Abstract
Background:
Social connections have a significant impact on health across age groups, including older adults. Loneliness and social isolation are known risk factors for Alzheimer’s disease and related dementias (ADRD). Yet, we did not find a review focused on meta-analyses and systematic reviews of studies that had examined associations of social connections with cognitive decline and trials of technology-based and other social interventions to enhance social connections in people with ADRD.
Study design:
We conducted a scoping review of 11 meta-analyses and systematic reviews of social connections as possible determinants of cognitive decline in older adults with or at risk of developing ADRD. We also examined eight systematic reviews of technology-based and other social interventions in persons with ADRD.
Study results:
The strongest evidence for an association of social connections with lower risk of cognitive decline was related to social engagement and social activities. There was also evidence linking social network size to cognitive function or cognitive decline, but it was not consistently significant. A number of, though not all, studies reported a significant association of marital status with risk of ADRD. Surprisingly, evidence showing that social support reduces the risk of ADRD was weak. To varying degrees, technology-based and other social interventions designed to reduce loneliness in people with ADRD improved social connections and activities as well as quality of life but had no significant impact on cognition. We discuss strengths and limitations of the studies included.
Conclusions:
Social engagement and social activities seem to be the most consistent components of social connections for improving cognitive health among individuals with or at risk for ADRD. Socially focused technology-based and other social interventions aid in improving social activities and connections and deserve more research.
Keywords: aging, digital, robots, loneliness, pets, marital status
Introduction
Social determinants of health (SDoHs) are social and structural factors that affect incidence, prevalence, and course of diseases as well as health inequities and reportedly account for 30–55% of health outcomes, exceeding the contribution from medical factors (World Health Organization, 2008). SDoHs impact physical, mental, and cognitive function and longevity among all age groups including older adults (Jeste, 2022; Jester et al., 2023). Over recent decades, the construct of social connections has acquired increasing attention as an SDoH. Social connection is a broad term that encompasses various structural, functional, and quality aspects of interpersonal relationships and interactions (National Academies of Sciences, Engineering and Medicine, 2020). Considerable scientific evidence shows that being embedded in close relationships and feeling socially connected to the people in one’s life is associated with a significantly reduced risk for a range of disease morbidities and all-cause mortality (Holt-Lunstad et al., 2017). In a meta-analysis of 148 studies with a total of 308,849 participants, the odds ratio (OR) for the strength of social relationships was 1.50 (95% confidence nterval 1.42–1.59), indicating a 50% [CI95% 42%–59%] increased likelihood of survival among participants with stronger social relationships (Holt-Lunstad et al., 2010). This finding remained consistent across a range of variables including age, sex, initial health status, cause of death, and follow-up period.
There is considerable literature on the relationship of social connections, isolation, and health (Jeste et al., 2023a). Social disconnection has become a global behavioral pandemic (Na et al., 2023). A National Academies of Sciences, Engineering, and Medicine Report highlighted how social isolation and loneliness are serious yet underappreciated public health risks that affect more than a quarter of the older adult population (National Academies of Sciences, Engineering and Medicine, 2020). Social isolation is a major risk factor for several disabling and life-shortening disorders including dementia. This has led to research on interventions to enhance social support. A review (Hogan et al., 2002) of 100 studies pointed to overall usefulness of social support interventions, although there was not enough evidence to conclude which interventions worked best for what problems. A more recent systematic review and meta-analysis of psychological interventions for loneliness, many of which involved cognitive behavioral therapy, found a significant reduction in loneliness compared to control groups, with a small to medium effect size (g = 0.43) (Hickin et al., 2021).
Growing evidence suggests that SDoHs can help explain heterogeneity in outcomes in Alzheimer’s disease and related dementias (ADRD). According to the World Health Organization (WHO), the number of individuals with ADRD worldwide is about 55 million today and will increase to 78 million by 2030 and 139 million by 2050 (World Health Organization, 2021). Recently, the national network of Alzheimer’s Disease Research Centers presented a framework for assessing SDoHs in ADRD (Stites et al., 2022). It proposed several specific SDoH domains that appear foundational to ADRD, and social support and social networks were prominent on that list. However, we found no published scholarly review that synthesized the findings of meta-analyses and systematic reviews focused on social connections as possible determinants of cognitive health in older adults with or at risk of developing ADRD. It will be useful to determine which components of social connections are more impactful than others. Similarly, there were no reviews synthesizing the findings of meta-analyses or systematic reviews on technology-based and other social interventions targeting social connections in people with ADRD. This review sought to address both those gaps in the literature and offer suggestions for interventions as well as future research. Scoping reviews aim at developing an overview of the published evidence when research objectives or review questions involve exploring, identifying, and discussing characteristics or concepts across a breadth of domains and sources (Munn et al., 2018; Peters et al., 2021). Given the heterogeneity of the published literature on SDoHs in ADRD, a scoping review was considered to be most appropriate.
Methods
We performed a scoping review of the literature on commonly listed social factors relevant to ADRD, as well as technology-based and other social interventions. Meta-analyses and systematic reviews were searched for inclusion, using the terms mentioned in Figure 1. This list was developed via consensus among the co-authors and was made to highlight potentially malleable major factors that could be assessed in a clinical setting (Figure 1). We used MeSH Trees to inform the specific search terms rather than searching for MeSH terms themselves. Many terms came directly from the following MeSH Tree: Anthropology, Education, Sociology and Social Phenomena Category -> Social Sciences -> Sociology -> Sociological Factors. After consulting this MeSH Tree, we found that many common terms were missing from the Nodes. Therefore, we sought additional guidance from a recently published review by Holt-Lunstad on how the many different facets of social connection may influence health in older age (e.g. social isolation vs. marital status vs. neighborhood and built environment) (Holt-Lunstad, 2022). We obtained a total of 2,513 articles from PubMED, PsycINFO, EMBASE, and CINAHL. After deleting 609 duplicates, we screened 1,904 articles based on the criteria of having all three elements in their title: (1) systematic review or meta-analysis, (2) Alzheimer’s disease or dementia, and (3) one of the following terms: social connection, social isolation, social support, social network, socioeconomic, social activities, social engagement or disengagement, social skills, neighborhood, social contact, social belonging, social fragmentation, pets, social robot, marriage, social environment, couple relationship, social functioning, social behavior, loneliness, social participation, social interaction, intergenerational, community, social or community resources, social class, social drift, social determinant, social measure, social stressor, social disparity, social positioning, social identity, sociocultural factors, and social cohesion.
Figure 1.
