Abstract
Objective:
Previous research has not objectively assessed patients’ comprehension of their pharmacogenomic test results. In this study we assessed understanding of patients who had undergone cytochrome P450 2C19 (CYP2C19) pharmacogenomic testing.
Methods:
31 semi-structured interviews with patients who underwent CYP2C19 testing after cardiac catheterization and had been sent a brochure, letter, and wallet card explaining their results. Answers to Likert and binary questions were summarized with descriptive statistics. Qualitative data were analyzed using a grounded theory approach, with particular focus on categorization.
Results:
No participants knew the name of the gene tested or their metabolizer status. Seven participants (23%) knew whether the testing identified any medications that would have lower effectiveness or increased adverse effects for them at standard doses (“Adequate Understanding”). Four participants (13%) read their results from the letter or wallet card they received but had no independent understanding (“Reliant on Written Materials”). Ten participants remembered receiving the written materials (32%).
Conclusion:
A majority of participants who had undergone CYP2C19 PGx testing did not understand their results at even a minimal level and would be unable to communicate them to future providers.
Practice implications:
Further research is necessary to improve patient understanding of PGx testing and their results, potentially through improving patient-provider communication.
Keywords: Pharmacogenomics, Patient understanding, CYP2C19, Clopidogrel (Plavix)
1. Introduction
Studies suggest that a majority of patients in some populations have pharmacogenomic (PGx) variants that could impact the metabolism of commonly prescribed medications. [1,2] Widespread efforts, including by a multi-site academic consortium, have identified promising ways to implement PGx testing in routine care, and multiple commercial companies offer PGx testing to patients. [3,4] These efforts raise the possibility that PGx will become the first type of genetic testing to be widely used in clinical medicine. Important barriers include developing and implementing ways to store results in the electronic health record as discrete data, to provide effective clinical decision support, and to increase understanding of providers and pharmacists. [5–8] Any successful implementation must also include steps to educate patients about their pharmacogenomics test results, to help them participate actively in their care and, when needed, alert providers about previous testing and results. [9]
Studies have found that a majority of patients who received PGx testing say that they feel that they understand their results, although some report feeling unsure about them. [10–13] However, these studies have only assessed patients feelings or beliefs about their understanding, not whether they actually did understand them. These studies did not measure whether patients who have actionable PGx results knew that there were certain medicines they should avoid or take at non-standard doses. The single example of an objective measurement of lay understanding of PGx is the Minnesota Assessment of Pharmacogenomic Literacy (MAPL) test, which consists of 13 true/false questions regarding genetics, basic pharmacology, the role of genetics on medicine, and the benefits and limitations of PGx.[14] While this instrument assesses understanding of PGx as a field of medicine, it does not measure understanding of a specific PGx test results, and there are no published reports of it being administered to patients who have undergone PGx testing.
The current study grew out of a university-funded project to implement CYP2C19 testing for patients undergoing cardiac catheterization at a partner healthcare system. Clopidogrel, an antiplatelet medication often prescribed to patients receiving cardiac stents, is a “pro-drug,” i.e., inactive and metabolized into the active form by the CYP2C19 enzyme. Individuals whose CYP2C19 genotype makes them “Poor metabolizers” or “Intermediate metabolizers” produce a reduced amount of the active drug, which results in a higher risk of major cardiovascular events. [15] The Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines recommend that these patients should be switched to other platelet inhibitors that are not metabolized by CYP2C19. [15] Individuals whose CYP2C19 genotype makes them a “Rapid metabolizer” or “Ultra-rapid metabolizer” produce an increased amount of the active drug, which does not affect the effectiveness or side effect profile of clopidogrel but reduces the effectiveness of other medicines that are metabolized by CYP2C19. [15–17] The implementation project at our partner healthcare system educated clinicians, worked with payers to cover testing, and updated the electronic health record system to record PGx results and provide alerts to providers through clinical decision support.
As part of the same project, a group of clinicians and experts in PGx, health communication, and bioethics developed written materials to be sent to patients to explain their CYP2C19 test results, including a tri-fold brochure, a letter, and a wallet card (examples provided in Appendix). The brochure provides a non-technical explanation of CYP2C19 and metabolizer status and explains the clinical relevance and importance of sharing the results with future providers. The letter reports the individual patient’s PGx results, including their CYP2C19 metabolizer status and a list of medicines predicted to be affected based on current CPIC guideline recommendations. The letter also informs patients that their provider has already received the result, instructs them to talk to a provider before making any medication changes, and encourages them to tell future healthcare providers about their test results. The wallet card lists the patient’s CYP2C19 allele and metabolizer status as well as medications that can be impacted.
After this system was implemented, we designed and carried out the first study to assess patient understanding of their pharmacogenomic results. We conducted a telephone survey and interview with patients who underwent CYP2C19 testing in order to answer the following five research questions:
RQ1.. Do participants know what PGx is and that they had PGx testing?
RQ2.. How well do participants understand their results?
RQ3.. How did participants learn their results?
RQ4.. Did participants receive and save the written materials? What were their opinions of the materials?
RQ5.. What are participants’ opinions of PGx testing?
2. Methods
2.1. Population and sampling
Between October 2020 and December 2021, 404 patients at [Redacted for Blind Review] underwent PGx testing and were mailed the written materials 14–136 days after testing (mean = 66 days, SD = 28 days). For this study, individuals were assigned a unique randomized number and classified by their metabolizer status. Patients were invited in order of their assigned number, with a goal of enrolling 20% Normal metabolizers and 80% not-Normal metabolizers (Poor, Intermediate, Rapid, and Ultra-rapid). The study team mailed introductory letters to the study between 1.5 and 15 months (average 8 months) after the patients had been sent the written materials. The letter described the study as examining participants’ attitudes regarding the care they had received, without disclosing the focus on PGx testing. Patients were called about a week later to assess interest and eligibility. Candidates were eligible if they were at least 18 years old, able to provide verbal consent, and expressed the ability to engage in a 30-minute phone interview in English. Interviews were conducted between October 2021 and April 2022, at which point thematic saturation had been achieved. The study was approved by the Institutional Review Board (IRB) of Indiana University.
