Abstract
A series of novel chromone derivatives of (N-(4-oxo-2-(trifluoromethyl)-4H-chromen-6-yl) benzamides) were synthesized by treating 7-amino-2-(trifluoromethyl)-4H-chromen-4-one with K2CO3 and/or NaH, suitable alkyl halides and acetonitrile and/or 1,4-dioxane. The obtained products are in high yields (87 to 96%) with various substituents in short reaction times with no more by-products and confirmed by FT-IR, 1H, and 13C-NMR Spectral data. The in vitro cytotoxic activity was examined against two human cancer cell lines, namely the human lung adenocarcinoma (A-549) and the human breast (MCF-7) cancer cell line. Compound 4h showed promising cytotoxicity against both cell lines with IC50 values of 22.09 and 6.40 ± 0.26 µg/mL respectively, compared to that of the standard drug. We also performed the in vitro antioxidant activity by DPPH radical, hydrogen peroxide, NO scavenging, and total antioxidant capacity (TAC) assay methods, and they showed significant activities. The possible binding interactions of all the synthesized chromone derivatives are also investigated against selective pharmacological targets of human beings, such as HERA protein for cytotoxic activity and Peroxiredoxins (3MNG) for antioxidant activity which showed closer binding free energies than the standard drugs and evidencing the above two types of activities.
Keywords: Chromones, Molecular docking study, Cytotoxic activity, Antioxidant activity
Subject terms: Biophysical chemistry, Chemical libraries, Synthetic biology
Introduction
A significant class of synthetic and natural compounds with pharmacological activity are chromone derivatives1–8. These substances displayed a variety of biological activities, including inhibitors of monoamine oxidase B (MAO-B), anticancer, antimycobacterial, antimicrobial, and inhibitors of the human immunodeficiency virus (HIV-1). These chromone derivatives played a significant role as intermediates in producing dyestuffs, agrochemicals, and pharmaceuticals3,9,10. As a result, the topic of study on the synthesis of chromone derivatives is quite interesting and has a lengthy history in the literature11. There are reports of certain chromones acting as anti-HIV agents12. The chromones klelen and 2,4-thiazolidinedione are utilised as antidiabetic agents to reduce peripheral insulin resistance in individuals with type II diabetes and as antispasmodic agents to treat anginapectoris13,14. Substantial anticancer properties were also reported by several phenyl-substituted chromones15.
The cyclodehydration of 1-(o-hydroxyaryl)-1,3-diketones or related intermediates, which can be assisted by strong bases or acids (Vilsmeier-Haack reaction), is a standard method for producing chromatones16. The Allan-Robinson synthesis, which involves acylation rearrangement and subsequent cyclization, has been widely used to produce them17. Additionally, 2-triflouromethylchromone electrophilic nitration was investigated. It has been observed that several lavendustin analogues based on chromone have cytotoxic effects on tumour cell lines18. The in vitro cytotoxic activity of novel substituted chromenopyridones was assessed against a range of HCLs, including PC-3 for the prostate, MCF-7 for the breast, IMR-32 for the central nervous system, Hela for the cervix, and Hep-G2 for the liver19. Studying cytotoxic effects, particularly on specific cancer cell lines like A-549 (lung cancer) and MCF-7 (breast cancer), serves several important purposes in biomedical research and drug development. These types of cancer are among the most prevalent and deadly worldwide, making them significant targets for drug development studies. Thus, A-549 and MCF-7 cells have become standard models for evaluating cytotoxicity in vitro due to their well-characterized properties and reproducible responses to anticancer agents.
On the other hand, a new class of chemicals known as phosphorus-containing chromone and coumarin derivatives exhibits significant cytotoxicity, alkylating, and anticancer action against specific tumour cell lines20. A series of derivatives of 4H-chromen-1,2,3,4-tetrahydropyrimidine-5-carboxylate were synthesized and evaluated for their in vitro anti-mycobacterial activity and cytotoxicity against three HCLs, SK-N-SH, A549, and MTB21.
Nowadays, Cancer is the primary cause of death for humans because of the unchecked, fast multiplication of aberrant cells22. Cancer remains a major issue despite significant advancements in its biology and pharmacology; consequently, a safe, effective, and targeted search for novel chemotherapeutic drugs is necessary for their treatment. Our search finds there is no report for the synthesis of N-(4-oxo-2-(trifluoromethyl)-4H-chromen-7-yl) benzamides and their biological activity. Therefore, herein, we synthesized a series of chromen benzamide derivatives (Scheme 1) and tested their biological activities. Furthermore, integrating in vitro cytotoxicity assays with computational modeling enhances study credibility and translational relevance. Molecular docking predictions correlate with experimental results, validating proposed molecular design strategies. Synthesized chromone derivatives exhibit strong cytotoxic potential, promising as anticancer agents with favorable pharmacological profiles. Supported by robust in vitro and computational evidence, they warrant clinical trial exploration. This research broadens bioactive chromone derivatives and underscores rational drug design's role in advancing novel anticancer therapies, significantly contributing to cancer research.
Scheme 1.
Synthesis of the N-(4-oxo-2-(trifluoromethyl)-4H-chromen-7-yl) benzamides (4a-k).
Results and discussion
Chemistry
The N-(4-oxo-2-(trifluoromethyl)-4H-chromen-7-yl) benzamides (4a-k) wαe synthesized by alkylation of 6-amino-2-(trifluoromethyl)-4H-chromen-4-one (3) with various alkyl/aryl halides in the presence 1.5 mol of K2CO3 or NaH and 10 mL acetonitrile or 1,4-dioxane stirred at room temperature under nitrogen (Scheme 1). The used amino chromen (3) was obtained by reductive amination reaction on the corresponding 6-nitro-2-(trifluoromethyl)-4H-chromen-4-one (2) in MeOH, with SnCl2.2H2O at 60 °C for 8 h and this nitro compound was previously synthesized by the nitration of the 2-(trifluoromethyl)-4H-chromen-4-one (1, 1 g) with nitration mixture in 4 mL of conc. H2SO4 at 75 °C for 1 h. The intermediate compound (1) was obtained by the reaction of 2-hydroxy acetophenone and trifluoroacetic anhydride in the presence of pyridine at 120 °C for 4 h (Table S1).
Biological evaluation
In vitro cytotoxicity by MTT assay
The in vitro anticancer screening of synthesized chromone derivatives (4a-k) was tested with MCF-7 and A-549 (these are exhibited good cytotoxic activity on various cell lines such as MCF-7 and A-549.So we have selected these cell lines, based on literature) using the methylthiazolyltetrazolium (MTT) assay method. The reference drug used in this method was Doxorubicin. The IC50 values of synthesized compounds against the two cell lines were determined from a graph of cytotoxicity and were shown in Table 1. The graphical representation of IC50 values of the title compounds against MCF-7 and A-549 cell lines was demonstrated. From the obtained results, it was observed that all the compounds exhibited significant cytotoxic activity against the A-549 cell line. Among the tested compounds, the compound 4h, having a p-fluorophenyl substitution at chromenbenzamide side chain, displayed high cytotoxicity with an IC50 value of 22.09 µg/mL, also followed by the compounds 4b (with p-nitrophenyl), 4e (with trifluoromethyl), 4c (with phenyl) and 4k (with propyl) which exhibited moderate inhibitory potentiality with IC50 values of 38.03, 41.99, 44.16 and 48.06 µg/mL respectively.
Table 1.
