Abstract
Spray drying of skim milk was evaluated as a means of preserving Lactobacillus paracasei NFBC 338 and Lactobacillus salivarius UCC 118, which are human-derived strains with probiotic potential. Our initial experiments revealed that NFBC 338 is considerably more heat resistant in 20% (wt/vol) skim milk than UCC 118 is; the comparable decimal reduction times were 11.1 and 1.1 min, respectively, at 59°C. An air outlet temperature of 80 to 85°C was optimal for spray drying; these conditions resulted in powders with moisture contents of 4.1 to 4.2% and viable counts of 3.2 × 109 CFU/g for NFBC 338 and 5.2 × 107 CFU/g for UCC 118. Thus, L. paracasei NFBC 338 survived better than L. salivarius UCC 118 during spray drying; similar results were obtained when we used confocal scanning laser microscopy and LIVE/DEAD BacLight viability staining. In addition, confocal scanning laser microscopy revealed that the probiotic lactobacilli were located primarily in the powder particles. Although both spray-dried cultures appeared to be stressed, as shown by increased sensitivity to NaCl, bacteriocin production by UCC 118 was not affected by the process, nor was the activity of the bacteriocin peptide. The level of survival of NFBC 338 remained constant at ∼1 × 109 CFU/g during 2 months of powder storage at 4°C, while a decline in the level of survival of approximately 1 log (from 7.2 × 107 to 9.5 × 106 CFU/g) was observed for UCC 118 stored under the same conditions. However, survival of both Lactobacillus strains during powder storage was inversely related to the storage temperature. Our data demonstrate that spray drying may be a cost-effective way to produce large quantities of some probiotic cultures.
Given that probiotic microorganisms play a role in promoting and maintaining health (29) has stimulated considerable interest in incorporating these into functional foods and pharmaceutical products. By definition, probiotics are “living microorganisms, which upon ingestion in certain numbers, exert health benefits beyond inherent basic nutrition” (13), and it is recommended that probiotic products contain at least 107 live microorganisms per g or per ml (15). Therefore, from a commercial point of view, an inexpensive method for large-scale production of cultures containing high levels of viable probiotic cells in a form suitable for product applications is highly desirable.
In previous studies researchers have investigated the production of freeze-dried powders and frozen concentrates of probiotic Bifidobacterium and Lactobacillus spp. (10, 12, 24). However, there are many disadvantages associated with this approach; freeze-drying is time-consuming and expensive, there are high transport and storage costs associated with frozen concentrated cultures, and the freeze-thaw process is associated with a loss of culture viability. In comparison, spray drying, one of the predominant processing tools used in the dairy industry, can be used to produce large amounts of dairy ingredients relatively inexpensively; it has been estimated that the cost of spray drying is six times lower per kilogram of water removed than the cost of freeze-drying (20). Spray-dried powders can be transported at a low cost and can be stored in a stable form for prolonged periods. However, there are obvious challenges associated with using spray drying to produce viable cultures, including the requirement that the microorganisms survive the relatively high temperatures used (5). While freeze-drying is more suitable than spray drying for some cultures (17), researchers have found that there is no difference in microbial viability between these methods (33).
In previous studies, workers have investigated the use of spray drying as a way to preserve yogurt with viable microorganisms (18) and dairy starter cultures, such as Lactobacillus bulgaricus and Streptococcus thermophilus (23, 33, 34), and as a way to attenuate adjunct cultures, such as Lactobacillus helveticus (16, 17). In addition to maintaining the viability of probiotic cultures, it is important that probiotic properties are maintained following the spray-drying process. Although spray-dried probiotic cultures are available commercially, the previously published data related to spray drying of such microorganisms is limited. Some studies have been undertaken to investigate the survival during spray drying of Lactobacillus acidophilus cultures chosen for their health-promoting properties (8, 27, 28). In addition, a process for spray drying probiotic lactic acid bacteria, including Lactobacillus and Leuconostoc spp. and Bifidobacterium spp., has been described in a patent application by Meister et al. (N. Meister, A. Sutter, and M. Vikas, 19 March 1998, European Patent Office), who developed a food powder that contained 109 CFU/g and exhibited at least 10% probiotic survival per year.
Because of the advantages of spray drying, in the present study we investigated the use of this method as a way to preserve human-derived Lactobacillus paracasei and Lactobacillus salivarius strains. The cultures which we used have been well characterized previously with respect to both their probiotic properties (4, 7, 19) and their behavior in dairy products, including Cheddar cheese and yogurt (11, 30; G. Gardiner, R. P. Ross, and C. Stanton, unpublished data; E. O'Sullivan, G. F. Fitzgerald, and J. K. Collins, unpublished data). The aim of the present study was to investigate spray drying as a method for pilot-scale production of dairy-based powders containing these probiotic Lactobacillus cultures.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Probiotic strains NFBC 338 and UCC 118, which were isolated previously from the human gastrointestinal tract (GIT) and were identified as members of L. paracasei subsp. paracasei and L. salivarius subsp. salivarius, respectively, by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of total cell proteins (26), were obtained from University College Cork, Cork, Ireland, under a restricted-materials transfer agreement. These strains were routinely cultured as previously described (11). For spray-drying purposes, both probiotic Lactobacillus strains were cultured as follows. Cells harvested by centrifugation (1,600 × g, 10 min) from overnight MRS broth (6) cultures were concentrated 10-fold in maximum-recovery diluent (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom). The resulting culture concentrates were then inoculated (1%) into 1.8 liters of heat-treated (90°C, 30 min) 20% reconstituted skim milk (RSM) supplemented with 0.5% (wt/vol) yeast extract (Merck, Darmstadt, Germany), which was added to aid culture growth. An additional 1% (wt/vol) sucrose (Sigma Chemical Co., Poole, Dorset, United Kingdom) was added for L. salivarius UCC 118, as this organism cannot utilize lactose. The inoculated milk preparations were incubated at 37°C, and fermentation was terminated just prior to coagulation by cooling on ice (i.e., after 3.5 and 6 h for L. salivarius UCC 118 and L. paracasei NFBC 338, respectively). The sensitive indicator strain Bacillus coagulans NCIMB 9365, which was used to detect bacteriocin production by L. salivarius UCC 118, was routinely grown in Trypticase soy broth (Oxoid) supplemented with 0.5% (wt/vol) yeast extract.
