphate (20) and proportions of the expected types of oxygen
| Compound | Proportion (%)
|
|||
|---|---|---|---|---|
| Determined by XPS
|
Expected
|
|||
| 532.4–532.9 eV | 530.9–531.5 eV | P—OH, P—O—C, C—OH | P=O, P—O−, C=O | |
| KH2PO4 | 40 | 60 | 50 | 50 |
| CaHPO4 | 27 | 73 | 25 | 75 |
| FePO4 | 15 | 85 | 0 | 100 |
| Glucose-6-phosphate | 76 | 24 | 78 | 22 |
In the case of bacteria, the excess of O531.2/C with respect to Nnonproton/C may thus be tentatively attributed to the oxygen of phosphate groups, involved in (lipo)teichoic acids or in phospholipids. In these moieties, two oxygen atoms of phosphate are singly bound to carbon and are expected to contribute to the component at 532.6 eV. One oxygen is doubly bound to phosphorus, and one is in the form P—O−; these are expected to contribute to the component at 531.2 eV, together with O=C. Consequently, the O=C/C ratio should be corrected as follows: O=C/C = [O531.2/C] − 2 P/C. Figure 2B shows that this correction brings O531.2/C closer to Nnonproton/C; O=C/C may thus be mainly assigned to proteins.
The above discussion of relationships between functional groups, as detected by XPS, supports the accuracy of the sensitivity factors and peak decomposition. Furthermore, it demonstrates the relevance of XPS for quantifying the main classes of molecular constituents at the surfaces of microorganisms.
Surface molecular composition.
The results of the modeling of XPS data in terms of proteins, polysaccharides, and hydrocarbonlike compounds (Fig. 3) may be discussed in the light of previous biochemical studies. The cell surface composition of L. lactis shows a large excess of polysaccharide with respect to protein. This is in line with the results (7) obtained with loosely associated cell surface material of another strain of L. lactis subsp. lactis bv. diacetilactis. Moreover, it is also in agreement with the fact that the phage receptors present at the surfaces of other strains of L. lactis were found to be located mainly on carbohydrate moieties (22, 35, 45, 50).
It is well established that the S-layer present at the surface of L. helveticus ATCC 12046 is made of unglycosylated proteins (30, 31, 36). According to the amino acid composition of the S-layer protein of L. helveticus determined by Lortal et al. (31), the ratio Nproton/C should be about 0.030 compared with the ratio of 0.013 ± 0.002 measured by XPS. The observation of polysaccharide material at the L. helveticus extreme surface may not be due to contribution of material present below the S-layer, as the latter is expected to have a thickness of about 9 nm (31). It may thus be attributed either to a fragmentation of the S-layer, allowing the underlying material to be at the surface, or to the protrusion of polysaccharides and/or (lipo)teichoic acids through the S-layer. The enrichment of the extreme surface in polysaccharide material for stationary-phase cells could be due either to their synthesis during growth or to a further fragmentation of the S-layer.
Relation between surface composition and physicochemical properties.
Water contact angle measurements (Table 1) indicated that the surfaces of L. lactis and L. helveticus were hydrophilic whatever the growth phase. This is in agreement with previous studies, based on microbial adhesion to solvents, which showed a hydrophilic character for other strains of L. lactis subsp. lactis bv. diacetilactis (7) and of Lactobacillus (40). In previous works, cell surface hydrophobicity, assessed by the water contact angle, was directly correlated with the concentration of nitrogen or carbon involved in hydrocarbon form and inversely correlated with the concentration of oxygen (3, 13, 16, 34, 37). In this study, the hydrophilic character of the two strains is in agreement with the low concentration of hydrocarbon and the high concentration of oxygen measured by XPS. However, the differences observed in the polysaccharide/protein ratios when the two strains or the two growth phases are compared are not accompanied by significant differences in surface hydrophilicity.