In vitro cytotoxic activity of title compounds (4a-k) against human lung adenocarcinoma (A-549) and human breast cancer (MCF-7) cell lines.
| Compound | IC50 (µg/mL) | |
|---|---|---|
| A-549 | MCF-7 | |
| 4a | 67.36 ± 0.22 | 12.30 ± 0.12 |
| 4b | 38.03 ± 0.28 | 9.46 ± 0.72 |
| 4c | 44.16 ± 0.18 | 9.32 ± 0.16 |
| 4d | 94.98 ± 0.41 | 12.72 ± 0.42 |
| 4e | 41.99 ± 0.33 | 10.22 ± 0.46 |
| 4f | 52.3 ± 0.37 | 9.86 ± 0.40 |
| 4g | 70.36 ± 0.14 | 13.02 ± 0.60 |
| 4h | 22.09 ± 0.26 | 6.40 ± 0.26 |
| 4i | 70.18 ± 0.11 | 11.90 ± 0.48 |
| 4j | 60.22 ± 0.21 | 11.48 ± 0.36 |
| 4k | 48.06 ± 0.46 | 10.72 ± 0.64 |
| Doxorubicin | 09.18 ± 1.12 | 15.06 ± 1.08 |
Similarly, the MCF-7 cell line was much more sensitive to test compounds when compared to that of the A-549 cell line. Most of the test compounds exhibited higher activity levels than the reference compound, Doxorubicin. The cell viability was inhibited significantly in a dose-dependent manner by all the synthesized compounds. When compared to reference drug doxorubicin, the compound 4h displayed promising cytotoxicity with an IC50 value of 6.40 µg/mL, followed by the compounds 4c, 4b, and 4f, which exhibited moderate inhibitory potential with IC50 values of 9.32, 9.46, and 9.86 µg/mL respectively against the MCF-7 cell line.
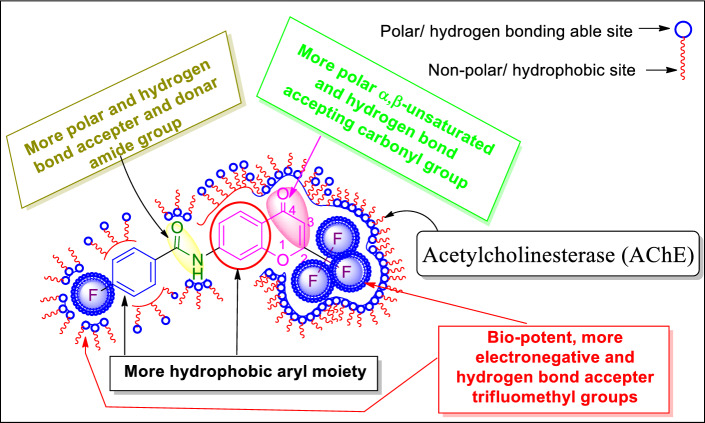
As seen in the literature, the 4H-chromobenzamide core unit itself has good bioactivity, and in addition, we have also designed and introduced another bio-potent trifluoromethyl group as the best anchoring group in pharmacology. With this, the bioactive potentiality of the core unit was enhanced and showed good to excellent in vitro cytotoxic activity in this work. This bioactivity was tentatively evidenced herein, as shown in Fig. 1.
Figure 1.
Possible and tentative SAR of compound 4h as a model for the titled compounds.
The present results clearly stated that the introduction of electron-withdrawing substitutions, like p-fluoro benzene, p-nitro benzene, and tri-fluoro methyl groups on the amide side chain of chromenbenzamide at position six are, markedly enhanced the cytotoxicity and while introduction of p-toluene sulphonyl, and methane sulphonyl moieties decreased the cytotoxic activity23,24. The inverted phase contrast microscope (Primo Vert, Carl Zeiss) was used to observe the cytomorphological abnormalities that occurred to the effect of test compounds for A-549 and MCF-7 cells. The A-549 and MCF-7 cells tested with the synthesized compounds were photographed after 48 h of drug exposure (Figs. 2 and 3). The Results indicated that the control group exhibited typical cobble-stone-like epithelial morphology without any cytological abnormalities. The cells treated with the test compounds showed morphological changes such as membrane blebbing, nuclear disintegration, rounding-off, and membrane degeneration are indicative of cellular apoptosis. Our results of chromone derivatives are further supporting that the findings of cell shrinkage, chromatin condensation, and cellular disintegration in K-562 human myeloid leukaemia cells25.
Figure 2.
Photomicrographs of human lung adenocarcinoma (A549) cells treated with the test compounds 4h, 4b, and 4e at concentrations of 12.5, 25, 50, and 100 µg/mL after 48 h of drug exposure (magnification 10X).
Figure 3.
Changes in cell viability during the treatment with title compounds and control in MCF-7 cell lines.
As a result, this investigation identified a novel class of N-(4-oxo-2-(trifluoromethyl)-4H-chromen-7-yl) benzamides (4a-k) with notable target-specific cytotoxicity on human breast cancer (MCF-7) and lung adenocarcinoma (A-549) cell lines. Therefore, most of the synthesized compounds are good therapeutic candidates that will likely be further optimized and developed into anticancer medications in the near future.
Antioxidant activity
Assay for DPPH radical scavenging activity
Table 2 displays the chemicals' inhibitory effects on the DPPH radical at various doses. The test results were compared with the standard ascorbic acid, and the antioxidant activity was represented in terms of IC50 value (μM), which is the effective concentration for scavenging 50% of the initial DPPH. The results of the titled compounds indicated that compounds 4b (p-nitrophenyl), 4h (p-fluorophenyl), and 4a (p-toluene sulfonyl) were displayed high radical scavenging activity among all the compounds with IC50 = 38.22 μM, 42.46 μM, 44.40 μM respectively. Compounds 4f and 4k exhibited better radical scavenging ability than positive control ascorbic acid. Compounds 4c and 4e, with IC50values 50.35 ± 0.06 μM, and 52.61 ± 0.27 μM respectively, showed comparable activity when compared to standard ascorbic acid (IC50 = 51.06 ± 0.11 μM). As deduced from the IC50 data, the chromone derivatives with the lowest anti-radical scavenging capacity were found to be derivatives of 4j (with IC50 = 113.24 μM) followed by 4d (IC50 = 92 μM) and 4i (IC50 = 90.60 μM).
Table 2.
In-vitro antioxidant activity of the newly synthesized chromone derivatives (4a-k) using DPPH free radical scavenging, total antioxidant capacity (TAC) assay, and Hydrogen peroxide and Nitric oxide scavenging methods.
| Compound | Antioxidant activity of the title compounds (4a-k) compounds | |||
|---|---|---|---|---|
| DPPH IC50 Conc. (µM) (Mean) | TAC Mean (Ascorbic acid equivalents) | H2O2 IC50 Conc. (µM)a | NO IC50 Conc. (µM)a | |
| 4a | 44.40 ± 0.00 | 72.84 ± 0.04 | 45.20 ± 0.08 | 50.44 ± 0.12 |
| 4b | 38.22 ± 0.04 | 102.72 ± 0.13 | 34.71 ± 0.01 | 41.23 ± 0.17 |
| 4c | 50.35 ± 0.06 | 61.37 ± 0.37 | 51.42 ± 0.16 | 55.07 ± 0.03 |
| 4d | 92.00 ± 0.30 | 34.76 ± 0.02 | 79.01 ± 0.02 | 92.11 ± 0.10 |
| 4e | 52.61 ± 0.27 | 58.62 ± 0.39 | 50.16 ± 0.18 | 39.22 ± 0.07 |
| 4f | 57.59 ± 1.63 | 34.33 ± 0.00 | 64.83 ± 0.04 | 61.62 ± 0.05 |
| 4g | 70.88 ± 2.52 | 24.47 ± 0.48 | 71.92 ± 0.11 | 74.01 ± 0.14 |
| 4h | 42.46 ± 0.49 | 93.67 ± 0.09 | 43.11 ± 0.06 | 38.40 ± 0.02 |
| 4i | 90.60 ± 0.10 | 41.05 ± 0.27 | 82.02 ± 0.12 | 77.28 ± 0.09 |
| 4j | 113.24 ± 22.99 | 32.92 ± 0.04 | 93.81 ± 0.01 | 101.54 ± 0.01 |
| 4k | 61.31 ± 0.46 | 35.89 ± 0.04 | 69.10 ± 0.13 | 62.30 ± 0.16 |
| Ascorbic acid | 51.06 ± 0.11 | – | 52.19 ± 0.05 | 50.86 ± 0.11 |
aIC50 values were calculated by using the data obtained in each test for % scavenging at different concentrations of (25, 50, 75,100 and 125 µM).