2.2. Data collection and measures
Participants were interviewed via telephone using a structured interview script containing open-ended, Likert, and binary questions. The full interview guide is available by request from the corresponding author. In order to identify whether participants were aware of implications of their PGx testing for choice of their anti-platelet medicines, participants were first asked about medicines that they take or have taken that “thin the blood,” such as clopidogrel, prasugrel, and ticagrelor. If participants said they had taken one of these, they were asked why they had been prescribed it, whether it had been stopped, and, if so, why.
Participants were then asked whether they were familiar with the term “pharmacogenomics.” If they said they were, they were asked to explain what they thought it meant (open-ended), and then whether they thought that it referred to a genetic test, whether their healthcare provider had been given their PGx results, and whether the results had been put in their electronic health record. Participants who did not know the term “pharmacogenomics” were told that PGx is a blood test to check which medicines work best for them.
All patients then were asked whether they remembered having PGx testing. Participants who recalled having PGx testing were asked what the testing found (open-ended). Follow-up questions included whether the testing showed that there were any medicines that might not work as well for them or have serious side effects, the name of the gene tested, and what metabolizer status they were found to have. They were asked how they learned about the results. Participants who did not know that they had PGx testing were informed that they had undergone this testing.
All participants were then asked whether they remembered receiving the written materials that were sent to them by mail. If participants remembered receiving the materials, they were asked Likert questions about acceptability and usability of the materials, and whether they had kept them, to gauge potential for future use. All participants were asked seven Likert questions about the importance of their PGx test results for their future healthcare and about provider access to the results. The interview ended with demographic questions, including a three-item health literacy measure, [18] to allow assessment of impact of health literacy on understanding of PGx results.
During the interview, interviewers entered participant responses into a REDcap database. For open-ended questions, the interviewer entered a summary of the response. An attempt was made to audio record all interviews, though six of 31 interviews were not recorded, due to patients refusing consent for recording or technical failure. Participants for whom recordings were not obtained are indicated in the results section.
2.3. Analysis
Likert style and binary questions were summarized with descriptive statistics. Patient agreement with the statements about usability and acceptability of the written materials were coded as 1–4 (Strongly Agree=4, Agree=3, Disagree=2, and Strongly Disagree=1), and means and standard deviations were calculated.
Qualitative data were analyzed using a grounded theory approach, with a particular focus placed on categorization. [19] Consensual validation among the study team was used to place participants into particular categories. A coding scheme was created using abductive analysis, [20] in which an active dialogue between data and theory was fostered. This resulted in the creation of three formal categories with robust inclusion and exclusion criteria, by which we describe participants’ understanding of their PGx results: Adequate Understanding, Reliant on Written Materials, and Inadequate Understanding. (These categories are described in more detail in the Results section.) After the completion of all interviews, two members of the research team (PHS, TAD) independently reviewed each participant’s answers. Cases of disagreement among the reviewers were resolved through consultation and consensus.
3. Results
3.1. Recruitment and demographics
One hundred and twenty patients were sent a letter introducing the study, and 31 participated. Forty-one patients were unreachable using their provided contact information, 11 refused eligibility assessment, five were not eligible, and 32 declined to participate, with the most common reason being that they were too busy. Of the 31 patients who participated, five were Normal metabolizers and 26 were not Normal metabolizers, with 18 being Poor or Intermediate metabolizers and 8 being Rapid or Ultra-rapid metabolizers. The average age was 65.7 years, and 61% of the participants were male. Eighty seven percent of participants received a high school diploma or GED, and 74% had completed trade school or some college or had received their Bachelor’s degree. For more information about our sample’s demographics, see Table 1.
Table 1.
Demographics of Participants.
| Age (years) | |
|---|---|
|
| |
| Mean (range) | 65.7 (44–88) |
| Sex | |
| Male | 19 (61%) |
| Female | 12 (39%) |
| Race | |
| White | 25 (81%) |
| Black | 5 (16%) |
| Native Hawaiian or Pacific Islander | 1 (3%) |
| Education | |
| Less than high school graduate | 4 (13%) |
| High school graduate or GED | 4 (13%) |
| Trade/technical school or some college | 12 (39%) |
| Bachelor’s degree or higher | 11 (35%) |
| Income | |
| Are comfortable | 16 (52%) |
| Have just enough to make ends meet | 10 (32%) |
| Do not have enough to make ends meet | 5 (16%) |
| Health Literacy a | |
| Average (range) | 2.07 (0–9) |
scores range from 0 to 12: higher score indicates lower health literacy
3.2. Knowledge of pharmacogenomics and awareness of being tested (Research Question 1)
Four participants (13%) recognized the term “pharmacogenomics” and were able to give a general definition (see Tables 2 and 3). A definition was considered acceptable if it included that PGx is a test whose goal is to determine whether certain medicines would work for the participant. Definitions did not need to specify that it was a genetic or “blood” test. Ten participants (32%) knew that they had undergone pharmacogenomic testing (Table 2). In follow-up questions to these ten participants, nine correctly answered “yes” when asked whether it is a genetic test, ten correctly answered “yes” when asked whether the results had been given to their healthcare provider, and nine correctly answered “yes” when asked whether the results were placed in their electronic health record.
Table 2.