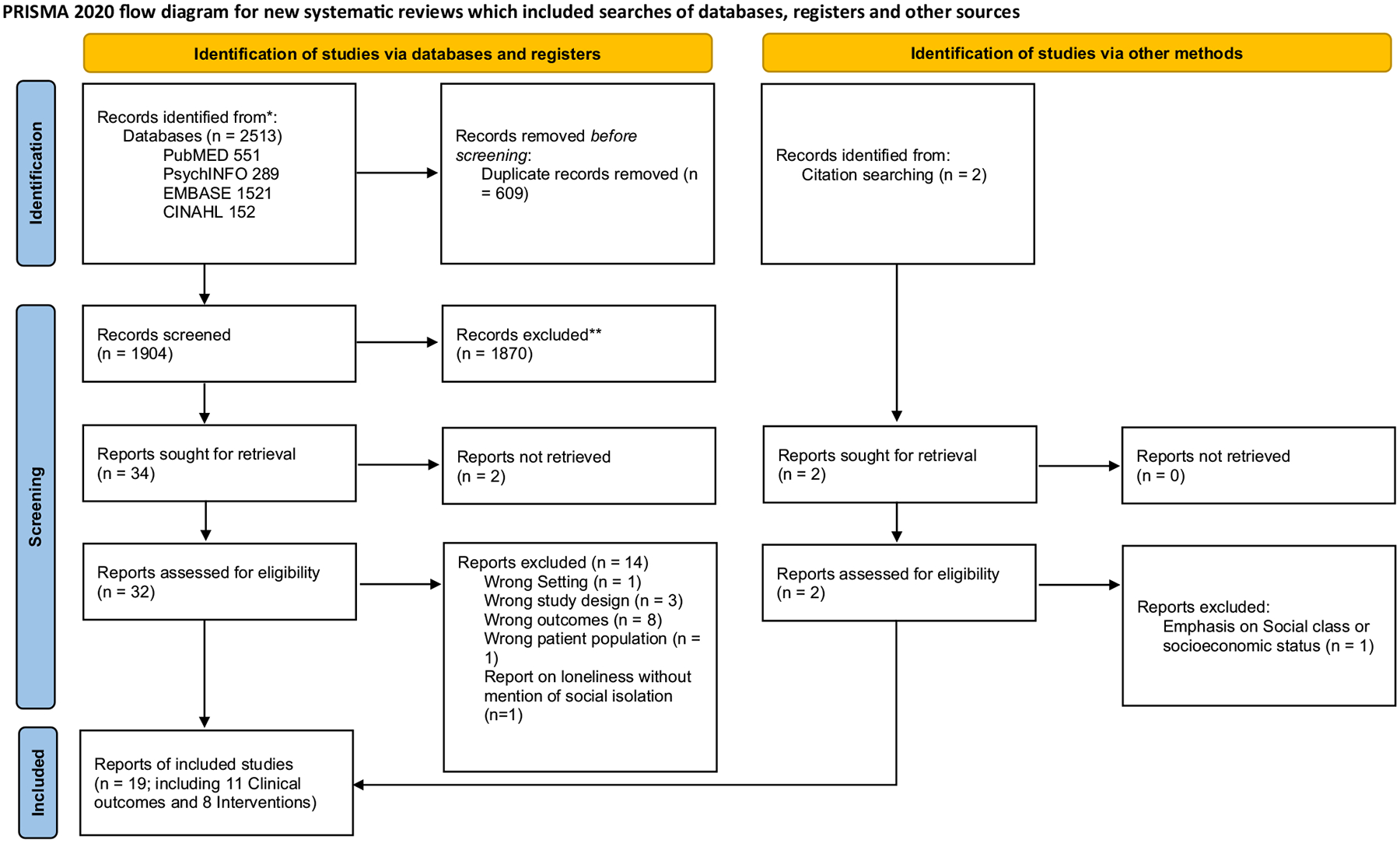
Prisma flow chart.
*Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).
**If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Articles were excluded if they had all three elements but referenced only caregivers or carers and not individuals with ADRD. A total of 1,872 records were excluded after title and abstract screening and 2 additional records were identified from other search methods and assessed for eligibility. Fifteen articles were excluded after full-text review. Nineteen studies (11 clinical outcomes studies and 8 intervention studies) were included in the final review (see Figure 1).
The data extracted included (1) author/year and study type, (2) number of studies included in the meta-analysis or systematic review, (3) sample size, (4) samples with or without ADRD at baseline, (5) study outcomes, (6) heterogeneity of findings, quality of study, publication bias, and sensitivity analysis, if provided, and (7) results of the meta-analysis with estimates and effect sizes when available – for example, OR (with 95% confidence intervals). Several articles also examined a few other potential risk factors for ADRD not related to social connectedness. These non-social factors are not discussed below.
Results
I. Associations of social connections with clinical outcomes
Table 1 lists main findings from 11 articles focused on meta-analyses and systematic reviews of the associations of various social connection-related factors, which included social engagement, social activities, social network, marital status, and social support, with clinical outcomes – primarily, change in cognitive function or risk of ADRD.
Table 1.
Meta-analyses and systematic reviews of studies on social connections related to cognitive decline and other clinical outcomes in Alzheimer’s disease
| AUTHOR (YEAR) STUDY TYPE | # OF ARTICLES INCLUDED | SAMPLE SIZE | SOCIAL DETERMINANT(s) OF HEALTH | outcome(s) | META-ANALYSIS METHODS FINDINGS | |
|---|---|---|---|---|---|---|
| Bougea et al. (2022): Meta-analysis and Systematic review | 13 studies; all longitudinal design; people with or without ADRD at baseline | N/A | Psychosocial stress: Work- related stress, SES inequalities, marital status, offspring status, PTSD, vital exhaustion or somatization of distress, and combined factors | Risk of ADRD (diagnosed by DSM-III,III-R, IV, V, NINCDS-ADRDA, NIN DS-AIREN, or ICD–8,9,10) Results split into risk of AD vs. risk of dementia of any cause. |
Heterogeneity: (1) forest plot visualization (2) null hypothesis of Cochrane’s Q χ2 test (p < 0.1) and (3) I2 = 82% (p < 0.01) Publication bias: publication bias indicated given uneven distribution of studies shown in funnel plot. Egger’s test was not performed. Quality Assessment: Quality of Prognosis Studies in Systematic Reviews (QUIPS): all but 7 included publications were rated as having adequate participation; all but 2 studies were noted to have 70% data on cognitive decline at follow-up, and all but 1 study were reported to have measured for potential cofounders. No studies met the quality statistics criterion for “no differences between participants and drop-outs” or “using sufficient methods” (though one study was listed as having insufficient information to judge this aspect). All but 2 studies failed to meet quality statistic criterion for “using sufficient methods.” Sensitivity: No analyses reported. |
|
| Edwards et al. (2018): Systematic Review | 23 publications on 20 prospective and retrospective cohort studies in people with ADRD | N/A | Quality of relationship between a person with ADRD and their family carer | Incidence of institutionalization, hospitalization, QoL, death, behavioral, and psychotic symptoms of ADRD. | N/A |
|
| Evans et al. (2019): Meta-analysis and Systematic Review | 65 articles with longitudinal design in persons without ADRD at baseline | 102,035 | Social isolation, assessed using measures of social network/contact, and social engagement/activity | Measures of global cognition (e.g. MMSE), episodic and semantic memory, and executive function. | Heterogeneity: Calculated using random effects model for (1) all social measures, (2) social activity, (3) social networks, and (4) measures that assess a combination of social activity and networks in relation to the cognitive measures assessed. All showed moderate or considerable heterogeneity other than combination of social activity and networks which had little heterogeneity. Publication bias: Egger’s test: b = 1.52, 95% CI: 0.746, 2.285, p < 0.001, suggesting that results seemed to be overestimated due to publication bias. Quality Assessment: Critical Appraisal Skills Program checklist where higher scores indicate greater methodological quality (ranging from 14 to 42). The mean score of included articles was 38.1 (range 28–41). No articles had poor methodological quality. Sensitivity: No analyses reported. |
|
| Lenart-Bugla et al. (2022): Systematic Review | 314 total studies; 17 SRs or MAs exploring 60 seperate“social factors”; people with or without ADRD at baseline | N/A | Social network (N = 13), social contacts (N = 12), social isolation, loneliness (N = 5), marital status (N = 9), social support (N = 6), participation in social activities (N = 5), satisfaction with social ties (N = 5), and social engagement (N = 5) | Various measures of cognitive health status including cognitive impairment and ADRD. Authors report the number of studies a given characteristic was reported as a risk factor, a protective factor, if inconsistent results were reported or if no association was found. Individual statistical measures were not reported. |
N/A | Associations based on reported results from individual articles without giving specific statistical analysis:
|
| Martyr et al., (2018): Meta-analysis and Systematic Review | 307 articles: 282 journal manuscripts, 16 conference abstracts, 3 health technology assessment reports, 1 book chapter, 5 PhD theses; includes longitudinal and cross-sectional studies of persons with ADRD | 37,639 | For systematic review: 159 factors related to persons with ADRD and 69 related to carers 43 factors for meta-analysis: 33 related to persons with ADRD and 10 related to carers Main factors: Social engagement, Marital status, Quality of current relationship with carer, religiosity, spirituality, Living in the community |