The previous studies evidenced that chromone derivatives with 2,3 double bonds, 4-oxo group, and substitutions at position 6 showed good antioxidant activity. The present study results were synchronized with the previous results26,27.
Total antioxidant capacity assay (TAC)
Table 2 provides the results of the TAC assay. The ascorbic acid equivalents were used to express the antioxidant capabilities. The obtained results of the TAC assay proved a high ascorbic acid equivalent (AAE) for compounds 4b with p-nitrophenyl, 4h with p-fluorophenyl, and 4a with para toluene sulfonyl substitutions. Hence, they have potent antioxidant activity and can scavenge free radicals efficiently compared to other compounds.
Hydrogen peroxide scavenging assay
The synthesized compounds were tested for hydrogen peroxide scavenging activity, and the results were expressed as IC50 values in Table 2. Among the tested compounds, the compounds 4b (p-nitrophenyl) and 4h (p-fluorophenyl) displayed high hydrogen peroxide scavenging activity with IC50 values 34.71, 43.11 μM respectively, when compared to standard antioxidant ascorbic acid (52.19 μM). The compound 4a with p-toluene sulfonyl substitution also showed good activity with a low IC50 value of 45.20 μM. Compounds 4c and 4e with phenyl trifluoromethyl substitutions exhibited comparable and equipotent activity to standard ascorbic acid with IC50 values 51.42 and 50.16 μM, respectively. The results clearly revealed that 2,3 double bonds, 4-oxo group, and the presence of electron-withdrawing substitutions on the amide side chain of chromen benzamide at position 6 enhanced the antioxidant activity28.
Nitric oxide scavenging activity
Table 2 presents the results of the evaluation of tilted compounds' nitric oxide scavenging ability. The titled compounds 4h, 4e, and 4b, possessing p-fluorophenyl, trifluoromethyl, and p-nitrophenyl substitutions, showed good activity with IC50 values 38.40, 39.22, 41.23 μM respectively when compared to standard ascorbic acid (50.86 μM). In comparison to the standard, the other tilting compounds likewise showed moderate to good nitric oxide scavenging efficacy. The current study's findings are consistent with earlier findings29.
Computational studies
Molecular properties prediction
Table S2 contains a tabulation of the title compound's computed molecular characteristics. The majority of synthesized derivatives have zero or one violation rate. The obtained results proved that every synthetic derivative exhibited good drug-like properties and complied with Lipinski's criteria. The compounds have good oral bioavailability, as shown by the TPSA of less than 140.
BBB (blood–brain barrier penetration) and the amount of intestinal absorption in humans (HIA%) were projected for each derivative, in addition to the other molecular predictions. All the compounds were found to be well absorbed and to have high HIA% values between 94.54% and 98.58%. Furthermore, it was discovered that they had a moderate BBB-to-CNS penetration rate (0.02–3.63). Therefore, theoretically, all of these parameters showed that the compounds had adequate oral absorption, bioavailability, and reasonable permeability through the blood–brain barrier.
Bioactivity score prediction
The synthesized compound's molecular properties prediction is shifted to SI as Table S2.
Molecular docking studies
Cytotoxic activity: molecular docking studies with HERA
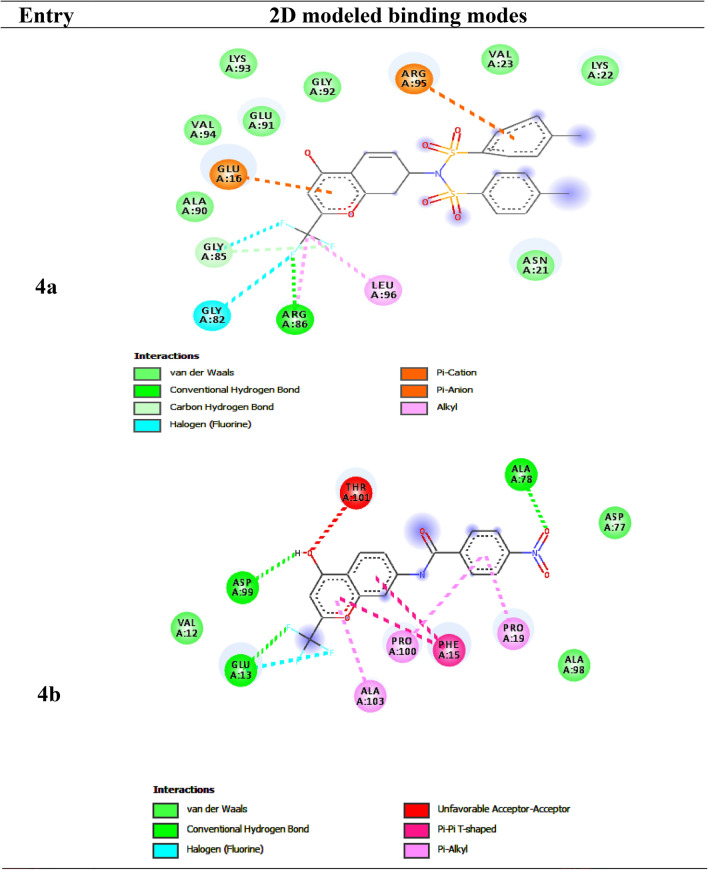
The synthesized chromone derivatives (4a–k) have substantial binding modes against the HERA protein, owing to the docking results. All the drugs, including Doxorubicin (− 6.7 kcal/mol), had dock scores that were higher than those of the reference chemical. The H-bonds, binding affinities, and energy profiles of compounds (4a-k) and Doxorubicin concerning the amino acids in the enzyme's active site are shown in Table 3. Figures 4 and S1 depict the best lead compounds' 3D and 2D modelled interactions with the HERA protein. The synthesized compounds have binding free energy in the range of − 9.6 to − 7.5 kcal/mol, whereas the standard drug Doxorubicin showed a score of − 6.7 kcal/mol. Compound 4h (p-fluorophenyl) has the best binding energy of − 9.6 kcal/mol and showed the highest affinity among all the synthesized compounds. Compound 4h with p-fluorophenyl substitution fits into the binding cleft of HERA protein and forms H-bonding between carbonyl group with side chain amino acids, Leu 346 with bond length 3.1 Å (Fig. 4). The compounds 4b with p-nitrophenyl and 4c with phenyl substitutions form H-bonding (binding energy of − 9.0 kcal/mol) between hydroxyl group with side chain amino acids Leu 384, Gly 521 of HERA protein with bond lengths 2.6, 2.3 Å respectively. The compounds 4e (trifluoromethyl), 4k (propyl), 4i (chloromethyl), and 4j (ethyl) also showed good binding affinities in the range of − 8.4, − 8.2, − 7.8, − 7.8 kcal/mol respectively (Fig. 4). All the synthesized compounds have shown hydrophobic interactions against HERA protein except 4a (p-toluene sulphonyl), 4c (phenyl), and 4d (ethoxy).
Table 3.
