Abstract
We examined how physical pain impacts the developmental construct of Awareness of Age-Related Change (AARC-gains and AARC-losses) and, in turn, how AARC mediates and moderates the association between pain and subsequent physical activity. We used longitudinal data from 434 participants of the UK PROTECT Study (mean age = 65.5 years; SD = 6.94 years). We found that pain in 2019 predicted higher AARC-losses (β =.07; p = .036) and less physical activity (β = −.13; p-value = .001) in 2020. Additionally, we found that AARC-losses partially mediated, but did not moderate, the association of pain in 2019 and physical activity in 2020. AARC-losses may explain physical inactivity in middle-aged and older adults experiencing pain. Incorporating developmental constructs such as AARC into theories and empirical studies on pain and pain management may be necessary to more fully capture people’s responses to pain.
Keywords: views on aging, Awareness of Age-Related Change, physical activity, pain, United Kingdom
A relevant proportion of middle-aged (50–64 years) and older (aged 65 and over) people in the United Kingdom experience persistent physical pain (Fayaz et al., 2016; Zimmer et al., 2022). Addressing pain is a global health priority because of its impact on people’s well-being and on their ability to carry out daily activities (Goldberg & McGee, 2011). In addition to being related to maintenance of physical and mental health in middle and older-age (DiPietro, 2001; Kekäläinen et al., 2020; Warburton et al., 2006), physical activity is also a key mechanism to reduce pain and pain-related activity limitations (Cary & Gyurcsik, 2021; da Silva Marques et al., 2022; Denche-Zamorano et al., 2022; Geneen et al., 2017; Smith et al., 2019). There is a long-standing literature base of people’s health promotion behavior when they have persistent pain, alongside an array of self-management programs and behavioral interventions aimed at improving physical activity amongst people with pain (Cary & Gyurcsik, 2021; Du et al., 2011; Hayden et al., 2005; National Institute for Health and Care Excellence, 2021; Searle et al., 2015). However, despite these strong translational research efforts, people with pain often do not meet physical activity recommendations (Kelley et al., 2020; Zadro et al., 2017). Therefore, expanding our understanding of physical activity in the presence of pain is necessary to ensure theoretical propositions of pain self-management are accurate, and that interventions are relevant and effective. In this study, we analyzed the role of Awareness of Age-Related Change (AARC; Diehl & Wahl, 2010)—a psychological construct capturing subjective views on aging—in the association between pain and physical activity for people in late midlife and older-age.
Views on Aging and Self-Regulatory Health Behavior
Views on aging are a broad, multi-faceted construct that captures people’s perceptions and appraisals of their own age and aging process. Frequently incorporated in psychosocial studies of adult development, views on aging are measured with various constructs, such as self-perceptions of aging (Levy et al., 2002), felt age (Montepare, 2020), future self-views (Kornadt et al., 2020), and the focus of this study, AARC (Diehl et al., 2014). There is a long-standing and robust literature base connecting people’s views on aging to their longer-term health outcomes, including mortality. For example, research suggests that people who feel older than their chronological age have a 25% higher risk of mortality (Stephan et al., 2018), and people’s self-perceptions of aging are associated with mortality up to 22 years later (Kotter-Grühn et al., 2009; Levy et al., 2002; Sargent-Cox et al., 2013).
Given the strong connections between various views on aging constructs and health, researchers have developed conceptual and theoretical models hypothesizing the connections between views on aging and health outcomes (Kotter-Grühn et al., 2016). These theories posit that views on aging shape lifespan developmental health through their impact on developmental self-regulation. For example, Wurm et al.’s (2017) model of views of aging and lifespan developmental regulation highlights the views on aging construct of self-perceptions of aging as having an influence on developmental regulation and personality. In turn, per the model, developmental regulation and personality shape health-related outcomes. Similarly, Diehl et al.’s (2014) model of Awareness of Aging in the context of lifespan developmental processes and outcomes depicts pre-conscious/implicit awareness of aging constructs (e.g., attitudes toward aging and age stereotypes) as interacting with conscious/explicit awareness of aging constructs (e.g., subjective age and age identity) to shape the self-regulatory processes that eventually impact developmental outcomes such as functional health and longevity. Indeed, people who have more positive views on aging tend to engage in more self-regulatory health-promotive behavior. Considering physical activity, feeling more positively about one’s age is associated with greater intention to engage in physical activity (Caudroit et al., 2012; Wienert et al., 2017) and more self-reported physical activity (Chen et al., 2019; Hooker et al., 2019; Steward & Hasche, 2022; Wienert et al., 2015).
Awareness of Age-Related Change, Physical Activity, and Pain
AARC is a views on aging construct that captures people’s perceptions and appraisals of the positive (AARC-gains) and negative (AARC-losses) age-related changes they may experience as a function of getting older (Diehl & Wahl, 2010). As are the other views on aging constructs, AARC is connected to self-regulatory health behavior (Dutt et al., 2016). Regarding physical activity, researchers have found that intervening to improve people’s AARC-losses can increase their physical activity levels (Diehl et al., 2020). Increased physical activity levels can, in turn, improve AARC (Brothers & Diehl, 2017; Klusmann et al., 2012; Nehrkorn-Bailey et al., 2023).
Moreover, recent research suggests that pain is associated with AARC. Sabatini et al. (2021), for example, found that higher levels of pain are cross-sectionally associated with more AARC-losses. Middle-aged and older adults who have arthritis, a leading cause of persistent pain, report more AARC-losses (Dunsmore & Neupert, 2022). Thus, it is plausible that AARC may be involved in the existing well-established connections between higher pain and lower physical activity.
Though there is a body of research connecting AARC to physical activity and a separate body of research connecting AARC to pain, to our knowledge there are no studies that simultaneously analyze the relationship between all three constructs. Elucidating how AARC and pain interact to influence physical activity can contribute to theoretical models on the role of views on aging in pain and pain management, and can inform physical activity-related pain self-management interventions. Further, understanding the extent to which pain is longitudinally associated with AARC and physical activity will not only further develop the literature on pain’s role in views on aging and developmental regulation over time, but will also inform intervention components such as how long a participant needs to participate in a program in order for the program to maintain efficacy.