QoL, well-being, or life satisfaction measures in persons with ADRD. QoL measured with self-rated (IE. QoL-AD), informant-rated or proxy-rated (DEMQOL-Proxy) scales. Well-being measures included (PWB-CIP). Life satisfaction measures varied but include Life Satisfaction Questionnaire weighted effect sizes </= to 0.09 considered negligible; 0.10–0.29 considered small; 0.30–0.49 considered moderate; and >/= to 0.5 considered large. |
Heterogeneity: I2 measurement of between-study heterogeneity moderate-to-large for most factors. Publication bias: No findings reported. Assessment: Measured using a study quality checklist adapted from existing measures. Authors labeled 16 studies as poor quality, while the remaining studies were labeled as satisfactory or good quality. Quality sensitivity analysis: through inserting correlation = 0 for p values deemed to be nonsignificant and then repeated meta-analysis. Additionally, 14 articles deemed to be of poor quality were removed from the analysis. Both measures reportedly had limited impact on overall results. |
|
| Penninkilampi et al. (2018): Meta-Analysis and Systematic Review | 33 studies (31 cohort and 2 case–control) in persons without ADRD at baseline | 2,370,452 | Social engagement characterized by marital status, living situation, social network size, degree of social support, degree of social satisfaction, frequency of social contacts, and frequency of participation in social activities. Also assessed loneliness and social isolation. |
Risk ratio of ADRD. (Meta-analysis showed significant heterogeneity among studies as well as potential publication bias.) | Heterogeneity: Substantial heterogeneity in association of dementia risk with poor social engagement (I2 = 94.3) but not with good social engagement (I2<75.00). Publication bias: Not for the association between poor social engagement and increased dementia risk (p = 0.13), but significant bias for the relationship between good social engagement and risk of dementia (p < 0.001). Quality Assessment using Newcastle–Ottawa Scale (9-star rating scale): In studies related to good social engagement, 9 were characterized as excellent and 8 as poor or adequate to good. In studies related to poor social engagement, 4 were characterized as excellent and 11 as poor or adequate to good. Sensitivity: No analyses reported. |
|
| Plassman et al. (2010): Systematic Review | 250 articles (127 observational studies, 22 RCTs, and 16 systematic reviews). 15 observational studies looking specifically at SDoH | 42,950 in studies assessing SDoH | Social engagement characterized by marital status (N = 16,565), social network (N = 10,926), and social support (N = 15,459) | Varied measures of cognitive decline, such as MMSE. | N/A |
|
| Samtani et al. (2022): Meta-analysis | 13 cohort studies in persons without ADRD at baseline | 38,614 | Social connections characterized by being in a relationship or married, living with others, weekly interactions with family and friends, weekly community group engagement, relationship satisfaction, having a confidante, degree of social support, and never feeling lonely | Annual rate of change in global cognition (usually with MMSE), and scores on some specific cognitive domains: memory, executive function, and language skills. | Heterogeneity: Assessed using I2 and tau2 statistics. Described as low for most statistically significant findings (I2 0–15%) though relationship between slower memory decline and living with others (I2 = 58.3%) and slower memory decline and community group engagement (I2 = 37.5–72.2%) had greater heterogeneity. Publication bias: Assessed using Egger’s test and funnel plot visualization. Individual statistics listed in supplementary material, but no overall results or summary statistics provided. Quality assessment: STROBE statement checklist used to assess quality of included cohort studies. Authors did not report summary statement or statistics regarding quality of included studies. Sensitivity Analyses: Using “complete cases”, authors reported that the results pattern was replicated. |
|
| Taniguchi & Ukawa (2022): Systematic Review | 7 longitudinal, cohort studies in persons without ADRD at baseline | N/A | Participation in social group activities, including voluntary work, artistic activities, attending religious activities, and participating in community organization or events | Individual study hazard ratios for ADRD at follow-up with different time intervals (<5 years, 5–10 years, >10 years). | N/A |
|
| Walker et al. (2020): Systematic Review | 13 studies: 3 review articles, 6 cross-sectional studies, 4 cohort studies; people with or without ADRD at baseline | N/A | Reports on associations and risk factors in Indigenous populations including cultural and community connections and marital status. Authors do not clearly define terms |
Measures of risk (OR, PAR) or prevalence of ADRD. Definition of how individual studies defined ADRD is not included. |
N/A |
|
| Wu-Chung et al. (2022): Systematic Review | 64 studies: including both cross-sectional and longitudinal studies; people with or without ADRD at baseline | N/A | Spousal caregiving (N = 11, including 5 longitudinal) and spousal bereavement (N = 53, including 30 longitudinal) | Cognitive function and risk of ADRD in widow(er)s and caregivers. Measures of cognitive impairment included studies using incident ADRD and subtype by clinician diagnoses or chart review and specific tests (MMSE, AVLT, AD8, LNST, CDR, word recall, serial 7s, etc.) reported as single score or as composite scores. |
N/A |
|
AD = Alzheimer’s disease, AD8 = The Eight-item Informant Interview to Differentiate Aging and Dementia, ADRD = Alzheimer’s disease and related disorders, AVLT = Auditory Verbal Learning Test, CDR = Clinical Dementia Rating Scale, DEMQOL-Proxy = Quality of Life in Dementia – Proxy Assessment, DSST = Digit Symbol Substitution Test, LNST = Letter-Number Sequencing Test, MCI = mild cognitive impairment, MMSE = Mini-Mental State Examination, OR = odds ratio, PAR = population attributable risk, PTSD = post-traumatic stress disorder, PWB-CIP = Psychological Well-being in Cognitively Impaired Persons Scale, QoL = quality of life, QoL-AD = Quality of Life in Alzheimer’s Disease, RR = relative risk, SES = socioeconomic status, VaD = vascular dementia.
Below, we summarize overall results regarding reported associations of social connections with cognitive and other outcomes from studies included in Table 1.