Bonding characterization of synthesized compounds (4a-k) and doxorubicin (Reference compound) against human estrogen receptor alpha protein.
| Compound | B E (kcal/mol) | Bonding interaction | Bond length (Å) | Bond angle (°) | Bond type |
|---|---|---|---|---|---|
| 4a | − 7.6 | Arg 515 CB ….OH | 2.5 | 88.1 | H-acc |
| Asn 455 CA ….HN | 2.7 | 96.0 | H-don | ||
| Asn 455 CA ….HN | 2.5 | 80.7 | H-don | ||
| 4b | − 9.0 | Leu 384 CZ …HO | 2.6 | 138.8 | H-don |
| 4c | − 9.0 | Gly 521 CZ ….HO | 2.3 | 91.4 | H-don |
| 4d | − 7.5 | Arg 394 CZ ….OC | 2.6 | 100.1 | H-acc |
| 4e | − 8.4 | Leu 387 CZ ….HO | 2.6 | 100.8 | H-don |
| 4f | − 7.7 | Leu 525 CB ….CO | 2.8 | 120.0 | H-acc |
| 4g | − 7.5 | Leu 387 CZ ….CF | 2.6 | 109.7 | H-acc |
| 4h | − 9.6 | Leu 346 CZ ….OC | 3.1 | 118.3 | H-acc |
| 4i | − 7.8 | Leu 525 CB ….OC | 3.1 | 130.5 | H-acc |
| 4j | − 7.8 | Ile 424 CB ….HO | 3.7 | 90.3 | H-don |
| 4k | − 8.2 | Met 343 CZ ….HO | 2.0 | 97.0 | H-don |
| Doxorubicin | − 6.7 | Leu 320 CZ ….HO | 2.0 | 122.7 | H-don |
Figure 4.

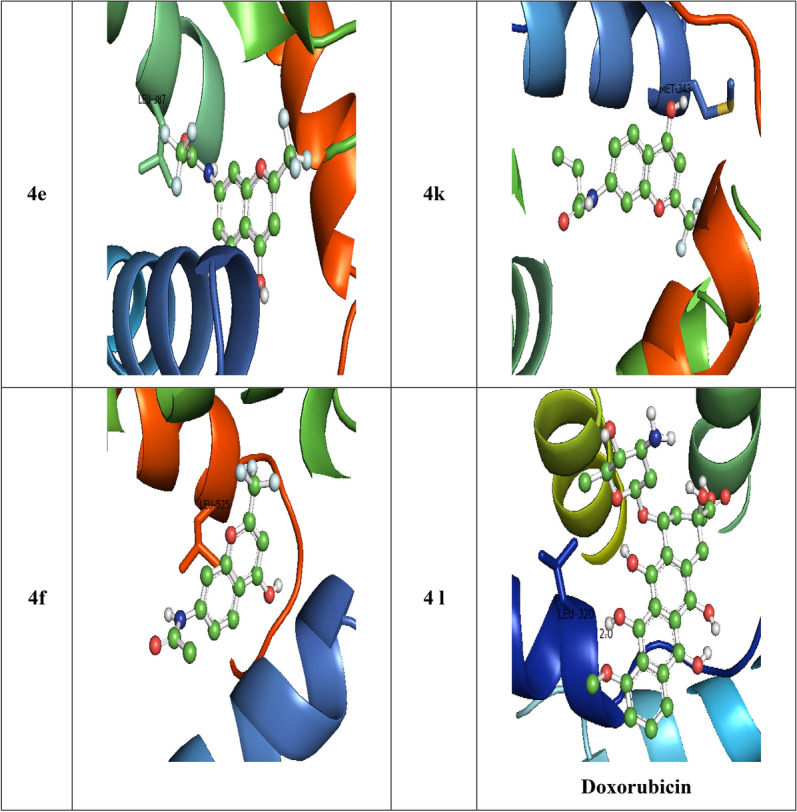
Diagrammatic representation of 3D modelled binding modes of the compounds and standard with the binding domain of Human estrogen receptor alpha protein.
The best binding affinity (ΔG, − 9.6 kcal/mol) towards HERA protein was displayed by the compound 4h, which exhibited marked in vitro cytotoxicity on both cell lines A549 and MCF-7 and this active compound 4h was found to establish six types of interactions: (i) halogen(fluorine): with LEU:387, GLU:353, (ii) Pi-sigma: with LEU:346, (iii) Pi-sulfur: with MET:421, (iv) Pi-Pi-T-shaped: with PHE:404, (v) alkyl: with ALA:350, (vi) pi-alkyl: with LEU:391 (Figure S1).
From the results of docking studies, it was found that the newly synthesized chromone-linked amide derivatives have a good binding affinity with HERA and confirmed the in vitro cytotoxicity in ERα positive cell line MCF-7 and A549. Present study results also support the previous results30,31. As a result, these interactions may provide supporting evidence for in vitro cytotoxic screening of the compounds on various cell lines.
Antioxidant activity: molecular docking studies with Peroxiredoxins (PDB: 3MNG)
The human 3MNG protein is a good target for antioxidant activity, and therefore, the synthetic compounds 4a–k was subjected to a selective pharmacological target for their molecular docking investigation. The compounds' docking results against the 3MNG protein showed that when compared to the control drugs tocopherol (− 5.0 kcal/mol) and ascorbic acid (− 5.4 kcal/mol), respectively, all the compounds in the title had significant binding modes, with dock scores ranging from − 5.9 to − 7.4 kcal/mol. The H-bonds, binding affinities, and energy profiles of compounds 4a-k and reference drugs towards the enzyme's active site amino acids are shown in Table 4. Figures S1 and 5 exhibit the compounds' 3D and 2D binding modes. The compounds 4b (p-nitrophenyl), 4h (p-fluorophenyl), 4a (p-toluene sulphonyl), and 4c (phenyl) have formed high dock with the target protein 3MNG, followed by the rest of the compounds. Out of all the synthesized compounds, 4b with p-nitrophenyl substitution showed the best binding affinity (− 7.4 kcal/mol) with the 3MNG protein when compared to standard drugs. Compound 4b fits into the binding cleft of 3MNG protein and forms an H-bonding between hydroxyl, a nitro group with side chain amino acids As 99, Ala 78 with bond length 2.3, 2.0 Å (Figure S2). Out of all of them, 4c and 4 k (propyl) have bonded hydrophobically to the 3MNG target protein.
Table 4.
Bonding characterization of standards and synthesized compounds (4a-k) against 3MNG protein.
| Compound | B E (kcal/mol) | Binding interaction | Bond length (Å) | Bond angle (o) | Bond type |
|---|---|---|---|---|---|
| Tocopherol | − 5.0 | Val 94 CB ….HO | 1.9 | 115.7 | H- don |
| Ascorbic acid | − 5.4 | Ala 90 CZ ….OC | 2.8 | 120.8 | H- acc |
| Glu 91 CZ ….HO | 2.7 | 118.0 | H- don | ||
| Gly 92 CZ ….OH | 2.3 | 100.1 | H- acc | ||
| 4a | − 6.6 | Val 94 CB ….OC | 2.3 | 121.8 | H- acc |
| 4b | − 7.4 | As 99 CZ ….HO | 2.3 | 70.8 | H- don |
| Ala 78 CZ ….ON | 2.0 | 81.9 | H- acc | ||
| 4c | − 6.4 | Glu 16 CB ….HN | 2.1 | 88.5 | H- don |
| 4d | − 5.9 | Asn 2 CZ ….OH | 2.3 | 91.7 | H- acc |
| Arg 86 CB ….OC | 2.3 | 103.0 | H- acc | ||
| 4e | − 6.1 | Glu 16 CB ….HN | 2.4 | 127.7 | H- don |
| Arg 86 CA ….OC | 2.8 | 133.8 | H- acc | ||
| 4f | − 6.1 | Glu 16 CB ….HN | 2.4 | 127.7 | H- don |
| Arg 86 CA ….OC | 2.8 | 133.8 | H- acc | ||
| 4g | − 6.0 | Gly 92CB ….HO | 2.2 | 84.7 | H- don |
| Asn 21 CB ….OS | 2.5 | 120.9 | H- acc | ||
| 4h | − 6.9 | Gly 92 CB ….OC | 2.2 | 138.1 | H- acc |
| 4i | − 5.9 | Glu 16 CB ….HN | 2.4 | 127.7 | H- don |
| Arg 86 CA ….OC | 2.8 | 133.8 | H- acc | ||
| 4j | − 5.9 | Asn 21 CB ….OC | 2.6 | 123.7 | H- acc |
| 4k | − 6.0 | Asn 21 CB ….OC | 2.8 | 114.0 | H- acc |
Figure 5.