Measurement Strengths.
Unlike many measurement tools for other views on aging constructs, AARC was originally conceptualized as a multi-dimensional construct, meaning that it separately measures positive views on aging (AARC-gains) alongside negative views on aging (AARC-losses); its associated surveys were intentionally designed to capture the co-existence of both gains and losses associated with getting older. Existing research suggests that positive and negative views on aging have independent influences on physical activity (Hooker et al., 2019). The AARC construct and its associated measurement tools are capable of capturing both positive and negative views on aging and how they may differentially relate to pain and physical activity, thus offering a comprehensive perspective on such possible connections. Additionally, AARC encompasses gains and losses across five life and behavior domains (health and physical functioning, cognitive functioning, interpersonal relationships, socio-cognitive functioning, and lifestyle/engagement). Because pain is known to impact multiple quality of life domains (Hadi et al., 2019), and because of the domain-specificity of views on aging (Rothermund & Kornadt, 2015), AARC may be a particularly valid tool for capturing how views on aging are connected to physical activity and pain.
The Present Study
Ultimately, the views on aging construct of AARC may be a factor in the existing established relationship between pain and physical activity. Pain may worsen a person’s views on aging in ways that reduce their motivation to engage in the self-regulatory health behavior of physical activity. However, this hypothesis has yet to be studied. Analyzing connections between pain, AARC-gains and AARC-losses, and physical activity—and doing so longitudinally to capture the potential longer-term impacts of pain—can offer insight into a possible psychosocial mechanism that can either threaten or facilitate physical activity among people with pain. In this study, we asked the following questions:
How is pain related to AARC-gains, AARC-losses, and physical activity one year later?
Do AARC-gains and/or AARC-losses mediate the association between pain at baseline and physical activity one year later?
Do AARC-gains and/or AARC-losses moderate the association between pain at baseline and physical activity one year later?
We hypothesized that pain would be related to more AARC-losses and less AARC-gains one year later, and that AARC-losses and AARC-gains would both mediate and moderate the association between pain and physical activity.
Method
Study Sample
This study is based on data collected online through the UK PROTECT study (https://www.protectstudy.org.uk). UK PROTECT is a 25-year longitudinal cohort study that started in November 2015 and aims to explore the role of genetic, lifestyle, and medical factors on cognition in individuals aged 50 years or over and living in the United Kingdom (UK). Participants were recruited through several channels including advertisement at King’s College London, invitation of participants enrolled in existing UK-based cohort studies (https://exetercrfnihr.org/about/exeter-10000/; https://www.joindementiaresearch.nihr.ac.uk/; https://bdr.alzheimersresearchuk.org), and information leaflets placed in general practitioners’ surgeries and memory clinics throughout the UK. In UK PROTECT inclusion criteria were being a UK resident, English speaker, aged 50+, having access to the internet, and lacking a clinical diagnosis of dementia at baseline assessment. At baseline, participants provided informed consent online on the UK PROTECT study platform. The UK PROTECT study has ethical approval from the London Bridge NHS Research Ethics Committee and Health Research Authority (Ref: 13/LO/1578).
UK PROTECT study participants are invited to take part in a follow-up assessment each year. In January 2019 and 2020, for the purpose of this study, participants were asked to fill in additional optional questions assessing AARC. In 2019 (baseline for the current study) 1,013 participants completed both the AARC and the pain questionnaires. Of these, 434 completed both the AARC and the pain questionnaires again one year later and comprised the current study sample. Differences between the current study analytic sample and people who did not provide longitudinal data for study analyses are reported in Supplemental Table S1. The two samples only differed in education achievement, with the sample of participants who did not provide longitudinal data comprising fewer individuals who completed university level education.
Measures
Socio-Demographic Variables
We included age, sex, and education (secondary education, post-secondary education, vocational qualifications, undergraduate degrees, post-graduate degrees, doctorates) as covariates.
Awareness of Age-Related Change
We used the 10-item short form of the AARC questionnaire (AARC-10 SF; Kaspar et al., 2019) comprising five items assessing AARC-gains and five items assessing AARC-losses. An item in each of the AARC-gains and AARC-losses subscales represents one of the five AARC life and behavioral domains. Each item starts with the stem: “With my increasing age, I realize that …”. Examples of items assessing AARC-losses and AARC-gains in the physical health domain are “I have less energy” and “I pay more attention to my health,” respectively. Respondents rate how much items apply to them (1 = “not at all” to 5 = “very much”). Scores are obtained for the AARC-gains and AARC-losses subscales by summing the five items within the respective subscales. Higher scores indicate higher AARC-gains/losses (range = 5–25). In this sample Cronbach’s alpha for internal consistency for the AARC-gains subscale was .75 and for the AARC-losses subscale was .81, indicating adequate reliability. The validity of the AARC-10 SF among UK adults aged 50 and over has previously been established (Sabatini et al., 2020). The AARC-10 SF is also valid for use with people in advanced old age (Kaspar et al., 2019, 2022).
Pain
We assessed pain with a 4-item scale adapted from the PROMIS pain scale, developed by the PROMIS health organization (Cella et al., 2010). Respondents report how much pain has interfered with their daily activities and social engagement over the past week. An example item is “In the past 7 days how much did pain interfere with your household chores?” (0 = “not at all” to 4 = “very much”). A total score is obtained by summing single item scores (range = 0–16); higher total scores indicate greater pain interference. In this sample Cronbach’s alpha for internal consistency for the scale was .93, indicating excellent reliability.
