Abstract
Hydrolytic exoenzymes as indicators of metabolically active bacteria were investigated in four consecutive sapropel layers collected from bathyal sediments of the eastern Mediterranean Sea. For comparison, the organic carbon-poor layers between the sapropels, sediment from the anoxic Urania basin, and sediments of intertidal mud flats of the German Wadden Sea were also analyzed. The sapropel layers contained up to 1.5 · 108 bacterial cells cm−3, whereas cell numbers in the intermediate layers were lower by a factor of 10. In sapropels, the determination of exoenzyme activity with fluorescently labeled substrate analogues was impaired by the strong adsorption of up to 97% of the enzymatically liberated fluorophores (4-methylumbelliferone [MUF] and 7-amino-4-methylcoumarin [MCA]) to the sediment particles. Because all established methods for the extraction of adsorbed fluorophores proved to be inadequate for sapropel sediments, we introduce a correction method which is based on the measurement of equilibrium adsorption isotherms for both compounds. Using this new approach, high activities of aminopeptidase and alkaline phosphatase were detected even in a 124,000-year-old sapropel layer, whereas the activity of β-glucosidase was low in all layers. So far, it had been assumed that the organic matter which constitutes the sapropels is highly refractory. The high potential activities of bacterial exoenzymes indicate that bacteria in Mediterranean sapropels are metabolically active and utilize part of the subfossil kerogen. Since a high adsorption capacity was determined not only for the low-molecular-weight compounds MUF and MCA but also for DNA, the extraordinarily strong adsorption of structurally different substrates to the sapropel matrix appears to be the major reason for the long-term preservation of biodegradable carbon in this environment.
Deep-sea sediments of the eastern Mediterranean Sea are characterized by the cyclic occurrence of dark, more than 1-cm-thick sediment layers with high organic carbon contents (>2% [wt/wt] total organic carbon [TOC]) (32). These so-called sapropel layers are embedded in light-gray to brown hemipelagic carbonate oozes which are poor in TOC (<0.5% [wt/wt]). Sapropel formation has been related to increased influxes of freshwater, which in turn was caused by changes in the climate of the northern hemisphere (28, 38). The resulting isolation and subsequent anoxia of deep Mediterranean bottom water (49, 54, 55, 57) and/or increased marine primary production (16, 17) may have caused the enhanced organic carbon preservation in the sapropels.
The total numbers of bacterial cells in sapropel layers are significantly enhanced compared to those in neighboring carbon-lean sediment layers. Dividing and divided bacterial cells have been detected even in 4.7-million-year-old sapropels and make up 10% of total cell numbers (23). Furthermore, porewater sulfate is enriched in 34S with respect to modern Mediterranean seawater (14). These observations indicate the presence of metabolically active bacteria in the sapropel layers. Physiologically active bacterial communities have been detected in diverse subsurface environments, e.g., >750 m below the sea floor in marine sediments (5, 48), in continental aquifer sediments (33, 36), in 230-million-year-old sandstone (62), and in continental petroleum reservoirs (37). Deep crystalline rock aquifers contain only traces of organic matter and harbor lithoautotrophic communities in which autotrophic methanogenesis predominates (60). Sulfate reduction has been demonstrated in samples from deep marine sediments (48) and deep subsurface sandstone (36). Sulfate-reducing bacteria have been isolated from deep marine sediments (5); a wider variety of physiological types was isolated from some continental deep subsurface environments (4). However, little is known about the physiological activity of the bacteria in situ and the origins of their substrates (12, 36). This is especially true for eastern-Mediterranean sapropels.
Sapropels differ from many other subsurface environments in that they contain high concentrations of TOC (up to 30.5% [dry weight]) (23). The organic matter in the sapropels is up to 5.3 million years old (25) and consists mainly of dark-brown amorphous kerogen (1). The estimated burial efficiencies for sapropel organic matter are high, ranging between 20 and 80% (50, 51). Isotopic and geochemical tracers indicate that this organic matter is of marine origin and was predominantly formed by prymnesiophytan (coccolithophoridan) and eustigmatophycean algae and—in some cases—diatoms (15, 25). Apart from the indirect evidence for postburial sulfate reduction, nothing is known of the degradation potential and the actual physiological state of natural bacterial communities in the subfossil sapropels.
A sensitive method for the detection of active microbial communities in natural environments is the analysis of exoenzyme activities using fluorescently labeled substrate analogues. From such measurements, the affinity and potential rate of extracellular hydrolysis of biopolymers can be inferred. Some exoenzymes, like alkaline phosphatase and β-glucosidase, are subject to substrate induction and catabolite repression (20). Therefore short-term measurements of the cell-specific hydrolysis rates of these exoenzymes also provide information on the actual availability of bacterial carbon substrates (20, 21, 22, 45). The present communication describes a method for measurement of exoenzyme activities in sapropels. The activities detected in the 124,000-year-old sapropels provide conclusive evidence for the presence of active bacteria in the subfossil sapropel layers.
MATERIALS AND METHODS
Sampling and sample preparation.
Gravity cores (average length, 5 m) were obtained between January 22 and 30, 1998, at three locations in the eastern Mediterranean during the R/V Meteor cruise, leg 40/4 (Fig. 1A and Table 1). The core obtained at station 66 contained two sapropels (S1 and S3); this sapropel material was used for method development. The second core, from station 69, contained four different sapropels (S1, S3, S4, and S5 [according to the numbering system in reference 57). From the latter core, the top of the sediment (Z0; 4 to 6 cm below the surface), each sapropel layer (S1, 25 to 27 cm; S3, 281 to 283 cm; S4, 337 to 339 cm; and S5, 387 to 389 cm below the surface), and three intermediate hemipelagic layers between the sapropels (denoted Z1, 89 to 91 cm; Z3, 304 to 306 cm; and Z4, 356 to 358 cm below the surface) were sampled. A third core was obtained with a multicorer in the hypersaline Urania basin, station 76, located between the crest of the Mediterranean Ridge and the Matapan Trench (40). The top 4 cm of surface sediment was used for measurements.
FIG. 1.
Location of sampling sites in the eastern Mediterranean (A) and in the German Wadden Sea (B). The asterisks denote sampling points. Station numbers in the eastern Mediterranean are given in italics.
TABLE 1.
Characteristics of sediment samples
| Sample designation | Sampling date (day.mo.yr) | Sampled layera | Ageb | Water depth (m) |
|---|---|---|---|---|
| Dangast | 17.11.97 | 0.035–0.085 | 0–1c | |
| Schillig sand | 17.11.97 | 0–0.05 | 0–1c | |
| Schillig clay | 17.11.97 | 0.06–0.11 | 0–1c | |
| M40/4-66-4SL | 22.01.98 | 3.34–3.51 (S3) | 81 | 550 |
| M40/4-69-2SL | 24.01.98 | 0.04–0.06 (Z0) | 2,150 | |
| 0.25–0.27 (S1) | 8 | |||
| 0.89–0.91 (Z1) | ||||
| 2.81–2.83 (S3) | 81 | |||
| 3.04–3.06 (Z3) | ||||
| 3.37–3.39 (S4) | 102 | |||
| 3.56–3.58 (Z4) | ||||
| 3.87–3.89 (S5) | 124 | |||
| M40/4-76-2MC (Urania basin) | 30.01.98 | 0–0.04 | 3,515 |
Each core was cut longitudinally, which left behind a potentially contaminated surface. In order to collect samples aseptically, the surface was covered first with Saran Wrap and then by a layer of powdered dry ice. After 5 min of incubation, the underlying 0.5-cm-thick portion of the sediment was frozen and could easily be lifted off with a sterile scalpel, leaving behind an uncontaminated area. Through this area, 5-cm3 subsamples were retrieved with sterile plastic syringes which had their ends cut off. A total of 30 cm3 of each layer was aseptically transferred into a sterile 100-ml serum flask, and 30 ml of filtered (0.2-μm pore size; Sartorius, Göttingen, Germany), autoclaved synthetic seawater was added. The synthetic seawater contained (per liter) NaCl, 24.3 g; MgCl2 · 6H2O, 10 g; CaCl2 · 2H2O, 1.5 g; KCl, 0.66 g; Na2SO4, 4 g; KBr, 0.1 g; H3BO3, 25 mg; SrCl2 · 6H2O, 40 mg; NH4Cl, 21 mg; KH2PO4, 5.4 mg; NaF, 3 mg; trace element solution SL12 (47), 1 ml; and selenite-tungstate solution (64), 1 ml. The pH was maintained at 7.3 by buffering the seawater with HEPES (10 mM).
The headspace of each slurry was immediately flushed with N2 for 3 min in order to create an anoxic atmosphere. The samples were stored at 4°C until the measurements of exoenzyme activities, which were performed on board within 48 h after sampling.