2D binding domains of lead compounds against 3MNG protein.
The compound 4b exhibited high binding affinity (− 7.4 kcal/mol) towards 3MNG protein and displayed potential invitro antioxidant activity. The active compound 4b was found to establish four types of interactions: (i) halogen (fluorine) with GLU:13, (ii) unfavourable acceptor-acceptor: with THR:101, (iii) Pi-Pi-T-shaped: with PHE:15, (iv) Pi-alkyl: with PRO:19, PRO:100, ALA:103 (Fig. 5).
From the results, it was clear that the presence of p-nitrophenyl substitutions containing chromone-linked amides was important for the antioxidant activity. The synthetic compounds' binding modes showed that they fit into the 3MNG protein's binding pocket more stably. The results of the present study were consistent with those from earlier studies31.
In this present study, chromone derivatives of N-(4-oxo-2-(trifluoromethyl)-4H-chromen-7-yl) benzamides (4a-k) were synthesized by short reaction times with high yields. The chromone derivatives showed good in vitro cytotoxicity and antioxidant activity. Upon in silico studies, titled compounds displayed good binding interactions with the target proteins (HERA and 3MNG). Thus, synthesized compounds might be useful cytotoxic and antioxidant drug candidates with good oral bioavailability.
Experimental section
Chemistry
HCl (7647-01-0), CH3COOC2H5 (141-78-6), CH2Cl2(75-09-2) are acquired from Sd fine, India, 2-Hydroxy acetophenone (118-93-4), Na2SO4(239,313), H2SO4 (7664-93-9), Hexane (110-54-3), HNO3(7697-37-2), are purchased from Mark India, and L-Glutamine–(J60573.A1), Streptomycin (15,465,739), are purchased from Alfa Aesar. The given melting points were calculated in open capillaries using the Stuart melting point equipment at °C and are uncorrected. The UV spectra were recorded using a Systronics UV–visible spectrometer, while the IR spectra were recorded in cm−1 using a Perkin-Elmer Spectrum BX-I Infrared Spectrophotometer utilizing the KBr pellet. The Jeol JNM-ECS400 model instrument is used to capture the NMR data at 400 MHz, with TMS serving as an external reference. Iodine vapour is utilized to see the sample spots on the Silica gel-G coated glass plates, which are used to verify the purity of the compounds mentioned.
Synthesis of 2-(trifluoromethyl)-4H-chromen-4-one (1)
2-Hydroxy acetophenone (1 eq.) was dissolved in trifluoroacetic anhydride (1.2 eq.), and pyridine (2 eq.) was added to the reaction mixture. The mixture was heated at 120 °C for 4h. After completion of the reaction, judged by TLC, the reaction mixture was treated with 1 M HCl, followed by water wash, and then the unreacted 2-hydroxy acetophenone was removed by washing with 1 M NaOH. After drying the obtained organic phase with anhydrous Na2SO4, the solvent was removed in a rotary evaporator to yield the crude compound, which was then pure 2-(trifluoro methyl)-4H-chromen-4-one (1). Using a 5% ethyl acetate/hexane eluent system, column chromatography was used to purify the resultant compound.
Synthesis of 7-nitro-2-(trifluoromethyl)-4H-chromen-4-one (2)
A chromone (1, 1 g) solution in 4 mL of concentrated H2SO4 was combined with 1 mL of conc. H2SO4 and 1 mL of 70% HNO3. The reaction mixture was cooled after one hour of heating at 75 °C and then poured over crushed ice while being stirred. When the precipitate was filtered, rinsed with water, and dried, it yielded the pure nitro compound 6-nitro-2-(trifluoromethyl)-4H-chromen-4-one (2). The obtained organic phase was dried with anhydrous Na2SO4, and the solvent was removed in a rotary evaporator to provide the crude compound of 2-(trifluoro methyl)-4H-chromen-4-one (1). Column chromatography with a 5% ethyl acetate/hexane eluent system was used to purify the resulting compound.
Synthesis of 7-amino-2-(trifluoromethyl)-4H-chromen-4-one (3)
After the reaction is completed, the solvent is removed in a rotary evaporator and diluted with C2H5COOCH3 before being quenched with NaHCO3. The resulting slurry was filtered through a celite bed, the organic layer was dried with anhydrous Na2SO4, and the solvent was removed in a rotary evaporator to yield the amine compound 6-amino-2-(trifluoromethyl)-4H-chromen-4-one (3).
General procedures for the synthesis of titled amides (4a-k)
In a round bottom flask, the following were added: 1 mol of an appropriate amine; 1.5 mol of K2CO3 (for 4a–4c synthesis) or NaH (for 4d–4 k synthesis); 1 mol of appropriate alkyl halides; and 10 mL of either acetonitrile (for 4a–4c synthesis) or 1,4–dioxane (for 4d–4 k synthesis). The mixture was then agitated at room temperature while being exposed to a nitrogen atmosphere. The reaction mixture was dried off under low pressure once the reaction was finished, which was observed by TLC. After dissolving the obtained compound in CH2Cl2, it was twice washed in water using a separating funnel. After repeatedly washing the last aqueous layer with new CH2Cl2, the collected organic fractions were mixed and dried over anhydrous Na2SO4. The solvent was then extracted under low pressure, yielding the crude as well as the titled compounds. Which, after being purified by column chromatography, yields huge quantities of the desired compounds (4a-k).
In this synthesis, the titled products are obtained by simple condensation and nitration, followed by the reduction of the nitro group. At first, the simple condensation reaction of o-hydroxy acetophenone was reacted with pyridine, and then the phenolic group underwent condensation with trifluoroacetic anhydride followed by the cyclisation of obtained intermediate (Scheme 2), resulting in 2-(trifluoromethyl)-4H-chromen-4-one (1).
Scheme 2.
Pictorial representation of the simple condensation reaction of chromone derivative (1).
In these reaction conditions, products are observed in high yields irrespective of the nature of the alkyl/aryl halides (Table 1).
Physical and spectral data of representative compounds, 4-methyl-N-(4-oxo-2-(trifluoromethyl)-4H-chromen-7-yl)-N-tosylbenzenesul fonamide (4a)
Yield: 95%; IR (KBr) νmax: 3078.8, 3030.3 (C=CStretch), 2926.2 & 2855.7 (C–HStretch), 1675.9 (C=O), 1602.4 (C–), 1476.1 & 1449.0 (C–HBend), 1380.1 (S=OStretch) cm−1; 1H NMR (CDCl3) δ: 7.87 (4H, d, 3JH-H = 8.0 Hz, Ar–H(14,14´,18&18´)), 7.78 (4H, d, 3JH-H = 8.0 Hz, Ar–H(15,15´,17&17´)), 7.58–7.56 (1H, d, 3JH-H = 10.0 Hz, Ar–H(5)), 7.54 (1H, s, = CH(3)), 6.85–6.76 (2H, m, Ar–H(6&8)); 13C NMR (CDCl3) δ: 177.1 (C-4), 160.4 (C-2), 149.2 (C-9), 145.3 (C-7), 124.8 (C-16&16´), 122.5 (C-13&13´), 119.4 (C-5), 109.4 (C-15,15´,17&17´), 103.6 (C-14,14´,18&18´), 98.9 (–CF3), 95.1 (C-8), 61.6 (C-3), 14.1 (–CH3); Mass [M + Na]+• = 560, [M + 1]+• = 537; Anal. Calcd. for C24H18F3NO6S2: C, 53.63; H, 3.38; N, 2.61; found: C, 53.60; H, 3.37; N, 2.60.