L. lactis has a lower isoelectric point and an electrophoretic mobility above pH 5 four times larger than L. helveticus; similar results were obtained on cells in exponential and stationary growth phases (Fig. 1). In previous works, positive correlations were found between the electrophoretic mobility at pH 4 and the phosphorus content of the extreme cell surface (37). For a set of Lactobacillus species, Millsap et al. (34) showed that the isoelectric point was directly correlated with the nitrogen surface concentration and inversely correlated with the oxygen surface concentration. The electrokinetic properties of bottom-fermenting brewing yeasts were shown to be controlled by phosphate, resulting in a low isoelectric point, while those of top-fermenting yeasts were determined by the balance between the protonated amino and carboxylate groups in proteins, giving a higher isoelectric point (11).
In order to tentatively relate the electrokinetic properties to the surface chemical composition, the surface charge concentration of the exponential L. helveticus cell was computed, as described previously for yeasts (11). This was done by using the surface chemical composition given by XPS and the amino acid composition of its S-layer protein, determined from biochemical analysis (31). Since the latter did not allow a distinction between aspartic acid and asparagine or between glutamic acid and glutamine, two extreme situations were considered: only aspartic and glutamic acid or only asparagine and glutamine. In this charge computation, three ionized groups were considered to determine the surface electrical properties: phosphate groups (pKa = 2.15), involved in (lipo)teichoic acids, and carboxylate (pKa = 3.95) and protonated amino (pKa = 9.67) groups of proteins.
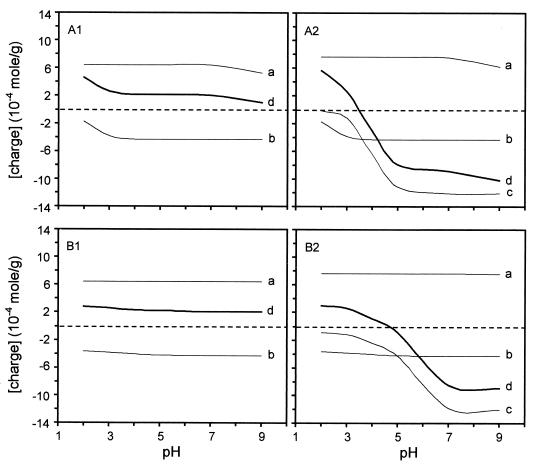
Figure 5A1 and A2 shows the variation of the concentration of ionized functions and of the resulting charge as a function of pH for the surface of L. helveticus in the exponential growth phase, considering the two extreme situations and the intrinsic acidity constants given above. Actually, the acidity constant should be replaced by an apparent constant, Kapp, obtained by multiplying the intrinsic acidity constant by exp (FΨ/RT), where F is the Faraday constant, Ψ is the local electrical potential, R is the gas constant, and T is the temperature. The electrical potential at the slip plane, the zeta potential, may be deduced from the electrophoretic mobility through multiplication of the latter by 12.85. Using Kapp and assuming that the local electrical potential was equal to the zeta potential provided curves (not shown) which were not significantly different from those in Fig. 5A1 and A2, respectively. In reality, the surface potential differs from the zeta potential due to specific ion adsorption and the separation between the surface and the slip plane at which the zeta potential is measured. In order to examine the sensitivity of the results to the local potential, the concentrations of the ionized groups and of the resulting charge were computed by assuming a local electrical potential equal to 10 times the zeta potential. The results obtained are given in Fig. 5B1 and B2. Comparison with Fig. 5A shows that, in the pH range of interest, the local potential has a strong influence on deprotonation of RCOOH; it has no effect on deprotonation of RNH3+ and hardly any on that of R2HPO4.
FIG. 5.
Plot of the concentrations of RNH3+ (a), R2PO4− (b), RCOO− (c), and resulting charges (d) as a function of pH for exponential-phase L. helveticus ATCC 12046 cells. The acid-base equilibria are assumed to be unaffected by electrical potential (A1 and A2) or affected by a local potential equal to 10 times the zeta potential (B1 and B2). The S-layer protein is assumed to contain no acidic amino acids (A1 and B1) or no asparagine or glutamine (A2 and B2).
A comparison of Fig. 1 and 5 shows that the S-layer protein of L. helveticus contains a certain proportion of aspartic and glutamic acids or that the polysaccharides protruding through the S-layer carry carboxylate groups. Otherwise (Fig. 5A1), the total charge concentration of the cell surface would remain positive over the whole pH range, in contradiction to negative electrophoretic mobilities above pH 3. Moreover, it appears in Fig. 5A2 that the carboxyl groups determine the shape of the charge density-versus-pH curve and, consequently, the shape of the electrophoretic mobility curve of L. helveticus.