Heat challenge experiments.
The heating menstrum used was heat-treated (90°C, 30 min) 20% (wt/vol) RSM supplemented with 0.5% (wt/vol) yeast extract; an additional 1% (wt/vol) sucrose was added for strain UCC 118. The heating method used was essentially the method described by Teixeira et al. (35). Two 50-ml portions of RSM in 100-ml bottles were agitated with magnetic stirrer bars and were placed in a water bath at the following test temperatures: 37°C (for the control and to obtain the initial count) and 55, 58, 59, 60, and 61°C. One bottle was used to monitor the temperature and, after temperature equilibration, a 1% inoculum of an overnight culture of either L. paracasei NFBC 338 or L. salivarius UCC 118 was added to the second bottle. At intervals (between 30 s and 4 min at the temperatures used), 1-ml samples were removed from the test bottles, serially diluted in maximum-recovery diluent, and pour plated onto MRS agar. The survivors were counted after 3 days of anaerobic incubation at 37°C. Duplicate tests were conducted at each temperature, and the mean log survivor counts were plotted as a function of heating time for each temperature. At each temperature a best-fit straight line was obtained by regression analysis, and decimal reduction times (D values) (the times required to kill 90% of the cells) were determined by determining the absolute value of the inverse of the slope of this line (31).
Spray drying of probiotic cultures.
The pH values of cultures of both probiotic strains grown in RSM as described above were adjusted to 6.8 with 4 N NaOH, and the cultures were warmed to ∼15°C and then spray dried with a laboratory-scale spray dryer (model B191 Buchi mini spray dryer; Flawil, Switzerland) by using a constant inlet air temperature of 170°C. The fermentate was atomized and sprayed into the drying chamber by using a two-fluid nozzle, and the product dried almost instantaneously; the residence time was very low. To investigate the effect of the outlet air temperature, the feed rate was varied to obtain outlet temperatures ranging from 60 to 120°C. Each trial was conducted in duplicate for both probiotic strains. The data obtained in these trials indicated that the optimal drying conditions for both Lactobacillus cultures were an air inlet temperature of 170°C and an air outlet temperature of 80 to 85°C, which yielded powders with moisture contents within the recommended range (21). The powders were then stored in sealed polyethylene bags at 4, 15, and 30°C, and probiotic viability was assessed over time.
Determination of probiotic viability in spray-dried powders.
We assessed the viability of the probiotic lactobacilli in the inoculated milk preparations before spray drying and in the resulting powders by examining duplicate MRS pour plates after 3 days of anaerobic incubation at 37°C. To 0.1 g of powder, 9.9 ml of maximum-recovery diluent was added (1:100 dilution); the preparation was allowed to rehydrate for ∼1 h and then diluted further with diluent, and appropriate dilutions were pour plated. The percent survival at each of the outlet temperatures tested was calculated as follows: % survival = (N/N0) × 100, where N0 is the number of bacteria per gram of dry matter before drying and N is the number of bacteria per gram of dry matter in the powder.
We also assessed the viability of the probiotic Lactobacillus cultures in spray-dried powders by using confocal scanning laser microscopy (CSLM) and LIVE/DEAD BacLight viability staining. With this technique, two nucleic acid stains, propidium iodide and SYTO 9, were used to differentiate viable and nonviable bacterial cells based on membrane permeability. A Zeiss model LSM310 confocal scanning laser microscope (Carl Zeiss Ltd., Welwyn Garden City, Herts, United Kingdom) was used to acquire digital images which were 525 by 512 pixels with a resolution of 0.2 μm/pixel. A 2× stock solution of the LIVE/DEAD BacLight viability stain (Molecular Probes Inc., Eugene, Oreg.) was prepared according to the manufacturer's instructions. One hundred microliters of the stain was then mixed with an equal volume of RSM powder. Following 1 h of incubation in the dark, 2.5 μl of the stained milk was placed on a microscope slide and covered with a coverslip. Simultaneous pseudocolor dual-channel CSLM imaging with 488-nm excitation was used to display green fluorescence and red fluorescence, which represented live and dead cells, respectively. An image analysis of the color CSLM images was then performed by using a Kontron model KS400 image analysis system (Imaging Associates Ltd., Thame, Oxon, United Kingdom) to separate the red and green fluorescence signals and to calculate the area of green fluorescence as a percentage of the total bacterial fluorescence. In this way, the effect of outlet temperature during spray drying on probiotic viability was determined. In addition, in order to observe the probiotic lactobacilli in situ in skim milk powder, a small amount (∼10 μg) of powder was mixed with ∼20 μl of 0.1% (wt/vol) Nile blue A stain (sulfite salt; catalog no. CI 51180; Sigma Chemical Co.) in a 90% (vol/vol) solution of polyethylene glycol (molecular weight, 200; Sigma Chemical Co.). CSLM was used to observe the powders 20 to 30 min after staining by using 633-nm excitation.
Electron microscopy of spray-dried powders.
Spray-dried powders were attached to brass stubs and coated with gold by using a model E5100 scanning electron microscopy coating system (Bio-Rad, Hercules, Calif.). Samples were then examined with a JEDL model JSM-35 scanning electron microscope by using an accelerating voltage of 20 kV. Micrographs were taken at various magnifications.