Social Engagement and Social Activities:
The strongest evidence for an association of social connections with lower risk of cognitive decline was related to social engagement and social activities. A meta-analysis (Jeste et al., 2023b) found that poor social engagement was significantly associated with increased risk of ADRD (risk ratio = 1.41), whereas good social engagement was negatively associated with risk of ADRD (RR = 0.81), its main individual components being many social contacts (see below), and a high level of social activity (RR = 0.62), but not high social satisfaction. Martyr et al. (2018) reported that greater social engagement (weighted effect r = 0.31) and better quality of current relationship with caregiver (weighted effect r = 0.38) had moderate associations with better quality of life (QoL) of persons with ADRD. Another (Samtani et al., 2022) meta-analysis investigated the associations between social connection markers and the rate of annual change in cognition (global and domain-specific). It revealed that living with others was associated with slower global cognitive decline (b = 0.007). In terms of specific cognitive functions, living with others (b = 0.017), weekly interactions with family and friends (b = 0.016), and weekly community group engagement (b = 0.030) predicted slower decline in memory; and living with others also predicted slower decline in language skills (b = 0.008). On the other hand, relationship satisfaction and having a confidante were not predictive of decline in global cognition or memory, language, or executive function. Lenart-Bugla et al. (2022) reported that less participation in social activities, having unsatisfying social ties, low social engagement, and social isolation “can contribute to an elevated risk of ADRD” and that frequent social contact “may confer some protection against cognitive decline and ADRD by reducing the risk or delaying the onset,” but no statistics were given.
A systematic review (Taniguchi and Ukawa, 2022) assessed the association between social participation in group activities and the risk of ADRD based on seven longitudinal cohort studies, five of which indicated that social participation in group activities was associated with slower cognitive decline. The investigators examined the association of the ADRD risk with three different types of activities: voluntary work, artistic activities, and participation in religious events. Older adults participating in voluntary work had a lower likelihood of having ADRD in two studies, with HR = 2.44 at 3-year follow-up and 2.46 at 5-year follow-up, while not participating in voluntary work increased the risk of ADRD at follow-up of <5 years (HR = 1.27), 5–10 years (HR = 1.10), and >10 years (HR: 0.96). Older adults participating in artistic activities had a lower likelihood of having ADRD at <10-year follow-up, and not participating in artistic activities was associated with an increased risk of ADRD at follow-up of <5 years (HR = 1.37) and 5–10 years (HR = 1.19), but not >10 years (HR = 1.04). Finally, in older adults without ADRD, those who attended religious events daily or almost daily were less likely to have ADRD at follow-up (HR = 0.66), and in adults aged 65–74 years, those regularly participating in community organizations/events were less likely to have ADRD at follow-up (HR = 0.75) as were those holding a leadership position the community (HR = 0.81).
Social Network:
There was evidence linking social network to cognitive function or cognitive decline, but it was not consistently significant. Penninkilampi et al. (2018) noted that having a poor social network was significantly associated with increased risk of ADRD (RR = 1.59), and having many social contacts was negatively associated with risk of ADRD (RR = 0.85); however, extensive social network was not associated with reduced risk of ADRD. Evans et al. (2019) reported that larger social networks were associated with marginally better late-life cognitive function (r = 0.054; 95% CI 0.043, 0.065). Lenart-Bugla et al. (2022) found that having a small social network “can contribute to an elevated risk of ADRD” but no statistics were provided. In studies of Indigenous communities, Walker et al. (2020) found that feeling connected to their community was associated with lower risk of ADRD (OR = 0.61), though these results were not statistically significant. A systematic review (Plassman et al., 2010) reported no statistically significant associations between social network and cognitive decline.
Social Support:
Evidence showing that social support reduces the risk of ADRD was weak. Two reviews (Penninkilampi et al., 2018; Plassman et al., 2010) found that strong social support was not associated with a reduced risk of ADRD or cognitive decline. Samtani et al.’s (2022) meta-analysis too found that the degree of social support was not predictive of decline in global cognition or in memory, language, or executive function. Lenart-Bugla et al. (2022) reported that greater social support “may confer some protection against cognitive decline and ADRD by reducing the risk or delaying the onset,” but the findings from individual studies were inconsistent.
Marital status:
Several studies reported a significant association of marital status with risk of ADRD. A meta-analysis (Samtani et al., 2022) revealed that being married or in a relationship was associated with slower global cognitive decline (b = 0.010). Penninkilampi et al. (2018) found that being unmarried was significantly associated with increased risk of ADRD (RR = 1.41) and being married was associated with a lower risk of ADRD (RR = 0.68). Bougea et al. (2022) observed that widowhood and divorce were associated with increased risk of overall ADRD but not AD. Wu-Chung et al. (2022) reported that in 14 of 23 cross-sectional studies and 21 of 30 longitudinal studies examining bereavement status, widow(er)s exhibited significantly poorer cognitive function or were more likely to be diagnosed with mild cognitive impairment (MCI) ADRD or than non-widowed subjects, but no overall statistics were provided. Lenart-Bugla et al. (2022) found that being single, divorced, or widowed “can contribute to an elevated risk of ADRD” but did not provide statistics. On the other hand, some studies reported no significant relationship of marital status to risk of ADRD. Martyr et al. (2018) found that being married had a small association with better QoL (weighted effect r = 08). Walker et al. (2020) noted that among Indigenous people of East Malaysia never being married was associated with higher risk of ADRD (OR = 1.85) but failed to reach statistical significance. Plassman et al. (2010) reported no statistically significant association between marital status and cognitive decline in older adults.
Other Factors:
Bougea et al. (2022) observed that a greater number of psychosocial stressors was related to a “progressively higher risk” of AD and other dementias. Wu-Chung et al. (2022) found that spousal caregivers had higher incidence of ADRD, higher risk for cognitive impairment, poorer cognitive function at follow-up, and more rapid decrease in cognitive function over time than non-caregivers in five longitudinal studies. Edwards et al. (2018) examined the association of the relationships between patient and caregiver and found a significant association between relationship with the family carer and global challenging behaviors (most p values <0.02), whereas caregiver relationship was not associated with QoL or with risk of institutionalization, hospitalization, or death. Martyr et al. (2018) reported that religious beliefs/spirituality (weighted effect r = 0.35) had moderate associations with better QoL, while living in the community showed small associations with better QoL (weighted effect r = 0.12). In Indigenous communities, Walker et al. (2020) found that noting culture as a source of strength (OR = 0.514) was associated with lower risk of ADRD, though these results were not significant statistically.
II. Technology-based and other social interventions to enhance social connections in people with (or without) ADRD
Table 2 includes eight systematic reviews of social interventions in persons with ADRD. (There were no meta-analyses of such interventions.) Two papers also included persons with MCI (Neal et al., 2021; Rai et al., 2022), while one included older people without ADRD (Heins et al., 2021). The eight articles are subdivided into (A) five on technology-based social interventions (Heins et al., 2021; Hirt et al., 2021; Neal et al., 2021; Pinto-Bruno et al., 2017; Rai et al., 2022), (B) two on other social interventions (Marks and McVilly, 2020; Scott et al., 2022), and (C) one on mixed technology-based and other social interventions (Han et al., 2016).
Table 2.