The Nnonproton/P ratio differs markedly between L. helveticus (14 and 16) and L. lactis (8 and 7). The two strains follow the correlation between N/P and the electrophoretic mobility at pH 4 reported for other microorganisms (1, 37). The more negative character of L. lactis compared to L. helveticus is thus related to the absence of an S-layer and a better exposition of the underlying materials at the surface. A more detailed interpretation in terms of the amount and nature (isoelectric point) of proteins (or polypeptides), of (lipo)teichoic acids, and of the amount of carboxylate borne by polysaccharides would require the examination of a larger collection of strains and biochemical data.
Conclusion.
The interpretation of the XPS data has been improved by showing that the oxygen peak component appearing around 531.2 eV contains a contribution of oxygen from phosphate (P=O and P—O−), together with oxygen making a double bond with carbon.
The surfaces of L. lactis and L. helveticus are essentially made of proteins (or polypeptides) and polysaccharides. The hydrophilic character of the two strains, as evidenced by the water contact angle, is in agreement with a high concentration of polysaccharide and with a low concentration of hydrocarbonlike compounds. For L. helveticus, the polysaccharide/protein ratio increases between the exponential and stationary growth phases, which is attributed to either a fragmentation of the S-layer or a synthesis of polysaccharides and/or (lipo)teichoic acids protruding through the S-layer. The polysaccharide/protein ratio is higher for L. lactis and does not change during culture.
The electrokinetic properties of L. helveticus reveal the influence of carboxyl groups involved in the protein of the S-layer or borne by polysaccharides. On the other hand, the more negative character of L. lactis is related to a lower N/P ratio at the surface. The approach followed here may be used to relate the behavior of lactic acid bacteria to their surface chemical compositions and to understand the influence of culture conditions, strains, and mutation.
ACKNOWLEDGMENTS
We thank C. Dupont-Gillain, M. Genet, Y. Dufrêne, and J. Delcour for valuable discussions and G. Vereecke for providing XPS spectra of phosphate-containing compounds.
This work was supported by the Foundation for Training in Industrial and Agricultural Research (FRIA), the National Foundation for Scientific Research (FNRS), the Federal Office for Scientific, Technical and Cultural Affairs (Interuniversity Poles of Attraction Programme), and the Research Department of Communauté Française de Belgique (Concerted Research Action).
REFERENCES
- 1.Amory D E, Mozes N, Hermesse M P, Leonard A J, Rouxhet P G. Chemical analysis of the surface of microorganisms by X-ray photoelectron spectroscopy. FEMS Microbiol Lett. 1988;49:107–110. [Google Scholar]
- 2.Busscher H J, van de Belt-Gritter B, van der Mei H C. Implications of microbial adhesion to hydrocarbons for evaluating cell surface hydrophobicity. 1. Zeta potentials of hydrocarbon droplets. Colloids Surf B: Biointerfaces. 1995;5:111–116. [Google Scholar]
- 3.Busscher H J, Bellon-Fontaine M-N, Mozes N, van der Mei H C, Sjollema J, Léonard A J, Rouxhet P G, Cerf O. An interlaboratory comparison of physico-chemical methods for studying the surface properties of microorganisms—application to Streptococcus thermophilus and Leuconostoc mesenteroides. J Microbiol Methods. 1990;12:101–115. [Google Scholar]
- 4.Cerning J. Production of exopolysaccharides by lactic acid bacteria and dairy propionibacteria. Lait. 1995;75:463–472. [Google Scholar]