Categorization and Knowledge of PGx.
| ID # | Metabolizer Status | Knows what “Pharmacogenomics” is | Recalls having PGx testing done | Recalls receiving written materials | Has kept Written Materials | Relies on Written Materials to Answer Knowledge Questions |
|---|---|---|---|---|---|---|
|
| ||||||
| Adequate Understanding | ||||||
| #14 | Rapid | Yes | Yes | Yes | Yes | No |
| #623 | Intermediate | Yes | Yes | Yes | Yes | No |
| #159 | Intermediate | No | Yes | No | No | No |
| #60 | Intermediate | No | Yes | Yes | Yes | No |
| #2 | Poor | No | Yes | No | No | No |
| #216 | Intermediate | No | Yes | Yes | No | No |
| #653 | Poor | Yes | Yes | Yes | Yes | No |
| Reliant on Written Materials | ||||||
| #107 | Rapid | No | No | Yes | Yes | Yes |
| #108 | Intermediate | No | Yes | Yes | Yes | Yes |
| #188 | Normal | No | No | Yes | Yes | Yes |
| #229 | Intermediate | Yes | Yes | Yes | Yes | Yes |
Table 3.
Descriptions of PGx and Testing Results by Participants with Adequate Understanding or Reliant on Written Materials.
| ID # and Metab Status | Descriptions of Pharmacogenomics Testing | Description of their Results, and Areas of Misunderstanding (if any) |
|---|---|---|
|
| ||
| Adequate Understanding | ||
| #14 Rapid | “It tests your blood to see how sensitive you are to medicines.” | “They did it to me and the test found that I’m less sensitive to pain meds and Plavix – so I need higher doses because of my decreased sensitivity.” Area of misunderstanding: Rapid metabolizers are less sensitive to some antidepressants, but not to clopidogrel or pain medications. |
| #623 Intermed | “That’s when they do genetic testing to find the right medications for you” | Participant Response: Mutant gene affects their metabolizing of clopidogrel |
| #159 Intermed | Participant was not familiar with the term. | “It looks like this DNA test told her that I couldn’t have that medicine. My doctor wanted to change Brilinta to something else but my body affected that” |
| #60 Intermed | Participant was not familiar with the term. | “That must’ve been why the doctor told me about [and switched] the clopidogrel. He called me up and said, ‘We discovered your system doesn’t absorb all the clopidogrel, only half. So, we’re switching you over to aspirin.’” |
| #2 Poor (No recording available) | Participant was not familiar with the term. After PGx testing was described to them, they remarked that the doctor had conducted a test that determined she could not take Brilinta. |
Participant response: Cannot metabolize Brilinta. Area of misunderstanding: Poor metabolizers cannot metabolize clopidogrel, but CYP2C19 phenotypes do not impact the metabolism of ticagrelor (Brilinta). |
| #216 Intermed | After having PGx testing described to them, participant states: ”I think I’ve had that done. I got a notice about DNA testing that was done about my drugs.” | Participant Response: Recalled receiving a letter that said that “due to my genetics, something would be better for me” and added, at a later point, “I didn’t fully understand other than which medicines would work best for my genetic make-up. That’s all I got out of it.” |
| #653 Poor | “They did a DNA test [...] it’s about certain medications that may not work well for me.” | “My body doesn’t absorb medicines like it should, absorbs it poorly.” Area of misunderstanding: CYP2C19 phenotypes affect drug metabolism, not drug absorption. |
| Reliant on Written Materials | ||
| #107 Rapid | Participant was not familiar with the term. | Patient initially did not recall having PGx testing or having received written materials. After the interviewer mentioned the wallet card, the participant checked their wallet and retrieved the card, and read that he is a “rapid metabolizer” and added that: “I have no idea what it means.” |
| #108 * Intermed (No Recording Available) | Participant could not define the term but thought that she had this sort of testing since it was “a genetic thing.” Later in the interview, participant found the brochure to confirm she had PGx testing. | Participant was only able to report their results by reading them off of written materials. They were unable to describe these results in further detail. Participant was able to identify that she was an Intermediate metabolizer through written materials. |
| #188 Normal | Participant was not familiar with the term. | Reads results off card, indicates that no medications are affected. |
| #229 Intermed | Participant read off information from the brochure to describe PGx, but stated: “I’m not sure of the importance [...] not sure how it affects my daily life.” | Read results off card, states that the results show: “Something to do with my genes does not metabolize my medicine.” |
3.3. Understanding of pharmacogenomic results (Research Question 2)
No participants knew the name of the gene that had been tested or could name their metabolizer status. The team classified participants into one of three categories based on the criteria established in the flow chart below (Fig. 1).
Fig. 1.