All the other compound’s physical and spectral data associated with this article are given as supplementary information.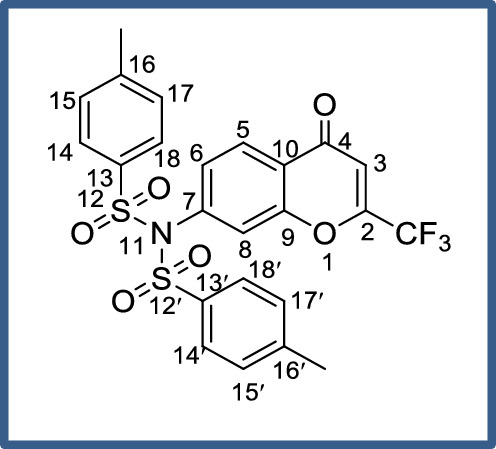
In vitro cytotoxicity studies
Maintenance of cell lines
The King Institute of Preventive Medicine in Chennai provided A549 and MCF-7 cells. The cells were cultured in a T25 mL vented flask with MEM supplemented with 10% foetal bovine serum, 3% L-Glutamine, Streptomycin (100 g/mL), Penicillin (100 IU/mL), and Amphotericin B along with 7.5% NaHCO3, and the flask was incubated at 37 °C in a 5% CO2 incubator. An inverted microscope confirmed the formation of an approximately 80–90% confluent monolayer (adherent) after 3 days. It was then subcultured and used for further research using a TPVG solution and a small amount of media.
Cytotoxicity assessment using methyl thiazolyltetarazolium (MTT) assay
The cytotoxicity of the drugs on A549 and MCF-7 cells was assessed by MTT assay32. After the cells were gathered and seeded on 96-well plates, they were incubated for 24h at 37 °C with 5% CO2 to promote cell attachment. The mixture was incubated for 48 h with varying concentrations of the test compounds after 12 h. Following incubation, phenol red and FBS-free media were added to each well, along with 15 mL of MTT (5 mg/mL) dye, and the plate was covered with Al foil and incubated for another 4h. The medium was aspirated after incubation, and each well received 100 L of DMSO to dissolve the formazan crystals33. At 570 nm, the optical density (OD) was measured. The percentage of cell inhibition was calculated using the formula below.
In vitro antioxidant studies
Antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay
The ability to scavenge the stable free radical DPPH was measured using a modified method34. Test compounds (50 L) at various concentrations (25, 50, 75, 100, and 125 M) were combined with 200 L of methanolic solution containing 100 M DPPH radical. The mixture was thoroughly mixed and left to stand in the dark for 10 min (until stable absorption readings were achieved). The absorbance at 517 nm was used to calculate the DPPH radical's decrease. The radical scavenging activity (In h%) was calculated as a % of DPPH discolouration using the following equation.
The graph of the inhibition percentage against test concentration was used to determine the test compound concentration that provided 50% inhibition (IC50 of DPPH), with vitamin C serving as the standard. There were three duplicates of each test run.
Total antioxidant capacity assay
This assay is based on the reduction of Mo (VI) to Mo(V) in the sample and the subsequent formation of a green phosphate/Mo(V) complex at an acidic pH. In test tubes containing 1 mL of test solutions (50 M), 1 mL of Mo(V) reagent solution (0.6 M H2SO4, 28 mM Na PO4, and 4 mM (HH4)6 Mo7O24) was added. Vortexed tubes were incubated at 90 °C for 90 min. After allowing the tubes to cool to room temperature, the absorbance of the samples was measured at 695 nm. The results are given in milligrams of ascorbic acid equivalent per gram of test compound35.
Hydrogen peroxide scavenging method
The modified approach was used for this method36. A solution of H2O2 (40 mM) was prepared in PO4−3 buffer (pH 7.4). At concentrations of 25, 50, 75, and 100 M, the test compounds were added to an H2O2 solution (0.6 mL, 40 mM) in 3.4 mL PO4−3 buffer. After 10 min, the test compounds' H2O2 scavenging activity was measured at 230 nm against a blank solution. Under similar conditions, the percentage of inhibition was calculated from the control without the test compound. The IC50 values were then calculated using regression analysis.
NO Scavenging method
The Griess reaction was used to calculate the amount of NO produced by Na2[Fe (CN)5NO]37. The test chemicals (25, 50, 75, 100, and 125 M) were dissolved in a suitable solvent (CH3OH) in standard PO4−3 buffer (0.2 M, pH 7.4) and incubated in the tubes for five hours at 25 °C. The control experiment was carried out similarly to the test experiment but with the same amount of solvent. After 5 h, 0.5 mL of the incubation solution was removed and diluted with 0.5 mL of Griess reagent. At 546 nm, the absorbance of the chromophore formed by nitrite diazotization with sulphanilamide followed by coupling with C12H14N2 was measured. The experiment was repeated three times with Ascorbic acid as the control. The nitric oxide scavenging activity was calculated using the following equation38.
Computational studies
Molecular properties prediction
The molecular characteristics of the synthesized compounds (4a-k) were predicted. Lipinski's rule (L-Rule) of five was used to forecast or estimate whether a chemical molecule predicted or included specific biological activity. L- Rule violations were calculated using topological polar surface area (TPSA), molecular weight, Log P, H-Bond acceptors and donors, number of rotatable bonds of synthesized compounds, and molinspiration online property tool kit39. To forecast ADME features such as HIA%, CaCO2 permeability, and blood–brain barrier (BBB), the Pre-ADMET web server, http://preadmet.bmdrc.org/, was used.
The ADMET process is vital in determining a medicine's therapeutic efficacy, and drug similarity appears as an important parameter of a compound that optimizes its ADME in the human body. L- Rule of five states that when there are more than 10 H, 5 H- bonds acceptors, and donors, 15 rotatable bonds, a molecular weight larger than 500, and a partition coefficient (Log p) greater than 5, poor absorption or penetration is more likely. Molecules that violate more than one of these characteristics may have issues with bioavailability and drug-likeness40. To estimate the drug bioavailability, TPSA is another crucial parameter. The TPSA ≥ 140 Å containing drugs were thought to have low bioavailability41.
Prediction of bioactivity score
The bioactivity of the synthesized compounds (4a-k) was assessed by using the molinsipartion server to calculate the activity score of drug targets ion channel, kinase, nuclear and GPCR, receptor ligands, protease, and enzyme inhibitors42.
Molecular docking studies
In silico cytotoxic activity
The Schrödinger software suite, which provides a wide range of computational chemistry and molecular modelling methods for studying protein–ligand interactions, was employed in this investigation. These include Glide and Piper molecular docking, FEP + relative binding free energy predictions, Macro Model and Desmond conformational searches, and Prime and Prime X structural refinement43.
The docking module in Schrödinger was used to conduct molecular docking studies against the 2IOG protein with compounds 4a-k and the reference Doxorubicin drug. Protein structures were protonated by adding polar hydrogens, and then energy was minimized using the MMFF94x force field to obtain the protein's stable conformer. The inhibitor binding site residues were softened and highlighted using the "Site Finder" module software, and flexible docking was used. The expected grid dimensions for 2IOG were X: 28.27, Y: 27.13, and Z: 28.51. The docking study was conducted with the default settings of placement: triangle matcher, recording 1: London dG, refinement: force field, and the ability to save up to 10 conformations of each compound in a separate mdb format database file43.