- 5.Champagne C P, Lacroix C, Sodini-Gallot I. Immobilized cell technologies for the dairy industry. Crit Rev Biotechnol. 1994;14:109–134. doi: 10.3109/07388559409086964. [DOI] [PubMed] [Google Scholar]
- 6.Chapot-Chartier M-P, Deniel C, Rousseau M, Vassal L, Gripon J-C. Autolysis of two strains of Lactococcus lactis during cheese ripening. Int Dairy J. 1994;4:251–269. [Google Scholar]
- 7.Crow V L, Gopal P K, Wicken A J. Cell surface differences of lactococcal strains. Int Dairy J. 1995;5:45–68. [Google Scholar]
- 8.Crow V L, Coolbear T, Gopal P K, Martley F G, McKay L L, Riepe H. The role of autolysis of lactic acid bacteria in the ripening of cheese. Int Dairy J. 1995;5:855–875. [Google Scholar]
- 9.De Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 10.Dengis P B, Rouxhet P G. Preparation of yeast cells for surface analysis by XPS. J Microbiol Methods. 1996;26:171–183. [Google Scholar]
- 11.Dengis P B, Rouxhet P G. Surface properties of top- and bottom-fermenting yeast. Yeast. 1997;13:931–943. doi: 10.1002/(SICI)1097-0061(199708)13:10<931::AID-YEA149>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Dengis P B, Gerin P A, Rouxhet P G. X-ray photoelectron spectroscopy analysis of biosurfaces: examination of performances with yeast cells and related model compounds. Colloids Surf B: Biointerfaces. 1995;4:199–211. [Google Scholar]
- 13.Dufrêne Y F, Rouxhet P G. Surface composition, surface properties, and adhesiveness of Azospirillum brasilense—variation during growth. Can J Microbiol. 1996;42:548–556. [Google Scholar]
- 14.Dufrêne Y F, Boonaert C J-P, Rouxhet P G. Role of proteins in the adhesion of Azospirillum brasilense to model substrata. In: Berthelin J, Huang P M, Bollag J-M, Andreux F, editors. Effect of mineral-organic-microorganism interactions on soil and freshwater environments. New York, N.Y: Kluwer Academic/Plenum Publishers; 1999. pp. 261–274. [Google Scholar]
- 15.Dufrêne Y F, Boonaert C J P, Rouxhet P G. Surface analysis by X-ray photoelectron spectroscopy in the study of bioadhesion and biofilms. Methods Enzymol. 1999;310:375–389. doi: 10.1016/s0076-6879(99)10030-2. [DOI] [PubMed] [Google Scholar]
- 16.Dufrêne Y F, van der Wal A, Norde W, Rouxhet P G. X-ray photoelectron spectroscopy analysis of whole cells and isolated cell walls of gram-positive bacteria: comparison with biochemical analysis. J Bacteriol. 1997;179:1023–1028. doi: 10.1128/jb.179.4.1023-1028.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flint S H, Bremer P J, Brooks J D. Biofilms in dairy manufacturing plant—description, current concerns and method of control. Biofouling. 1997;11:81–97. [Google Scholar]
- 18.Forde A, Fitzgerald G F. Analysis of exopolysaccharide (EPS) production mediated by the bacteriophage adsorption blocking plasmid, pCI658, isolated from Lactococcus lactis ssp. cremoris HO2. Int Dairy J. 1999;9:465–472. [Google Scholar]
- 19.Gasson M J. Progress and potential in the biotechnology of lactic acid bacteria. FEMS Microbiol Rev. 1993;12:3–20. [Google Scholar]
- 20.Gerin P A, Dengis P B, Rouxhet P G. Performances of XPS analysis of model biochemical compounds. J Chim Phys. 1995;92:1043–1065. [Google Scholar]
- 21.Gerin P A, Dufrene Y, Bellon-Fontaine M-N, Asther M, Rouxhet P G. Surface properties of the conidiospores of Phanerochaete chrysosporium and their relevance to pellet formation. J Bacteriol. 1993;175:5135–5144. doi: 10.1128/jb.175.16.5135-5144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal P K, Reilly K I. Molecular architecture of the lactococcal cell surface as it relates to important industrial properties. Int Dairy J. 1995;5:1095–1111. [Google Scholar]
- 23.Gusils C, Pérez Chaia A, González S, Oliver G. Lactobacilli isolated from chicken intestines: potential use as probiotics. J Food Prot. 1999;62:252–256. doi: 10.4315/0362-028x-62.3.252. [DOI] [PubMed] [Google Scholar]
