Abstract
BACKGROUND
Lipomyelomeningocele associated with an ulnar club hand in the spectrum of VACTERL association ([costo-]vertebral abnormalities; anal atresia; cardiac defects; tracheal-esophageal abnomalities, including atresia, stenosis, and fistula; renal and radial abnormalities; limb abnormalities; single umbilical artery) is a very rare and infrequently reported phenomenon. Within the fat mass of the lipoma, it is not common to find a well-defined cartilaginous mass with no attachments to the surrounding tissue.
OBSERVATIONS
The authors present the case of a 3-month-old male with low-back swelling that was off-center to the left, accompanied by a left short forearm displaying outward bowing. Echocardiography showed an atrial septal defect. This rare VACTERL association comprises lipomyelomeningocele, atrial septal defect, and ulnar longitudinal deficiency syndrome. During surgical intervention for the lipoma, a well-defined cartilaginous mass was discovered within the adipose tissue.
LESSONS
The manifestation of VACTERL association can be partially explained by the Shh/Gli and Wnt pathway defects. It is prudent to screen children with neural tube defects to be aware of any associated syndromes. This case is very rare, and the literature has contained no prior report on the VACTERL association of lipomyelomeningocele, atrial septal defect, and ulnar longitudinal deficiency.
Keywords: VACTERL association, ulnar longitudinal deficiency syndrome, lipomyelomeningocele, illustrative case
ABBREVIATIONS: ASD = atrial septal defect, CSF = cerebrospinal fluid, DV = dorsoventral, EA = esophageal atresia, LMMC = lipomyelomeningocele, Shh = Sonic hedgehog, STIR = short tau inversion recovery, TEF = tracheoesophageal fistula, T1 = T1 (longitudinal relaxation time) weighted image, T2 = T2 (transverse relaxation time) weighted image
The acronym VATER (for vertebral defects, anal atresia, tracheoesophageal fistula with esophageal atresia, radial and renal dysplasia) was used for the first time in 1973 to establish the association specificity of a group of congenital malformations including vertebral defects, anal atresia, tracheoesophageal fistula with esophageal atresia (TEF/EA), and radial and renal dysplasia. The diagnosis of a VACTERL association is usually made if at least three of the defects (vertebral defects, anal atresia, cardiac defects, TEF/EA, renal defects, and limb abnormalities) are present.1
Lipomyelomeningocele (LMMC) is a neurulation defect with controversies of primary and secondary. Dorsal-type LMMC is a primary neurulation defect with the problem occurring at the time of surface ectoderm disjunction from the neuroectoderm. For the transitional type, the problem occurs at the transition from primary to secondary, and for caudal and filar types, it seems to be a secondary neurulation defect. According to the new classification of spinal lipoma, type 1 is purely primary, type 2 is a transition from primary to secondary, and types 3 and 4 have early and late secondary neurulation defects.2
In this illustrative case, we discuss the novel occurrence of VACTERL association with LMMC, atrial septal defect (ASD), and ulnar longitudinal deficiency syndrome, all of which use Shh/Gli3 and Wnt pathways for their development.
Illustrative Case
A 3-month-old infant, born to a para 1 mother, presented to our neurosurgical outpatient department with back swelling and a left upper-extremity deformity present since birth. Upon examination, there was 4 × 5 cm, skin-covered swelling on the lower back, off-center to the left side. He also had a short left upper extremity with radial bowing of his forearm and only four digits, missing an index finger (Fig. 1).
FIG. 1.

A: Swelling on the back, more on the left side. B: Normal hand. C: Short outward-bowed hand with four digits.
Magnetic resonance imaging (MRI) of the spine showed a T1 (longitudinal relaxation time) weighted image (T1)- and T1 (longitudinal relaxation time) weighted image (T2)-hyperintense and short tau inversion recovery (STIR)–hypointense mass, which was in continuation with subcutaneous fat and covered a T1-hypointense and T2-hyperintense cyst, which was in direct contact and covered the protruded spinal cord. There was also a separate T1-isointense to -hypointense, T2-isointense to -hyperintense, and STIR-hyperintense well-defined mass in the fat. The distal cord had a syrinx (Fig. 2). An upper-extremity radiograph showed the complete absence of ulnar bone with a bowed radial bone and four metacarpal bones. In addition, humeral joint dislocation was visible (Fig. 3). Upon investigation for any cardiac abnormality, echocardiography showed an ASD. In all panels, the patient’s complete blood count was within the normal range for his age. With the diagnosis of VACTERL syndrome association, as he fulfilled three criteria (cardiac, vertebral, and limb), we counseled the family, and surgical management was planned for the LMMC.
FIG. 2.