Cores from intertidal mudflats of the German Wadden Sea in Jade Bay near Dangast (53°27′N, 8°07′E) and Schillig (53°42′N, 8°01′E) (Fig. 1B and Table 1) were obtained in November 1997. The cores were sampled with 25-cm-long, 4.5-cm-diameter plexiglass tubes. The silty Dangast sediment was homogenous and black. The sediment from Schillig consisted of a light-brown 5-cm-thick sandy top layer overlying a gray silty bottom sediment. After transport to the laboratory, subsamples of each type of sediment were taken aseptically, and sediment slurries were prepared using the methods described above.
Development of a method for the determination of exoenzyme activities in sapropels.
Because we observed a strong adsorption of free fluorophores, we initially determined the time required to reach adsorption equilibrium for the two fluorophores 4-methylumbelliferone (MUF) and 7-amino-4-methylcoumarin (MCA). A series of slurries was prepared for each sediment type (sapropels, intermediate layers, and intertidal sediments), and MUF or MCA was added to a final concentration of 37 or 7.5 μM, respectively. At regular intervals between 5 and 480 min after the addition, a subset of three samples was centrifuged (5 min at 10,000 × g), and the supernatants were transferred to fresh Eppendorf tubes. The pHs of samples containing MUF were increased to 11 by the addition of NaOH (final concentration, 40 mM). Precipitation of carbonates was prevented by the addition of Na4EDTA (1.7 M; final concentration, 0.1 M). Initial experiments demonstrated that EDTA did not affect the fluorescence intensities of the samples. The concentrations of free fluorophores in the supernatant were determined by fluorometry (RF1501 spectrofluorometer; Shimadzu, Duisburg, Germany) at an excitation wavelength of 360 nm and an emission wavelength of 450 nm. For calibration, MUF and MCA standards in artificial seawater at concentrations of 10 to 2,500 nM were used. The amount of adsorbed fluorophore was calculated as the difference between the total amount added and the amount remaining in the supernatant.
At equilibrium, the amount of a substance adsorbed {S; in nanomoles · (gram [dry weight])−1} depends on the concentration of the substance remaining in solution (Ce; in nanomoles milliliter−1) according to the Freundlich equation (44):
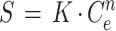 |
1 |
Here, K is the affinity coefficient {in milliliters · (gram [dry weight])−1} and n is a dimensionless exponent. S was calculated from the total concentration of fluorophore added, CT (in nanomoles milliliter−1), and the dry weight content of the slurry, DSlurry (in grams [dry weight] · milliliter−1).
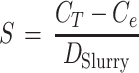 |
2 |
Equilibrium adsorption isotherms were determined by adding five different concentrations of MUF or MCA to a set of slurry aliquots of the different sediment types. After an incubation time of 8 h, the concentrations of free fluorophores were measured in the supernatant, and the amount of fluorophores adsorbed per gram (dry weight) of sediment was calculated. Equation 1 was then fitted to the data points using the nonlinear-regression tool of SigmaPlot 5.0 (Jandel Scientific, Erkrath, Germany), which yielded the two parameters K and n. For comparative purposes, we also measured the equilibrium adsorption isotherms of MUF and MCA with montmorillonite and cellulose as adsorbers.
The total concentration of fluorophore, CT, liberated during the exoenzyme assays (see below) could be calculated from the equilibrium concentrations (Ce) of MUF or MCA in the supernatants of the sediment slurries and from the values of the parameters K, n, and DSlurry determined for each type of sediment:
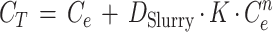 |
3 |
Comparison with established methods.
Our new method for the quantification of exoenzyme activities was compared with established methods, which rely on a quantitative desorption of the bound fluorochromes from the sediment matrix (6, 13). Duplicate samples of sediment slurries were first incubated for 8 h with MUF (final concentration, 10 μM) in order to load the sediment with fluorochromes. A third aliquot was incubated without MUF as a control for autofluorescence. In one series of experiments, 0.5 ml of sterile synthetic seawater and EDTA (final concentration, 0.1 M; in order to prevent a subsequent precipitation of carbonates) was added to the slurries. For desorption, NaOH was added to a final concentration of 90 mM, and the samples were stirred for 20 min. Subsamples (1.5 ml) were centrifuged (5 min; 10,000 × g) in a microcentrifuge, and the concentration of liberated MUF was measured fluorophotometrically as described above. In a second series of desorption experiments, 0.5 ml of synthetic seawater and ethanol (final concentration, 80% [vol/vol]) was added to the slurries after they were loaded with MUF. Following centrifugation, EDTA (final concentration, 0.1 M) and NaOH (final concentration, 0.04 N) were added to the supernatants, and the fluorescence was measured. A third series of sediment slurries was incubated for 8 h with MCA at a final concentration of 8 μM, again leaving a parallel devoid of MCA as a control for autofluorescence. After incubation, 0.5 ml of synthetic seawater and acetone (final concentration, 10% [vol/vol]) was added, the samples were centrifuged, and the fluorescence in the supernatant was determined.
Michaelis-Menten kinetics of exoenzymes in natural sediments.
Our newly established correction method was used to determine the Michaelis-Menten kinetics of the hydrolytic exoenzymes alkaline phosphatase (EC 3.1.3.1), β-glucosidase (EC 3.1.21), and leucine aminopeptidase (EC 3.4.1.1). Alkaline phosphatase and β-glucosidase were assayed with MUF-phosphate and MUF-β-d-glucoside (Sigma, Deisenhofen, Germany). Aminopeptidase activity was measured with MCA-labeled leucine (Sigma). Since the hydrolytic activities of aminopeptidase and β-glucosidase in up to 4,920-m-deep sediments have been demonstrated to be independent of hydrostatic pressure (52), we did not use in situ pressure during the incubation of exoenzyme assays.
Duplicate 1.4-ml aliquots of the sediment slurry were transferred to autoclaved 10-ml serum vials containing stirring bars. The enzymatic reaction was started by the addition of 75 μl of substrate analogue solution to yield final concentrations of 5, 12, 24, 60, and 120 μM. Each vial was sealed with a sterile butyl rubber stopper and flushed with N2 for 1 min. During incubation, all slurries were stirred at 225 rpm and incubated at a temperature of 15°C (the in situ temperature). Sapropel sediments and hemipelagic samples of cores 69 and 76 were incubated for 8 h. Samples from intertidal mudflat sediments had to be incubated for only 30 min (alkaline phosphatase), 140 min (β-glucosidase), and 85 min (aminopeptidase) due to the significantly higher enzyme activities.
For each concentration, three different blanks (B1, B2, and B3) were incubated in parallel. One blank (B1), used to assess nonenzymatic hydrolytic cleavage of the substrate analogues, was boiled for 30 min prior to incubation in order to inhibit the enzyme. This method of inactivation was chosen because of the adsorptive capacities of the sapropels. Mercuric chloride fails to block exoenzyme activity in sediments or in aquatic samples containing particulate material (6, 19). Similarly, various detergents, denaturing agents, and organic solvents do not completely inhibit aminopeptidase activity (6). However, boiling sediment slurries prior to the addition of substrate represents a reliable control for abiotic cleavage of fluorogenic substrates in various samples (6, 30, 34). In order to correct for the fluorescent compounds released from the sediments during boiling, a second blank (B2) was incubated without substrate analogues. The fluorescence caused by the compounds extracted from the sediments without boiling was determined in a third blank (B3) which was also incubated without substrate analogues. After incubation, the slurries were transferred to microcentrifuge tubes and centrifuged for 5 min at 10,000 × g, and the concentrations of free dissolved fluorophores were determined fluorometrically as detailed above. Using this new approach, the detection limit for the concentration of free fluorophores was 0.024 nmol · ml−1 for the intermediate layers and 0.12 nmol · ml−1 for the sapropel layers as a result of the higher autofluorescence in these samples.
The resulting Michaelis-Menten plot was linearized by the formula of Wright and Hobbie (65), and the maximum enzyme activity, Vmax (in nanomoles centimeter−3 sediment hour−1), and Km + Sn (the sum of the half saturation constant plus the concentration of natural substrate [in micromolar units]) were estimated:
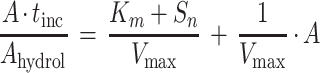 |
4 |
Here, tinc is the incubation time (in hours) and Ahydrol is the concentration of substrate analogue enzymatically hydrolyzed during the incubation; A denotes the total concentration of substrate analogue added.
Vertical distribution of exoenzyme activities.