- 24.James A M. Charge properties of microbial cell surfaces. In: Mozes N, Handley P S, Busscher H J, Rouxhet P G, editors. Microbial cell surface analysis: structural and physicochemical methods. New York, N.Y: VCH Publishers; 1991. pp. 221–262. [Google Scholar]
- 25.Jeurnink T J M, Brinkman D W. The cleaning of heat exchangers and evaporators after processing milk or whey. Int Dairy J. 1994;4:347–368. [Google Scholar]
- 26.Landis W J, Martin J R. X-ray photoelectron spectroscopy applied to gold-decorated mineral standards of biological interest. J Vacuum Sci Technol A. 1984;2:1108–1111. [Google Scholar]
- 27.Latrache H, Mozes N, Pelletier C, Bourlioux P. Chemical and physicochemical properties of Escherichia coli: variation among three strains and influence of culture conditions. Colloids Surf B: Biointerfaces. 1994;2:47–56. [Google Scholar]
- 28.Lindblad T, Rebenstorf B, Yan Z-G, Andersson S L T. Characterization of vanadia supported on amorphous AlPO4 and its properties for oxidative dehydrogenation of propane. Appl Catalysis A. 1994;112:187–208. [Google Scholar]
- 29.Lortal S, Lemée R, Valence F. Autolysis of thermophilic lactobacilli and dairy propionibacteria: a review. Lait. 1997;77:133–150. [Google Scholar]
- 30.Lortal S, Rousseau M, Boyaval P, van Heijenoort J. Cell wall and autolytic system of Lactobacillus helveticus ATCC 12046. J Gen Microbiol. 1991;137:549–559. [Google Scholar]
- 31.Lortal S, van Heijenoort J, Gruber K, Sleytr U B. S-layer of Lactobacillus helveticus ATCC 12046: isolation, chemical characterization and reformation after extraction with lithium chloride. J Gen Microbiol. 1992;138:611–618. [Google Scholar]
- 32.Mercenier A. Lactic acid bacteria as live vaccines. In: Tannock G, editor. Probiotics: a critical review. Wymondham, United Kingdom: Horizon Scientific Press; 1999. pp. 113–127. [Google Scholar]
- 33.Messner P, Allmaier G, Schäffer C, Wugeditsch T, Lortal S, König H, Niemetz R, Dorner M. Biochemistry of S-layers. FEMS Microbiol Rev. 1997;20:25–46. doi: 10.1111/j.1574-6976.1997.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 34.Millsap K W, Reid G, van der Mei H C, Busscher H J. Cluster analysis of genotypically characterized Lactobacillus species based on physicochemical cell surface properties and their relationship with adhesion to hexadecane. Can J Microbiol. 1997;43:284–291. [Google Scholar]
- 35.Monteville M R, Ardestani B, Geller B. Lactococcal bacteriophages require a host cell wall carbohydrate and a plasma membrane protein for adsorption and ejection of DNA. Appl Environ Microbiol. 1994;60:3204–3211. doi: 10.1128/aem.60.9.3204-3211.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mozes N, Lortal S. X-ray photoelectron spectroscopy and biochemical analysis of the surface of Lactobacillus helveticus ATCC 12046. Microbiology. 1995;141:11–19. [Google Scholar]
- 37.Mozes N, Léonard A J, Rouxhet P G. On the relations between the elemental surface composition of yeasts and bacteria and their charge and hydrophobicity. Biochim Biophys Acta. 1988;945:324–334. doi: 10.1016/0005-2736(88)90495-6. [DOI] [PubMed] [Google Scholar]
- 38.Mozes N, Marchal F, Hermesse M P, Van Haecht J L, Reuliaux L, Leonard A J, Rouxhet P G. Immobilization of microorganisms by adhesion: interplay of electrostatic and nonelectrostatic interactions. Biotechnol Bioeng. 1987;30:439–450. doi: 10.1002/bit.260300315. [DOI] [PubMed] [Google Scholar]
- 39.Nordström K M, Mozes N. Analysis of wild-type and plasmid-cured Thermus spp. by X-ray photoelectron spectroscopy. Colloids Surf B: Biointerfaces. 1994;2:67–72. [Google Scholar]