Accession of target protein and doxorubicin
HERA and Doxorubicin three-dimensional structures were obtained from the RCSB Protein Data Bank and Drug Bank, respectively. The protein's atomic coordinates were separated, and geometry optimization was performed with Argus Lab 4.0.144. Figure 6 (A and B) depict the structures of the target protein and the reference chemicals, 2IOG and Doxorubicin.
Figure 6.
3D structures of (a) HERA and (b) Doxorubicin.
Ligand preparation
Chem Bio Draw45 was used to generate compound molecular structures, which converted all ligands into Pdbqt file format and calculated atomic coordinates using the Schrödinger module.
Analysis of target active binding sites
The target protein's active sites are represented by the ligand's coordinates in the original target protein grids. These target protein binding sites were examined using the virtual instruments 3D Ligand Site and Drug Discovery Studio version 3.046,47.
Structural analysis and visualization
Protein and ligand interactions have been studied and visualized using the Pymol Viewer tool (www.pymol.org)48.
In Silico Antioxidant activity: Accession of the target protein and reference drugs
Peroxiredoxins (PDB: 3MNG) and reference medications such as tocopherol (Pub Chem ID 14,986) and ascorbic acid (Pub Chem ID 54,670,067) were downloaded from the RCSB Protein Data Bank and Pub Chem. After separating the atomic coordinates of the protein, Argus Lab 4.0.1 was used to optimize its geometry44. Figure 7 (A, B, and C) depicts the enzyme 3MNG as well as reference medications such as Tocopherol and Ascorbic acid.
Figure 7.
Structures of the target protein and reference compounds.
Molecular docking analysis
The 3MNG protein was molecular docked utilizing compounds 4a-k and the reference medication, Ascorbic acid and tocopherol, as well as the Schrödinger docking module. The protein structures were protonated by adding polar hydrogens, and then energy was reduced using the MMFF94x force field to obtain the protein's stable conformer. Using the "Site Finder" module software, the inhibitor binding site residues were softened and highlighted, and flexible docking was used. Aromatase's grid dimensions were predicted to be X: 28.27, Y: 27.13, and Z: 28.51. The default parameters for the docking research were placement: triangle matcher, recording 1: London dG, refinement: force field, and a maximum of 10 conformations of each compound allowed to be saved in a separate database file in mdb format43.
Conclusion
Biologically potent chromone derivatives of N-(4-oxo-2-(trifluoromethyl)-4H-chromen-7-yl) benzamides (4a-k) were synthesized at good yields with shorter reaction times. The titled compounds were assessed for cytotoxic activity against cell lines, such as the human lung adenocarcinoma cell line (A-549) and breast cancer cell line (MCF-7). Among the synthesized compounds, compound 4h having a p-fluorophenyl substitution at the chromenbenzamide side chain showed good cytotoxic activity, good DPPH radical scavenging, TAC, nitric oxide scavenging, hydrogen peroxide scavenging activities. The SAR studies performed on the new chromone derivatives revealed that the 4H-chromo benzamide core unit itself has a good bioactivity and, in addition to this present study designed and introduced another bio-potent trifluoromethyl group on it as a best anchoring group in pharmacology, which might be useful as an anticancer candidate with good oral bioavailability. Comparative analysis with previous or contemporary studies reinforces the novelty and significance of the findings. The results align well with emerging trends in drug design, emphasizing the importance of rational molecular modifications to enhance therapeutic efficacy. Furthermore, the molecular docking studies conducted on the synthesized compounds revealed promising binding interactions with specific targets (HERA and 3MNG), thereby providing valuable insights into the potential mechanisms of action. Silico studies titled compounds showed good binding interactions with the targets (HERA and 3MNG), suggesting a good correlation between molecular docking and in vitro studies. The integration of in vitro cytotoxicity assays with computational modelling enhances the credibility and translational relevance of the study findings. The observed correlation between molecular docking predictions and experimental results underscores the reliability of the proposed molecular design strategies. The synthesized chromone derivatives show great potential as cytotoxic drugs, poised for further development into effective anticancer agents. Their favourable pharmacological profile, supported by strong in vitro and computational evidence, makes them promising candidates for clinical trials. This research expands the range of bioactive chromone derivatives and emphasizes the role of rational drug design in advancing novel anticancer therapies, contributing significantly to cancer research.
Supplementary Information
Acknowledgements
The Indian Institute of Chemical Technology (IICT), Hyderabad, Telangana, and IISC Bangalore are also thanked by the authors for their assistance in obtaining some analytical data and helpful discussions.
Author contributions
Sumalathaa Jorepalli: Conceptualization, Investigation, Writing -Original Draft. Sreedevi Adikay: Writing -Original Draft, Funding Acquisition. Radha Rani Chinthaparthi: Data Analysis and Validation. Chandra Sekhar Reddy Gangireddy: Data Analysis and Validation. Janardhan Reddy Koduru: Writing review-editing. Rama Rao Karri: Writing review-editing. All authors reviewed the manuscript.
Data availability
No data other than those reported in this manuscript are available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sreedevi Adikay, Email: sreedevi@spmvv.ac.in.
Janardhan Reddy Koduru, Email: reddyjchem@gmail.com.
Rama Rao Karri, Email: kramarao.iitd@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-59166-5.
References
- 1.Ellis GP. Chromenes, Chromanones and Chromones—Introduction. In: Weissberger A, Taylor EC, editors. Chemistry of Heterocyclic Compounds: Chromenes, Chromanones, and Chromones. John Wiley and Sons; 1977. pp. 1–10. [Google Scholar]
- 2.Patil VM, Masand N, Verma S, Masand V. Chromones: Privileged scaffold in anticancer drug discovery. Chem. Biol. Drug Des. 2021;98(5):943–953. doi: 10.1111/cbdd.13951. [DOI] [PubMed] [Google Scholar]
- 3.Mohsin NUA, Irfan M, Hassan SU, Saleem U. Current strategies in development of new chromone derivatives with diversified pharmacological activities: A review. Pharm. Chem. J. 2020;54(3):241–257. doi: 10.1007/s11094-020-02187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mays JR, Hill SA, Moyers JT, Blagg BS. The synthesis and evaluation of flavone and isoflavone chimeras of novobiocin and derrubone. J. Bioorg. Med. Chem. 2010;18:249–266. doi: 10.1016/j.bmc.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Ma J, Bao Y, Yang P, Zou L, Li K, Sun X. Nitrogen-containing flavonoid analogues as CDK1/cyclin B inhibitors: Synthesis, SAR analysis, and biological activity. Bioorg. Med. Chem. 2008;16:7127–7132. doi: 10.1016/j.bmc.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 6.Chniti S, Pongrácz P, Kollár L, Bényei A, Dornyei A, Takács A. Synthesis of chroman-2, 4-diones via ring-opening/ring-closing reaction involving palladium-catalyzed intramolecular Aryloxycarbonylation. J. Org. Chem. 2024;89:1175–1183. doi: 10.1021/acs.joc.3c02337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ipe RS, Kumar S, Benny F, Jayan J, Manoharan A, Sudevan ST, George G, Gahtori P, Kim H, Mathew B. A concise review of the recent structural explorations of chromones as MAO-B inhibitors: Update from 2017 to 2023. Pharmaceuticals. 2023;16:1310. doi: 10.3390/ph16091310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Zhang Y, Lv Y, Guo J, Gao B, Lu Y, Zang A, Zhu X, Zhou T, Xie Y. Chromone-based monoamine oxidase B inhibitor with potential iron-chelating activity for the treatment of Alzheimer’s disease. J. Enzyme Inhibition Med. Chem. 2023;38(1):100–117. doi: 10.1080/14756366.2022.2134358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harnisch H. Chromon-3-carbaldehyde. Justus Liebigs Annalen der Chemie. 1972;765:8–14. doi: 10.1002/jlac.19727650103. [DOI] [Google Scholar]