Determination of moisture contents of spray-dried powders.
The moisture contents of spray-dried skim milk powders were determined in duplicate by oven drying the powders at 102°C, determining the difference in weight, and expressing the weight loss as a percentage of the powder weight (14). A moisture content of 4% is recommended for skim milk powder (21).
Salt tolerance test.
In order to investigate possible cellular damage resulting from the spray-drying process, we determined the sensitivity of L. paracasei NFBC 338 and L. salivarius UCC 118 cultures to NaCl before and after spray drying as follows. Fresh overnight MRS broth cultures and culture-containing spray-dried powders (prepared by using air inlet and outlet temperatures of 170 and 80 to 85°C, respectively) were pour plated onto MRS agar supplemented with 4 to 5% NaCl (Prolabo, Paris, France). The plates were examined after 3 to 6 days of anaerobic incubation at 37°C, and the colony sizes and numbers were compared with the colony sizes and numbers on MRS plates without NaCl.
Bacteriocin assay and activity.
Prior to spray drying, L. salivarius UCC 118 was grown in RSM as described above and filter sterilized by using 0.45-μm-pore-size filters. Five-microliter portions of the cell-free supernatant were then spotted onto seeded indicator plates, which were incubated overnight at 37°C. These indicator plates were prepared by overlaying 3 ml of soft agar seeded with 300 μl of an overnight culture of the B. coagulans indicator strain on Trypticase soy agar supplemented with 0.6% (wt/vol) yeast extract. Bacteriocin production was revealed by the formation of a clear zone of inhibition in the indicator lawn after overnight incubation. MRS broth cultures grown from spray-dried powders were assayed for bacteriocin production in a similar manner. Protease sensitivity was determined by spotting 5 μl of a 5-mg/ml solution of proteinase K (Sigma Chemical Co.) close to the cell-free supernatant spot on an indicator plate. Protease sensitivity was indicated by inhibition of the zone of clearing. Bacteriocin activity in the cell-free supernatant was determined as previously described (25). In addition, bacteriocin production was detected and bacteriocin activity was determined in spray-dried powders containing strain UCC 118 as follows. One gram of powder was rehydrated in 10 ml of maximum-recovery diluent, and a cell-free supernatant was obtained as described above. Protease sensitivity and bacteriocin activity were determined as described above. Activity was expressed in activity units per gram of powder.
RESULTS AND DISCUSSION
In the present study, we investigated the use of spray drying as a way to prepare dairy-based powders harboring high numbers of viable cells of the human-derived strains L. paracasei NFBC 338 and L. salivarius UCC 118. This study involved assessing the heat resistance of the Lactobacillus strains and subsequent spray drying at a range of outlet temperatures.
Heat resistance of the probiotic strains.
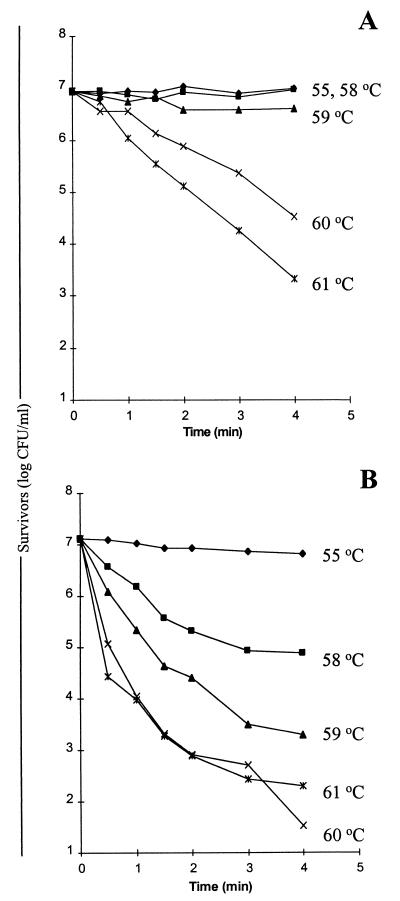
One of the principal factors that govern microbial survival during spray drying is the ability of a strain to withstand high temperatures, and previous studies have shown that different Lactobacillus spp. vary in this respect (9, 32). Our initial experiments involved determining the heat resistance of the probiotic Lactobacillus strains in the skim milk medium subsequently used for spray drying. The results showed that L. paracasei NFBC 338 and L. salivarius UCC 118 did not differ in the ability to survive at 55°C, but at temperatures above 58°C, differences in the thermal tolerance of these strains became apparent (Fig. 1). For instance, when NFBC 338 was heated at 58°C, 100% of the culture survived, while there was a 46% reduction in cell numbers (from 8.6 × 106 to 4 × 106 CFU/ml) following incubation at 59°C for 4 min (Fig. 1A). In contrast, the viability of L. salivarius UCC 118 cells decreased 100- and 1,000-fold after incubation for 4 min at 58 and 59°C, respectively (Fig. 1B). Although at temperatures above 59°C the viability of NFBC 338 decreased more dramatically (from 1.3 × 107 to 3.3 × 104 CFU/ml at 60°C) (Fig. 1A), at these temperatures the reduction in the number of strain UCC 118 cells was considerably greater than the reduction in the number of strain NFBC 338 cells (Fig. 1). These data demonstrate that L. paracasei NFBC 338 exhibited greater heat resistance than L. salivarius UCC 118.
FIG. 1.
Survival of L. paracasei NFBC 338 (A) and L. salivarius UCC 118 (B) heated in 20% (wt/vol) RSM supplemented with 0.5% (wt/vol) yeast extract and in 20% (wt/vol) RSM supplemented with 0.5% (wt/vol) yeast extract and 1% (wt/vol) sucrose, respectively, at 55°C (⧫), 58°C (■), 59°C (▴), 60°C (×), and 61°C (✠). The results are means based on data from duplicate heat challenge experiments.