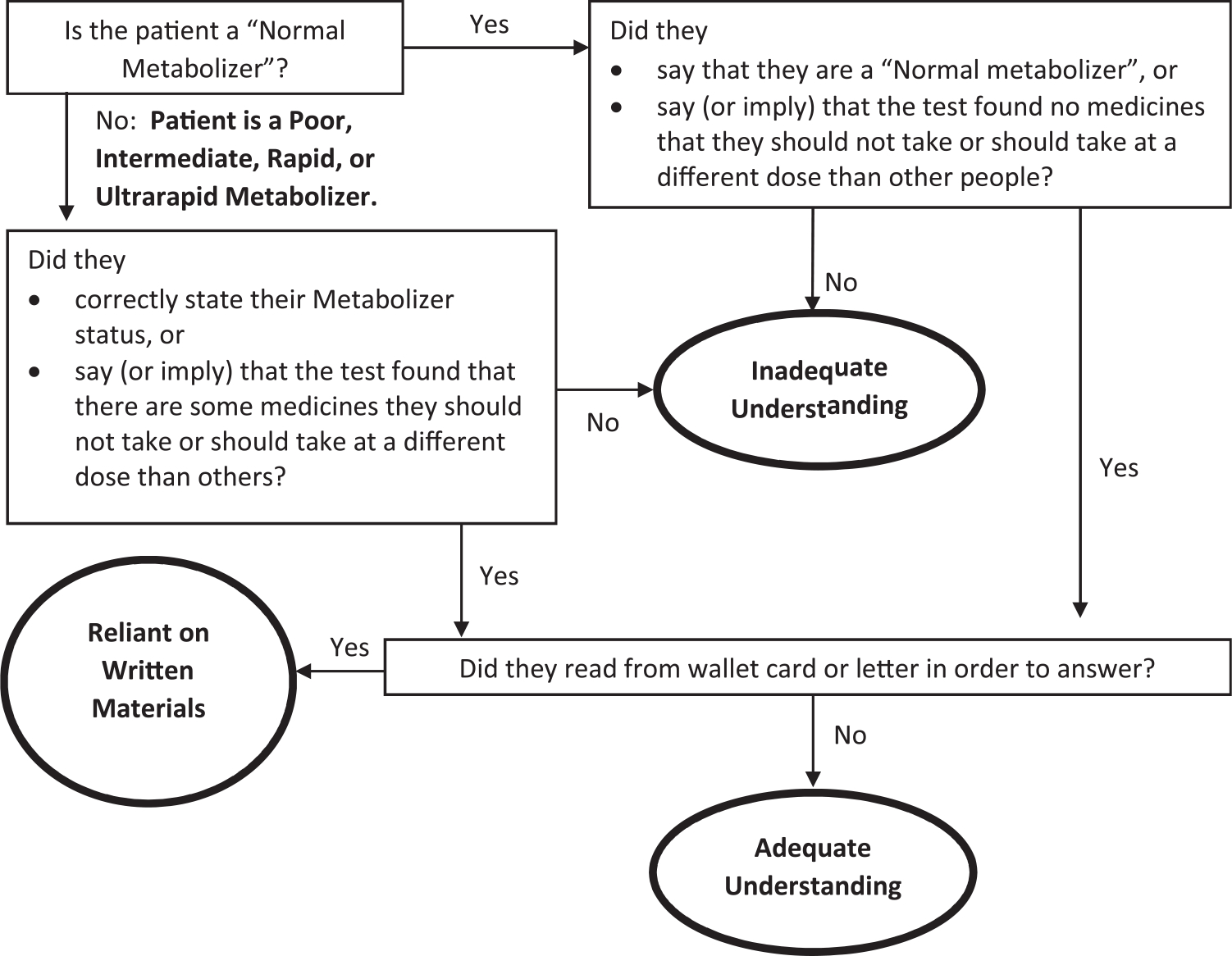
Classifying Levels of PGx Understanding.
Seven participants (23%) were categorized as having “Adequate Understanding” according to these criteria. All seven were not Normal metabolizers, and two correctly named at least one medicine that had altered recommendations based on their PGx results. Four participants (13%) who did not have Adequate Understanding were classified as “Reliant on Written Materials,” since they read their results from the letter or wallet card they had received and showed no independent recall or understanding of their results (Table 2).
The remaining 20 participants (65%) were categorized as having “Inadequate Understanding.” Of the participants who had Inadequate Understanding, only two participants (2/20, 10%) provided a correct definition of “pharmacogenomics,” and only one (5%) knew that she had been tested. One participant with Inadequate Understanding who was a Normal Metabolizer incorrectly stated that the test showed that she “didn’t metabolize Plavix correctly.” Participants with each level of understanding had a variety of metabolizer statuses.
Participants who had Adequate Understanding or were Reliant on Written Materials described PGx as a “genetic” or “DNA” test that was related to medications (Table 3). Some participants were able to identify specific drugs they could not take due to their PGx results, or were able to describe how their metabolizer status would impact their response to some medications. Two participants with Adequate Understanding exhibited important areas of misunderstanding: while they were able to identify that their PGx results indicated they could not take certain drugs, they named specific drugs that are not actually impacted by their metabolizer statuses (Table 3, IDs #2 and #14).
3.4. How participants learned their results (Research Question 3)
Of the seven participants with Adequate Understanding, four learned their results from a healthcare provider and three from the written materials. One of these three participants (ID #216) expressed irritation that he learned that he was tested and about the results through the written materials: “It was all out of the blue. What do you mean my DNA says I should be taking this drug and not this kind of drug?” Of the four participants who were Reliant on Written Materials, all four learned of their PGx results from the written materials Table 4.
Table 4.
How Participants Learned About Their Results.
| ID # and Metab status | How Did Participant Learn About Results? | Comments about value, clarity, and use of written materials |
|---|---|---|
|
| ||
| Adequate Understanding | ||
| #14 Rapid | Written materials | “I didn’t know anything about the test until I got the letter” |
| #623 Intermed | Provider | “I probably just tossed [the letter] aside” |
| #159 Intermed | Provider | Does not recall receiving written materials |
| #60 Intermed | Provider | “I read the card, I realized what it was, and I shoved it my wallet” |
| #653 Poor | Written materials | “Prior to receiving the letter, I didn’t know that I had gotten tested” |
| #2 * (No Recording Available) Poor | Provider | Does not recall receiving written materials |
| #216 Intermed | Written materials | Participant was unable to describe a use for the written materials; “If I kept that letter, I have no idea where it is.” |
| Reliant on Written Materials | ||
| #107 Rapid | Written materials | After reading off metabolizer status: “I have no idea what it means” |
| #108 * Intermed (No Recording Available) | Written materials | Participant states that they would use the written materials when talking with their doctor. |
| #188 Normal | Written materials | Card “told me my metabolizer status and that no medications were affected.” Keeps the care in her wallet. “If I were in an accident and unconscious/unable to communicate, it would be good to have it on me. Otherwise, I can just remember and verbalize the information if needed.” |
| #229 Intermed | Written materials | Participant was unable to describe a use for the written materials. |
3.5. Receipt, use, and opinions of written materials (Research Question 4)
Ten participants (32%) remembered receiving at least some of the written materials. These ten participants provided high ratings for the acceptability and usability of these written materials (Table 5). Of the seven participants with Adequate Understanding, five remembered receiving at least some of the materials, and all five had saved them. Of the ten participants who reported saving written materials, three were unsure where they were. Only one of the 20 participants who had Inadequate Understanding remembered receiving written information.
Table 5.
