Abstract
Background and Objectives
Real-world clinical data, outside of clinical trials and expert centers, on adverse events related to the use of SyncCardia total artificial heart (TAH) remain limited. We aim to analyze adverse events related to the use of SynCardia TAH reported to the Food and Drug Administration (FDA)’s Manufacturers and User Defined Experience (MAUDE) database.
Methods
We reviewed the FDA’s MAUDE database for any adverse events involving the use of SynCardia TAH from 1/01/2012 to 9/30/2020. All the events were independently reviewed by three physicians.
Results
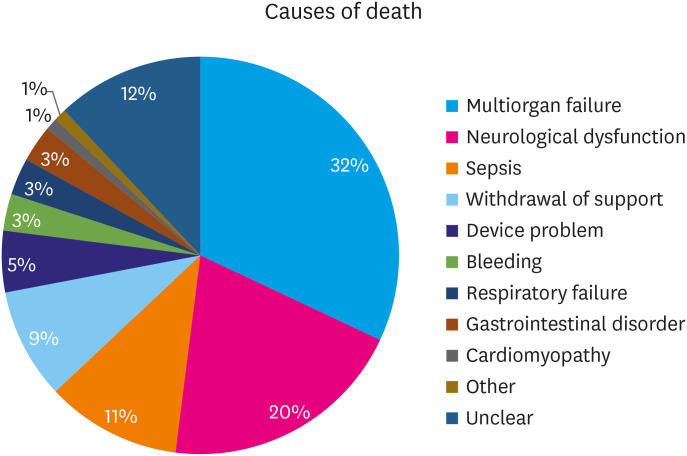
A total of 1,512 adverse events were identified in 453 “injury and death” reports in the MAUDE database. The most common adverse events reported were infection (20.2%) and device malfunction (20.1%). These were followed by bleeding events (16.5%), respiratory failure (10.1%), cerebrovascular accident (CVA)/other neurological dysfunction (8.7%), renal dysfunction (7.5%), hepatic dysfunction (2.2%), thromboembolic events (1.8%), pericardial effusion (1.8%), and hemolysis (1%). Death was reported in 49.4% of all the reported cases (n=224/453). The most common cause of death was multiorgan failure (n=73, 32.6%), followed by CVA/other non-specific neurological dysfunction (n=44, 19.7%), sepsis (n=24, 10.7%), withdrawal of support (n=20, 8.9%), device malfunction (n=11, 4.9%), bleeding (n=7, 3.1%), respiratory failure (n=7, 3.1%), gastrointestinal disorder (n=6, 2.7%), and cardiomyopathy (n=3, 1.3%).
Conclusions
Infection was the most common adverse event following the implantation of TAH. Most of the deaths reported were due to multiorgan failure. Early recognition and management of any possible adverse events after the TAH implantation are essential to improve the procedural outcome and patient survival.
Keywords: Total artificial heart, Heart failure
INTRODUCTION
Heart failure is a common disease among the United States (U.S.) population, and the prevalence is projected to increase by 46% in 2030.1) Despite advances in the medical management of heart failure, many patients with end-stage heart failure end up requiring a heart transplant.2) The wait time for a heart transplant could be long, and up to 40% of adults on the heart transplant waitlist failed to receive the heart in the first year.3) A recent retrospective cross-sectional study by Bakhtiyar et al.4) revealed that 36.1% of patients who were on the heart transplant waitlist died within the first year. Hence, mechanical circulatory support devices such as ventricular assist devices and total artificial heart (TAH) may serve as a bridge to heart transplant in end-stage heart failure patients or as a destination therapy for certain groups of patients. While TAH may remain a less common choice among those listed for heart transplantation, the percentage of TAH recipients that survived and received heart transplantation was as high as 85.9%5). However, the data on adverse events related to the use of SynCardia TAH outside of clinical trials and expert centers are limited. Our study aims to describe the adverse events associated with the use of TAH reported to the Food and Drug Administration (FDA) Manufacturers and User Defined Experience (MAUDE) database.