Systematic reviews of technology-based and other social interventions to enhance social connections
| AUTHOR (YEAR) STUDY TYPE | # OF ARTICLES INCLUDED | TYPE OF INTERVENTION | OUTCOME(S) | FINDINGS |
|---|---|---|---|---|
| Technology-based interventions | ||||
| Pinto-Bruno et al. (2017): Systematic Review | 6 articles using 10 different interventions in people with ADRD | Information and Communication Technology (ICT)-based applications | Social health and social participation in older adults with ADRD. |
|
| Rai et al. (2022): Systematic Review | 10 studies in people with ADRD or MCI | Digital technologies | QoL, depression, emotional responses, agitation, anxiety, loneliness, sleep quality, and perceived social support. N varied for different outcome measures. |
|
| Hirt et al. (2021): Systematic Review | 15 articles on 16 studies included people with ADRD | Social robot interventions separated into Pet robot, Humanoid robot, and Telepresence (social presence) robot studies | Behavioral, emotion-related, well-being, and QoL, medication-related, functional, and cognitive outcomes in people with ADRD. |
|
| Heins et al. (2021): Systematic Review | 37 articles on 36 studies included people with or without ADRD | Technological interventions targeting social participation or social isolation in older adults with and without ADRD | Loneliness, perceived social support, social isolation, social network size, social integration, social connectedness, social interaction, and social participation Scales varied by study and included UCLA Loneliness Scale, De Jong Gierveld Loneliness Scale, Multidimensional Scale of Perceived Social Support, Duke Social Support Index, Interpersonal Support Evaluation List, Lubben Social Network Index, and Medical Outcomes Study Social Support Survey. |
|
| Neal et al. (2021): Systematic Review | 9 studies; 4 related to social participation; included people with ADRD or MCI | Technological interventions (virtual reality-based, wearable technology based, or software application based) |
Self-management (measured by Instrumental Activities of Daily Living scale (LIADL), Adults and Older Adults Functional Assessment Inventory (IAFAI), among others); social participation (measured by self-reported satisfaction with social contacts, Dementia Quality of Life (DQoL), 36-Item Short-Form Health Survey (SF-36), Medical Outcome Social Support Survey (MOSS) among others) Caregiver outcomes (measured by Beck Depression Inventory (BDI-II), Care of Older People in Europe (COPE-index), or World Health Organization Quality of Life BREF (WHOQOLBREF)). |
|
| Non-technology-based social interventions | ||||
| Scott et al. (2022): Systematic Review | 8 articles (3 mixed methods studies, 2 qualitative studies, 2 quantitative studies, and 1 case study); 5 articles assessing social interactions, participants were people with ADRD living in the community | Horticulture-based activities | Social interactions or socialization, cognitive function, memory, physical function, well-being, and QoL 5 studies reported a standardized assessment tool (MMSE, Geriatric Depression Scale-15, Wechsler Memory Scale-Revised, Five-item Observed Emotion Rating Scale, dementia care mapping tool, Lubben Social Network Scale 6, etc.). |
|
| Marks and McVilly (2020): Systematic Review | 24 articles in people with ADRD | Trained assistance dogs | Mood, prosocial behaviors, daily activity/quality of life, cognitive impairment, existential function measured using Scales (MMSE, Geriatric Depression Scale, Cornell Depression in Dementia Scale, etc.), physiologic measures of stress (change in cortisol levels, salivary chromogranin A (CgA), or subjective observation. |
|
| Mixed technology-based and other social interventions | ||||
|
Han et al. (2016): Systematic Review |
32 studies in people with ADRD | Individualized social and leisure activities in people with ADRD, simulated presence therapy (SPT), and individualized reminiscence therapy (IRT) in people with ADRD | Varied outcomes: agitation, disruptive or withdrawn behaviors, social interactions, overall QoL, depression, and cognition. |
|
AD = Alzheimer’s disease, ADRD= Alzheimer’s disease and related disorders, MCI = mild cognitive impairment, MMSE = Mini-Mental State Examination, OR = odds ratio, QoL = quality of life; RCT = randomized controlled trial.
Impact on Social Connections and Other Outcomes:
Five of the included systematic reviews found that, to varying degrees, technology-based interventions designed to reduce or prevent social isolation or loneliness in people with ADRD improved social behavior and QoL, reduced loneliness and social exclusion, and enhanced social interaction. Pinto-Bruno et al. (2017) reported that Information and Communication Technology (ICT)-based interventions produced benefit for people with ADRD in maintaining, facilitating, and creating social networks. There were statistically significant improvements in increasing positive social activities and behaviors such as making more choices, spending less time asking direct questions, initiating conversation, and engaging in more singing. Rai et al. (2022) noted some improvements on measures of QoL in individuals with ADRD, including outcomes related to social connectedness, with technology-based interventions. A systematic review (Heins et al., 2021) of studies of technological interventions targeting social participation or social isolation in older adults with and without ADRD found that participants with cognitive impairment showed initial improvement at 6 weeks with significantly higher social interaction, but it did not persist at 12 weeks. Older adults without cognitive impairment largely reported no statistically significant changes. Qualitative studies in older adults found that technological interventions promoted development or maintenance of social connections, companionship, social interactions, and communication, and decrease in loneliness. In mixed-method studies, a minority reported statistically significant positive effects on social participation. Hirt et al. (2021) obtained mixed results relating to behavioral outcomes, including neuropsychiatric symptoms, disturbing behavior, QoL, and activities of daily living in persons with ADRD. Neal et al. (2021) noted significant positive effect of technological intervention on social participation in ADRD in only one of the four studies reviewed.
Two other reviews (Marks and McVilly, 2020; Scott et al., 2022) reported that other social interventions such as horticulture-based activities and pet-based interventions led to positive impacts on social engagement, social interactions, and mental and physical well-being for individuals with ADRD. Scott et al., (2022) reviewed five studies of horticulture-based activities in community-dwelling people with ADRD. Four of those studies reported improved social interaction, including promotion of shared communication about experiences living with ADRD, development of new social bonds, creation of a shared sense of identity, and development of intimate relationships. Another systematic review (Marks and McVilly, 2020) noted that the use of trained assistance dogs resulted in a statistically significant increase in social interaction in the experimental group in one study and a significant decrease in behavioral pathology in another. Qualitative studies reported enhanced communication with “volunteers”, increased trust and self-determination, and the ability to reflect. Results were inconsistent regarding irritability and agitation, mood, daily activities, and QoL.
One review (Han et al., 2016) of mixed technology-based and other social interventions included 32 studies investigating possible benefits of individualized social and leisure activities, particularly technology-based simulated presence therapy (SPT) and non-technology-based individualized reminiscence therapy (IRT), in people with ADRD. The SPT, which consists of individually tailored audio or video simulations of social contact with a family member, produced reduction in agitation and disruptive or withdrawn behaviors and increase in social interactions, compared to alternative interventions or standard care. However, one-on-one interactions had superior benefit in decreasing agitation than SPT, and audio SPT could be unsuitable for people with ADRD and a history of hallucinations. The IRT, consisting of facilitated activity where participants spoke with others about memories and life experiences to promote direct social interactions, improved overall QoL and depressive symptoms.