The heat resistance of microorganisms can also be defined by a thermotolerance parameter, the D value (31). A comparison of the D values obtained for the two Lactobacillus strains showed that at all of the temperatures investigated, the D values for L. paracasei NFBC 338 were greater than the D values for L. salivarius UCC 118 (68 to 1.1 and 13.4 to 0.5 min, respectively, at 55 to 61°C), which reflected the greater heat tolerance of the former microorganism. It should also be noted that, prior to the experiment, both cultures were in the stationary phase, which may have resulted in increased heat resistance as it has been demonstrated previously that stationary-phase cultures are more resistant to heat stress than cells in the exponential phase of growth (32). Although the D values obtained in the present study for the L. paracasei and L. salivarius strains were lower than the D values previously reported for a strain of L. bulgaricus in skim milk (32), they were higher than the D values previously reported for mesophilic lactobacilli (9). However, it should be noted that the heat resistance experiments conducted by Franz and von Holy (9) were performed with Ringers solution, whereas skim milk, which previously has been shown to have a protective effect during heat treatment (32), was used in the present study; these results highlight the difficulty of comparing data obtained in different studies.
Spray drying of probiotic cultures.
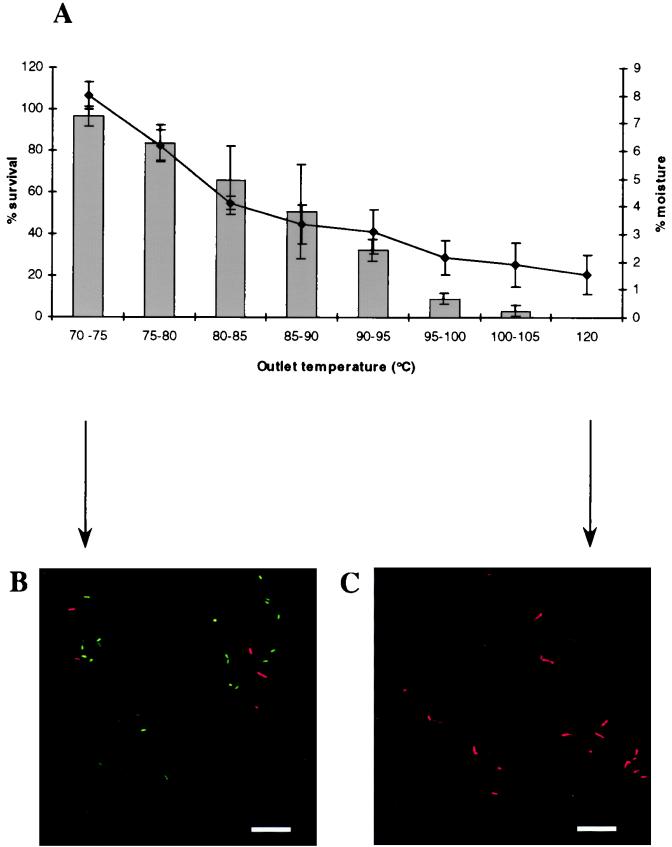
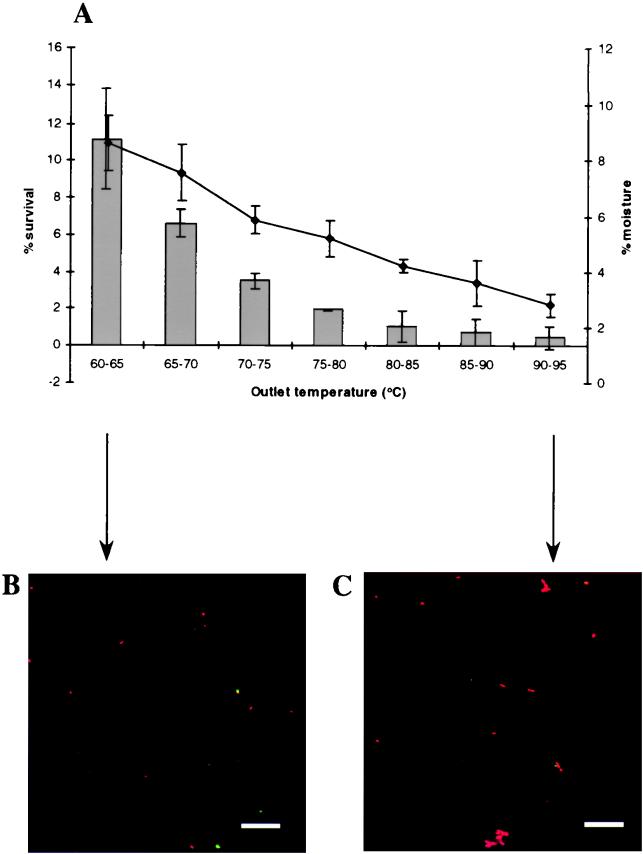
The initial spray-drying experiments were performed to determine the outlet temperature which was optimum for probiotic viability and yielded powders with moisture contents that were not greater than 4% (21). Since L. salivarius UCC 118 was more heat sensitive than L. paracasei NFBC 338 (Fig. 1), lower outlet temperatures (60 to 95°C) were used when strain UCC 118 was spray dried. The probiotic survival rate decreased during spray drying as the outlet temperature increased for both NFBC 338 (r = −0.93) and UCC 118 (r = −0.91) (Fig. 2A and 3A), as previously observed in studies performed with other microorganisms (8, 16, 18). The survival rates for L. paracasei NFBC 338 during spray drying ranged from 97% at an outlet temperature of 70 to 75°C to 0% at 120°C (Fig. 2A); these survival rates were better than the survival rates for UCC 118 (only 11% even at the lowest outlet temperature investigated, 60 to 65°C) (Fig. 3A). These findings may be attributed to the greater thermal tolerance of strain NFBC 338 (Fig. 1). Indeed, the survival rate of NFBC 338 during spray drying was considerably higher than the survival rate previously obtained for L. acidophilus or Lactobacillus curvatus cultures spray dried under similar conditions, while the highest survival rate obtained for UCC 118 (11%) was similar to or lower than previously reported values (22, 28). Growth studies revealed that both cultures were in the exponential phase prior to spray drying (data not shown), which may have increased their sensitivity to the process given that Teixeira et al. (33) have previously shown that exponential-phase cells of L. bulgaricus are more susceptible to spray drying than cells in the stationary phase of growth. It may be possible to increase the probiotic counts in spray-dried powders (which ranged from <1 × 101 to 4.8 × 109 CFU/g for NFBC 338 and from 9.1 × 106 to 6.5 × 108 CFU/g for UCC 118) by using more concentrated cultures for spray drying. In the case of strain UCC 118, using encapsulation techniques (3) or carriers such as dextrin (16) may increase the survival rate during spray drying.