Physical Activity
We calculated caloric expenditure as indicator of physical activity using the Community Health Activities Model Program for Seniors (CHAMPS) physical activity questionnaire (Stewart et al., 2001). Participants were presented with 28 physical exercises (e.g., dance, jog or run) and asked whether they engage in them in a typical week (0 = “never”, 1 = “once”, 2 = “twice or more”). For those physical exercises participants reported endorsing; they were asked to indicate how many total hours they engaged in the selected activity in a typical week (1 = “less than 1 hr,” 2 = “1–2 ½ hr,” 3 = “3–4 ½ hr,” 4 = “5–6 ½ hr,” 5 = “7–8 ½ hr,” 6 = “9 or more hr”). For each activity, we created a weighted duration variable by multiplying hours per week in the given variable by corresponding metabolic equivalent of task value (Supplemental Table S2). Activities not endorsed or missing received a score of 0. For each activity, we created a caloric expenditure per week variable by multiplying the given weighted duration variable by 3.5 and by 60 (to convert METs/minute to METs/hour) and by weight in kg/200. We used the sum of the caloric expenditure per week of all given activities to create caloric expenditure/week for all activities. The CHAMPS physical activity questionnaire is valid for use with people aged 50 and older (Stewart et al., 2001).
Study Analyses
To analyze how pain at baseline predicted AARC-gains, AARC-losses, and physical activity one year later (Research Question 1), we fit three linear regression models with pain at baseline as the predictor and AARC-gains (Model 1), AARC-losses (Model 2), or physical activity (Model 3) at follow-up as the outcome. In each model, we adjusted for age, sex, educational level, and each outcome variable’s corresponding value at baseline. For Research Question 2, we tested the mediating role of AARC-gains and AARC-losses in the association between pain at baseline and physical activity at one-year follow-up by using the sem interface in STATA. We adjusted for age, sex, educational level, and baseline physical activity. For Research Question 2, we use linear regression models with an interaction term between AARC-gains (or AARC-losses) and pain to test the moderating role of AARC-gains and AARC-losses at baseline in the association of pain at baseline with physical activity at one-year follow-up. We conducted all analyses in STATA Version 17 using complete case analyses (StataCorp, 2021).
Results
Descriptive Statistics
At baseline, participants’ mean age was 65.5 years (SD = 6.94). The majority of participants were women (86.4%), and 58.6% had a university degree. Almost all participants were of White ethnicity (99.3%). Around 20% of participants reported experiencing any level of pain interference and among all participants, on average, pain interfered with an average of one activity over the past week. On average, participants reported awareness of moderate age-related gains and few age-related losses, and expended an average of 2,775.64 (SD = 2208.38) calories per week. Table 1 provides descriptive statistics for all study variables at baseline and one year follow-up.
Table 1.
Descriptive Statistics.
| Sample who reported data on AARC and pain in 2019 and 2020 (n = 434) |
||
|---|---|---|
| Baseline (2019) | Follow-up (2020) | |
|
| ||
| Age, M (SD), range | 65.50 (6.94), 51–89 | |
| Women, n (%) | 375 (86.4) | |
| Education, n (%) | ||
| Secondary education | 47 (10.8) | |
| Post-secondary education | 47 (10.8) | |
| Vocational qualification | 86 (19.8) | |
| Undergraduate degree | 160 (36.9) | |
| Post-graduate degree | 74 (17.1) | |
| Doctorate | 20 (4.6) | |
| White ethnicity, n (%) | 431 (99.3) | |
| Pain, M (SD), rangea | 1.02 (2.72), 0–17 | 1.63 (3.39), 0–17 |
| Awareness of age-related losses, M (SD), rangeb | 9.85 (3.27), 5–25 | 10.12 (3.34), 5–22 |
| Awareness of age-related gains, M (SD), rangeb | 18.74 (3.67), 6–25 | 18.37 (3.73), 7–25 |
| Caloric expenditure per week, M (SD), range | 2,775.64 (2,208.38), 0–17,614.22 | 3,325.45 (2,331.22), 0–14,394.19 |
Note. Data are from participants who reported data on AARC and pain in 2019 and 2020 (n = 434).
Possible score range = 0–16.
Possible score range = 5–25.
Pain as Predictor of AARC and Physical Activity (Research Question 1)
In the regression model adjusted for age, sex, educational level, and baseline AARC-gains, pain at baseline was not a statistically significant predictor of AARC-gains at one-year follow-up. In the regression model adjusted for age, sex, educational level, and baseline AARC-losses, pain at baseline predicted higher AARC-losses at one-year follow-up (β = .07; .95% CI: .01; .14). In the regression model adjusted for age, sex, educational level, and baseline caloric expenditure per week, higher pain at baseline predicted less caloric expenditure per week at one-year follow-up (β = −.13; 95% CI: −.21; −.05).
AARC as a Mediator of the Association of Pain and Engagement in Physical Activity (Research Question 2)
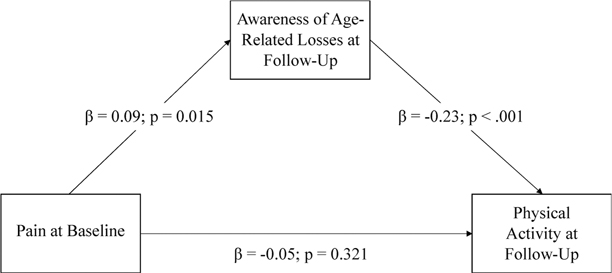
While controlling for baseline levels of AARC-losses, AARC-losses at follow-up partially mediated the association of pain with caloric expenditure per week. Greater pain predicted greater AARC-losses (β = .09; 95% CI .02; .15) and higher AARC-losses predicted less caloric expenditure per week (β = −.23; 95% CI −.34; −.13). In the mediation model, however, pain no longer directly predicted AARC-losses (Figure 1).
Figure 1.
Mediation model for the relationship between pain at baseline and engagement in physical activity at follow-up as mediated by AARC-losses at follow-up.
Note. Statistics are standardized beta coefficients. Results are adjusted for age, sex, education, and baseline AARC-losses.
Because pain at baseline was not a significant predictor of AARC-gains at follow-up, we did not test the mediating role of AARC-gains in the association of pain at baseline with caloric expenditure per week at follow-up.