Impact on Cognition:
In general, the intervention studies that assessed cognition did not find a significant improvement in cognition. Thus, Han et al. (2016) found that a randomized controlled trial (RCT) of IRT in people with ADRD showed no significant effect on cognition. Hirt et al. (2021) also reported no significant effect of social robots including pet robots, humanoid robots, and telepresence (social presence) robots on cognition in people with ADRD. While Marks and McVilly (2020) noted that the use of trained assistance dogs produced “generally positive results” on “cognitive impairment outcomes” in people with ADRD, the form and quality of the studies varied considerably, and no statistical analyses were presented.
Discussion
In the US healthcare practice for older adults, healthy lifestyle including physical activity and better nutrition is emphasized along with adherence to prescribed medications for persons with hypertension, diabetes, heart disease, etc. Yet, little attention is paid to the assessment and interventions related to the single most important evidence-based determinant of health and longevity – viz., social connections. Countless investigations have shown that social relationships are highly significant predictors of mental and physical well-being and reduce the risk of multiple illnesses including ADRD (Holt-Lunstad et al., 2017). Yet, we did not find a review that synthesized the estimates and findings from meta-analyses and systematic reviews of studies on the association of social connections with cognitive decline, or of trials of technology-based and other social interventions seeking to enhance social connections in people with ADRD.
This scoping review examined the associations between social connections and outcomes primarily related to cognitive decline in older adults with or at risk for ADRD, as well as technology-based and other social interventions to promote social connections. Social connection is a broad construct, and it will be useful to determine which components are more impactful than others. The strongest evidence for an association of social connections with lower risk of cognitive decline was related to social engagement and social activities. There was also evidence linking social network size to cognitive function or cognitive decline, but it was not consistently significant. A number of, though not all, studies reported a significant association of being married or in a relationship with lower risk of ADRD. However, evidence showing that social support reduces the risk of ADRD was weak. Social support is known to improve well-being in people with mental and physical illnesses but may not delay the progression of cognitive decline because it is not associated with increased physical and psychosocial activities. To varying degrees, technology-based and other social interventions designed to reduce or prevent social isolation or loneliness in people with ADRD were found to improve social behavior and QoL and enhance social interaction and social activities; however, they had no significant impact on cognition.
This review has several limitations. Despite our best efforts, we might have missed a few relevant meta-analyses or systematic reviews on social connections in ADRD. Also, there are other social factors affecting individuals with ADRD that we did not include here (e.g. healthcare access, stigma, macroeconomic policies that affect access to resources, etc.), but our focus was on social connections as these have been shown to have some of the largest effects on health and longevity (Holt-Lunstad et al., 2017). There are also a number of noteworthy limitations of the individual studies that were included in the meta-analyses and systematic reviews. For example, many investigations were cross-sectional and, therefore, do not show causality. There was also considerable heterogeneity in the measured constructs, some of which had not been validated in persons with ADRD, and in study samples of patients and comparison groups, restricting generalizability of the results. The definitions of terms such as social engagement were often lacking or variable across studies. The assessment of heterogeneity of study findings, scientific quality, publication bias, and sensitivity analysis varied across the meta-analyses and was usually not performed in systematic reviews, as can be seen from Table 1. A number of studies did not adjust for relevant confounders including overlapping SDoHs such as socioeconomic status and disadvantaged neighborhoods. Most studies were predominantly conducted in high-income countries. Future studies should expand the research to low- and middle-income countries and also include immigrants who face additional problems in connecting with their newly adopted society.
This scoping review was conducted to characterize the developing literature on social connections and ADRD through the lens of meta-analyses and systematic reviews. We found a variety of outcomes through 11 meta-analyses and systematic reviews of longitudinal, case–control, and cross-sectional studies (e.g. social engagement, social activities, social network, social support, social isolation, and marriage) and eight interventions seeking to improve social health and social connections. As a greater number of meta-analyses and systematic reviews will be published in the near future on each of these unique topics, umbrella reviews will be needed to synthesize the effect sizes, examine the study quality, and determine future directions.
While we present findings in terms of social connections’ impact on ADRD, it may also be that persons predisposed to cognitive decline and ADRD may have poorer social connections or may socially isolate themselves years before a clinical diagnosis. A majority of the studies included in this paper were cross-sectional, and therefore, limited in establishing a cause-and-effect relationship. Data from the US Health and Retirement Study showed that loneliness at baseline predicted accelerated cognitive decline, and poor baseline cognition predicted greater loneliness over time, pointing to the potential bidirectional relationship between aspects of social relations and cognitive function (Donovan et al., 2017). Data from the National Health and Aging Trends Study 2011–2018 surveys showed different trajectories of social isolation and dementia risk (Xiang et al., 2021). Two-thirds of the low-risk dementia group were in the rarely isolated group. The high-risk dementia group had the most overlap with the decreasing social isolation group, followed by the persistently isolated group. A study of 5,753 older dementia-free Americans over 8 years reported that social isolation was associated with subsequent cognitive functioning and lower cognitive functioning was associated with greater subsequent social isolation (Qi et al., 2023). Sleep disturbance partially mediated the effect of social isolation on cognitive functioning. Shen et al. examined bidirectional relationships between social isolation/social interaction and AD using Mendelian randomization method for assessing potential causal inference (Shen et al., 2021). Of the five types of social engagement examined, only one showed evidence of an association with the risk of AD. Attendance at a gym or sports club was inversely associated with the risk of AD, suggesting that gym/sports club attendance may lead to a reduced risk of AD. Thus, the overall evidence for a direct causal link between social participation and dementia is not definitive due to limitations in observational research but is somewhat consistent and biologically plausible. Further studies are warranted to elucidate potential mechanisms.
Another confounding factor related to the association between social connections and cognitive function among persons with ADRD is the well-being and functioning of the family caregivers on whom they rely (Jeste et al., 2021). As stressed by Van Orden and Heffner, social connection is an understudied target of intervention for the health of ADRD patients’ family caregivers, and there is an urgent need for developing mechanism-informed and principle-driven behavioral interventions to promote social connection in these individuals (Van Orden and Heffner, 2022). A systematic review (Jeste et al., 2021) focused on social support interventions for caregivers of persons with ADRD concluded that while multicomponent social support interventions may improve caregiver well-being, there was insufficient evidence to conclude whether a change in social support was the underlying mediating factor. Another systematic review reported preliminary evidence to support the acceptability of psychosocial interventions by dementia caregivers, although the available supporting evidence was limited (Dam et al., 2016). A third systematic review of dementia care programs commented on limitations of the literature, although there seemed to be some positive effect on providing support and improving outcomes for persons living with dementia and their caregivers (Demanes et al., 2021).
Our review did not include studies of risk for MCI. The reason is the marked heterogeneity in the course of cognitive decline in persons with MCI, indicating that that the risk factors for MCI may be different from those for ADRD. According to Peterson, the annual rate at which MCI progresses to dementia varies between 8 and 15% per year (Petersen, 2016). In a study of 739 participants with MCI from the National Alzheimer’s Coordinating Center Uniform Data Set, after 3 years, 238 participants (33.6%) progressed to dementia, while 90 (12.2%) reverted to normal cognition (McGirr et al., 2022). Thus, a majority of the persons with MCI do not develop ADRD at least over a period of several years. Nonetheless, future studies should examine risk factors for MCI.