METHODS
The MAUDE database is a publicly available database regulated by the FDA. Mandatory (user facilities, manufacturers, and distributors) and voluntary (health care professionals, patients, and customers) will report suspected device-related issues (including adverse events, injuries and deaths) to the database. FDA will monitor the collection and make necessary corrections to the device-related safety issue. Each adverse event report contains the following information: device name, manufacturer name, event type (malfunction, injury, or death), report date; and human-written event description and manufacturer narrative fields. This valuable database offers important insights on incidents that affect patient safety in real world settings. These data are de-identified, neither informed consent nor institutional board review was required to access the data. Although this database cannot determine the standard event rates, it offers important insight into the frequently encountered complications associated with the use of SynCardia TAH in the real-world setting.
We queried the database for any available adverse events associated with the use of SynCardia TAH which were reported in the category of “injury” and “death” from January 2012 through September 2020. All the adverse events were reviewed independently by two physicians. In case of disagreement, a third physician casts the deciding vote. All the events that represented complications during or after TAH implantation and resulted in adverse patient outcomes were considered for further analysis. Duplicate submissions or data were excluded from the report. All the relevant adverse events were analyzed and reported as counts and percentages.
RESULTS
There were a total of 469 ‘injury and death’ reports related to the use of SynCardia TAH in the MAUDE database between January 2012 and September 2020. Sixteen reports were excluded because of duplication or incomplete data. After further analyzing the reports in detail, there were 1512 adverse events reported in relation to the use of SynCardia TAH device. The most common adverse event reported to MAUDE during our study period was infection (n=306, 20.2%) (Table 1). Pulmonary infection was the most common infection among all the infectious events (n=101/306, 33%), followed by bacteremia (n=36/306, 11.8%), gastrointestinal infection (n=31/306, 10.1%), device-related infection (n=25/306, 8.2%), mediastinal infection (n=17/306, 5.6%), and fungemia (n=12/306, 3.9%). Device malfunctioning was the second most common adverse event reported (n=304, 20.1%), and they included device thrombosis, driveline disconnection, driver failure, fault alarms, small air leaks, and others. The annual number of device malfunctioning is illustrated in Supplementary Figure. The third most common adverse event was bleeding (n=249, 16.5%), followed by respiratory failure (n=152, 10.1%), cerebrovascular accident (CVA)/other non-specific neurological dysfunction (n=132, 8.7%), renal dysfunction (n=114, 7.5%), hepatic dysfunction (n=34, 2.2%), pericardial effusion (n=27, 1.8%), other thromboembolic events (n=27, 1.8%), and hemolysis (n=15, 1.0%). Other few reported adverse events were wound dehiscence (n=9), hypertensive events (n=4), pulmonary edema (n=2), anastomotic leak (n=1), arrhythmia (n=1), abdominal migraine (n=1), and other nonspecific adverse events. Table 2 depicts the timing of those adverse events among the reported TAH patients.
Table 1. Adverse events following the implantation of total artificial heart.
| Adverse events | No. (%) | |
|---|---|---|
| Infection | 306 (20.2) | |
| Pulmonary infection | 101 | |
| Bacteremia | 36 | |
| Gastrointestinal infection | 31 | |
| Device-related infection | 25 | |
| Mediastinal infection | 17 | |
| Fungemia | 12 | |
| Non-specified infection | 84 | |
| Device malfunction | 304 (20.1) | |
| Freedom driver-related | 44 | |
| Line-related | 27 | |
| Non-specified/Device thrombosis | 233 | |
| Bleeding events | 249 (16.5) | |
| Respiratory failure | 152 (10.1) | |
| Neurological dysfunction | 132 (8.7) | |
| Cerebrovascular accident | 110 | |
| Non-specified neurological dysfunction | 22 | |
| Renal dysfunction | 114 (7.5) | |
| Hepatic dysfunction | 34 (2.2) | |
| Other thromboembolic events | 27 (1.8) | |
| Venous thromboembolism | 12 | |
| Arterial thromboembolism (non-CVA) | 8 | |
| Non-specified thromboembolism | 7 | |
| Pericardial effusion | 27 (1.8) | |
| Hemolysis | 15 (1.0) | |
| Others | 152 (10.1) | |
| Wound dehiscence | 9 | |
| Hypertensive events | 4 | |
| Pulmonary edema | 2 | |
| Anastomotic leak | 1 | |
| Arrhythmia | 1 | |
| Abdominal migraine | 1 | |
| Non-specified | 134 | |
| Total | 1,512 (100.0) | |
CVA = cerebrovascular accident.