T1-hypointense (A) and T2-hyperintense (B) cystic mass with a T1-hypointense, T2-isointense to -hyperintense, and STIR-hyperintense mass (C) within the fat.
FIG. 3.
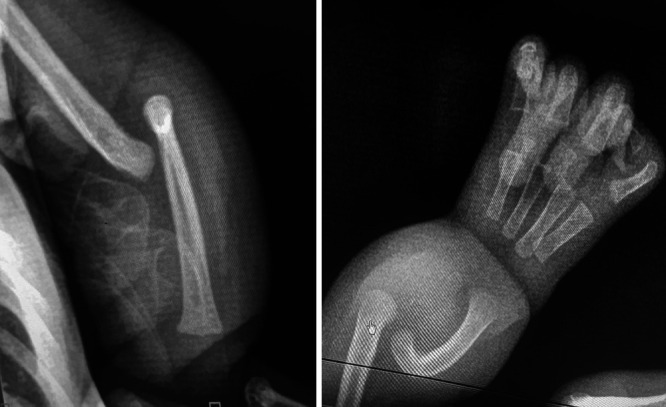
Radiographs showing a dislocated elbow (left) and a bowed radius with four metacarpal bones (right).
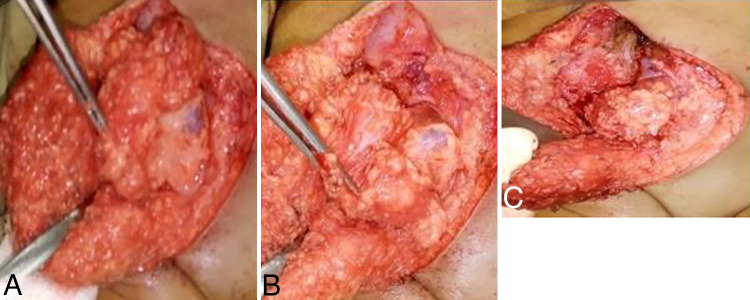
After the patient was positioned prone and prepared for surgery, standard washing and draping procedures were performed. A skin incision was made over the swelling, and the fat was dissected, revealing a distinct mass embedded within it. This mass exhibited a soft to firm consistency and was found to have no attachment to the surrounding fascia or bone (Fig. 4). Upon resection of the fatty mass, the cerebrospinal fluid (CSF)–filled sac was opened and the fat attachment to the placoid was visible, which makes fat dissection relatively easier, but some fat tissue was left on the placoid, and watertight dural closure was achieved with duraplasty (Fig. 5). Then we left a drain, and the skin was closed in layers. There was no postoperative CSF leakage, the drain was removed on the 10th postoperative day, and the child was discharged without complications. During the first outpatient visit at the neurosurgical clinic 1 month postoperation, the wound had fully healed. Plans were made to refer the patient to a pediatric orthopedic physician for the management of the ulnar club hand. Histopathological analysis of the discrete mass within the fatty tissue revealed multiple tissue fragments, including striated muscle fibers, mature adipose tissue, and a matrix containing lacuna and chondrocytes. Additionally, nerve bundles and separated annuli of obliquely oriented collagen fibers were identified within the specimen.
FIG. 4.

Cartilage mass with firm consistency. Intraoperatively opened to see the characteristics.
FIG. 5.
A: Fatty mass after dissection. B: Glistening dura on the lateral side of fat filled with CSF. C: The fat mass as it extends intradural to attach to the low-lying cord.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Neural tube defects are the most common problem in low-income countries. LMMC is one of the primary neurulation abnormalities with premature disjunction of surface ectoderm from the neuroectoderm, resulting in mesenchymal tissue attachment on the developing neural tissue, which develops into fatty tissue.3 There have been few reports of LMMC with an accessory limb attached to the mass and anomalous bone,4–12 but there is no, to the best of our knowledge, published case of LMMC with well-developed cartilage, atrial septal defect, and ulnar longitudinal deficiency in a form of VACTERL association.
The critical period of ulnar longitudinal deficiency is earlier than that for other anomalies, and it corresponds to the period of a high mortality rate for fetuses. The most critical period for the development of limb anomalies is 24 to 36 days of embryonic life. Ulnar deficiency is rarely associated with a syndrome, unlike radial clubbed hand.13
LMMC is characterized by a subcutaneous lipoma that is generally located in the lumbar or sacral region. The subcutaneous lipoma extends through a defect in the lumbodorsal fascia, vertebral neural arch, and dura, attaching to an elongated and tethered spinal cord. The most common presenting symptom is a fatty mass positioned in the midline or just off the midline in the lumbosacral region,3 which is how our patient presented.