AARC as a Moderator of the Association of Pain and Engagement in Physical Activity (Research Question 3)
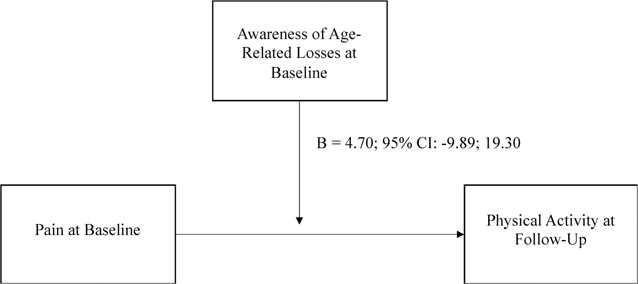
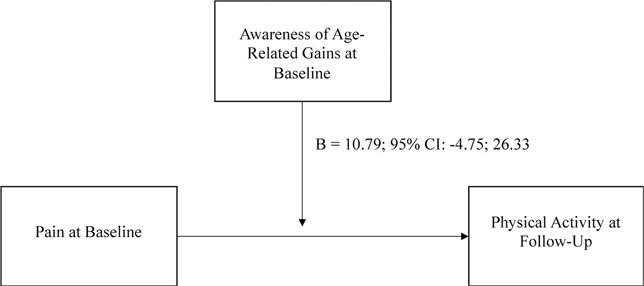
After adjusting for age, sex, education, and physical activity at baseline, the interactions between AARC-gains at baseline and pain at baseline and between AARC-losses at baseline and pain at baseline were not statistically significant predictors of caloric expenditure per week (p-values of .527 and .173, respectively; see Figures 2 and 3).
Figure 2.
Moderation model predicting physical activity with pain and AARC-losses.
Note. This moderation model predicts participants’ physical activity in 2020 from pain in 2019, with the moderating effect of awareness of age-related losses in 2019. Statistics are unstandardized beta coefficients.
Figure 3.
Moderation model predicting physical activity with pain and AARC-gains.
Note. This moderation model predicts participants’ physical activity in 2020 from pain in 2019, with the moderating effect of awareness of age-related gains in 2019. Statistics are unstandardized beta coefficients.
Discussion
Physical activity can help reduce pain symptoms among people who experience persistent and/or activity-limiting pain (Geneen et al., 2017). As such, physical activity is often recommended as a non-pharmacological treatment for pain (Ambrose & Golightly, 2015). However, people with pain are often physical inactive (Kelley et al., 2020; Zadro et al., 2017). In this study, we hypothesized that people’s views on aging, specifically their AARC, may be one of the factors explaining the connections between their pain and physical activity, and we conducted the first study to analyze longitudinal associations between pain, physical activity, and AARC.
Expanding on the existing literature that suggests pain and AARC are cross-sectionally correlated (Sabatini et al., 2021), our study suggests that pain can predict increased levels of AARC-losses one year later. However, pain did not predict levels of AARC-gains, suggesting that even people with higher levels of pain can experience and report positive age-related changes. Additionally, unsurprisingly given existing literature, pain predicted less engagement in physical activity one year later. Our results suggest that high AARC-losses may be an explanation as to why people with greater pain engage less in physical activity.
This initial study testing the mediating and moderating role of AARC in the association of pain with physical activity offers a new salient psychosocial mechanism connecting pain and subsequent physical activity. Study results can add to conceptual models of health-promotive behavior in the presence of physical pain. Because AARC plays a role in how people respond to their pain, it is possible that AARC (and, possibly, other views on aging constructs) should be included in pain and health behavior theoretical models. As just one example, per the fear-avoidance model of pain (Vlaeyen & Linton, 2000) people may interpret experiences of pain by catastrophizing it, which can spiral into pain-related fear and then avoidance behavior. It is this hypervigilant avoidance behavior that facilitates disuse of parts of the body with pain (e.g., disengaging in physical activity) and eventual disability. One explanation for why AARC-losses mediated the relationship between pain and subsequent physical activity in our study could be that poorer AARC contributes to more pain catastrophizing. We encourage future research on possible correlations between AARC and pain catastrophizing in ways that can add to or otherwise extend the fear-avoidance model of pain.
We also invite health behavior interventionists to consider incorporating components of views on aging interventions into pain management interventions. Existing research suggests that older adults perceive pain as an inevitable part of aging (Makris et al., 2015). Physical activity interventions for older adults in pain might benefit from helping older adults reframe their pain so that they do not discount it as an expected part of aging. Older adults who do consider pain as simply a sign that they are getting old may begin to think they should not be exercising anymore anyway or that exercising is not going to resolve their inevitable age-related pain. Physical activity interventions might be even more efficacious if they include psychosocial components that help middle-aged and older adults with pain think more positively about their aging. In Wolff et al.’s (2014) randomized controlled trial, older adults who participated in an intervention that paired physical activity with a views on aging component engaged in more physical activity compared to older adults who participated in an intervention that only included physical activity. Yoga is a common pain management intervention, and may be particularly adaptable to integrating intervention components that reframe participants’ thoughts on aging because yoga already combines physical movement and exercise with meditation. For example, the “MY-Skills” (Merging Yoga and self-management to develop Skills; Gibson et al., 2021) intervention encourages people with persistent pain to return to yoga mantras, such as “I choose health,” while centering their emotions and alongside performing yoga poses. Including mantras that reframe aging might increase the efficacy of the intervention, and we encourage future researchers to test these possibilities.
Additionally, existing scholarship has analyzed AARC-losses and pain (Sabatini et al., 2021). Ours was the first to also include AARC-gains. We found that pain at baseline predicted follow-up AARC-losses, but not AARC-gains. Moreover, we found that AARC-losses, but not AARC-gains, partially mediated the relationship between pain and weekly caloric expenditure. That AARC-losses were more strongly connected to physical health constructs than AARC-gains is in contrast with existing scholarship showing that positive dimensions of views on aging are equally, if not more, connected to physical activity than negative dimensions (Hooker et al., 2019). Further research exploring how AARC-losses and AARC-gains are differentially connected to physical activity can help ensure approaches to intervene on AARC are efficacious. Some existing interventions focus on increasing positive views on aging whereas other focused on decreasing negative views on aging (Diehl et al., 2022; Knight et al., 2021; Wolff et al., 2014). It is possible that interventions being designed to improve AARC may be more effective if they focus on reducing AARC-losses rather than increasing AARC-gains. Recent research suggests that the impact of AARC-gains on health is dependent on AARC-losses, and that the connections between AARC-gains and AARC-losses should be considered in parallel, rather than separately (Sabatini et al., 2022). Analyzing AARC-gains and AARC-losses in this way could be a fruitful avenue for future research exploring connections between AARC, pain, and physical activity.