Acceptability and Usability of Written Materials.
| Statement | Agreement Score (1–4 scale) | ||
|---|---|---|---|
|
|
|||
| Brochure | Letter | Card | |
|
| |||
| You read the [brochure / letter / card] carefully. | 2.75 (n = 4) | 3.33 (n = 3) | 3.14 (n = 7) |
| You found the [brochure / letter / card] helpful. | 2.75 (n = 4) | 3.5 (n = 4) | 3 (n = 7) |
| Other people who are getting pharmacogenomic testing should get a [brochure / letter / card] like this. | 3.75 (n = 4) | 3.5 (n = 4) | 3.57 (n = 7) |
| The [brochure / letter / card] made sense to you. | 2.67 (n = 3) | 3.2 (n = 5) | 3 (n = 8) |
| The [brochure / letter / card] contained information you found was important. | 3 (n = 3) | 3.4 (n = 5) | 3.43 (n = 7) |
| The [brochure / letter / card] will help you talk to your healthcare providers in the future. | 3 (n = 3) | 3 (n = 4) | 3.4 (n = 5) |
| The [brochure / letter / card] contained too much information. (Reverse Coding)* | 2.33 (n = 3) | 2.2 (n = 5) | 1.86 (n = 7) |
| The [brochure / letter / card] contained information that you already knew. (Reverse Coding)* | 1.33 (n = 3) | 1.8 (n = 5) | 2 (n = 6) |
| The [brochure / letter / card] was confusing. (Reverse Coding)* | 1.67 (n = 3) | 1.75 (n = 4) | 1.83 (n = 6) |
Higher scores indicate a positive reception of written materials. Lower scores for reverse coded questions indicate positive reception of written materials.
3.6. Opinions about PGx testing (Research Question 5)
Near the end of the interview, after participants were given a definition of PGx and told that they had had PGx testing, participants expressed a belief in the benefit of PGx testing, confidence that providers would have access to their results, and belief that knowing their own PGx results would have a positive impact on their healthcare (See Table 6). A minority expressed concerns: Twelve of 31 participants (39%) agreed or strongly agreed with the statement “I am worried that my PGx test results in the electronic health record could be accessed by the wrong people in the future.”
Table 6.
Participant Perception of Future Use of PGx Results.
| Question | Strongly Agree | Agree | Neither Agree or Disagree | Disagree | Strongly disagree |
|---|---|---|---|---|---|
|
| |||||
| It is important for you to tell your future healthcare providers that you had PGx testing done. | 14 (45%) | 16 (52%) | 0 | 1 (3%) | 0 |
| Your PGx testing does not apply to your future healthcare. | 1 (3%) | 8 (26%) | 0 | 11 (36%) | 9 (29%) |
| Your healthcare providers know your results without you telling them anything. | 9 (29%) | 13 (42%) | 0 | 3 (10%) | 3 (10%) |
| Other healthcare providers can see your PGx test results in the electronic health record. | 7 (23%) | 20 (65%) | 0 | 1 (3%) | 2 (7%) |
| Making PGx test results available to my healthcare providers in the future will help them make better decisions about prescribing medications for me. | 15 (48%) | 15 (48%) | 0 | 1 (3%) | 0 |
| I am worried that my PGx test results in the electronic health record could be accessed by the wrong people in the future. | 4 (13%) | 8 (26%) | 0 | 13 (42%) | 6 (19%) |
| Letting me know the results of my PGx testing would help me improve my healthcare in the future. | 14 (45%) | 14 (45%) | 2 (7%) | 1 (3%) | 0 |
4. Discussion
This paper is the first to report patients’ objective understanding of their PGx test results. We found that only a minority of participants knew what PGx is, knew that they had this kind of testing, or understood their results at even a minimal level. Most participants did not remember receiving the written materials that explained the testing and their results. Those who did remember gave the materials high ratings for usability and acceptability, and some participants relied on the letter or wallet card to answer questions about their results. Once participants were told what PGx is and that they had undergone PGx testing, a majority believed that it would improve their healthcare if they and their providers were aware of the results. This study demonstrates significant problems in patient understanding of PGx and PGx test results and emphasizes the importance of developing and implementing tools to educate patients and to help them alert their providers about the existence of PGx test results.
4.1. Patient understanding
The small percentage of our participants who recognized the term “pharmacogenomics” or knew they had this testing when it was described to them suggests that only a minority could tell a future provider that they had undergone this sort of testing. Many providers may not know about previous PGx testing, since systems to alert providers are still being developed and implemented. [5,6] Also, since patients often receive healthcare from multiple healthcare systems, their providers may not have access to electronic health records containing the previous results. If providers do not know about previous PGx results, they may repeat the same tests or, more importantly, may prescribe medications predicted to have reduced efficacy or a high risk of adverse effects for their patients.
Even among the participants who recalled having PGx testing, none knew the name of the gene that had been tested or their metabolizer status. Participants met Adequate Understanding criteria as long as they knew whether the testing identified medicines that affected their recommended medications or dosing. At this level of understanding, a patient could at least tell a future provider that they had undergone testing whose results affected medication recommendations, which in turn could lead a provider to look up the results and respond appropriately. It is worrisome that just seven of the 26 participants who were not Normal metabolizers had this level of knowledge, and just two could correctly identify at least one medicine predicted to be affected. This indicates a significant lack of understanding about PGx test results that again can lead to significant and unnecessary dangers.
Four participants located the written materials they had been sent and read the results from them (“Reliant on Written Materials”), which is another potentially effective way for patients to alert providers about their PGx results. One advantage of written materials is that they provide a large amount of information, including the name of the gene tested, alleles identified, metabolizer status, and some of the affected medicines. Scholars advocating a view of the “extended mind” have emphasized the importance of systems for encoding and retrieving information beyond the brain, including written documentation. [21] At the same time, just four participants referred to their letter or card in this way, and just two explicitly stated they would show it to a healthcare provider.