Table 2. Timing of adverse event from total artificial heart implantation.
| Adverse events | Median, day | Interquartile range, day | |
|---|---|---|---|
| Early onset (median 30 days) | |||
| Renal dysfunction | 7 | 17 | |
| Respiratory failure | 9 | 15 | |
| Thromboembolic events | 9 | 45 | |
| Pericardial effusion | 11 | 6 | |
| Neurological dysfunction | 14 | 39 | |
| Bleeding events | 15 | 27 | |
| Infection | 20 | 51 | |
| Hepatic dysfunction | 20 | 20 | |
| Late onset (median >30 days) | |||
| Hemolysis | 92 | 243 | |
| Device malfunction | 95 | 157 | |
Death was reported as an end-result in 49.4% of all the reported patients (n=224) during our study period. Figure 1 depicts the major causes of death in our analysis. The most common cause of death was multiorgan failure (n=73, 32.6%), followed by CVA/other non-specific neurological dysfunction (n=44, 19.7%), sepsis (n=24, 10.7%), withdrawal of support (n=20, 8.9%), device malfunction (n=11, 4.9%), bleeding (n=7, 3.1%), respiratory failure (n=7, 3.1%), gastrointestinal disorder (n=6, 2.7%), and cardiomyopathy (n=3, 1.3%). The other causes of death (n=2, 0.9%) included disseminated intravascular coagulation and pulmonary embolism. Figure 2 demonstrates the annual adverse events reported to MAUDE database from 2012 to 2020.
Figure 1. Causes of death reported to Manufacturers and User Defined Experience after total artificial heart implantation.
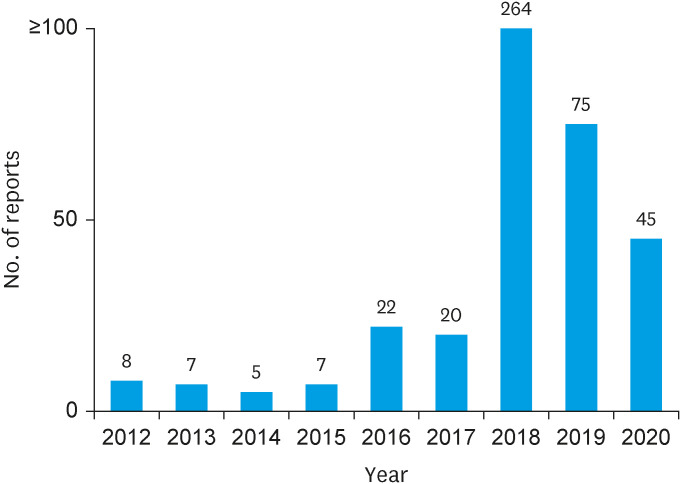
Figure 2. The annual adverse events reported to Manufacturers and User Defined Experience after total artificial heart implantation during the study period.
DISCUSSION
Our study revealed that 1) infection was the most common adverse events reported to the MAUDE database, followed by device malfunction; and 2) death occurred in close to half of the ‘injury and death’ reports, with multiorgan failure and CVA or other neurological dysfunction being the leading causes of death. Our analysis results are in line with the study by Copeland et al.6) and Razumov et al.7) which revealed that infection was the most common adverse events reported and multiorgan failure was the main cause of death post-TAH implantation.