Future Directions
AARC’s role in the health-promotive behaviors of people in pain should also be explored among other health behaviors beyond physical activity. For example, existing research suggests that people with more negative views on their own aging delay seeking healthcare (Sun & Smith, 2017). Identifying differences in healthcare-seeking behaviors among people with pain depending on their AARC is an important avenue to continue bolstering research on AARCs’ role in the overall health and people who experience pain. Specifically, analysis of whether AARC mediates the relationship between pain and seeking healthcare for that pain would be beneficial. Moreover, efforts to reframe pain beliefs should be extended to healthcare providers, who often perpetuate the ageist assertation that old age is synonymous with pain (Ouchida & Lachs, 2015). In their 2011 study, Davis et al. (2011) found that nearly 64% of primary care clinicians reported that pain was an accepted part of aging. If a healthcare provider conveys that an older adult patient’s pain is inevitable or a normal part of aging, the older adult may perceive even greater age-related losses and worsening overall views on their own aging. In turn, these poorer views on their own aging may restrict health-promotive behaviors that would otherwise relieve their pain symptoms.
Relatedly, it is important that efforts to promote positive views on aging among older adults in pain do not encourage older adults to defy, or triumph over, their pain. One major criticism of healthy aging literature is that it conveys ableist ideas that older adults must remain youthful and non-disabled in order to have aged healthfully (Gibbons, 2016). In this way, healthy aging discourses perpetuate ageism. Indeed, though pain is not a normal part of aging, it is highly correlated with increasing age and many older adults experience pain and chronic conditions with pain symptoms (e.g., arthritis). The best way to move forward may be to help people realize that their pain does not mean they are aging poorly, nor that old age is bad. In so doing, they may report fewer AARC-losses.
Limitations
There are a few key limitations of this study that we would like to highlight. First, although analyses are based on a comprehensive self-report of physical activity, we lacked objective assessment of physical activity and self-reports may not always coincide with objective indicators. Second, a significant proportion of participants was lost to follow-up, though those who remained and those excluded from the study did not significantly differ in key demographic characteristics at baseline. It is possible that attrition was due to the COVID-19 pandemic. Relatedly, the follow-up data were collected during the COVID-19 pandemic. Research suggests that the COVID-19 pandemic impacted physical activity (Stockwell et al., 2021); the physical activity declines we found may be attributed to the pandemic. Third, research suggests that pain and its impact on health behavior changes on a daily basis (Turner et al., 2021). The analyses we conducted in this study may not fully represent the day-to-day experience of pain, AARC, physical activity (and the connection between all three), and it is possible that our findings may be different if we conducted a microlongitudinal daily study.
Fourth, a further limitation of this study is the limited diversity of its sample. Almost all of participants were of White ethnicity and about 86% of participants were women. Research suggests that experiences of aging, pain, and engagement with physical activity all vary across different ethnic groups (Green et al., 2003; North & Fiske, 2015; Schönstein et al., 2021, 2022; Williams et al., 2011). Likewise, there is also some evidence suggesting that levels of perceived age-related gains and losses, and of pain, vary between men and women (Sabatini et al., 2021; Wiesenfeld-Hallin, 2005). As such, it is possible that with a more diverse sample, our results would be different. However, we did control for sex in our models and found that sex was not a significant covariate. Of note, however, is that women (M = 1.28; SD = 3.11) in our sample had higher levels of pain than men (M = 0.86; SD = 2.62), which is in line with existing literature in the UK suggesting that women are more likely to have pain than men (Fayaz et al., 2016). Thus, even though the connections between pain, AARC, and physical activity may not differ between men and women, it is possible that the connections are more salient to women as they experience more pain.
Conclusions
Our study suggests that pain leads to increased AARC-losses in ways that render older adults less physically active. Hence, views on aging may be a key theoretical mechanism for changing the health behavior of older adults in pain, and behavioral interventions that aim to promote physical activity engagement for people with pain may be more efficacious if they target participants’ views on aging. Ultimately, given physical activity is a key strategy for managing pain and pain symptoms, we encourage future theoretical and translational research to consider views on aging as a way to support physical activity engagement for the growing number of middle-aged and older adults experiencing pain.
Supplementary Material
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This paper represents independent research coordinated by the University of Exeter and King’s College London and is funded in part by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. This research was also supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula and the National Institute for Health Research (NIHR) Exeter Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Serena Sabatini was supported by an ESRC (Economic and Social Research Council) Postdoctoral fellowship (Grant Ref: ES/X007766/1). Shelbie G. Turner was supported by a grant from the United States National Institute on Aging (T32AG049666). UK PROTECT data is available upon request and approval from the UK PROTECT steering committee.
Biographies
Author Biographies
Shelbie G. Turner is a postdoctoral fellow at Weill Cornell Medicine in New York City (US). She studies health behavior motivation in later life, especially among populations at high-risk for poor health outcomes such as older adults with persistent pain and dementia family caregivers.
Helen Brooker is the managing director at Ecog Pro Ltd (Bristol, UK). Her work focuses on the assessment of cognitive functioning in older age.
Clive Ballard is professor of Age Related Diseases at the University of Exeter (UK). His research focuses on dementia, and especially on non-pharmacological treatments for people with dementia.
Anne Corbett is a professor in Dementia Research at the University of Exeter (UK). She leads the PROTECT study. Her research focuses on cognitive health in aging and on care homes.
Adam Hampshire is a professor at Imperial College London (UK). His research focuses on human cognition.