FIG. 2.
(A) Survival of L. paracasei NFBC 338 during spray drying in 20% (wt/vol) RSM supplemented with 0.5% (wt/vol) yeast extract at different air outlet temperatures (bar graph). The line shows the moisture contents of the resulting powders. The air inlet temperature was maintained at 170°C. The results are means based on data from duplicate spray-drying trials, and standard deviations are indicated by vertical bars. (B and C) CSLM micrographs of NFBC 338-containing powders produced at air outlet temperatures of 70 to 75°C (B) and 120°C (C). The powders were stained with the LIVE/DEAD BacLight viability stain; live cells are green, and dead cells are red. Bars = 10 μm.
FIG. 3.
(A) Survival of L. salivarius UCC 118 during spray drying in 20% (wt/vol) RSM supplemented with 0.5% (wt/vol) yeast extract and 1% (wt/vol) sucrose at different air outlet temperatures (bar graph). The line indicates the moisture contents of the resulting powders. The air inlet temperature was maintained at 170°C. The results are means based on data from duplicate spray-drying trials, and standard deviations are indicated by vertical bars. (B and C) CSLM micrographs of UCC 118-containing powders produced at air outlet temperatures of 60 to 65°C (B) and 90 to 95°C (C). The powders were stained with the LIVE/DEAD BacLight viability stain; live cells are green, and dead cells are red. Bars = 10 μm.
The moisture contents of the powders increased as the outlet temperature decreased (Fig. 2A and 3A) (r = −0.87 and r = −0.99 for the NFBC 338 and UCC 118 powders, respectively), as reported previously during preparation of spray-dried powders containing other microorganisms (8, 16). In general, an air outlet temperature of 80 to 85°C was necessary in order to obtain powders with moisture contents that did not exceed the level required for prolonged powder storage life and stability (4%) (Fig. 2A and 3A) (21). At this outlet temperature, the survival rates for NFBC 338 and UCC 118 were 66 and 1%, respectively, which represented viable counts of 3.2 × 109 and 5.2 × 107 CFU/g, respectively. Skim milk powders prepared in this way could be used to incorporate these probiotic microorganisms into a wide range of food and pharmaceutical products. Indeed, the NFBC 338 powder described here could be diluted up to 200-fold and still have a viable count of ≥107 CFU/ml, which would satisfy recommendations regarding the level of viable cells in a probiotic food (15).
CSLM of probiotic powders.
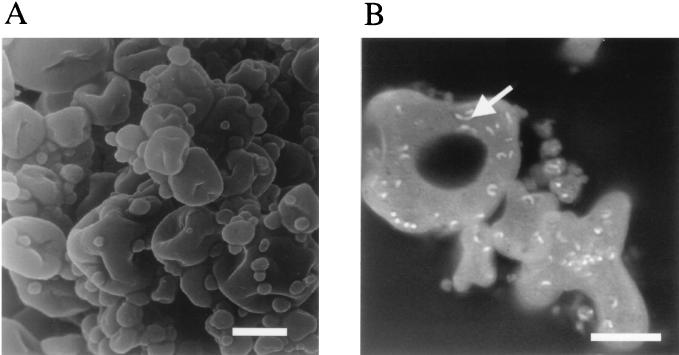
CSLM micrographs were obtained after powders were stained with the LIVE/DEAD BacLight viability stain, which stained dead cells red and live cells green; skim milk powders containing both probiotic strains prepared at the highest and lowest outlet temperatures were examined (Fig. 2 and 3). The fact that L. paracasei NFBC 338 survived better than L. salivarius during spray drying was apparent. The influence of the air outlet temperature during spray drying on probiotic viability was also evident in the micrographs; powders of both cultures dried at the maximum outlet temperatures (120 and 90 to 95°C for NFBC 338 and UCC 118, respectively) contained only dead cells (Fig. 2C and 3C). The UCC 118 powder prepared at an outlet temperature of 90 to 95°C contained 9.1 × 106 CFU/g, while previous work has shown that the limit of detection of the CSLM method is 107 CFU/g (M. A. E. Auty and G. Gardiner, unpublished data). In addition, although scanning electron microscopy of the NFBC 338 powders did not reveal that the probiotic lactobacilli were present (Fig. 4A), the optical sectioning capability of the CSLM technique revealed that the probiotic Lactobacillus cells were encapsulated in the milk powder particles (Fig. 4B), which may have protected the culture during spray drying.
FIG. 4.
(A) Transmission electron micrograph of L. paracasei NFBC 338-containing spray-dried skim milk powder. Bar = 500 μm. (B) CSLM micrograph of the same NFBC 338-containing spray-dried powder stained with Nile blue A stain. Lactobacillus cells encapsulated in a powder particle are indicated by an arrow. Bar = 10 μm.
Salt tolerance of cultures as an indicator of stress damage.