Several studies have shown that post-implantation infection can occur in up to 85% of TAH patients.6,8) Among all the infectious events, pulmonary infection (33%) was the most frequently reported infection. A retrospective study by Roussel et al.8) suggested that the extended stay in the intensive care unit after TAH implantation might predispose the patients to infection, especially pneumonia. A short-term outcome (1-month post-TAH implantation) study by Chambers et al.9) showed that bacteremia and driveline infections were the two most common types of infectious events within one month of TAH implantation. Further research is needed to determine and develop multi-faceted approaches to help lower the infection risk. This may include pharmacological methods like appropriate prophylactic antibiotics and non-pharmacological measures such as minimizing ventilatory support, appropriate selection of TAH recipient, careful respiratory care, and early mobilization.10,11)
The second most common adverse event in our analysis was device malfunction. Device malfunction has been reported commonly after TAH implantation.12,13) The approval of Freedom Portable Driver System by FDA in 2014, a portable device that could provide pneumatic power to TAH, has been associated with many of the device malfunction reports. In our analysis, a change of external drive to a hospital drive was required in many device malfunction reports. An increase in the annual number of device malfunctioning reports especially after 2014 has further validated it. A study by Arabia et al. suggested that the high device malfunction rate within the first two years post-TAH implantation was due to frequent Freedom driver changes.12) Another mechanism of device malfunction proposed by Demondion et al.14) revealed that shear stress on the driveline device during patient mobility could result in air leaks on the pneumatic pipeline. Other relatively uncommon malfunction events include membrane rupture, device thrombosis, and valve dysfunction.8,15,16)
The bleeding event, which has been reported as one of the most common adverse events post-TAH implantation in existing literatures, was the third most common adverse event reported in our analysis.6,16,17) The incidence of bleeding was reported to be 21–62% in several retrospective studies.6,16,17) The common sites of bleeding included intracranial, gastrointestinal, and mediastinal. A study suggested that dysfunction of the liver, renal failure, and anti-coagulant therapy in TAH patients predispose this patient population to bleeding events.18) While some bleeding cases were managed conservatively, a substantial number of cases required surgical intervention.6,16,17)
Death occurred in 49.4% of all the ‘injury and death’ reports. Multiorgan failure was the most common cause of death, which accounted for 32% of total deaths. In a retrospective study by Arabia et al.,12) 80% of the patients were either in critical cardiogenic shock status (INTERMACS profile 1) or in progressive decline status despite the intravenous inotropic support (INTERMACS profile 2) prior to TAH implantation.19) A multicenter study by Carrier et al.13) revealed that up to 20% of liver dysfunction and 32% of renal dysfunction did not improve from TAH support. Study has suggested that poor preoperative status and irreversible preimplant multiorgan failure were responsible for the majority of deaths in TAH recipients.16) Worse preimplant conditions would result in a relatively poorer prognosis.20) With the findings of our analysis where multiorgan failure remained the most common cause of reported deaths, it is thus crucial to select the appropriate TAH recipient prior to implant to optimize the post-implant outcomes. These also emphasize the need for multidisciplinary collaboration for optimum post-implant care, which may include close and frequent monitoring with a lower threshold for other interventions like hemodialysis.