Because of the limited amount of sapropel material which was available from core 69, only exoenzyme activities at saturating substrate concentrations were determined for the vertical series of sapropel layers. Based on the Michaelis-Menten kinetics determined in sapropel S3 of core 66 and in the Dangast and Schillig sediments (see Table 4), a final concentration of 250 μM was chosen for the substrate analogues. For each sample, measurements were performed in duplicate and included the three blanks (B1, B2, and B3; see above). The rates of enzymatic hydrolysis were calculated from the equilibrium concentrations of the free fluorophores and from the adsorption isotherms for MUF and MCA in the respective sediment material (equation 3).
TABLE 4.
Estimate of the half saturation constant, Km + Sn, and of the maximum velocity, Vmax, of the three hydrolytic enzymes determined for the different sediment types
| Sediment type | Alkaline
phosphatase
|
β-Glucosidase
|
Aminopeptidase
|
|||
|---|---|---|---|---|---|---|
| Km + Sn (μM) | Vmax (nmol · cm−3 · h−1) | Km + Sn (μM) | Vmax (nmol · cm−3 · h−1) | Km + Sn (μM) | Vmax (nmol · cm−3 · h−1) | |
| Dangast | 26.8 ± 5.8 | 115.3 ± 12.0 | 21.1 ± 6.8 | 20.6 ± 1.9 | 26.2 ± 11.1 | 6.1 ± 1.1 |
| Schillig sand | 23.0 ± 20.7 | 55.4 ± 15.3 | 53.0 ± 16.6 | 16.6 ± 2.8 | 42.9 ± 7.0 | 86.0 ± 25.7 |
| Schillig clay | 20.6 ± 16.4 | 15.1 ± 3.7 | 12.1 ± 2.0 | 5.1 ± 0.2 | 36.8 ± 12.8 | 33.1 ± 7.5 |
| Sapropel S3 core 66 | 27.5 ± 5.2 | 1.7 ± 0.1 | NDa | ND | ND | ND |
ND, not determined.
Acridine orange direct cell count.
For staining of sediment samples, screw-cap glass tubes (22 ml) containing one glass bead each were heat sterilized at 160°C for 4 h. Sediment slurries (1:10 dilution) were prepared with sterile filtered (0.1-μm pore size; Sartorius) artificial sea water, and 5 μl of the slurries was added to 9.9 ml of a sterile filtered (0.1-μm pore size) formaldehyde solution (4% [vol/vol] in artificial seawater). After the mixture was vortexed for 1 min, 0.1 ml of sterile filtered (0.1-μm pore size) acridine orange staining solution (200 μg · ml−1) was added. Staining proceeded for 10 min in the dark, after which the entire sample was filtered with a black polycarbonate filter (0.2-μm pore size; Millipore, Eschborn, Germany). Bacterial cells were counted by epifluorescence microscopy (Zeiss Axiolab, Jena, Germany) at a magnification of ×1,000. As a contamination control, sterile filtered and autoclaved artificial seawater was used instead of sediment slurries.
Most probable numbers of chemoorganotrophic bacteria.
Plate counts of aerobic chemoorganoheterotrophic bacteria were performed on CPSm agar. This medium contains (per liter of artificial seawater [see above]) casein peptone, 0.5 g; Bacto Peptone, 0.5 g; soluble starch, 0.5 g; glycerol, 1 ml; SL12, 1 ml; selenite-tungstate solution, 1 ml; and washed agar, 15 g. The pH was set to 7.4.
The most probable numbers of anaerobic chemotrophic bacteria were determined in liquid dilution series which were set up in polystyrene microtiter plates. All manipulations were carried out within an anaerobic chamber on board the research vessel. The medium MM consisted of artificial seawater containing a suite of carbon substrates (glucose, cellobiose, N-acetyl glucosamine, glycerol, pyruvate, 2-oxoglutarate, succinate, fumarate, formate, acetate, propionate, butyrate, ethanol, mannitol, salicylate, dimethyl sulfide, alanine, arginine, asparagine, isoleucine, cysteine, tyrosine, and glutamine; final concentrations, 200 μM each), as well as soluble starch, 0.1% (wt/vol); Tween 80, 0.01% (wt/vol); SL10 (64), 1 ml · liter−1; selenite-tungstate solution, 1 ml · liter−1; and 10-vitamin solution (3), 10 ml · liter−1. The liquid medium was reduced by the addition of sulfide to a final concentration of 400 μM, and the pH was set to 7.4. After inoculation, the microtiter plates were transferred to incubation bags containing an oxygen removal and CO2 generation system (Anaerocult C mini; Merck, Darmstadt, Germany). The microtiter plates were incubated at a temperature of 20°C for 3 months.
After incubation, each well was monitored for bacterial growth. Subsamples (20 μl) were transferred to a 96-well dot blot apparatus containing one sheet of black polycarbonate filter membrane (0.2-μm pore size; Millipore). To each well, 180 μl of sterile filtered (0.1-μm pore size) autoclaved water and 10 μl of sterile filtered acridine orange staining solution were added. After 10 min of incubation in the dark, the acridine orange-stained microtiter plate samples were blotted onto the polycarbonate membrane, and each dot was screened for the presence of bacterial cells by epifluorescence microscopy at a magnification of ×1,000.
TOC in sediments.
For carbon analyses, all sediment samples were ground in a bead mill. Total carbon was measured with an elementary analyzer (SC444; Leco, Kirchheim, Germany), inorganic carbon was measured with a CO2-coulometer (CM 5012; UIC Coulometrics, Joliet, Ill.), and organic carbon in the sediments was calculated as the difference. The water content was determined by weighing each type of sediment before and after drying it for 24 h at a temperature of 70°C, after which the sediments had reached a constant weight.
RESULTS
Adsorption kinetics.
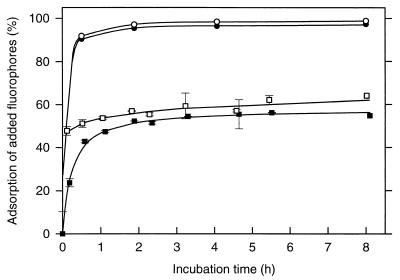
Initially, we determined the time course for the adsorption of MUF and MCA to the different types of sediments. The adsorption of both fluorophores reached saturation as early as after 1 h of incubation (Fig. 2). After this time interval, 95.4% of the MUF and 97.1% of the MCA which had been added to the sapropel sediment slurries had disappeared from the dissolved phase. In contrast, only 52% of the MUF and 59% of the MCA adsorbed to Dangast sediments (Fig. 2). Since the measurement of exoenzyme activities in sapropels requires an incubation time of 8 h (see Materials and Methods), the major fraction of the liberated fluorophores will rapidly adsorb to the sapropel matrix concomitant to their enzymatic liberation from the substrate analogues and thus will not be detectable at the end of the experiments.
FIG. 2.
Time course of fluorophore adsorption in slurries of sapropel S3 of core 66 (● and ○) and of Dangast sediment (■ and □). For both sediments, the adsorption kinetics of MUF (● and ■; final concentration, 36 μM) and of MCA (○ and □; final concentration, 7.5 μM) are shown. The error bars indicate standard deviations.
Equilibrium adsorption isotherms of fluorophores.
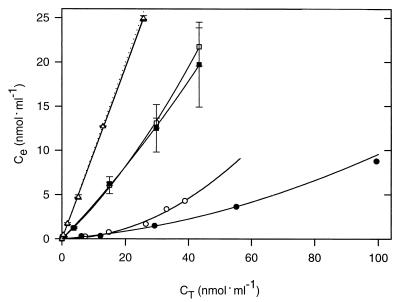
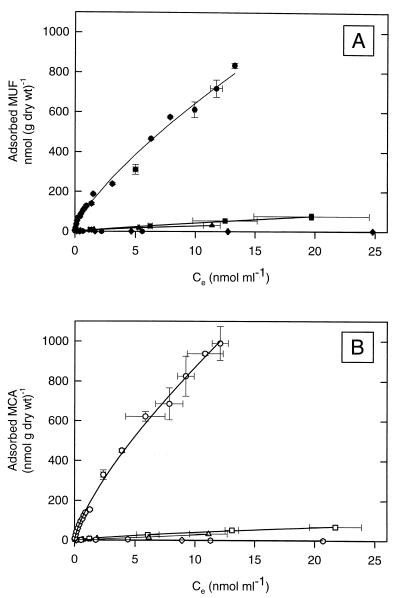
For each type of sediment, MUF or MCA was added at five different concentrations and the equilibrium concentrations in solution were determined (Fig. 3). The adsorption of fluorochromes to sediments from Dangast and Schillig and to the carbonaceous intermediate sediment layers from the eastern Mediterranean was significantly lower than the absorption to the sapropels (compare Fig. 3 and 4). The equilibrium adsorption isotherms of fluorophores for sapropel S3 of core 66 followed the Freundlich adsorption model (equation 1) rather than Langmuir adsorption kinetics (44). The adsorptive affinity, K, of MUF to sapropel S3 was (69.5 ± 7.9) ml · g−1 and thus was comparable to that of pure montmorillonite (Table 2). However, the affinity of sapropel material for MCA (37.4 ± 3.3 ml · g−1) was lower than that for MUF.