A potential disadvantage of spray drying as a way to preserve cultures is the damage caused to bacterial cells during the process. One of the most susceptible sites in bacterial cells is the cytoplasmic membrane, which is affected by spray drying (33) and is also sensitive to other stresses, such as freeze-drying (2) and heat treatment (22, 35). In addition, increased sensitivity of sublethally injured bacteria to NaCl has been associated with cell membrane damage (2, 33, 35). Both probiotic Lactobacillus strains investigated in the present study became sensitive to NaCl following spray drying at an air outlet temperature of 80 to 85°C. This was shown by the fact that prior to spray drying, there were no differences between the viable counts of the probiotic strains on MRS alone and the viable counts of the strains on MRS containing 5% NaCl, while after spray drying, decreases in cell numbers were observed in the presence of 5% NaCl. Prior to spray drying, strain NFBC 338 exhibited only 4% sensitivity to NaCl, but 70% sensitivity was observed following spray drying. This higher level of sensitivity to NaCl indicates that cell membrane damage occurred as a result of the spray-drying process. Furthermore, when the NFBC 338-containing spray-dried powder was plated in the presence of high concentrations of NaCl, colony size was found to be markedly reduced compared to the colony size prior to spray drying (data not shown). This morphological change that occurred in the presence of NaCl indicates that the spray-drying process stressed the cells. The results obtained for L. salivarius UCC 118 were even more dramatic; this strain exhibited no sensitivity to 5% NaCl before spray drying but 100% sensitivity following spray drying, suggesting that it was damaged to a greater extent by the spray-drying process than NFBC 338. This finding is supported by the lower survival rate of this strain during spray drying compared with the survival rate of NFBC 338. Other possible sites in the cell where damage may occur as a result of spray drying or heat stress include the cell wall and DNA (34, 35). Cellular injury as a result of spray drying is a cause for concern, particularly in the case of probiotic strains, which subsequently must survive adverse conditions, such as those encountered both in fermented foods and in the GIT, in order to be active at the target site (4).
Effect of spray drying on bacteriocin production.
It has been shown previously that L. salivarius UCC 118 produces a broad-spectrum bacteriocin that exhibits activity against microorganisms such as Bacillus, Staphylococcus, and Listeria spp. (7). Bacteriocin production is a desirable trait for probiotic cultures (4) and may be used to competitively exclude undesirable microorganisms in the GIT, thereby playing a role in probiotic persistence in the host. We investigated the effect of spray drying on the ability of UCC 118 to produce bacteriocin and on the activity of the bacteriocin peptide. Following isolation from spray-dried powders produced at a range of outlet temperatures, strain UCC 118 retained its ability to produce bacteriocin, showing that even at outlet temperatures as high as 95°C, this potential probiotic trait was not affected. Furthermore, the spray-drying process did not affect the activity of the bacteriocin peptide, as shown by the presence of antimicrobial activity (32,000 activity units/g of bacteriocin) before and after spray drying at all outlet temperatures. The fact that the antimicrobial activity was due to the UCC 118 bacteriocin was confirmed by the sensitivity of the activity to proteolytic action and its failure to inhibit the producing strain (strain UCC 118). It has been well documented that bacteriocin peptides retain their activity following spray drying; this has been shown for nisin (Nisaplin; Aplin and Barrett, Towbridge, Wiltshire, United Kingdom), lacticin 3147 (25), and bacteriocins produced by both lactobacilli and lactococci (22). The presence of active bacteriocin, as well as viable probiotic microorganisms, in the UCC 118 powder produced in this study means that in addition to providing the potential to add viable probiotic microorganisms to food products, the powder may also play a role in inhibiting spoilage and pathogenic microorganisms in food systems (7). Although we found that the spray-drying process did not affect bacteriocin production by L. salivarius UCC 118, survival of the spray-dried cultures under conditions that are present in the GIT must also be investigated.
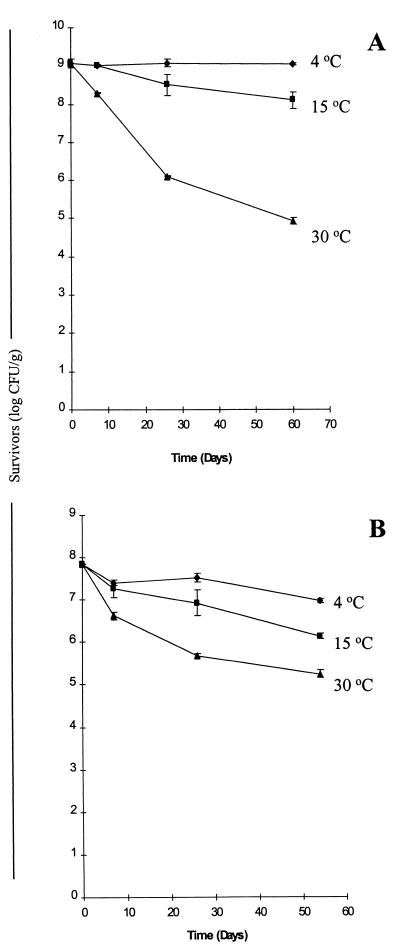
Probiotic survival in spray-dried powders during storage.