During primary neurulation, anterior and posterior neuropore closure occurs at approximately 24 to 26 postovulatory days, which lies within the critical time frame for the development of limb anomalies. There are few case reports mentioning LMMC with limb anomalies in the lower extremities, but there is no report that mentions upper limb anomalies with LMMC. Adding to the rarity of this case is the conjunction of LMMC with an uncommon ulnar longitudinal deficiency, typically nonsyndromic, and presenting as part of the VACTERL association syndrome.
Ulnar longitudinal deficiency pathoembryologically is attributed to the underexpression or inexpression of Sonic hedgehog (Shh) ligand,14 which is paradoxically required in spina bifida pathoembryology.15 Shh/Gli3 and Wnt act on dorsoventral (DV) patterning in neural tube formation. Shh/Gli3 act on ventralization and Wnt on the dorsal/roof of the neural tube. The Wnt pathway regulates the dorsal Gli3 expression. Wnt maintains progenitor cell cycling and restricts Shh/Gli graded activity to ensure the generation of the required amount of progenitor populations at their precise spatial DV locations.16 On the other hand, abnormalities in the Wnt7a pathway (located in the dorsal ectoderm) produce several clinically relevant conditions like ulnar ray deficiency, which explains the associated limb anomalies in VACTERL association, because Shh also plays a major role in limb formation.17,18 Along with this, ASD, which is also part of the VACTERL association and one of the anomalies in our patient, is another congenital problem associated with disrupted Shh signaling.19
Shh/Gli3 and Wnt signaling pathway have a huge impact on the dorsoventral patterning in neural tube development and neural folding at the medial hinge point and dorsolateral hinge point, respectively.
According to Yang et al.20 on the pathoembryogenesis of LMMC, especially transitional, caudal, and filar types, a potential cause of error lies in “neuro-mesenchymal adhesion” during secondary neurulation. Their explanation is based on the fact that the attachment of the lipoma fat with the subcutaneous fat in almost all LMMCs, along with this, the multipotent cell in the caudal cell mass can differentiate into a mesenchymal component like bone, cartilage, muscle, and even renal tissue, which is the case in our patient with discrete cartilage in the fatty tissue with muscle as well as nerve fibers, which can evolve from adjacent skin and neural crest cells.20 Gardner21 proposed a theory of “over distension of the neural tube causing anomalies in a non-neural organ” that suggests that the subsequent rupture of the neural tube disperses and infiltrates mesodermal tissues, leading to anomalous development.
VACTERL is a nonrandom association of congenital anomalies and is an acronym for vertebral or vascular anomalies (V), anal (A), cardiac (C), tracheoesophageal fistula (TE), renal anomalies (R), and limb abnormalities (L). At least three components should be fulfilled to make the diagnosis, and in our case, the patient fulfilled three of the six criteria.22 Notably, there are no reported instances of VACTERL association with both ulnar deficiency and LMMC, rendering this case exceptionally unique and rare.
Gupta and Singh22 described a case of VACTERL association with a closed neural tube defect LMMC. However, the limb anomaly observed in that case was clubfoot with radial hypoplasia. The diagnosis of LMMC was made by the mere absence of a cranial sign on the ultrasound examination and a tethered cord with a lumbosacral mass. In contrast, in our case, the diagnosis was confirmed through MRI, and during surgery, we directly observed fat tissue attached to the low-lying cord, along with the presence of cartilage within the fat mass. Additionally, the limb abnormality observed in our patient was an ulnar deficiency, which is uncommon and typically not associated with syndromes.
Lessons
VACTERL association is a rare phenomenon, but its association with both LMMC and ulnar longitudinal deficiency is exceptionally uncommon and has never been documented. The involvement of the Shh/Gli and Wnt ligand pathways in this association is noteworthy, as these pathways are implicated in most of the anomalies observed. It is wise to screen children with neural tube defects for other congenital malformations.
Acknowledgments
We would like to thank our patient’s family for their permission to publish this case and use his diagnostic image.
Author Contributions
Conception and design: Shimekit. Drafting the article: Shimekit. Critically revising the article: Shimekit, Teferi, Lemma. Reviewed submitted version of manuscript: Shimekit, Yesuf. Approved the final version of the manuscript on behalf of all authors: Shimekit.