Serena Sabatini is a lecturer in Psychology at the University of Surrey (UK). She is an associate editor in Frontiers in Aging Psychiatry and in Frontiers in Neuroinfectious Diseases. Her work focuses on self-perceptions of aging, dementia, and caregiving.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- Ambrose KR, & Golightly YM (2015). Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Practice & Research Clinical Rheumatology, 29(1), 120–130. 10.1016/j.berh.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers A, & Diehl M (2017). Feasibility and efficacy of the Aging(Plus) Program: Changing views on aging to increase physical activity. Journal of Aging and Physical Activity, 25(3), 402–411. 10.1123/japa.2016-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary MA, & Gyurcsik NC (2021). Differences in adaptive and maladaptive psychosocial responses to chronic pain among adults with varying physical activity levels. British Journal of Pain, 15(3), 259–269. 10.1177/2049463720942535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudroit J, Stephan Y, Chalabaev A, & Le Scanff C (2012). Subjective age and social-cognitive determinants of physical activity in active older adults. Journal of Aging and Physical Activity, 20(4), 484–496. 10.1123/japa.20.4.484 [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone AA, Rothrock N, Reeve BB, Yount SE, Amtmann DA, Bode RK, Buysse DJ, Choi SW, Cook K, DeVellis RF, Dewalt DA, Fries JF, Gershon RC, Hahn EA, Lai J-S, Pilkonis P,, Revicki DA, …, PROMIS Cooperative Group. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Farrell ME, Moore W, & Park DC (2019). Actual memory as a mediator of the amyloid-subjective cognitive decline relationship. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 11, 151–160. 10.1016/j.dadm.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Marques RL, de Oliveira Rezende AT, Junger AL, Noll M, de Oliveira C, & Silveira EA (2022). What is the relationship between physical activity and chronic pain in older adults? A systematic review and meta-analysis protocol. BMJ Open, 12(11), e062566. 10.1136/bmjopen-2022-062566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Bond LA, Howard A, & Sarkisian CA (2011). Primary care clinician expectations regarding aging. The Gerontologist, 51(6), 856–866. 10.1093/geront/gnr017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denche-Zamorano Á, Franco-García JM, Pastor-Cisneros R, Salas-Gómez D, Collado-Mateo D, Olivares PR, & Adsuar JC (2022). Relationships between physical activity level and pain in the spanish population: A cross-sectional study. Journal of Personalized Medicine, 12(10), 1591. 10.1136/bmjopen-2022-062566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M, Nehrkorn-Bailey A, Thompson K, Rodriguez D, Li K, Rebok GW, Roth DL, Chung SE, Bland C, Feltner S, Forsyth G, Hulett N, Klein B, Mars P, Martinez K, Mast S, Monasterio R, Moore K, Schoenberg H, …, Tseng HY (2020). The Aging (PLUS) trial: Design of a randomized controlled trial to increase physical activity in middle-aged and older adults. Contemporary Clinical Trials, 96, 106105. 10.1016/j.cct.2020.106105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M, Nehrkorn-Bailey A, & Tseng H-Y (2022). Psychological interventions targeting adults’ subjective views of aging. In Palgi ASY & Diehl M (Eds.), Subjective views of aging: Theory, research, and practice (pp. 309–327). Springer. 10.1007/978-3-031-11073-3-17 [DOI] [Google Scholar]
- Diehl MK, & Wahl H-W (2010). Awareness of age-related change: Examination of a (mostly) unexplored concept. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65B(3), S340–S350. 10.1093/geronb/gbp110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl MK, Wahl H-W, Barrett AE, Brothers AF, Miche M, Montepare JM, Westerhof GJ, & Wurm S (2014). Awareness of aging: Theoretical considerations on an emerging concept. Developmental Review, 34(2), 93–113. 10.1016/j.dr.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro L (2001). Physical activity in aging. Changes in patterns and their relationship to health and function. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 56(suppl_2), 13–22. 10.1093/gerona/56.suppl_2.13 [DOI] [PubMed] [Google Scholar]
- Du S, Yuan C, Xiao X, Chu J, Qiu Y, & Qian H (2011). Self-management programs for chronic musculoskeletal pain conditions: A systematic review and meta-analysis. Patient Education and Counseling, 85(3), e299–e310. 10.1016/j.pec.2011.02.021 [DOI] [PubMed] [Google Scholar]
- Dunsmore VJ, & Neupert SD (2022). No pain, no gain? Personality associations with awareness of aging depend on arthritis. Frontiers in Psychology, 13. 10.3389/fpsyg.2022.863152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt AJ, Gabrian M, & Wahl H-W (2016). Awareness of age-related change and depressive symptoms in middle and late adulthood: Longitudinal associations and the role of self-regulation and calendar age. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(6), 944–953. 10.1093/geronb/gbw095 [DOI] [PubMed] [Google Scholar]
- Fayaz A, Croft P, Langford RM, Donaldson LJ, & Jones GT (2016). Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open, 6(6), e010364. 10.1136/bmjopen-2015-010364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, & Smith BH (2017). Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database of Systematic Reviews (4). 10.1002/14651858.CD011279.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons HM (2016). Compulsory youthfulness: Intersections of ableism and ageism in “successful aging” discourses. Review of Disability Studies: An International Journal, 12(2–3), 1–19. https://www.rdsjournal.org/index.php/journal/article/view/574 [Google Scholar]
- Gibson LP, Magnan RE, Kramer EB, & Bryan AD (2021). Theory of planned behavior analysis of social distancing during the COVID-19 pandemic: Focusing on the intention–behavior gap. Annals of Behavioral Medicine, 55(8), 805–812. 10.1093/abm/kaab041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DS, & McGee SJ (2011). Pain as a global public health priority. BMC Public Health, 11(1), 770. 10.1186/1471-2458-11-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kaloukalani DA, Lasch KE, Myers C, & Tait RC (2003). The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Medicine, 4(3), 277–294. 10.1046/j.1526-4637.2003.03034.x [DOI] [PubMed] [Google Scholar]