Our participants’ understanding was poor even though 87% had at least a high school diploma or GED, 74% had completed at least some college or trade school or had a Bachelor’s degree, and they had a mean score for health literacy of 2.07 on a 9 point scale (with a lower score indicating higher health literacy). In addition, the providers in the cardiology clinic where the testing occurred are familiar with CYP2C19 PGx testing and agreed to the implementation project, and patients were sent written materials about the results. Our participants’ lack of understanding may partly stem from the limited consent process before the testing. They had given merely verbal consent to testing, often after a very short conversation, and possibly during an acute health event requiring cardiac catheterization.
Problems with patient understanding have been documented widely, including in various areas of clinical care [22–24] and informed consent for research participation. [25,26] Within genetics, studies have shown that patients often fail to understand key test results. For instance, one study found that patients who were provided with results of genetic testing for hypertrophic cardiomyopathy often misunderstood the risk of themselves or their children developing the condition. [27] Another study documented misunderstanding of test results among participants who had or were at risk for developing Huntington’s disease, breast cancer, or Alpha-1 antitrypsin deficiency. [28] One might anticipate that PGx test results would be easier to understand, since they primarily involve specific medicines that should be avoided rather than disease risk, but our study does not support this hypothesis.
The percentage of participants who met the criteria for Adequate Understanding of PGx results in our study was significantly lower than the percentage self-reporting understanding in earlier studies. [10–13] It is possible that the patients in the previous studies were expressing over-confidence bias, which has been found widely, including regarding understanding of health information. [29] The potential disconnect between self-reported (subjective) and objective levels of understanding emphasizes the importance of directly measuring each in studies of PGx implementation.
4.2. Learning results from providers and written materials
Participants who were able to report their results in some way (Adequate Understanding or Reliant on Written Materials), learned of their results either from their healthcare provider or the written materials. These methods of communication failed to adequately inform the majority of our participants, indicating that these methods need to be improved or supplemented. While studies show that patients like to receive genetic results from their healthcare providers, [11,30–34] previous studies have found that providers face significant difficulties in conveying genetic test results to their patients. [35] Beyond improving clinician understanding of PGx, [36–38] it may be important to provide training on conveying PGx results to patients. Communication techniques might be helpful, such as the “teach-back” method (i.e., asking a patient to explain their results in their own words). [39].
It is unclear why only a minority of participants in our study remembered receiving the written materials they were sent. It is possible, but unlikely, that there was some problem with the mailing system, which was contracted to a highly regarded private company. It is also possible that participants did not open the materials or opened them but subsequently forgot about them. It is unlikely that many of our participants could not understand the written materials, since the letter was written at an eighth-grade level and 87% of our patients had graduated from high school.
Some participants who remembered receiving the materials reported not knowing where they were, so would not be able to rely on them to remind themselves or inform future providers about the results. While studies show that written materials can improve patient understanding, [40–43] minimal work has been done to evaluate whether patients save materials for future use and reference. One study found that 42 of 44 participants (95.5%) kept leaflets with information about medicines for future use, a significantly higher percentage than in our study. [44] The leaflets in that previous study, though, were provided by pharmacists along with verbal counseling during the process of filling a prescription. Information that is sent electronically, such as through a patient portal of an electronic health record patient, might be more easily accessed by patients in the future. It should be noted, however, that many patients face significant barriers to using health informatics technology such as patient portals. [45].
4.3. Opinions of PGx
It is notable that once participants were given a definition of PGx and told that they had undergone this testing, the vast majority agreed with statements about its importance to their healthcare and disagreed with statements downplaying its importance (Table 5). This finding is consistent with previous studies that have shown that laypeople who receive education about PGx are supportive of its importance and potential benefit. [46–48] There is a great opportunity to inform patients about PGx testing and their results in ways that can help them engage actively in directing and supporting their care.
4.4. Limitations
Our study has several limitations. First, the study was conducted at a single healthcare center, based on PGx testing of a single gene, with patients receiving one set of written materials to explain those results. Second, the study population had a mean age over 65 years old often had PGx testing while or shortly after undergoing an urgent or even emergent procedure (e.g., stent placement) that may have taken place after a major health event (e.g., myocardial infarction). It is uncertain if a younger population undergoing PGx testing in a different clinical setting would exhibit a similar lack of understanding. Third, judgments about adequacy of patient understanding were made based on an interpretation of qualitative data, introducing potential uncertainty. Fourth, the small sample size limits the ability to interpret and generalize from the quantitative data.
5. Conclusion
Our study identified significant limitations in patient understanding of PGx testing and PGx results. A majority of participants lacked understanding and awareness of their PGx results, despite being provided with written materials that explained PGx and these results. These limitations in understanding may increase the danger of a future provider prescribing medications that are ineffective or dangerous for the patient.
5.1. Practice implications
Implementation of PGx testing in clinical practice must effectively support the ability of patients to understand their results and share information with their providers. Potential approaches include explaining PGx to patients more clearly at the time of testing, possibly with a general information brochure about PGx, and potentially following up with a call from clinic staff to review results and alert the patient to watch for the arrival of written materials explaining those results. Other approaches could involve messaging through the patient portal of the electronic health record and educating providers to help them better explain PGx and the results to patients.
Supplementary Material
Acknowledgements
This study was funded by the Precision Health Initiative of the Indiana University Grand Challenges Program.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
Ethics declaration
The study was approved by the Institutional Review Board (IRB) of Indiana University.
CRediT authorship contribution statement
Doyle Tom A: Writing – review & editing, Writing – original draft, Formal analysis. Karen K. Schmidt: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Colin M.E. Halverson: Writing – review & editing, Methodology. Jesus Olivera: Writing – review & editing, Methodology, Investigation, Data curation. Abigail Garcia: Writing – review & editing, Methodology, Investigation, Data curation. Tyler A. Shugg: Writing – review & editing, Project administration, Data curation. Todd C. Skaar: Writing – review & editing, Methodology, Funding acquisition, Conceptualization. Peter H. Schwartz: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pec.2023.107904.