FIG. 3.
Concentrations of dissolved fluorophores in sediment slurries of sapropel S3 (● and ○), Dangast sediment (■ and □), and Schillig surface sediment (▴ and ▵) after 8 h of incubation. The solid symbols represent adsorption of MUF, and the open symbols represent that of MCA. The error bars indicate standard deviations. The concentrations of free fluorophores expected in the absence of adsorption are indicated by the dotted line.
FIG. 4.
Equilibrium adsorption isotherms of MUF (A) and MCA (B) for each type of sediment fitted to the Freundlich equation (lines). Sapropel S3, core 66 (● and ○); Dangast sediment (■ and □), Schillig silty bottom sediment (▴ and ▵), Schillig sandy top sediment (⧫ and ◊), and the top layer, Z0, of core 66 ( and ) are shown. The error bars indicate standard deviations.
TABLE 2.
Parameters of the Freundlich equilibrium adsorption isotherms for fluorophores and different adsorbers
| Adsorber | Fluorophore | n | K (ml · g−1) | r2 |
|---|---|---|---|---|
| Sapropel S3 core 66 | MUF | 0.54 ± 0.05 | 69.45 ± 7.85 | 0.987 |
| Sapropel S3 core 69 | MUF | 0.76 ± 0.03 | 59.63 ± 3.16 | 0.998 |
| Hemipelagic Z0 core 69 | MUF | 1.05 ± 0.10 | 0.268 ± 0.049 | 0.853 |
| Dangast | MUF | 0.85 ± 0.03 | 5.95 ± 0.55 | 0.999 |
| Schillig silt | MUF | 1.11 ± 0.20 | 1.94 ± 0.85 | 0.960 |
| Schillig sand | MUF | 1.97 ± 0.98 | 0.002 ± 0.008 | 0.859 |
| Mg2+-montmorillonite | MUF | 0.69 ± 0.02 | 73.54 ± 7.60 | 0.998 |
| Mg2+-cellulose | MUF | 0.95 ± 0.04 | 0.24 ± 0.01 | 0.997 |
| Sapropel S3 core 66 | MCA | 0.60 ± 0.07 | 37.36 ± 3.26 | 0.978 |
| Hemipelagic Z0 core 69 | MCA | 0.91 ± 0.05 | 1.64 ± 0.07 | 0.998 |
| Dangast | MCA | 0.72 ± 0.04 | 7.68 ± 0.86 | 0.997 |
| Schillig silt | MCA | 0.68 ± 0.04 | 5.91 ± 0.48 | 0.996 |
| Schillig sand | MCA | 0.94 ± 0.52 | 0.050 ± 0.080 | 0.738 |
| Mg2+-montmorillonite | MCA | 1.07 ± 0.09 | 367.9 ± 68.09 | 0.993 |
| Mg2+-cellulose | MCA | 1.64 ± 0.17 | 15.06 ± 1.20 | 0.985 |
Desorption experiments.
MUF which has been adsorbed to estuarine sediments has been shown to be completely extracted with 90 mM NaOH. As an alternative extraction method, 80% ethanol has been used successfully to recover MUF adsorbed to a freshwater sediment rich in organic carbon (13). Adsorbed MCA can be desorbed with 10% acetone (6). For comparison with our newly established method, we assessed the efficiencies of the three extraction methods described above with slurries of sapropel S3 and with intertidal sediments from Dangast.
Treatment with 90 mM NaOH resulted in a complete extraction of MUF from the particulates in Dangast sediment, but only 34% of the MUF was desorbed from the sapropel sample (Table 3). Treatment with 80% ethanol increased the recovery of MUF, but the desorption was unsatisfactory (54%). Similarly, the efficiency of extraction of MCA with diluted acetone was high (83%) for Dangast sediment. However, this method was not suitable for sapropel slurries, from which only 16% of the MCA could be liberated. A second drawback of the extraction method was the large amounts of autofluorescent compounds which were concomitantly extracted from the sapropel matrix. This autofluorescence interferes with the fluorometric quantification of MUF or MCA.
TABLE 3.
Efficiency of established extraction methods in liberating adsorbed fluorophores from two types of sedimentsa
| Sediment sample | % MUF desorbed
|
% MCA desorbed
|
|||
|---|---|---|---|---|---|
| With 90 mM NaOH | With 80% ethanol | Supernatant without extraction | With 20% (vol/vol) acetone | Supernatant without extraction | |
| Sapropel S3 M40/4-66-4SL | 34.1 ± 1.5 | 54.2 ± 5.7 | 5.7 ± 0.1 | 16.1 ± 0.4 | 3.8 ± 0.5 |
| Dangast | 100.9 ± 1.9 | 64.2 ± 1.9 | 36.7 ± 2.1 | 82.6 ± 0.5 | 7.9 ± 0.4 |
The fluorescence measured in the supernatants of the different treatments was corrected for the autofluorescence of sediment compounds liberated during incubation.
Obviously, the efficiency of extraction by NaOH, ethanol, or acetone depends critically on the composition of the sediment, and an alternative method is required for the quantification of exoenzyme activities in sediments containing large amounts of kerogen, like the sapropels from the eastern Mediterranean. For this reason we established and applied a correction procedure based on equilibrium adsorption isotherms for the two fluorochromes.
Michaelis-Menten kinetics of the three exoenzymes.
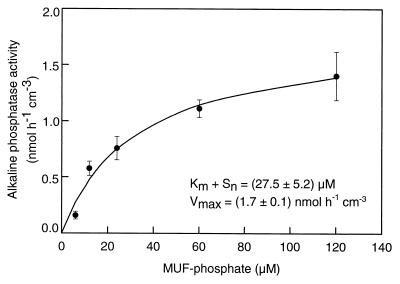
Because of the limited amount of core material available, the saturation characteristics of only one enzyme, alkaline phosphatase, could be determined for sapropel material (Fig. 5). For the other two exoenzymes, the maximum rates were measured. Alkaline phosphatase in sapropel S3 exhibited a Km + Sn of 27.5 μM, which is in the same range as the values determined for the other sediments (Table 4).
FIG. 5.
Michaelis-Menten kinetics of alkaline phosphatase activity measured in sapropel S3 of core 66. The error bars indicate standard deviations.
Vertical distribution of exoenzyme activities.
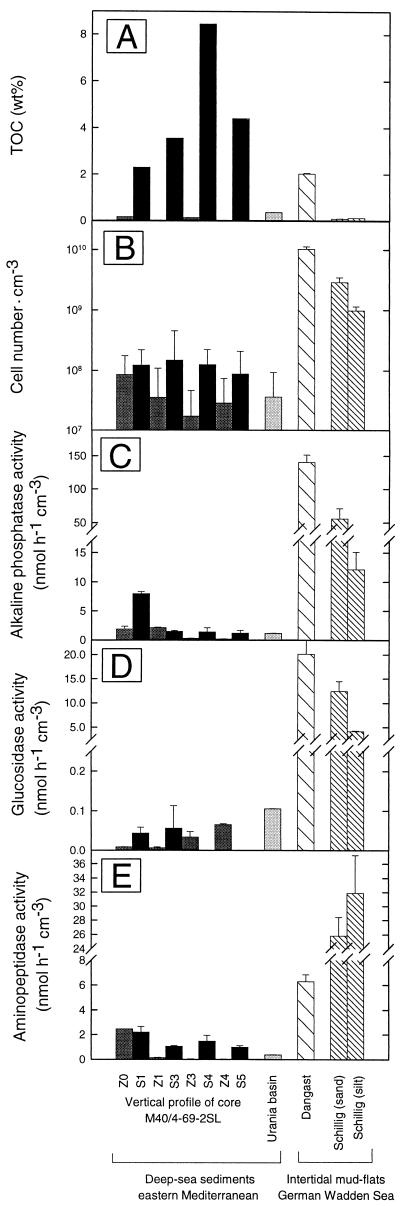
In core 69, the highest values for alkaline phosphatase were detected in the youngest sapropel, S1 (6.55 ± 0.19 nmol cm of sediment−3 h−1) (Fig. 6), but activities were only threefold lower in the oldest sapropels. The activity of alkaline phosphatase was significantly higher within the sapropels than in the intermediate hemipelagic layers. In the anoxic surface sediment of the Urania basin, alkaline phosphatase activity was lower by a factor of 2 to 5 than that in the sapropels, while the activities in Dangast and Schillig sediments were 60 and 30 times higher.