Neurological events (both CVA and non-specific neurological dysfunction) accounted for 20% of the reported deaths in our analysis, and this was the second most common cause of death. Several studies have suggested that CVAs may occur in 7–22.7% of the patients post-TAH implant.12,21) The prevalence of ischemic stroke (7.9–13.5%) was higher as compared to hemorrhagic stroke (7.9–10.2%) among the TAH patients.6,12) It was presumed that constant contact between the bloodstream and the foreign body of SynCardia TAH may predispose a person to a pro-inflammatory state, which subsequently activated the coagulation cascade and thrombus formation. This may increase the bleeding risk and CVA events despite the appropriate use of anticoagulation.22) While there is no definite consensus on antithrombotic regimens among experts, multi-disciplinary and multi-targeted anti-thrombotic therapy has been suggested to mitigate the prothrombotic state of TAH recipients and reduce the risk of CVA.23,24,25)
The TAH remains a bridge to heart transplantation given the limited number of available heart donors.26) While there have been studies suggesting the predictors such as baseline functional status, neurological disease, and dependence on a ventilator which affected the risk of mortality after TAH implantation,5,27) a careful and more individualized patient selection prior to TAH implantation is crucial to improve the procedural outcomes and patient survival.28)
Firstly, the MAUDE database is a collection of voluntarily (albeit mandatory) reported data on procedures performed with specific devices, therefore the data collected are likely to be incomplete, and the actual “denominator” is unknown, which limits our exploration in the incidence of complications reported. Second, the reporting bias is likely to be present, with the life-threatening adverse events being reported more frequently as compared to the minor adverse events, which might be underreported to the FDA’s MAUDE database. In addition, the adverse events might be underreported to a certain degree due to passive method of adverse events surveillance, and data on the individual level such as patients’ comorbidities and TAH implant center are not available, which limits our understanding in the causal relationship of these adverse events. Data on the timing of adverse event from TAH implantation was also not consistently available in all the reports. Lastly, there is a risk of over-reporting adverse events because the exclusion of duplicates cannot always be reliable due to the lack of identifiers such as personal health information and serial number.
Our study described the common adverse events and causes of death among the patients who received the SynCardia TAH devices. The two most common adverse events reported to MAUDE after TAH implantation were infection and device malfunction. Death occurred in 49.4% of all the reported patients, with multiorgan failure and CVA or other neurological dysfunction being the leading causes of death. Early recognition and management of possible adverse events after the TAH implant are important to improve procedural outcomes and post-implantation survival.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Lee JZ.
- Data curation: Tan MC, Tham JW, Lee JZ.
- Supervision: Tan MC, Lee JZ.
- Validation: Lee JZ.
- Visualization: Tan MC.
- Writing - original draft: Tan MC, Yeo YH, Tham JW, Tan JL, Fong HK, Tan BE, Lee KS, Lee JZ.
- Writing - review & editing: Tan MC, Tham JW, Fong HK, Tan BE, Lee KS, Lee JZ.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Metra M, Ponikowski P, Dickstein K, et al. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9:684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2016 annual data report: heart. Am J Transplant. 2018;18(Suppl 1):291–362. doi: 10.1111/ajt.14561. [DOI] [PubMed] [Google Scholar]
- 4.Bakhtiyar SS, Godfrey EL, Ahmed S, et al. Survival on the heart transplant waiting list. JAMA Cardiol. 2020;5:1227–1235. doi: 10.1001/jamacardio.2020.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyan GN, Huckaby LV, Diaz-Castrillon CE, Miguelino AM, Kilic A. Trends and outcomes following total artificial heart as bridge to transplant from the UNOS database. J Card Surg. 2022;37:1215–1221. doi: 10.1111/jocs.16329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copeland JG, Copeland H, Gustafson M, et al. Experience with more than 100 total artificial heart implants. J Thorac Cardiovasc Surg. 2012;143:727–734. doi: 10.1016/j.jtcvs.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Razumov A, Deutsch M, Lauenroth V, et al. Biventricular support with SynCardia total artificial heart: long-term single-center experience. J Heart Lung Transplant. 2020;39(Suppl):S336–S337. [Google Scholar]