References
- 1. Carli D, Garagnani L, Lando M, Fairplay T, Bernasconi S, Landi A, et al. VACTERL (vertebral defects, anal atresia, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies) association: disease spectrum in 25 patients ascertained for their upper limb involvement. J Pediatr. 2014;164(3):458–462.e2. doi: 10.1016/j.jpeds.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 2. Tominey S, Kaliaperumal C, Gallo P. External validation of a new classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 2020;25(4):1–8. doi: 10.3171/2019.11.PEDS19575. [DOI] [PubMed] [Google Scholar]
- 3. Sarris CE, Tomei KL, Carmel PW, Gandhi CD. Lipomyelomeningocele: pathology, treatment, and outcomes. Neurosurg Focus. 2012;33(4):E3. doi: 10.3171/2012.7.FOCUS12224. [DOI] [PubMed] [Google Scholar]
- 4. Bayri Y, Tanrıkulu B, Ekşi MŞ, Dağçınar A. Accessory lower limb associated with spina bifida: case report. Childs Nerv Syst. 2014;30(12):2123–2126. doi: 10.1007/s00381-014-2475-7. [DOI] [PubMed] [Google Scholar]
- 5. Dogra L, Bhayana A, Dakshayini HS, Bagri N. Anomalous osseous limb: a sneak peek into rare association with lipomyelomeningocele—a case report. Case Rep Clin Radiol. 2023;1(1):47–50. [Google Scholar]
- 6. Krishra A, Chandna S, Mishra NK, Gupta AK, Upadhyaya P. Accessory limb associated with spinal bifida. J Pediatr Surg. 1989;24(6):604–606. doi: 10.1016/s0022-3468(89)80517-2. [DOI] [PubMed] [Google Scholar]
- 7. Krishna A, Lal P. Accessory limbs associated with spina bifida - a second look. Pediatr Surg Int. 1999;15(3–4):248–250. doi: 10.1007/s003830050568. [DOI] [PubMed] [Google Scholar]
- 8. McAlister WH, Siegel MJ, Shackelford GD. A congenital iliac anomaly often associated with sacral lipoma and ipsilateral lower extremity weakness. Skeletal Radiol. 1978;3:161–166. [Google Scholar]
- 9. Murphy RF, Cohen BH, Muhlbauer MS, et al. An accessory limb with lipomyelomeningocele in a male. Pediatr Surg Int. 2013;29(7):749–752. doi: 10.1007/s00383-013-3269-9. [DOI] [PubMed] [Google Scholar]
- 10. Parkinson D. Accessory limbs and spinal dysraphism. J Neurosurg. 1991;75(3):498–499. doi: 10.3171/jns.1991.75.3.0498. [DOI] [PubMed] [Google Scholar]
- 11. Wasnik AP, Shinagare A, Lalchandani UR, Gujrathi R, Pai BU. Rudimentary third lower limb in association with spinal dysraphism: Two cases. Indian J Orthop. 2007;41(1):72–75. doi: 10.4103/0019-5413.30530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilkes SL, Choi JJ, Rooks VJ. Lumbosacral lipomyelomeningocele with anomalous osseous limb in a 3-month-old female. Radiol Case Rep. 2015;10(1):1051. doi: 10.2484/rcr.v10i1.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdulkadir AY, Adigun IA. Ulnar hemimelia with oilgodactyly: report of two cases. Radiol Case Rep. 2016;4(1):240. doi: 10.2484/rcr.v4i1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al-Qattan MM, Al-Sahabi A, Al-Arfaj N. Ulnar ray deficiency: a review of the classification systems, the clinical features in 72 cases, and related developmental biology. J Hand Surg Eur Vol. 2010;35(9):699–707. doi: 10.1177/1753193409358240. [DOI] [PubMed] [Google Scholar]
- 15. Murdoch JN, Copp AJ. The relationship between sonic Hedgehog signaling, cilia, and neural tube defects. Birth Defects Res A Clin Mol Teratol. 2010;88(8):633–652. doi: 10.1002/bdra.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Martí E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135(2):237–247. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- 17. Al-Qattan MM. WNT pathways and upper limb anomalies. J Hand Surg Eur Vol. 2011;36(1):9–22. doi: 10.1177/1753193410380502. [DOI] [PubMed] [Google Scholar]
- 18. Velasquez Restrespo S, Oboli VN, Kumar D, Marino-Villamizar C, Khanna S. Ulnar longitudinal deficiency: a case report. Cureus. 2023;15(6):e40111. doi: 10.7759/cureus.40111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ngan ES, Kim KH, Hui CC. Sonic hedgehog signaling and VACTERL association. Mol Syndromol. 2013;4(1–2):32–45. doi: 10.1159/000345725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang J, Lee JY, Kim KH, Wang KC. Disorders of secondary neurulation: mainly focused on pathoembryogenesis. J Korean Neurosurg Soc. 2021;64(3):386–405. doi: 10.3340/jkns.2021.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gardner WJ. Hypothesis; overdistention of the neural tube may cause anomalies of non-neural organs. Teratology. 1980;22(2):229–238. doi: 10.1002/tera.1420220212. [DOI] [PubMed] [Google Scholar]
- 22. Gupta N, Singh P. A novel co-occurrence of VACTERL and closed neural tube defect. J Foetal Med. 2020;7(3):253–258. [Google Scholar]