FIG. 6.
Comparison of TOC levels (A), total cell numbers (B), and exoenzyme activities of alkaline phosphatase (C), β-glucosidase (D), and aminopeptidase (E) for each type of sediment. The error bars indicate standard deviations.
Of the three exoenzyme activities, β-glucosidase exhibited by far the lowest activities in the sapropels and did not exceed 0.034 nmol cm of sediment−3 h−1 (Fig. 6 and Table 5). The β-glucosidase activity in the Urania sediment was three times higher than those in the sapropels and intermediate layers of the core from station M40/4-69-2SL. The two intertidal sediments exhibited β-glucosidase activities exceeding those in the upper sapropel sediments by a factor of 490 to 670 (Fig. 6 and Table 5). With a detection limit for free fluorophores of 0.01 μM MUF, the minimum detectable β-glucosidase activity after correction for the adsorption and for the backround fluorescence of the sapropel sediments was 0.015 nmol h−1 cm−3. At this detection limit, no significant β-glucosidase activity could be determined in the older sapropel layers, S4 and S5.
TABLE 5.
Comparison of exoenzyme activities in eastern Mediterranean sapropels with values from the literature
| Study area | Water depth (m) | Sediment depth (cmbsf)a |
Vmax
(nmol cm−3 sediment h−1)
|
Reference | |
|---|---|---|---|---|---|
| β-Glucosidase | Aminopeptidase | ||||
| NE Atlantic | |||||
| Biotrans area; 47°N, 19°W | 4,500 | 0–1 | 0.089 | 15 | 8 |
| NE Atlantic; 47°N, 19°W | 4,555–4,919 | 0–10 | <0.002–0.10 | 10.0–23.0 | 52 |
| Celtic Sea; 50°N, 10°W | 580–1,680 | 1–2 | 0.03–0.43 | 9.5–45 | 53 |
| NW Mediterranean; 40°N, 3°E | 1,230 | 1–2 | NDd | 576 | 61 |
| Eastern Mediterranean | |||||
| Ionean Sea; 37°N, 18°E | 993–4,617 | 0–1 | 0.33–2.13 | 109–284 | 11 |
| Levantine Sea; core M40/4-69/2SL; hemipelagic layers | 2,150 | 4–6 (Z0) | 0.0084 ± 0.0005 | 2.52 ± 0.00 | This work |
| 89–91 (Z1) | 0.0060 ± 0.0022 | 0.134 ± 0.031 | |||
| 304–306 (Z3) | 0.0335 ± 0.0143 | 0.036 ± 0.002 | |||
| 356–358 (Z4) | 0.0653 ± 0.0024 | 0.022 ± 0.001 | |||
| Levantine Sea, core M40/4-69/2SL; sapropel layers | 2,150 | 25–27 (S1) | 0.0309 ± 0.0107 | 1.78 ± 0.05 | This work |
| 281–283 (S3) | 0.0282 ± 0.0282 | 0.78 ± 0.04 | |||
| 337–339 (S4) | <0.015b | 1.20 ± 0.07 | |||
| 387–389 (S5) | <0.015b | 1.04 ± 0.13 | |||
| Urania basin; eastern Mediterranean | 3,600 | 0–4 | 0.120 ± 0.001 | 0.323 ± 0.002 | This work |
| Levantine Sea; 33°N, 29°E | 990–4,260 | 0–1 | 0.08–1.24 | 14–118 | 11 |
| Arctic circle | |||||
| Barents Sea; 82°N, 42°E | 1,013 | 0–4.5 | 0.2–0.4 | 67–144 | 10 |
| Laptev Sea | 796–3,427 | 0–1 | 0.02–0.36 | 21–116 | 9 |
| Amundsen Basin; 87°N, 68°E | 4,480 | 0–1 | 0.017 | 73.4 | 9 |
| Lomosov Ridge; 87°N, 153°E | 3,413 | 0–1 | 0 | 19.6 | 9 |
| Intertidal mudflats | |||||
| Lowes Cove, Maine | 0 | 0–2 | 23.2 | ND | 34 |
| Lowes Cove, Maine | 0 | 0–1 | ND | 12c | 39 |
| Dangast, German Wadden Sea; 53°27′N, 8°7′E | 0 | 3.5–8.5 | 20.6 ± 1.9 | 6.07 ± 1.06 | This work |
| 0 | 0–5 | 16.6 ± 2.8 | 86.0 ± 25.7 | This work | |
| Schillig, German Wadden Sea; 53°42′N, 8°01′E | 0 | 6–11 | 5.12 ± 0.16 | 33.1 ± 7.5 | This work |
cmbsf, centimeters below surface. The layer number is in parentheses.
Due to the high autofluorescence of sapropel supernatants, the detection limit for exoenzyme activity was 0.015 nmol · cm−3 · h−1. The detection limit for exoenzyme activity in the hemipelagic carbon-poor layers was 0.003 nmol · cm−3 · h−1.
Exoenzyme activities were available only in units of nanomoles · (grams of sediment · hour)−1.
ND, not detected.
The highest aminopeptidase activity was measured in the uppermost sediment layer (Z0) of core 69. In the intermediate layers below, the activity of this exoenzyme declined rapidly from 2.52 to 0.022 nmol cm−3 h−1. In contrast, the aminopeptidase activity remained almost constant in sapropels S1 through S5. Values in the Urania basin sediment were significantly lower than those in the sapropel layers, whereas the activities in the intertidal sediments of Schillig exceeded those in the sapropels by 25 and 66 times (Fig. 6 and Table 5).
TOC and total and culturable bacterial cell numbers.
The sapropels investigated in the present study had TOC contents between 2.3 and 8.5% (Fig. 6A). The TOC content of surface sediment from Dangast was comparable to that of sapropel S1. In contrast, the intermediate hemipelagic layers of the Mediterranean sediment core 69, as well as the surface sediments obtained from the Urania basin and from Schillig, all contained TOC at concentrations of less than 0.5% (dry weight) of the sediment.
Numbers of bacteria were higher in the surface sediment of core 69 (8.5 · 107 cells · cm−3) than in deeper hemipelagic layers (1.7 · 107 to 3.5 · 107 cells · cm−3). The bacterial numbers in all four sapropels investigated (0.87 · 108 to 1.48 · 108 cells · cm−3) exceeded those in the carbon-poor layers (Fig. 6B). The frequency of dividing and divided cells in Mediterranean sediments ranged between 2.1 and 5.2%; no significant differences were found between the sapropels and adjacent carbon-poor layers. In the present study, the highest concentration of bacterial cells was observed in Dangast sediment.
Plate counts of aerobic chemoorganoheterotrophic bacteria on CPSm agar yielded positive results only in the case of two out of eight layers of core 69. The numbers of culturable cells were very low, reaching a total of 1.5 · 102 CFU per ml in the uppermost carbon-poor layer, Z0, which corresponds to a culturability of 0.00017%. The second positive sample was sapropel S3, which contained 3.6 · 101 CFU ml−1 (culturability, 0.000024%). In the liquid dilution series, no growth of bacteria from the sediment layers of core 69 could be detected even after 3 months of incubation under anoxic conditions. For comparison, plate counts of aerobic chemoorganoheterotrophic bacteria from the intertidal sediment layers of Dangast and Schillig were much higher and ranged from 2.3 · 106 to 2.3 · 108 CFU ml−1 (culturability, 0.23 to 2.3%). Similarly, the culturability of anaerobic bacteria in the liquid medium MM was much higher when samples from Dangast (0.006%) and other surface sediments from the Wadden Sea (0.8% [S. Droege, H. Cypionka, J. Overmann, and H. Sass, unpublished data]) were used. This indicates that specific physiological features of the bacteria in Mediterranean sapropels are the reason for their low culturability.
Correlation analysis and cell-specific exoenzyme activity.
For Mediterranean sapropels, no correlation was found between bacterial cell numbers and TOC (r2 = 0.002) or between TOC and the three exoenzyme activities (r2 ≤ 0.072).