Data Availability
Given the potentially identifiable nature of qualitative data, the data are not available for public use. De-identified, relevant data are available upon request for academic purposes.
References
- [1.].Heise CW, Gallo T, Curry SC, Woosley RL. Identification of populations likely to benefit from pharmacogenomic testing. Pharm Genom 2020;30(5):91–5. 10.1097/FPC.0000000000000400. [DOI] [PubMed] [Google Scholar]
- [2.].Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level a drugs prescribed among us veterans health administration pharmacy users. JAMA Netw Open 2019;2(6):e195345. 10.1001/jamanetworkopen.2019.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3.].IGNITE Network. Implementation Guides. IGNITE. Accessed September 20, 2022. 〈https://gmkb.org/implementation-guides/〉. [Google Scholar]
- [4.].Filipski K, Murphy J, Helzlsouer K. Updating the landscape of direct-to-consumer pharmacogenomic testing. Pharm Pers Med 2017;Volume 10:229–32. 10.2147/PGPM.S140461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5.].Wake DT, Smith DM, Kazi S, Dunnenberger HM. Pharmacogenomic clinical decision support: a review, how-to guide, and future vision. Clin Pharm Ther 2022;112(1):44–57. 10.1002/cpt.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6.].Herr TM, Peterson JF, Rasmussen LV, Caraballo PJ, Peissig PL, Starren JB. Pharmacogenomic clinical decision support design and multi-site process outcomes analysis in the eMERGE network. J Am Med Inf Assoc JAMIA 2019;26(2):143–8. 10.1093/jamia/ocy156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7.].Guy JW, Patel I, Oestreich JH. Clinical application and educational training for pharmacogenomics. Pharmacy 2020;8(3):163. 10.3390/pharmacy8030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8.].Haga S, Burke W, Ginsburg G, Mills R, Agans R. Primary care physicians’ knowledge of and experience with pharmacogenetic testing: Primary care physicians’ and pharmacogenetic testing. Clin Genet 2012;82(4):388–94. 10.1111/j.1399-0004.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9.].Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. In: Mathes T, editor. PLOS ONE, 15; 2020, e0231350. 10.1371/journal.pone.0231350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10.].Haga SB, Mills R, Moaddeb J, Allen Lapointe N, Cho A, Ginsburg GS. Patient experiences with pharmacogenetic testing in a primary care setting. Pharmacogenomics 2016;17(15):1629–36. 10.2217/pgs-2016-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11.].Olson JE, Rohrer Vitek CR, Bell EJ, et al. Participant-perceived understanding and perspectives on pharmacogenomics: the Mayo Clinic RIGHT protocol (Right Drug, Right Dose, Right Time. Genet Med 2017;19(7):819–25. 10.1038/gim.2016.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12.].Haga SB, Liu Y. Patient characteristics, experiences and perceived value of pharmacogenetic testing from a single testing laboratory. Pharmacogenomics 2019;20(8):581–7. 10.2217/pgs-2019-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13.].Lemke AA, Hulick PJ, Wake DT, et al. Patient perspectives following pharmacogenomics results disclosure in an integrated health system. Pharmacogenomics 2018;19(4):321–31. 10.2217/pgs-2017-0191. [DOI] [PubMed] [Google Scholar]
- [14.].Allen JD, Zhang L, Johnson ANK, et al. Development and validation of the minnesota assessment of pharmacogenomic literacy (MAPL). J Pers Med 2022;12(9):1398. 10.3390/jpm12091398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15.].Lee CR, Luzum JA, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharm Ther 2022;112(5):959–67. 10.1002/cpt.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16.].Lima JJ, Thomas CD, Barbarino J, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin Pharm Ther 2021;109(6):1417–23. 10.1002/cpt.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17.].Hicks J, Sangkuhl K, Swen J, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharm Ther 2017;102(1):37–44. 10.1002/cpt.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18.].Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36(8):588–94. [PubMed] [Google Scholar]
- [19.].Corbin JM, Strauss AL. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Fourth edition. SAGE,; 2015. [Google Scholar]
- [20.].Tavory I, Timmermans S. Abductive Analysis: Theorizing Qualitative Research. The University of Chicago Press,; 2014. [Google Scholar]
- [21.].Slaby J, Gallagher S. Critical Neuroscience and Socially Extended Minds. Theory Cult Soc 2015;32(1):33–59. 10.1177/0263276414551996. [DOI] [Google Scholar]
- [22.].Horwitz LI, Moriarty JP, Chen C, et al. Quality of discharge practices and patient understanding at an academic medical center. JAMA Intern Med 2013;173(18):1715–22. 10.1001/jamainternmed.2013.9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23.].Engel KG, Buckley BA, Forth VE, et al. Patient understanding of emergency department discharge instructions: where are knowledge deficits greatest? Acad Emerg Med J Soc Acad Emerg Med 2012;19(9):E1035–44. 10.1111/j.1553-2712.2012.01425.x. [DOI] [PubMed] [Google Scholar]
- [24.].Schwartz PH, Edenberg E, Barrett PR, Perkins SM, Meslin EM, Imperiale TF. Patient understanding of benefits, risks, and alternatives to screening colonoscopy. Fam Med 2013;45(2):83–9. [PubMed] [Google Scholar]
- [25.].Beskow LM, Weinfurt KP. Exploring understanding of “understanding”: the paradigm case of biobank consent comprehension. Am J Bioeth AJOB 2019;19(5):6–18. 10.1080/15265161.2019.1587031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26.].Kasperbauer TJ, Schmidt KK, Thomas A, Perkins SM, Schwartz PH. Incorporating biobank consent into a healthcare setting: challenges for patient understanding. AJOB Empir Bioeth 2021;12(2):113–22. 10.1080/23294515.2020.1851313. [DOI] [PubMed] [Google Scholar]