Notwithstanding the limitations, this review supports the critical role of specific types of social connections – that is, social engagement and social activities, in reducing the risk of cognitive decline in older adults. Furthermore, the availability of efficacious technology-based and other interventions that enhance social connections suggests that promotion of social connections is a feasible and promising strategy to reduce the risk of ADRD. Below, we offer a number of suggestions for research and interventions for older people with cognitive impairments of different severity.
Methodologically sound research on SDoHs related to cognitive decline in older adults is urgently warranted. It should include the development and testing of pragmatic, reliable, and valid measures of social connections both at individual and community levels (Sturm et al., 2023). Other relevant measures and possible confounding factors such as socioeconomic characteristics should also be examined. Prospective longitudinal studies in randomly selected large and diverse samples are recommended. A further direction for future research is examining the neurobiological effects of social connections as well as those of prosocial interventions in people with ADRD that might improve brain function and alter biomarkers of cognition, aging, and stress (Jeste et al., 2023a). Higher levels of social engagement in older adults are reportedly associated with increased total brain and gray matter volumes as well as greater gray matter integrity in regions relevant to social cognition (Krivanek et al., 2021). Also, some interventions that enhanced social engagement seemed to have a potential to boost brain health and cognitive reserve. An RCT of an intergenerational social health promotion program labeled Experience Corps showed that, compared to the control group, the purposeful activity embedded within the intervention arm of that program halted in women, and reversed in men, declines in brain volume in regions vulnerable to ADRD (Carlson et al., 2015). Thus, a comprehensive biopsychosocial perspective should be employed to carry out meaningful research projects on social connections.
With the rapid growth and dissemination of digital technology as well as artificial intelligence, there is an unquestionable need to develop and test new interventions to promote social connections in older adults including those with ADRD. Hirt et al. (2021) reported on studies evaluating pet robots which resulted in improvement in behavioral emotion-related well-being, QoL, and functioning. While the results of the technology-based interventions were mixed, they hold promise for robots and digital interventions to improve social connectedness in people with ADRD. Rigorous studies with larger sample sizes are needed to evaluate the long-term effects of such strategies. While the research on digital interventions is nascent, it does show the potential of scalable support. The predominance of robot/animatronic-based interventions is likely related to their low cognitive load and a high degree of usability, which stands in contrast to most current smartphone apps, virtual reality systems, and wearables. Implementation of technology-based interventions in routine healthcare practice is challenging for several reasons including low usability of internet-based interventions. The System Usability Scale has been increasingly applied to measure usability of industrial products (Mol et al., 2020). Similarly, the Technology Acceptance Model is the most widely employed theory to explain the user acceptance of a particular technology (Feng et al., 2021). It is based on the hypothesis (Davis et al., 1989) that both perceived usefulness and perceived ease of use form the users’ beliefs and intent regarding technology use. Today very few studies in geriatric neuropsychiatry employ the System Usability Scale or the Technology Acceptance Model. New efforts to capture, quantify, and design around any barriers to technology-based interventions in older adults, utilizing these methods, will help advance the next generation of technology-informed approaches.
In sum, empirical evidence supports the significant contribution of social engagement and social activities to reducing the risk of ADRD in older adults. As the number of people with ADRD will nearly double within a few decades (Jeste et al., 2023b), innovative and scalable strategies are urgently needed to address this serious public health problem. Digital and other technology-based social interventions are likely to play an important role in this area.
Source of Funding
This work was supported by grants from the National Institute of Mental Health (T32MH019934, PI: Twamley) and K23 MH129684-01, PI: Dr Ku.). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. All the authors have declared that they have no conflicts of interest in relation to the subject of this study.
Footnotes
Conflict of interest
None of the other authors have any conflicts of interest to disclose.
References
- Bougea A, Anagnostouli M, Angelopoulou E, Spanou I and Chrousos G (2022). Psychosocial and trauma-related stress and risk of dementia: a meta-analytic systematic review of longitudinal studies. Journal of Geriatric Psychiatry and Neurology, 35, 24–37. 10.1177/0891988720973759. [DOI] [PubMed] [Google Scholar]
- Carlson MC et al. (2015). Impact of the Baltimore Experience Corps Trial on cortical and hippocampal volumes. Alzheimer’s & Dementia, 11, 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam AE, de Vugt ME, Klinkenberg IP, Verhey FR and van Boxtel MP (2016). A systematic review of social support interventions for caregivers of people with dementia: are they doing what they promise? Maturitas, 85, 117–130. 10.1016/j.maturitas.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Davis FD, Bagozzi RP and Warshaw PR (1989). User acceptance of computer technology: a comparison of two theoretical models. Management Science, 35, 982–1003. 10.1287/mnsc.35.8.982. [DOI] [Google Scholar]
- Demanes A, Ward KT, Wang AT and Hess M (2021). Systematic review of dementia support programs with multicultural and multilingual populations. Geriatrics (Basel), 7, 8. 10.3390/geriatrics7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan NJ, Wu Q, Rentz DM, Sperling RA, Marshall GA and Glymour MM (2017). Loneliness, depression and cognitive function in older U.S. adults. International Journal of Geriatric Psychiatry, 32, 564–573. 10.1002/gps.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards HB et al. (2018). Quality of family relationships and outcomes of dementia: a systematic review. BMJ Open, 8, e015538. 10.1136/bmjopen-2016-015538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans IEM, Martyr A, Collins R, Brayne C and Clare L (2019). Social isolation and cognitive function in later life: a systematic review and meta-analysis. Journal of Alzheimer’s Disease: JAD, 70, S119–S144. 10.3233/JAD-180501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng GC et al. (2021). Determinants of technology acceptance: two model-based meta-analytic reviews. Journalism & Mass Communication Quarterly, 98, 83–104. 10.1177/1077699020952400. [DOI] [Google Scholar]
- Han A, Radel J, McDowd JM and Sabata D (2016). The benefits of individualized leisure and social activity interventions for people with dementia: a systematic review. Activities, Adaptation & Aging, 40, 219–265. 10.1080/01924788.2016.1199516. [DOI] [Google Scholar]
- Heins P et al. (2021). The effects of technological interventions on social participation of community-dwelling older adults with and without dementia: a systematic review. Journal of Clinical Medicine, 10, 2308. 10.3390/jcm10112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickin N, Käll A, Shafran R, Sutcliffe S, Manzotti G and Langan D (2021). The effectiveness of psychological interventions for loneliness: a systematic review and meta-analysis. Clinical Psychology Review, 88, 102066. 10.1016/j.cpr.2021.102066. [DOI] [PubMed] [Google Scholar]