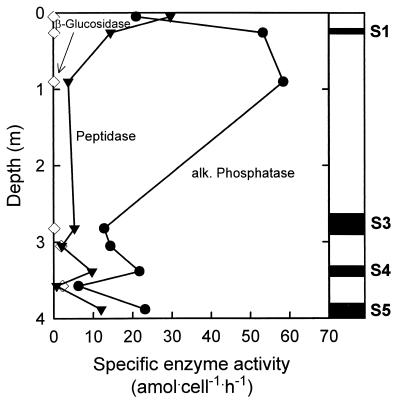
The cell-specific alkaline phosphatase activities were comparable in the sapropels (1.28 · 10−17 to 5.3 · 10−17 mol · cell−1 · h−1) and intermediate layers (0.21 · 10−17 to 5.85 · 10−17 mol · cell−1 · h−1) (Fig. 7). The specific activities of β-glucosidase were much lower and reached only 25.1 · 10−20 mol · cell−1 · h−1 in the sapropels and 9.9 · 10−20 to 228 · 10−20 mol · cell−1 · h−1 in the intermediate layers. Interestingly, a consistent trend with depth was observed for the specific aminopeptidase activity. We determined a maximum value of the specific activity at the surface of the Mediterranean sediments (2.97 · 10−17 mol · cell−1 · h−1). In the hemipelagic layers below, the activity continuously declined from 0.38 · 10−17 mol · cell−1 · h−1 (Z1) to 0.08 · 10−17 mol · cell−1 · h−1 (Z4). In contrast to the organic carbon-poor layers, however, the specific aminopeptidase activities remained at a higher level even in the oldest subfossil sapropel layers (0.53 · 10−17 to 1.45 · 10−17 mol · cell−1 · h−1) (Fig. 7).
FIG. 7.
Specific activities of alkaline (alk.) phosphatase (●), peptidase (▾), and β-glucosidase (◊) in sapropels S1 and S3 to S5 (vertical positions are indicated by black rectangles) and organic carbon-poor layers of core 69. The values were calculated from maximum enzyme activities and total bacterial cell counts and represent potential rates (see Discussion).
DISCUSSION
Methodology.
In the present investigation, exoenzyme activities were determined in sediment slurries. The injection of substrate analogues into intact sediment cores has been proposed to yield more realistic values of enzyme activities (41). However, because of the extraordinarily high adsorption capacity, it appears to be very difficult to create a homogenous distribution of substrate analogues in sapropels by the core injection technique. In addition, the small amount of sediment material which was available from the Mediterranean sapropels made subsampling and the preparation of sediment slurries inevitable. The disruption of in situ microstructures during the preparation of sediment slurries can cause an increase in exoenzyme activity (27, 41, 43); this effect may be especially pronounced in ancient sapropels. Consequently, our values should be taken as potential rather than actual rates of exoenzyme activity. An adsorption of substrate analogues could slow down their degradation in the sediment slurries. Since uncleaved fluorescently labeled substrate analogues cannot be detected by the present methods, we did not attempt to quantify their adsorption to the sapropel matrix. However, adsorption of substrate analogues would lead to an underestimation of exoenzyme activities, and the potential rates determined in the present study should thus be taken as minimum values.
In contrast to other sediments with even higher organic-matter content (i.e., 16% of the dry weight [13]), a desorption of bound fluorophores by chemical treatment was not feasible in the case of the sapropels. Obviously, the extremely high adsorption of the fluorophores is caused by the chemical properties of the kerogen in sapropels. Furthermore, adsorption of the fluorophores MUF and MCA depends on their concentrations in a nonlinear manner. Consequently, a linear correction for adsorption (9, 34, 61) was not adequate in the present study. Rather, equilibrium adsorption isotherms had to be recorded prior to measurements of exoenzyme activities in subfossil Mediterranean sapropels.
Exoenzymes as indicators of physiologically active bacteria.
Since the culturability of bacteria in the sapropels was extraordinarily low, the majority of bacterial cells counted in this habitat may be physiologically inactive. At present, no method is available to distinguish between exoenzymes associated with live bacterial cells (so-called ectoenzymes [20]) and liberated extracellular enzymes which have been immobilized by adsorption to the nonliving particulate fraction of sapropel sediments. In the adsorbed state, peroxidase, catalase, urease, and phosphatase can withstand proteolysis at least for several days (42, 58). Nothing is known about the long-term stability of extracellular enzymes in the natural environment; however, a persistence for 124,000 years appears highly unlikely.
Indeed, several lines of evidence indicate that the functional exoenzymes detected in the present study are associated with live bacterial cells. Firstly, alkaline phosphatase immobilized on soil particles is extremely heat stable and retains its full activity even during 2 h of incubation at 80°C (42). In contrast, the alkaline phosphatase activity measured in Mediterranean sapropels and other sediments was significantly reduced (e.g., to 5.9% of the initial activity in sapropel S1) by heating. Secondly, even the 124,000-year-old sapropel harbors bacteria which are still capable of uptake and degradation of glucose (46). Since glucose is liberated by β-glucosidase, this finding matches the presence of the active exoenzyme. Thirdly, a parallel investigation of the bacterial diversity in the sapropel layers revealed the presence of 16S rRNA sequences of exclusively gram-negative bacteria, most of them (92%) being members of the Chloroflexus subdivision (M. J. L. Coolen, A. Smock, H. Sass, H. Cypionka, and J. Overmann, unpublished data). Gram-negative cells, unlike their gram-positive counterparts, generally release only a little of their periplasmic enzymes (18). Based on this cumulative evidence, we conclude that the exoenzyme activities determined in the present study are associated with extant bacterial populations.
Implications for the microbial ecology of Mediterranean sapropels.
Previously, the presence of active bacterial populations in Mediterranean sapropels has been inferred from elevated numbers of bacterial cells and the high frequency of dividing bacteria (reference 23 and this study). The exoenzymes alkaline phosphatase and β-glucosidase are inducible by their respective substrates (organic phosphoesters and cellobiose) and are subject to catabolite repression by their products glucose and phosphate, respectively (20, 59). Since the cell-specific activities of both enzymes may thus be used as indicators for the presence of degradable biopolymers (20, 21, 22, 45), our data provide new insights into the physiological state of the bacteria present in the sapropel layers.
Compared to the cell-specific phosphatase activity determined for bacteria in the sapropels, that of phosphate-deficient natural bacterial communities reaches much higher values (3 · 10−15 to 7.7 · 10−15 mol · cell−1 · h−1 [45]). Although phosphorus is the main limiting inorganic nutrient in the ultraoligotrophic eastern Mediterranean (66), neither the bacteria in sapropels nor those in the intermediate layers appear to be limited by inorganic phosphate. The specific activities of β-glucosidase determined in the present study fall within the range observed for marine sediments (17.8 · 10−20 to 232 · 10−20 mol · cell−1 · h−1 [8, 34]) and pelagic water samples (50 · 10−20 mol · cell−1 · h−1 [29]). Thus, a complete anaerobic food chain still proceeds in most if not all of the sapropels investigated, despite the great age of the bulk sapropel organic matter. Based on the observation that almost all of the bacterial 16S rRNA sequences recovered from sapropel layers are affiliated with the Chloroflexus subdivision (Coolen et al., unpublished), the primary steps of the anaerobic microbial food chain in Mediterranean sapropel sediments may be mediated by unknown mesophilic chemoorganoheterotrophic members of this bacterial division.
The activity of leucine aminopeptidase is exclusively associated with heterotrophic bacteria (20). This exoenzyme, in contrast to alkaline phosphatase and β-glucosidase, is not induced by its natural substrate (polypeptides or proteins) in marine sediments; it is inhibited by glycine and other amino acids (10). The specific aminopeptidase activity at the surfaces of Mediterranean sediments (2.97 · 10−17 mol · cell−1 · h−1) was comparable to that in other sediments (0.5 · 10−17 to 2.94 · 10−17 mol · cell−1 · h−1 [8, 29]) but continuously declined with depth in the hemipelagic layers, whereas specific activities were elevated in the subfossil sapropel layers. The specific aminopeptidase activity increases during initial stages of energy and nutrient starvation and declines thereafter (2); hence, starvation may be less pronounced for bacteria living in the sapropels than for those in intermediate layers.
Extracellular enzymatic hydrolysis of biopolymers is the rate-limiting step for the utilization of organic matter in surface aquatic environments (7, 20, 56). In most marine sediments, exoenzyme activities decrease rapidly with depth (8, 9, 11, 35, 41, 53), thus following the gradients of easily degradable substances. This general pattern was also observed for aminopeptidase and alkaline phosphatase in the successive hemipelagic layers. However, localized maxima of specific exoenzyme activities were confined to the sapropels and thus cannot be explained by a percolation of easily degradable organic carbon into subfossil sapropel layers. Compared to other deep-sea sediments, those from the eastern Mediterranean contain much lower concentrations of labile organic compounds (24). We conclude that organic carbon provided by the sapropel layers themselves must support bacterial metabolism in situ.