- [27.].Bonner C, Spinks C, Semsarian C, Barratt A, Ingles J, McCaffery K. Psychosocial impact of a positive gene result for asymptomatic relatives at risk of hypertrophic cardiomyopathy. J Genet Couns 2018;27(5):1040–8. 10.1007/s10897-018-0218-8. [DOI] [PubMed] [Google Scholar]
- [28.].Klitzman RL. Misunderstandings concerning genetics among patients confronting genetic disease. J Genet Couns 2010;19(5):430–46. 10.1007/s10897-010-9307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29.].Canady BE, Larzo M. Overconfidence in managing health concerns: the dunning–kruger effect and health literacy. Published online June 29 J Clin Psychol Med Settings 2022. 10.1007/s10880-022-09895-4. Published online June 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30.].Lee YM, McKillip RP, Borden BA, Klammer CE, Ratain MJ, O’Donnell PH. Assessment of patient perceptions of genomic testing to inform pharmacogenomic implementation. Pharm Genom 2017;27(5):179–89. 10.1097/FPC.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31.].Maloney KA, Alaeddin DS, von Coelln R, et al. Parkinson’s disease: patients’ knowledge, attitudes, and interest in genetic counseling. J Genet Couns 2018;27(5):1200–9. 10.1007/s10897-018-0239-3. [DOI] [PubMed] [Google Scholar]
- [32.].Nusbaum R, Leventhal KG, Hooker GW, et al. Translational genomic research: protocol development and initial outcomes following SNP testing for colon cancer risk. Transl Behav Med 2013;3(1):17–29. 10.1007/s13142-012-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33.].Smit AK, Keogh LA, Hersch J, et al. Public preferences for communicating personal genomic risk information: a focus group study. Health Expect 2016;19(6):1203–14. 10.1111/hex.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34.].Veilleux S, Bouffard M, Bourque Bouliane M. Patient and health care provider needs and preferences in understanding pharmacogenomic and genomic testing: a meta-data analysis. Qual Health Res 2020;30(1):43–59. 10.1177/1049732319858325. [DOI] [PubMed] [Google Scholar]
- [35.].Rosas-Blum E, Shirsat P, Leiner M. Communicating genetic information: a difficult challenge for future pediatricians. BMC Med Educ 2007;7(1):17. 10.1186/1472-6920-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36.].Nickola TJ, Green JS, Harralson AF, O’Brien TJ. The current and future state of pharmacogenomics medical education in the USA. Pharmacogenomics 2012;13(12):1419–25. 10.2217/pgs.12.113. [DOI] [PubMed] [Google Scholar]
- [37.].Rohrer Vitek CR, Abul-Husn NS, Connolly JJ, et al. Healthcare provider education to support integration of pharmacogenomics in practice: the eMERGE Network experience. Pharmacogenomics 2017;18(10):1013–25. 10.2217/pgs-2017-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38.].Green JS, O’Brien TJ, Chiappinelli VA, Harralson AF. Pharmacogenomics instruction in US and Canadian medical schools: implications for personalized medicine. Pharmacogenomics 2010;11(9):1331–40. 10.2217/pgs.10.122. [DOI] [PubMed] [Google Scholar]
- [39.].Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed Pr Health Care Prof VA DoD PHS 2019;36(6):284–9. [PMC free article] [PubMed] [Google Scholar]
- [40.].Adepu R, Swamy MK. Development and evaluation of patient information leaflets (PIL) usefulness. Indian J Pharm Sci 2012;74(2):174–8. 10.4103/0250-474X.103857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41.].Akour A, Bardaweel S, Awwad O, Al-Muhaissen S, Hussein R. Impact of a pharmacist-provided information booklet on knowledge and attitudes towards oral contraception among Jordanian women: an interventional study. Eur J Contracept Reprod Health Care J Eur Soc Contracept 2017;22(6):459–64. 10.1080/13625187.2017.1412425. [DOI] [PubMed] [Google Scholar]
- [42.].Hill J, Bird H. The development and evaluation of a drug information leaflet for patients with rheumatoid arthritis. Rheuma Oxf Engl 2003;42(1):66–70. 10.1093/rheumatology/keg032. [DOI] [PubMed] [Google Scholar]
- [43.].Piredda M, Migliozzi A, Biagioli V, Carassiti M, De Marinis M. Written information improves patient knowledge about implanted ports. Clin J Oncol Nurs 2016;20(2):E28–33. 10.1188/16.CJON.E28-E33. [DOI] [PubMed] [Google Scholar]
- [44.].Mai A, Aslani P. Impact of Vietnamese written and verbal medicine information on Vietnamese-speaking Australians’ knowledge and satisfaction. Br J Clin Pharm 2007;64(4):527–35. 10.1111/j.1365-2125.2007.02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45.].Zhao JY, Song B, Anand E, et al. Barriers, facilitators, and solutions to optimal patient portal and personal health record use: a systematic review of the literature. AMIA Annu Symp Proc AMIA Symp 2017;2017:1913–22. [PMC free article] [PubMed] [Google Scholar]
- [46.].Haga SB, O’Daniel JM, Tindall GM, Lipkus IR, Agans R. Survey of US public attitudes toward pharmacogenetic testing. Pharm J 2012;12(3):197–204. 10.1038/tpj.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47.].Haga SB, Tindall G, O’Daniel JM. Public perspectives about pharmacogenetic testing and managing ancillary findings. Genet Test Mol Biomark 2012;16(3):193–7. 10.1089/gtmb.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48.].Rogausch A, Prause D, Schallenberg A, Brockmöller J, Himmel W. Patients ‘ and physicians’ perspectives on pharmacogenetic testing. Pharmacogenomics 2006;7(1):49–59. 10.2217/14622416.7.1.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Given the potentially identifiable nature of qualitative data, the data are not available for public use. De-identified, relevant data are available upon request for academic purposes.