Powders of both probiotic cultures that were produced by spray drying at a constant air outlet temperature of 80 to 85°C were stored at different temperatures (4, 15, and 30°C), and probiotic viability was assessed over a 2-month period. We found that following 2 months of storage, the maximum survival rates for both L. paracasei NFBC 338 and L. salivarius UCC 118 in the skim milk powders (92 and 13%, respectively) occurred at 4°C (Fig. 5). The survival rates of both strains decreased more rapidly during storage at 15 or 30°C, and the survival rates were 11 and 2% after 2 months of storage at 15°C for the NFBC 338 and UCC 118 powders, respectively (Fig. 5). Previous studies have also shown that temperature is critical for microbial survival during storage, and higher survival rates have been obtained at lower storage temperatures (1, 16, 34). It is apparent from the results of this study and other studies (1, 16, 34) that although refrigerated storage is impractical from a commercial point of view, it is necessary for optimal culture viability in spray-dried powders over time; this finding means that applications of the probiotic products are more limited. NFBC 338 exhibited higher survival rates than UCC 118 during storage at 4 and 15°C but not during storage at 30°C (Fig. 5). The lower survival rates of UCC 118 during storage may be related to the more extensive cell damage observed in this strain as a result of spray drying (see above). It is also interesting that UCC 118 exhibited lower survival rates in Cheddar cheese and yogurt than NFBC 338 (11; Gardiner et al., unpublished data; O'Sullivan et al., unpublished data). It may be useful to evaluate the effect of adding protectants, such as dextrin and antioxidants like ascorbic acid and monosodium glutamate, during spray drying. These additives have improved culture viability during powder storage (1, 33), although other studies have shown that dextrin has little effect (16) and antioxidants and oxygen absorbers have detrimental effects on culture stability during storage (3, 34).
FIG. 5.
Survival of L. paracasei NFBC 338 (A) and L. salivarius UCC 118 (B) in spray-dried skim milk powders during storage at 4°C (⧫), 15°C (■), and 30°C (▴). The results are means based on data from two replicates, and standard deviations are indicated by vertical bars.
Conclusions.
In this study, we found that two Lactobacillus strains, which were selected on the basis of their probiotic properties, varied considerably in their ability to survive during the spray-drying process. Our findings highlight the need to take into consideration the technological properties of probiotic strains and emphasize the importance of strain selection with regard to processing, as well as health-promoting properties. In this respect, when spray drying was considered, determining thermotolerance parameters proved to be useful for predicting the behavior of the probiotic strains during subsequent processing. Although we found that both probiotic Lactobacillus cultures remained viable during spray drying and determined an optimal outlet temperature for cell viability and moisture content of the powders, future work should include investigations of techniques which prevent cell damage and optimize viability during spray drying. In order for probiotic powders to be useful, storage at room temperature is desirable, and further work is also needed in this area. Furthermore, although the spray-drying process did not affect bacteriocin production by L. salivarius UCC 118, further evaluation of both of our dried cultures is necessary in order to determine if other probiotic properties remain following processing. Although the laboratory-scale experiments conducted in this study provide some indication of the performance of the probiotic Lactobacillus cultures during the spray-drying process, further studies should evaluate their performance during pilot-scale and ultimately industrial spray drying. In conclusion, spray drying is potentially a useful process for large-scale production of some human probiotic Lactobacillus strains in a form suitable for transport and storage. Furthermore, given the numerous applications of skim milk powders, not only in dairy products but also in foods such as instant desserts, mayonnaise, and confectionery products, it is possible that the resulting culture-containing powders could be used in a wide range of functional food applications.
ACKNOWLEDGMENTS
The technical assistance of Helen Slattery and Joe Roche is gratefully acknowledged. We thank William Reville and Myriam Cotter, University College Cork, for electron microscopy.
G.E.G. was supported by a Teagasc Walsh Fellowship. This work was also supported by the European Research and Development Fund.
REFERENCES
- 1.Abd El Gawad I A, Metwally M M, El Nockrashy S A, Ahmed K E. Spray drying of lactic acid cultures. II. The effect of culture conditions and storage on microorganism survival. Egypt J Dairy Sci. 1989;17:273–281. [Google Scholar]
- 2.Brennan M, Wanismail B, Johnson M C, Ray B. Cellular damage in dried Lactobacillus acidophilus. J Food Prot. 1986;49:47–53. doi: 10.4315/0362-028X-49.1.47. [DOI] [PubMed] [Google Scholar]
- 3.Champagne C P, Raymond Y, Mondou F, Julien J P. Studies on the encapsulation of Bifidobacterium longum cultures by spray-coating or cocrystallization. Bifidobacteria Microflora. 1995;14:7–14. [Google Scholar]
- 4.Collins J K, Thornton G, O'Sullivan G. Selection of probiotic strains for human applications. Int Dairy J. 1998;8:487–490. [Google Scholar]
- 5.Daemen A L H, van der Stege H J. The destruction of enzymes and bacteria during the spray drying of milk and whey. 2. The effect of the drying conditions. Neth Milk Dairy J. 1982;36:211–229. [Google Scholar]