In continental aquifers and deep marine sediments, microbial biomass correlates with total organic carbon content (23, 33). This correlation has not been observed for sapropels (23), suggesting that either the bioavailability of organic carbon substrates or the supply of electron-accepting substrates limits bacterial population size within the sapropels. Bacteria in Miocene subsurface sediments appear to be limited by the supply of electron acceptors rather than organic carbon substrates (26, 33). This does not apply to Mediterranean sediments, including sapropels, in which the concentrations of sulfate range between 20 and 50 mM (14; H.-J. Brumsack, personal communication). Consequently, a low bioavailability of organic carbon most likely limits bacterial metabolism in the sapropel layers. Adsorption of organic matter to mineral surfaces slows down the degradation rates by 5 orders of magnitude (31). Even in geologically young sediment layers, a major fraction (20 to 100%) of the detectable acetate is not bioavailable (63). As shown here for the first time, the sapropel matrix exhibits an extraordinarily high adsorption capacity for low-molecular-weight organic carbon compounds, as exemplified by MUF and MCA and also demonstrated for DNA (Coolen et al., unpublished). Hence, a strong adsorption of organic carbon substrates to the sapropel kerogen may be the major limiting factor for bacterial growth in Mediterranean sapropels.
Despite the significant exoenzyme activities and cell numbers, we were able to cultivate only a minute fraction of the bacteria from the various Mediterranean sapropels by conventional isolation techniques or by employing improved liquid media. Taken together, our results indicate that (i) survival and metabolism of nonsporulating bacteria can be supported even by 124,000-year-old sediment organic matter and (ii) new cultivation approaches will have to be developed for the isolation of bacteria from such extreme environments.
ACKNOWLEDGMENTS
We are indebted to Andrea Smock for help with determination of cell numbers and the TOC contents of the various sediments and Henrike Oertel for help with the initial experiments. Heribert Cypionka and Henrik Sass are gratefully acknowledged for their support during the cruise, and the master and crew of the R/V Meteor for their help during collection of the sediment cores.
The present work was supported by grants from the Deutsche Forschungsgemeinschaft to H. Cypionka and J.O. (Cy 1/8-1 and Cy 1/10-1).
REFERENCES
- 1.Aksu A E, Abrajano T, Mudie P J, Yasar D. Organic geochemical and palynological evidence for terrigenous origin of the organic matter in Aegean Sea sapropel S1. Mar Geol. 1999;153:303–318. [Google Scholar]
- 2.Albertson N H, Nyström T, Kjelleberg S. Exoprotease activity of two marine bacteria during starvation. Appl Environ Microbiol. 1990;56:218–233. doi: 10.1128/aem.56.1.218-223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch W E, Fox G E, Magrum L J, Woese C R, Wolfe R S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979;43:260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkwill D L, Fredrickson J K, Thomas J M. Vertical and horizontal variations in the physiological diversity of the aerobic chemoheterotrophic bacterial microflora in deep southeast coastal plain subsurface sediments. Appl Environ Microbiol. 1989;55:1058–1065. doi: 10.1128/aem.55.5.1058-1065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes S P, Bradbrook S D, Cragg B A, Marchesi J R, Weightman A J, Fry J C, Parkes R J. Isolation of sulfate-reducing bacteria from deep sediment layers of the Pacific Ocean. Geomicrobiol J. 1998;15:67–83. [Google Scholar]
- 6.Bélanger C, Desrosiers B, Lee K. Microbial extracellular enzyme activity in marine sediments: extreme pH to terminate reaction and sample storage. Aquat Microb Ecol. 1997;13:187–196. [Google Scholar]
- 7.Billen G. Modelling the process of organic matter degradation and nutrient recycling in sedimentary systems. In: Nedwell D B, Brown C M, editors. Sediment microbiology. London, United Kingdom: Academic Press; 1982. pp. 15–52. [Google Scholar]
- 8.Boetius A. Microbial hydrolytic enzyme activities in deep-sea sediments. Helgoländer Meeresunters. 1995;49:177–187. [Google Scholar]
- 9.Boetius A, Damm E. Benthic oxygen uptake, hydrolytic potentials and microbial biomass at the Arctic continental slope. Deep-Sea Res Ser I. 1998;45:239–275. [Google Scholar]
- 10.Boetius A, Lochte K. Effect of organic enrichments on hydrolytic potentials and growth of bacteria in deep-sea sediments. Mar Ecol Prog Ser. 1996;140:239–250. [Google Scholar]
- 11.Boetius A, Scheibe S, Tselepides A, Thiel H. Microbial biomass and activities in deep-sea sediments of the eastern Mediterranean: trenches are benthic hotspots. Deep-Sea Res Ser I. 1996;43:1439–1460. [Google Scholar]
- 12.Boivin-Jahns V, Ruimy R, Bianchi A, Daumas S, Christen R. Bacterial diversity in a deep-subsurface clay environment. Appl Environ Microbiol. 1996;62:3405–3412. doi: 10.1128/aem.62.9.3405-3412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boschker H T S, Cappenberg T E. A sensitive method using 4-methylumbelliferyl-β-cellobiose as a substrate to measure (1,4)-β-glucanase activity in sediments. Appl Environ Microbiol. 1994;60:3592–3596. doi: 10.1128/aem.60.10.3592-3596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Böttcher M E, Brumsack H-J, de Lange G J. Sulfate reduction and related stable isotope (34S, 18O) variations in interstitial waters from the eastern Mediterranean. In: Robertson A H F, Emeis K-C, Richter C, Camerlenghi A, editors. Proceedings of the ocean drilling program, scientific results. Vol. 160. Tex: Ocean Drilling Program, College Station; 1998. pp. 365–373. [Google Scholar]
- 15.Bouloubassi I, Rullkötter J, Meyers P A. Origin and transformations of organic matter in Pliocene-Pleistocene Mediterranean sapropels: organic geochemical evidence reviewed. Mar Geol. 1999;153:177–197. [Google Scholar]
- 16.Calvert S E. Geochemistry of Pleistocene sapropels and associated sediments from the Eastern Mediterranean. Oceanol Acta. 1983;6:255–267. [Google Scholar]
- 17.Calvert S E, Nielsen B, Fontugne M R. Evidence from nitrogen isotope ratios for enhanced productivity during formation of eastern Mediterranean sapropels. Nature. 1992;359:223–225. [Google Scholar]
- 18.Cembella A D, Antia N J, Harrison P J. The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective. Part 1. Crit Rev Microbiol. 1984;10:317–391. doi: 10.3109/10408418209113567. [DOI] [PubMed] [Google Scholar]
- 19.Christian J R, Karl D M. Bacterial ectoenzymes in marine waters: activity ratios and temperature responses in three oceanographic provinces. Limnol Oceanogr. 1995;40:1041–1049. [Google Scholar]
- 20.Chróst R J. Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In: Chróst R J, editor. Microbial enzymes in aquatic environments. New York, N.Y: Springer; 1991. pp. 29–59. [Google Scholar]
- 21.Cotner J B, Wetzel R G. Bacterial phosphatases from different habitats in a small hardwater lake. In: Chróst R J, editor. Microbial enzymes in aquatic environments. New York, N.Y: Springer; 1991. pp. 187–205. [Google Scholar]
- 22.Coveney M F, Wetzel R G. Effects of nutrients on specific growth rate of bacterioplankton in oligotrophic lake water cultures. Appl Environ Microbiol. 1992;58:150–156. doi: 10.1128/aem.58.1.150-156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cragg B A, Law K M, Cramp A, Parkes R J. The response of bacterial populations to sapropels in deep sediments of the eastern Mediterranean (site 969) In: Robertson A H F, Emeis K C, Camerlenghi A, editors. Proceedings of the ocean drilling program, scientific results. Vol. 160. 1998. pp. 303–307. [Google Scholar]
- 24.Danovaro R, Marrale D, Della Croce N, DellÁnno A, Fabiano M. Heterotrophic nonflagellates, bacteria, and labile organic compounds in continental shelf and deep-sea sediments of the eastern Mediterranean. Microb Ecol. 1998;35:244–255. doi: 10.1007/s002489900080. [DOI] [PubMed] [Google Scholar]
- 25.Emeis K-C, Robertson A H F, Richter C. Proceedings of the ocean drilling program, initial reports. Vol. 160. 1996. Paleoceanography and sapropel introduction; pp. 21–28. [Google Scholar]