- Hadi MA, McHugh GA, & Closs SJ (2019). Impact of chronic pain on patients’ quality of life: A comparative mixed-methods study. Journal of Patient Experience, 6(2), 133–141. 10.1177/2374373518786013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden J, Tulder M, & Tomlinson G (2005). Systematic review: Strategies for using exercise therapy to improve outcomes in chronic low back pain. Annals of Internal Medicine, 142, 776–785. 10.7326/0003-4819-142-9-200505030-00014 [DOI] [PubMed] [Google Scholar]
- Hooker K, Mejía ST, Phibbs S, Tan EJ, & Stevens J (2019). Effects of age discrimination on self-perceptions of aging and cancer risk behaviors. The Gerontologist, 59(Suppl. 1), S28–S37. 10.1093/geront/gny183 [DOI] [PubMed] [Google Scholar]
- Kaspar R, Gabrian M, Brothers AF, Wahl H-W, & Diehl MK (2019). Measuring awareness of age-related change: Development of a 10-item short form for use in large-scale surveys. The Gerontologist, 59(3), e130–e140. 10.1093/geront/gnx213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar R, Wahl H-W, Diehl MK, & Zank S (2022). Subjective views of aging in very old age: Predictors of 2-year change in gains and losses. Psychology and Aging, 37(4), 503–516. 10.1037/pag0000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekäläinen T, Freund AM, Sipilä S, & Kokko K (2020). Cross-sectional and longitudinal associations between leisure time physical activity, mental well-being and subjective health in middle adulthood. Applied Research in Quality of Life, 15, 1099–1116. 10.1007/s11482-019-09721-4 [DOI] [Google Scholar]
- Kelley GA, Kelley KS, & Callahan LF (2020). Number of physically inactive adults with arthritis in the United States who could improve physical function and pain control by exercising. Preventing Chronic Disease, 17, E99. 10.5888/pcd17.200121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, & Heuser I (2012). Views on aging and emotional benefits of physical activity: Effects of an exercise intervention in older women. Psychology of Sport and Exercise, 13(2), 236–242. 10.1016/j.psychsport.2011.11.001 [DOI] [Google Scholar]
- Knight RL, Chalabaev A, McNarry MA, Mackintosh KA, & Hudson J (2021). Do age stereotype-based interventions affect health-related outcomes in older adults? A systematic review and future directions. British Journal of Health Psychology, 27(2), 338–373. 10.1111/bjhp.12548 [DOI] [PubMed] [Google Scholar]
- Kornadt AE, Hess TM, & Rothermund K (2020). Domain-specific views on aging and preparation for age-related changes—Development and validation of three brief scales. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(2), 303–307. 10.1093/geronb/gby055 [DOI] [PubMed] [Google Scholar]
- Kotter-Grühn D, Kleinspehn-Ammerlahn A, Gerstorf D, & Smith J (2009). Self-perceptions of aging predict mortality and change with approaching death: 16-Year longitudinal results from the Berlin aging study. Psychology and Aging, 24(3), 654–667. 10.1037/a0016510 [DOI] [PubMed] [Google Scholar]
- Kotter-Grühn D, Kornadt AE, & Stephan Y (2016). Looking beyond chronological age: Current knowledge and future directions in the study of subjective age. Gerontology, 62, 86–93. 10.1159/000438671 [DOI] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Kunkel SR, & Kasl SV (2002). Longevity increased by positive self-perceptions of aging. Journal of Personality and Social Psychology, 83(2), 261–270. 10.1037/0022-3514.83.2.261 [DOI] [PubMed] [Google Scholar]
- Makris UE, Higashi RT, Marks EG, Fraenkel L, Sale JEM, Gill TM, & Reid MC (2015). Ageism, negative attitudes, and competing co-morbidities–why older adults may not seek care for restricting back pain: A qualitative study. BMC Geriatrics, 15(1), 1–9. 10.1186/s12877-015-0042-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montepare JM (2020). An exploration of subjective age, actual age, age awareness, and engagement in everyday behaviors. European Journal of Ageing, 17(3), 299–307. 10.1007/s10433-019-00534-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2021). Chronic pain (primary and secondary) in over 16s: Assessment of all chronic pain and management of chronic primary pain. https://www.nice.org.uk/guidance/ng193/resources/chronic-pain-primary-and-secondary-in-over-16s-assessment-of-all-chronic-pain-and-management-of-chronic-primary-pain-pdf-66142080468421 [PubMed]
- Nehrkorn-Bailey AM, Rodriguez D, Forsyth G, Braun B, Burke K, & Diehl M (2023). Change in views of aging, physical activity, and physical health over 8 weeks: Results from a randomized study. Journal of Aging and Physical Activity, 1–13. 10.1123/japa.2022-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North MS, & Fiske ST (2015). Modern attitudes toward older adults in the aging world: A cross-cultural meta-analysis. Psychological Bulletin, 141(5), 993–1021. 10.1037/a0039469 [DOI] [PubMed] [Google Scholar]
- Ouchida KM, & Lachs MS (2015). Not for doctors only: Ageism in healthcare. Generations (san Francisco, Calif ), 39(3), 46–57. https://www.jstor.org/stable/10.2307/26556135 [Google Scholar]
- Rothermund K, & Kornadt AE (2015). Views on aging: Domain-specific approaches and implications for developmental regulation. Annual Review of Gerontology and Geriatrics, 35(1), 121–144. 10.1891/0198-8794.35.121 [DOI] [Google Scholar]