- 8.Roussel JC, Sénage T, Baron O, et al. CardioWest (Jarvik) total artificial heart: a single-center experience with 42 patients. Ann Thorac Surg. 2009;87:124–129. doi: 10.1016/j.athoracsur.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Chambers HE, Pelish P, Qiu F, Florescu DF. Perioperative prophylaxis for total artificial heart transplantation. Transplant Proc. 2017;49:2169–2175. doi: 10.1016/j.transproceed.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Seidelman JL, Mantyh CR, Anderson DJ. Surgical site infection prevention: a review. JAMA. 2023;329:244–252. doi: 10.1001/jama.2022.24075. [DOI] [PubMed] [Google Scholar]
- 11.Zukowska A, Zukowski M. Surgical site infection in cardiac surgery. J Clin Med. 2022;11:6991. doi: 10.3390/jcm11236991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arabía FA, Cantor RS, Koehl DA, et al. Interagency registry for mechanically assisted circulatory support report on the total artificial heart. J Heart Lung Transplant. 2018;37:1304–1312. doi: 10.1016/j.healun.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Carrier M, Moriguchi J, Shah KB, et al. Outcomes after heart transplantation and total artificial heart implantation: a multicenter study. J Heart Lung Transplant. 2021;40:220–228. doi: 10.1016/j.healun.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Demondion P, Fournel L, Niculescu M, Pavie A, Leprince P. The challenge of home discharge with a total artificial heart: the La Pitie Salpetriere experience. Eur J Cardiothorac Surg. 2013;44:843–848. doi: 10.1093/ejcts/ezt146. [DOI] [PubMed] [Google Scholar]
- 15.Torregrossa G, Morshuis M, Varghese R, et al. Results with SynCardia total artificial heart beyond 1 year. ASAIO J. 2014;60:626–634. doi: 10.1097/MAT.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 16.El-Banayosy A, Arusoglu L, Morshuis M, et al. CardioWest total artificial heart: Bad Oeynhausen experience. Ann Thorac Surg. 2005;80:548–552. doi: 10.1016/j.athoracsur.2005.02.084. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen A, Pellerin M, Perrault LP, et al. Experience with the SynCardia total artificial heart in a Canadian centre. Can J Surg. 2017;60:375–379. doi: 10.1503/cjs.003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copeland J, Copeland H, Nolan P, Gustafson M, Slepian M, Smith R. Results with an anticoagulation protocol in 99 SynCardia total artificial heart recipients. ASAIO J. 2013;59:216–220. doi: 10.1097/MAT.0b013e318288a390. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28:535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Shah KB, Thanavaro KL, Tang DG, et al. Impact of INTERMACS profile on clinical outcomes for patients supported with the total artificial heart. J Card Fail. 2016;22:913–920. doi: 10.1016/j.cardfail.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KE, Prieto M, Joyce LD, Pritzker M, Emery RW. Summary of the clinical use of the Symbion total artificial heart: a registry report. J Heart Lung Transplant. 1992;11:103–116. [PubMed] [Google Scholar]
- 22.Joyce LD, Joyce DL. Total artificial heart: neurological complications. Ann Cardiothorac Surg. 2020;9:121–123. doi: 10.21037/acs.2020.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ensor CR, Cahoon WD, Crouch MA, et al. Antithrombotic therapy for the CardioWest temporary total artificial heart. Tex Heart Inst J. 2010;37:149–158. [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez A, Riley JB, Joyce LD. Multi-targeted antithrombotic therapy for total artificial heart device patients. J Extra Corpor Technol. 2016;48:27–34. [PMC free article] [PubMed] [Google Scholar]
- 25.Volod O, Lam LD, Arabia FA. Antithrombotic therapy for patients with total artificial hearts. Ann Cardiothorac Surg. 2020;9:110–112. doi: 10.21037/acs.2019.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsich EM. Matching the market for heart transplantation. Circ Heart Fail. 2016;9:e002679. doi: 10.1161/CIRCHEARTFAILURE.115.002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramu B, Cogswell RJ, Reding MT, John R, Martin CM. Plasma exchange to remove heparin-induced thrombocytopenia antibodies and the use of heparin during cardiopulmonary bypass in critically ill cardiac patients. J Heart Lung Transplant. 2018;37:1038–1040. doi: 10.1016/j.healun.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Senage T, David CH, Nanjaiah P, Roussel JC. Total artificial heart: patient selection and risk factors. Ann Cardiothorac Surg. 2020;9:118–120. doi: 10.21037/acs.2020.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]