- 6.de Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 7.Dunne C, Murphy L, Flynn S, O'Mahony L, O'Halloran S, Feeney M, Morrissey D, Thornton G, Fitzgerald G, Daly C, Kiely B, Quigley E M M, O'Sullivan G C, Shanahan F, Collins J K. Probiotics; from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Leeuwenhoek. 1999;76:279–292. [PubMed] [Google Scholar]
- 8.Espina F, Packard V S. Survival of Lactobacillus acidophilus in a spray-drying process. J Food Prot. 1979;42:149–152. doi: 10.4315/0362-028X-42.2.149. [DOI] [PubMed] [Google Scholar]
- 9.Franz C M A P, von Holy A. Thermotolerance of meat spoilage lactic acid bacteria and their inactivation in vacuum-packaged vienna sausages. Int J Food Microbiol. 1996;29:59–73. doi: 10.1016/0168-1605(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 10.Gagne J, Roy D, Gauthier S F. Production of frozen and freeze-dried concentrates of Bifidobacterium infantis by membrane filtration. Milchwissenschaft. 1993;48:501–505. [Google Scholar]
- 11.Gardiner G, Ross R P, Collins J K, Fitzgerald G, Stanton C. Development of a probiotic Cheddar cheese containing human-derived Lactobacillus paracasei strains. Appl Environ Microbiol. 1998;64:2192–2199. doi: 10.1128/aem.64.6.2192-2199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilliland S E, Lara R C. Influence of storage at freezing and subsequent refrigeration temperatures on β-galactosidase activity of Lactobacillus acidophilus. Appl Environ Microbiol. 1988;54:898–902. doi: 10.1128/aem.54.4.898-902.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarner F, Schaafsma G J. Probiotics. Int J Food Microbiol. 1998;39:237–238. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 14.International Dairy Federation. Dried milk and dried cream. Determination of water content. International Dairy Federation standard 26A. Brussels, Belgium: International Dairy Federation; 1993. [Google Scholar]
- 15.Ishibashi N, Shimamura S. Bifidobacteria: research and development in Japan. Food Technol. 1993;46:126–135. [Google Scholar]
- 16.Johnson J A C, Etzel M R. Inactivation of lactic acid bacteria during spray drying. In: Barbosa-Canovas G V, Okos M R, editors. Food dehydration. New York, N.Y: Institute of Chemical Engineering; 1993. pp. 98–107. [Google Scholar]
- 17.Johnson J A C, Etzel M R. Properties of Lactobacillus helveticus CNRZ-32 attenuated by spray-drying, freeze-drying, or freezing. J Dairy Sci. 1995;78:761–768. [Google Scholar]
- 18.Kim S S, Bhowmik S R. Survival of lactic acid bacteria during spray drying of plain yogurt. J Food Sci. 1990;55:1008–1010. [Google Scholar]
- 19.Kirjavainen P V, Ouwehand A C, Isolauri E, Salminen S J. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol Lett. 1998;167:185–189. doi: 10.1111/j.1574-6968.1998.tb13226.x. [DOI] [PubMed] [Google Scholar]
- 20.Knorr D. Technology aspects related to microorganisms in functional foods. Trends Food Sci Technol. 1998;9:295–306. [Google Scholar]
- 21.Masters K. Analytical methods and properties of dried dairy products. In: Hansen R, editor. Evaporation, membrane filtration and spray drying in milk powder and cheese production. 1985. pp. 393–403. North European Dairy Journal, Vanlose, Denmark. [Google Scholar]
- 22.Mauriello G, Aponte M, Andolfi R, Moschetti G, Villani F. Spray-drying of bacteriocin-producing lactic acid bacteria. J Food Prot. 1999;62:773–777. doi: 10.4315/0362-028x-62.7.773. [DOI] [PubMed] [Google Scholar]
- 23.Metwally M M, Abd El Gawad I A, El Nockrashy S A, Ahmed K E. Spray drying of lactic acid culture. I. The effect of spray drying conditions on the survival of microorganisms. Egypt J Dairy Sci. 1989;17:35–43. [Google Scholar]
- 24.Misra A K, Kuila R K. Intensified growth of Bifidobacterium and preparation of Bifidobacterium bifidum for a dietary adjunct. Cult Dairy Prod J. 1991;26:4–6. [Google Scholar]
- 25.Morgan S M, Galvin M, Kelly J, Ross R P, Hill C. Development of a lacticin 3147-enriched whey powder with inhibitory activity against foodborne pathogens. J Food Prot. 1999;62:1011–1016. doi: 10.4315/0362-028x-62.9.1011. [DOI] [PubMed] [Google Scholar]
- 26.Pot B, Hertel C, Ludwig W, Descheemaeker P, Kersters K, Schleifer K H. Identification and classification of Lactobacillus acidophilus, L. gasseri, and L. johnsonii strains by SDS-PAGE and rRNA-targeted oligonucleotide probe hybridization. J Gen Microbiol. 1993;139:513–517. doi: 10.1099/00221287-139-3-513. [DOI] [PubMed] [Google Scholar]
- 27.Prajapati J B, Shah R K, Dave J M. Nutritional and therapeutic benefits of a blended-spray dried acidophilus preparation. Cult Dairy Prod J. 1986;21:16–21. [Google Scholar]
- 28.Prajapati J B, Shah R K, Dave J M. Survival of Lactobacillus acidophilus in blended-spray dried acidophilus preparations. Aust J Dairy Technol. 1987;42:17–21. [Google Scholar]
- 29.Salminen S, Ouwehand A C, Isolauri E. Clinical applications of probiotic bacteria. Int Dairy J. 1998;8:563–572. [Google Scholar]
- 30.Stanton C, Gardiner G, Lynch P B, Collins J K, Fitzgerald G, Ross R P. Probiotic cheese. Int Dairy J. 1998;8:491–496. [Google Scholar]
- 31.Stumbo C R. Death of bacteria subjected to moist heat. In: Anson M L, Chichester C O, Mrak E M, Stewart G F, editors. Thermobacteriology in food processing. New York, N.Y: Academic Press Inc.; 1965. pp. 55–78. [Google Scholar]
- 32.Teixeira P, Castro H, Kirby R. Inducible thermotolerance in Lactobacillus bulgaricus. Lett Appl Microbiol. 1994;18:218–221. [Google Scholar]
- 33.Teixeira P, Castro H, Kirby R. Spray drying as a method for preparing concentrated cultures of Lactobacillus bulgaricus. J Appl Bacteriol. 1995;78:456–462. [Google Scholar]
- 34.Teixeira P C, Castro M H, Malcata F X, Kirby R M. Survival of Lactobacillus delbrueckii ssp. bulgaricus following spray drying. J Dairy Sci. 1995;78:1025–1031. [Google Scholar]
- 35.Teixeira P, Castro H, Mohacsi-Farkas C, Kirby R. Identification of sites of injury in Lactobacillus bulgaricus during heat stress. J Appl Microbiol. 1997;83:219–226. doi: 10.1046/j.1365-2672.1997.00221.x. [DOI] [PubMed] [Google Scholar]