- Hirt J et al. (2021). Social robot interventions for people with dementia: a systematic review on effects and quality of reporting. Journal of Alzheimer’s Disease, 79, 773–792. 10.3233/JAD-200347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BE, Linden W and Najarian B (2002). Social support interventions: do they work? Clinical Psychology Review, 22, 381–440. 10.1016/S0272-7358(01)00102-7. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J (2022). Social connection as a public health issue: the evidence and a systemic framework for prioritizing the “social” in social determinants of health. Annual Review of Public, 43, 193–213. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Robles TF and Sbarra DA (2017). Advancing social connection as a public health priority in the United States. American Psychologist, 72, 517–530. 10.1037/amp0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB and Layton JB (2010). Social relationships and mortality risk: a meta-analytic review. PLOS Medicine, 7, e1000316. 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV et al. (2023a). Review of major social determinants of health in schizophrenia-spectrum psychotic disorders: III. Biology. Schizophrenia Bulletin, 49, 867–880. 10.1093/schbul/sbad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV (2022). Non-medical social determinants of health in older adults. International Psychogeriatrics, 34, 755–756. 10.1017/S1041610222000709. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Mausbach B and Lee EE (2021). Caring for caregivers / care partners of persons with dementia. International Psychogeriatrics, 33, 307–310. 10.1017/S1041610221000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Nguyen TT and Donovan NJ (2023b). Loneliness: Science and Practice. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Jester DJ et al. (2023). Differences in social determinants of health underlie racial/ethnic disparities in psychological health and well-being: study of 11,143 older adults. The American Journal of Psychiatry, 180, 483–494. 10.1176/appi/ajp.20220158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivanek TJ, Gale SA, McFeeley BM, Nicastri CM and Daffner KR (2021). Promoting successful cognitive aging: a ten-year update. Journal of Alzheimer’s Disease, 81, 871–920. 10.3233/JAD-201462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart-Bugla M et al. (2022). What do we know about social and non-social factors influencing the pathway from cognitive health to dementia? A systematic review of reviews. Brain Sciences, 12, 1214. 10.3390/brainsci12091214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks G and McVilly K (2020). Trained assistance dogs for people with dementia: a systematic review. Psychogeriatrics, 20, 510–521. 10.1111/psyg.12529. [DOI] [PubMed] [Google Scholar]
- Martyr A et al. (2018). Living well with dementia: a systematic review and correlational meta-analysis of factors associated with quality of life, well-being and life satisfaction in people with dementia. Psychological Medicine, 48, 2130–2139. 10.1017/S0033291718000405. [DOI] [PubMed] [Google Scholar]
- McGirr A, Nathan S, Ghahremani M, Gill S, Smith EE and Ismail Z (2022). Progression to dementia or reversion to normal cognition in mild cognitive impairment as a function of late-onset neuropsychiatric symptoms. Neurology, 98, e2132–e2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol M et al. (2020). Dimensionality of the System Usability Scale among professionals using internet-based interventions for depression: a confirmatory factor analysis. BMC Psychiatry, 20, 218. 10.1186/s12888-020-02627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A and Aromataris E (2018. Nov). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Medical Research Methodology, 18, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na P, Jeste DV and Pietrzak R (2023). Social disconnection as a global behavioral epidemic: a call to action to address social disconnection in health policy, education, research, and clinical practice. JAMA Psychiatry, 80, 101–102. 10.1001/jamapsychiatry.2022.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering and Medicine (2020). Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, D.C: The National Academies Press. [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2020). Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: The National Academies Press. 10.17226/25663 [DOI] [PubMed] [Google Scholar]
- Neal D et al. (2021). Can use of digital technologies by people with dementia improve self-management and social participation? A systematic review of effect studies. Journal of Clinical Medicine, 10, 1–23. 10.3390/jcm10040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninkilampi R, Casey A-N, Singh MF and Brodaty H (2018). The association between social engagement, loneliness, and risk of dementia: a systematic review and meta-analysis. Journal of Alzheimer’s Disease, 66, 1619–1633. 10.3233/JAD-180439. [DOI] [PubMed] [Google Scholar]
- Peters MDJ et al. (2021). Scoping reviews: reinforcing and advancing the methodology and application. Systematic Reviews, 10, 263. 10.1186/s13643-021-01821-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2016). Mild cognitive impairment. Continuum (Minneap Minn), 22, 404–418. 10.1212/CON.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Bruno AC, García-Casal JA, Csipke E, Jenaro-Río C and Franco-Martín M (2017). ICT-based applications to improve social health and social participation in older adults with dementia: a systematic literature review. Aging & Mental Health, 21, 58–65. 10.1080/13607863.2016.1262818. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Williams JW, Burke JR, Holsinger T and Benjamin S (2010). Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine, 153, 182–193. 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Qi X, Pei Y, Malone SK and Wu B (2023). Social isolation, sleep disturbance, and cognitive functioning (HRS): a longitudinal mediation study. The Journals of Gerontology: Series A, 78, 1826–1833. 10.1093/gerona/glad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai HK, Kernaghan D, Schoonmade L, Egan KJ and Pot AM (2022). Digital technologies to prevent social isolation and loneliness in dementia: a systematic review. Journal of Alzheimer’s Disease: JAD, 90, 513–528. 10.3233/JAD-220438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samtani S et al. (2022). Associations between social connections and cognition: a global collaborative individual participant data meta-analysis. The Lancet. Healthy Longevity, 3, e740–e753. 10.1016/S2666-7568(22)00199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TL et al. (2022). Well-being benefits of horticulture-based activities for community dwelling people with dementia: a systematic review. International Journal of Environmental Research and Public Health, 19, 10523. 10.3390/ijerph191710523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LX et al. (2021). Social isolation, social interaction, and Alzheimer’s disease: a Mendelian randomization study. Journal of Alzheimer’s Disease, 80, 665–672. 10.3233/JAD-201442. [DOI] [PubMed] [Google Scholar]
- Stites SD et al. (2022). Establishing a framework for gathering structural and social determinants of health in Alzheimer’s disease research centers. Gerontologist, 62, 694–703. 10.1093/geront/gnab182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm ET et al. (2023). Review of major social determinants of health in schizophrenia-spectrum psychotic disorders: II. Assessments. Schizophrenia Bulletin, 49, 851–866. 10.1093/schbul/sbad024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi R and Ukawa S (2022). Participation in social group activities and risk of dementia: a systematic review. Open Public Health Journal, 15. 10.2174/18749445-v15-e2204141. [DOI] [Google Scholar]
- Valentí Soler M et al. (2015). Social robots in advanced dementia. Frontiers in Aging Neuroscience, 7, 133. 10.3389/fnagi.2015.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Orden KA and Heffner KL (2022). Promoting social connection in dementia caregivers: a call for empirical development of targeted interventions. The Gerontologist, 62, 1258–1265. 10.1093/geront/gnac032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JD, Spiro G, Loewen K, Jacklin K (2020). Alzheimer’s Disease and related dementia in indigenous populations: a systematic review of risk factors. Journal of Alzheimer’s Disease, 78, 1439–1451. 10.3233/JAD-200704. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2008). WHO Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health. Final report of the Commission on Social Determinants of Health. Geneva: World Health Organization. [Google Scholar]
- World Health Organization. (2021). Fact sheets of dementia [Internet] [cited 2022 Apr 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
- Wu-Chung EL, Leal SL, Denny BT, Cheng SL and Fagundes CP (2022). Spousal caregiving, widowhood, and cognition: a systematic review and a biopsychosocial framework for understanding the relationship between interpersonal losses and dementia risk in older adulthood. Neuroscience and Biobehavioral Reviews, 134, 104487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X et al. (2021). Dual trajectories of social isolation and dementia in older adults: a population-based longitudinal study. Journal of Aging and Health, 33, 63–74. 10.1177/0898264320953693. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]