- Sabatini S, Ukoumunne OC, Ballard C, Brothers AF, Kaspar R, Collins R, Kim S, Corbett A, Aarsland D, Hampshire A, Brooker H, & Clare L (2020). International relevance of two measures of awareness of age-related change (AARC). BMC Geriatrics, 20(1), 359. 10.1186/s12877-020-01767-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Ukoumunne OC, Ballard C, Collins R, Corbett A, Brooker H, & Clare L (2021). The cross-sectional relationship between pain and awareness of age-related changes. British Journal of Pain, 15(3), 335–344. 10.1177/2049463720961798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Ukoumunne OC, Brothers A, Diehl MK, Wahl H-W, Ballard C, Collins R, Corbett A, Brooker H, & Clare L (2022). Differences in awareness of positive and negative age-related changes accounting for variability in health outcomes. European Journal of Ageing, 19(4), 1087–1097. 10.1007/s10433-021-00673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent-Cox KA, Anstey KJ, & Luszcz MA (2013). Longitudinal change of self-perceptions of aging and mortality. The Journals of Gerontology: Series B, 69(2), 168–173. 10.1093/geronb/gbt005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönstein A, Ngo DTT, Stephan Y, Siè A, Harling G, Bärnighausen T, & Wahl H-W (2021). Feeling younger in rural Burkina Faso: Exploring the role of subjective age in the light of previous research from high-income countries. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(10), 2029–2040. 10.1093/geronb/gbag151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönstein A, Schlomann A, Wahl H-W, & Bärnighausen T (2022). Awareness of age-related change in very different cultural-political contexts: A cross-cultural examination of aging in Burkina Faso and Germany. Frontiers in Psychiatry, 13. 10.3389/fpsyt.2022.928564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle A, Spink M, Ho A, & Chuter V (2015). Exercise interventions for the treatment of chronic low back pain: A systematic review and meta-analysis of randomised controlled trials. Clinical Rehabilitation, 29(12), 1155–1167. 10.1177/0269215515570379 [DOI] [PubMed] [Google Scholar]
- Smith BE, Hendrick P, Bateman M, Holden S, Littlewood C, Smith TO, & Logan P (2019). Musculoskeletal pain and exercise—Challenging existing paradigms and introducing new. British Journal of Sports Medicine, 53(14), 907–912. 10.1136/bjsports-2017-098983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. (2021). Stata Statistical Software: Release 17.
- Stephan Y, Sutin AR, & Terracciano A (2018). Subjective age and mortality in three longitudinal samples. Psychosomatic Medicine, 80(7), 659–664. 10.1097/psy.0000000000000613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward A, & Hasche L (2022). Exploring lifestyle activities to reduce internalized ageism: Self-efficacy as a mediator between exercise, volunteering, computer use, and self-perceptions of aging. The International Journal of Aging and Human Development, 94(3), 255–272. 10.1177/00914150211024175 [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis DAWN, & Ritter PL (2001). CHAMPS Physical activity questionnaire for older adults: Outcomes for interventions. Medicine & Science in Sports & Exercise, 33(7), 1126–1141. 10.1097/00005768-200107000-00010 [DOI] [PubMed] [Google Scholar]
- Stockwell S, Trott M, Tully M, Shin J, Barnett Y, Butler L, McDermott D, Schuch F, & Smith L (2021). Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: A systematic review. BMJ Open Sport & Exercise Medicine, 7(1), e000960. 10.1136/bmjsem-2020-000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JK, & Smith J (2017). Self-perceptions of aging and perceived barriers to care: Reasons for health care delay. The Gerontologist, 57(Suppl. 2), S216–S226. 10.1093/geront/gnx014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SG, Hooker K, & Stawski RS (2021). Women’s self-relevant goal pursuit in the presence of physical pain: An intraindividual variability approach. The Journals of Gerontology: Series B, 76(8), 1565–1573. 10.1093/geronb/gbaa151 [DOI] [PubMed] [Google Scholar]
- Vlaeyen JWS, & Linton SJ (2000). Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain, 85(3), 317–332. 10.1016/s0304-3959(99)00242-0 [DOI] [PubMed] [Google Scholar]
- Warburton DER, Nicol CW, & Bredin SSD (2006). Health benefits of physical activity: The evidence. Canadian Medical Association Journal, 174(6), 801–809. 10.1503/cmaj.051351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienert J, Gellert P, & Lippke S (2017). Physical activity across the life-span: Does feeling physically younger help you to plan physical activities? Journal of Health Psychology, 22(3), 324–335. 10.1177/1359105315603469 [DOI] [PubMed] [Google Scholar]
- Wienert J, Kuhlmann T, & Lippke S (2015). Direct effects of a domain-specific subjective age measure on self-reported physical activity—Is it more important how old you are or how old you feel? Health Psychology Report, 3(2), 131–139. 10.5114/hpr.2015.51450 [DOI] [Google Scholar]
- Wiesenfeld-Hallin Z (2005). Sex differences in pain perception. Gender Medicine, 2(3), 137–145. 10.1016/S1550-8579(05)80042-7 [DOI] [PubMed] [Google Scholar]
- Williams ED, Stamatakis E, Chandola T, & Hamer M (2011). Assessment of physical activity levels in South Asians in the UK: Findings from the Health Survey for England. Journal of Epidemiology & Community Health, 65(6), 517–521. 10.1136/jech.2009.102509 [DOI] [PubMed] [Google Scholar]
- Wolff JK, Warner LM, Ziegelmann JP, & Wurm S (2014). What do targeting positive views on ageing add to a physical activity intervention in older adults? Results from a randomised controlled trial [psychol health]. Psychology & Health, 29(8), 915–932. 10.1080/08870446.2014.896464 [DOI] [PubMed] [Google Scholar]
- Wurm S, Diehl M, Kornadt AE, Westerhof GJ, & Wahl H-W (2017). How do views on aging affect health outcomes in adulthood and late life? Explanations for an established connection. Developmental Review, 46, 27–43. 10.1016/j.dr.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadro JR, Shirley D, Amorim A, Pérez-Riquelme F, Ordoñana JR, & Ferreira PH (2017). Are people with chronic low back pain meeting the physical activity guidelines? A co-twin control study. The Spine Journal, 17(6), 845–854. 10.1016/j.spinee.2017.01.015 [DOI] [PubMed] [Google Scholar]
- Zimmer Z, Fraser K, Grol-Prokopczyk H, & Zajacova A (2022). A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain, 163(9), 1740–1750. 10.1097/j.pain.0000000000002557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.