- 26.Fredrickson J K, McKinley J P, Nierzwicki-Bauer S A, White D C, Ringelberg D B, Rawson S A, Li S-M, Brockman F J, Bjornstad B N. Microbial community structure and biogeochemistry of Miocene subsurface sediments: implications for long-term microbial survival. Mol Ecol. 1995;4:619–626. [Google Scholar]
- 27.Hall K J, Kleiber P M, Yesaki I. Heterotrophic uptake of organic solutes by microorganisms in the sediment. Mem Ist Ital Idrobiol. 1972;29(Suppl.):441–471. [Google Scholar]
- 28.Hilgen F J. Astronomical calibration of Gauss to Matuyama sapropels in the Mediterranean and implication for the geomagnetic polarity time scale. Earth Planet Sci Lett. 1991;104:226–244. [Google Scholar]
- 29.Hoppe H G. Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates. Mar Ecol Prog Ser. 1983;11:299–308. [Google Scholar]
- 30.Hoppe H G. Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria. In: Kemp P F, Sherr B F, Shaw E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 423–431. [Google Scholar]
- 31.Keil R G, Montluçon D B, Prahl F G, Hedges J I. Sorptive preservation of labile organic matter in marine sediments. Nature. 1994;370:549–552. [Google Scholar]
- 32.Kidd R B, Cita M B, Ryan W B F. Stratigraphy of eastern Mediterranean sapropel sequences recovered during DSDP Leg 42A and their paleoenvironmental significance. In: Hsü K J, et al., editors. Initial Reports. Washington, D.C.: Deep Sea Drilling Program, U.S. Government Printing Office; 1978. pp. 421–443. [Google Scholar]
- 33.Kieft T L, Fredrickson J K, McKinley J P, Bjornstad B N, Rawson S A, Phelps T J, Brockman F J, Pfiffner S M. Microbiological comparisons within and across contiguous lacustrine, paleosol, and fluvial subsurface sediments. Appl Environ Microbiol. 1995;61:749–757. doi: 10.1128/aem.61.2.749-757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King G M. Characterization of β-glucosidase activity in intertidal marine sediments. Appl Environ Microbiol. 1986;51:373–380. doi: 10.1128/aem.51.2.373-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Köster M, Dahlke S, Meyer-Reil L A. Microbiological studies along a gradient of eutrophication in a shallow coastal inlet in the southern Baltic Sea (Nordrügensche Bodden) Mar Ecol Prog Ser. 1997;152:27–39. [Google Scholar]
- 36.Krumholz L R, McKinley J P, Ulrich G A, Suflita J M. Confined subsurface microbial communities in Cretaceous rock. Nature. 1997;386:64–66. [Google Scholar]
- 37.L'Haridon S, Reysenbach A-L, Glénat P, Prieur D, Jeanthon C. Hot subterranean biosphere in a continental oil reservoir. Nature. 1995;377:223–224. [PubMed] [Google Scholar]
- 38.Lourens L J, Hilgen F J, Gudjonsson L, Zachariasse W J. Late Pliocene to early Pleistocene astronomically forced sea surface productivity and temperature variations in the Mediterranean. Mar Micropaleontol. 1996;19:49–78. [Google Scholar]
- 39.Mayer L M. Extracellular proteolytic enzyme activity in sediments of an intertidal mudflat. Limnol Oceanogr. 1989;34:973–981. [Google Scholar]
- 40.MEDRIFF Consortium. Three brine lakes discovered in the seafloor of the eastern Mediterranean. Eos. 1995;76:315–320. [Google Scholar]
- 41.Meyer-Reil L A. Measurement of hydrolytic activity and incorporation of dissolved organic substrates by microorganisms in marine sediments. Mar Ecol Prog Ser. 1986;31:143–149. [Google Scholar]
- 42.Nannipieri P, Ceccanti B, Conti C, Bianchi D. Hydrolases extracted from soil: their properties and activities. Soil Biol Biochem. 1982;14:257–263. [Google Scholar]
- 43.Novitsky J A. Microbial activity at the sediment-water interface in Halifax Harbor, Canada. Appl Environ Microbiol. 1983;45:1761–1766. doi: 10.1128/aem.45.6.1761-1766.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogram A, Sayler G S, Gustin D, Lewis R J. DNA adsorption to soils and sediments. Environ Sci Technol. 1988;22:982–984. doi: 10.1021/es00173a020. [DOI] [PubMed] [Google Scholar]
- 45.Overmann J, Beatty J T, Hall K J. Purple sulfur bacteria control the growth of aerobic heterotrophic bacterioplankton in a meromictic salt lake. Appl Environ Microbiol. 1996;62:3251–3258. doi: 10.1128/aem.62.9.3251-3258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overmann J, Coolen M, Smock A, Sass H, Cypionka H. Microbial activities and populations in upper sediment and sapropel layers. In: Hieke W, Hemleben C, Linke P, Türkay M, Weikert H, editors. METEOR-Berichte 99-2, Mittelmeer 1997/1998 cruise no. 40. Leitstelle METEOR. Hamburg, Germany: Institut für Meereskunde der Universität Hamburg; 1999. pp. 148–157. [Google Scholar]
- 47.Overmann J, Fischer U, Pfennig N. A new purple sulfur bacterium from saline littoral sediments, Thiorhodovibrio winogradskyigen. nov. and sp. nov. Arch Microbiol. 1992;157:329–335. [Google Scholar]
- 48.Parkes R J, Cragg B A, Bale S J, Getliff J M, Gooman K, Rochelle P A, Fry J C, Weightman A J, Harvey S M. Deep bacterial biosphere in Pacific Ocean sediments. Nature. 1994;371:410–413. [Google Scholar]
- 49.Passier H F, Bosch H J, Nijenhuis I A, Lourens L J, Böttcher M E, Leenders A, Sinninghe Damsté J S, de Lange G J, de Leeuw J W. Sulphidic Mediterranean surface waters during Pliocene sapropel formation. Nature. 1999;397:146–149. [Google Scholar]
- 50.Passier H F, Middelburg J J, van Os B J H, de Lange G J. Diagenetic pyritisation under eastern Mediterranean sapropels caused by downward sulphide diffusion. Geochim Cosmochim Acta. 1996;60:751–763. [Google Scholar]
- 51.Passier H F, Middelburg J J, de Lange G J, Böttcher M E. Models of sapropel formation in the eastern Mediterranean: some constraints based on pyrite properties. Mar Geol. 1999;153:199–219. [Google Scholar]
- 52.Poremba K. Hydrolytic enzymatic activity in deep-sea sediments. FEMS Microbiol Ecol. 1995;16:213–222. [Google Scholar]
- 53.Poremba K, Hoppe H G. Spatial variation of benthic microbial production and hydrolytic enzymatic activity down the continental slope of the Celtic Sea. Mar Ecol Prog Ser. 1995;118:237–245. [Google Scholar]
- 54.Rohling E J, Hilgen F J. The eastern Mediterranean climate at times of sapropel formation: a review. Geol Mijnb. 1991;70:253–264. [Google Scholar]
- 55.Rossignol-Strick M. Mediterranean quaternary sapropels, an immediate response of the African monsoon to variation of isolation. Palaeogeogr Palaeoclimatol Palaeoecol. 1985;49:237–263. [Google Scholar]
- 56.Ruddy G. An overview of carbon and sulphur cycling in marine sediments. In: Jickells T D, Rae J E, editors. Biogeochemistry of intertidal sediments. Cambridge, United Kingdom: Cambridge University Press; 1997. pp. 99–118. [Google Scholar]
- 57.Ryan W B F. Stratigraphy of late Quaternary sediments in the eastern Mediterranean. In: Stanley D J, editor. The Mediterranean Sea: a natural sedimentation laboratory. Stroudsberg, Pa: Dowden, Hutchinson and Ross; 1972. pp. 146–169. [Google Scholar]
- 58.Serban A, Nissenbaum A. Humic acid association with peroxidase and catalase. Soil Biol Biochem. 1986;18:41–44. [Google Scholar]
- 59.Siuda W, Güde H. A comparative study on 5′-nucleotidase (5′-nase) and alkaline phosphatase (APA) activities in two lakes. Arch Hydrobiol. 1994;131:211–229. [Google Scholar]
- 60.Stevens T O, McKinley J P. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science. 1995;270:450–454. [Google Scholar]
- 61.Tholosan O, Bianchi A. Bacterial distribution and activity at the water-sediment boundary layer on NW Mediterranean continental margin. Mar Ecol Prog Ser. 1998;168:273–283. [Google Scholar]
- 62.Tseng H-Y, Onstott T C. A tectogenetic origin for the deep subsurface microorganisms of Taylorsville Basin: thermal and fluid flow model constraints. FEMS Microbiol Rev. 1997;20:391–397. [Google Scholar]
- 63.Wellsbury P, Parkes R J. Acetate bioavailability and turnover in an estuarine sediment. FEMS Microbiol Ecol. 1995;17:85–94. [Google Scholar]
- 64.Widdel F, Kohring G W, Mayer F. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov., sp. nov., and Desulfonema magnumsp. nov. Arch Microbiol. 1983;134:286–293. [Google Scholar]
- 65.Wright R T, Hobbie J E. Use of glucose and acetate by bacteria and algae in aquatic ecosystems. Ecology. 1966;47:447–464. [Google Scholar]
- 66.Zohary T, Roberts R D. Experimental study of microbial P limitation in the eastern Mediterranean. Limnol Oceanogr. 1998;43:387–395. [Google Scholar]