- 40.Pelletier C, Bouley C, Cayuela C, Bouttier S, Bourlioux P, Bellon-Fontaine M-N. Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Appl Environ Microbiol. 1997;63:1725–1731. doi: 10.1128/aem.63.5.1725-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid G. The scientific basis for probiotic strains of Lactobacillus. Appl Environ Microbiol. 1999;65:3763–3766. doi: 10.1128/aem.65.9.3763-3766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rijnaarts H H M, Norde W, Bouwer E J, Lyklema J, Zehnder A J B. Reversibility and mechanism of bacterial adhesion. Colloids Surf B: Biointerfaces. 1995;4:5–22. [Google Scholar]
- 43.Rouxhet P G, Genet M J. Chemical composition of the microbial cell surface by X-ray photoelectron spectroscopy. In: Mozes N, Handley P S, Busscher H J, Rouxhet P G, editors. Microbial cell surface analysis: structural and physicochemical methods. New York, N.Y: VCH Publishers; 1991. pp. 173–220. [Google Scholar]
- 44.Rouxhet P G, Mozes N, Dengis P B, Dufrêne Y F, Gerin P A, Genet M J. Application of X-ray photoelectron spectroscopy to microorganisms. Colloids Surf B: Biointerfaces. 1994;2:347–369. [Google Scholar]
- 45.Sijtsma L, Wouters J T M, Hellingwerf K J. Isolation and characterization of lipoteichoic acid, a cell envelope component involved in preventing phage adsorption, from Lactococcus lactis subsp. cremoris SK110. J Bacteriol. 1990;172:7126–7130. doi: 10.1128/jb.172.12.7126-7130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sijtsma L, Jansen N, Hazeleger W C, Wouters J T M, Hellingwerf K J. Cell surface characteristics of bacteriophage-resistant Lactococcus lactis subsp. cremoris SK110 and its bacteriophage-sensitive variant SK112. Appl Environ Microbiol. 1990;53:3230–3233. doi: 10.1128/aem.56.10.3230-3233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valence F, Richoux R, Thierry A, Palva A, Lortal S. Autolysis of Lactobacillus helveticus and Propionibacterium freudenreichii in Swiss cheeses: first evidence by using species-specific lysis markers. J Dairy Res. 1998;65:609–620. [Google Scholar]
- 49.Valyasevi R, Sandine W E, Geller B L. A membrane protein is required for bacteriophage c2 infection of Lactococcus lactis subsp. lactis C2. J Bacteriol. 1991;173:6095–6100. doi: 10.1128/jb.173.19.6095-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valyasevi R, Sandine W E, Geller B L. Lactococcus lactis ssp. lactis C2 bacteriophage sk1 receptor involving rhamnose and glucose moieties in the cell wall. J Dairy Sci. 1994;77:1–6. [Google Scholar]
- 51.van der Mei H C, van de Belt-Gritter B, Busscher H J. Implications of microbial adhesion to hydrocarbons for evaluating cell surface hydrophobicity. 2. Adhesion mechanisms. Colloids Surf B: Biointerfaces. 1995;5:117–126. [Google Scholar]
- 52.van der Mei H C, Rosenberg M, Busscher H J. Assessment of microbial cell surface hydrophobicity. In: Mozes N, Handley P S, Busscher H J, Rouxhet P G, editors. Microbial cell surface analysis: structural and physicochemical methods. New York, N.Y: VCH Publishers; 1991. pp. 263–287. [Google Scholar]
- 53.van Loosdrecht M C M, Lyklema J, Norde W, Schraa G, Zehnder A J B. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Loosdrecht M C M, Lyklema J, Norde W, Schraa G, Zehnder A J B. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987;53:1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Velraeds M M C, van der Mei H C, Reid G, Busscher H J. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol. 1996;62:1958–1963. doi: 10.1128/aem.62.6.1958-1963.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe K, Ishibashi K, Iki K, Nakashima Y, Hayashida M, Amako K. Cell surface characteristics of some phage-resistant strains of Lactobacillus casei. J Appl Bacteriol. 1987;63:197–200. [Google Scholar]