- 10.Kamboj S, Singh R. Chromanone-A prerogative therapeutic scaffold: an overview. Arabian J. Sci. Eng. 2022;47:75–111. doi: 10.1007/s13369-021-05858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horton DA, Bourne GT, Smythe ML. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 12.Yu D, Brossi A, Kilgore N, Wild C, Allaway G, Lee KH. Anti-HIV Agents. Part 55: 3′ R, 4′ R-Di-(O)-(−)-camphanoyl-2′, 2′-dimethyldihydropyrano [2, 3-f] chromone (DCP), a Novel Anti-HIV agent. Bioorg. Med. Chem. Lett. 2003;13(9):1575–1576. doi: 10.1016/S0960-894X(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 13.Rauwald HW, Brehm O, Odenthal KP. The involvement of a Ca2+ channel blocking mode of action in the pharmacology of ammi visnaga Fruits1. Planta Med. 1994;60:101–105. doi: 10.1055/s-2006-959426. [DOI] [PubMed] [Google Scholar]
- 14.Singh G, Singh L, Ishar MPS. 2-(N-Methylanilino)-3-formylchromone—a versatile synthon for incorporation of chromone moiety in a variety of heterocyclic systems and macrocycles through reactions with bifunctional nucleophiles. Tetrahedron. 2002;58:7883–7890. doi: 10.1016/S0040-4020(02)00908-0. [DOI] [Google Scholar]
- 15.Maicheen C, Phosrithong N, Ungwitayatorn J. Docking study on anticancer activity of chromone derivatives. Med. Chem. Res. 2013;22:45–56. doi: 10.1007/s00044-012-0009-y. [DOI] [Google Scholar]
- 16.Borrell JI, Teixidó J, Schuler E, Michelotti E. Solid-supported synthetic equivalents of 3-formylchromone and chromone. Tetrahedron Lett. 2001;42(31):5331–5334. doi: 10.1016/S0040-4039(01)00999-6. [DOI] [Google Scholar]
- 17.Rossollin V, Lokshin V, Samat A, Guglielmetti R. Reinvestigation of prototropic photochromism: 3-benzoyl-2-benzylchromones. Tetrahedron. 2003;59:7725–7731. doi: 10.1016/S0040-4020(03)01211-0. [DOI] [Google Scholar]
- 18.Nam DH, Lee KY, Moon CS, Lee YS. Synthesis and anticancer activity of chromone-based analogs of lavendustin A. Eur. J. Med. Chem. 2010;45(9):4288–4292. doi: 10.1016/j.ejmech.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Singh B, Sharma V, Singh G, Kumar R, Arora S, Ishar MPS. Synthesis and in vitro cytotoxic activity of chromenopyridones. Inter. J. Medi. Chem. 2013;2013:984329. doi: 10.1155/2013/984329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budzisz E. Synthesis, reactions and biological activity of phosphorus-containing derivatives of chromone and coumarin. Phosphorus, Sulfur Silicon. 2004;179:2131–2147. doi: 10.1080/10426500490475139. [DOI] [Google Scholar]
- 21.Raju BC, Rao RN, Suman P, Yogeeswari P, Sriram D, Shaik TB, Kalivendi SV. Synthesis, structure–activity relationship of novel substituted 4H-chromen-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylates as potential anti-mycobacterial and anticancer agents. Bioorg. Med. Chem. Lett. 2011;21:2855–2859. doi: 10.1016/j.bmcl.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Wang Z, Li CM, Lu Y, Vaddady PK, Meibohm B, Dalton JT, Miller DD, Li W. Discovery of novel 2-aryl-4-benzoyl-imidazoles targeting the colchicines binding site in tubulin as potential anticancer agents. J. Med. Chem. 2010;53:7414–7427. doi: 10.1021/jm100884b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, Ding Y, Miao Y, Liu MZ, Li Y, Yang GF. Synthesis and antitumor activity of novel dithiocarbamate substituted chromones. Eur. J. Med. Chem. 2009;44:3687–3696. doi: 10.1016/j.ejmech.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Simon L, Salam AAA, Kumar SM, Shilpa T, Srinivasan KK, Byrappa K. Synthesis, anticancer, structural, and computational docking studies of 3-benzylchroman-4-one derivatives. Bioorg. Medi. Chem. Lett. 2017;27:5284–5290. doi: 10.1016/j.bmcl.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Jiao R, Xu F, Huang X, Li H, Liu W, Cao H, Zang L, Li Z, Hua H, Li D. Antiproliferative chromone derivatives induce K562 cell death through endogenous and exogenous pathways. J. Enz. Inhibit. and Medi. Chem. 2020;35(1):759–772. doi: 10.1080/14756366.2020.1740696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phosrithong N, Samee W, Nunthanavanit P, Ungwitayatorn J. In vitro antioxidant activity study of novel chromone derivatives. Chem. Biol. Drug Des. 2012;79:981–989. doi: 10.1111/j.1747-0285.2012.01368.x. [DOI] [PubMed] [Google Scholar]
- 27.Pallavi K, Sailesh W. Synthesis, in vitro antioxidant and antimicrobial evaluation of 3-hydroxy chromone derivatives. Int. J. ChemTech Res. 2018;11(2):63–76. doi: 10.20902/IJCTR.2018.110209. [DOI] [Google Scholar]
- 28.Singh G, Thakur K. Synthesis and investigations on antioxidant behaviour of chromone based semicarbazones. Oriental J. Chem. 2018;34(6):3095–3099. doi: 10.13005/ojc/340653. [DOI] [Google Scholar]
- 29.Demetgül C, Beyazit N. Synthesis, characterization and antioxidant activity of chitosan-chromone derivatives. Carbohydrate Polym. 2018;181:812–817. doi: 10.1016/j.carbpol.2017.11.074. [DOI] [PubMed] [Google Scholar]
- 30.Kaushik S, Rikhi M, Bhatnagar S. Docking and cytotoxicity studies of 2-vinylchromone derivatives on human breast cancer cell lines. Int. J. Pharm. Pharm. Sci. 2015;7(12):113–117. [Google Scholar]
- 31.Ramanjaneyulu K, Hima Bindhu J, Umema Naaz T, Rajendra Prasad VVS, Satya BL. Synthesis, antioxidant, antibacterial and cytotoxic activity of novel chromone derivatives. Der Pharma Chemica. 2017;9(17):78–89. [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhou B, Li B, Yi W, Bu X, Ma L. Synthesis, antioxidant, and antimicrobial evaluation of some 2-arylbenzimidazole derivatives. Bioorg. Med. Chem. Lett. 2013;23:3759–3763. doi: 10.1016/j.bmcl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 36.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 37.Marcocci L, Maguire JJ, Droylefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vistoli G, Pedretti A, Testa B. Assessing drug-likeness–what are we missing? Drug Discov. Today. 2008;13:285. doi: 10.1016/j.drudis.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Zhao YH, Abraham MH, Le J, Hersey A, Luscombe CN, Beck G, Sherborne B, Cooper I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002;19(10):1446–1457. doi: 10.1023/A:1020444330011. [DOI] [PubMed] [Google Scholar]
- 41.Walkinshaw G, Waters CM. Neurotoxin-induced cell death in neuronal PC12 cells is mediated by induction of apoptosis. Neuro Sci. 1994;63:975–987. doi: 10.1016/0306-4522(94)90566-5. [DOI] [PubMed] [Google Scholar]
- 42.Jeelani A, Muthu S, Narayana B. Molecular structure determination, bioactivity score, spectroscopic and quantum computational studies on (E)-N'-(4-Chlorobenzylidene)-2-(napthalen-2-yloxy) acetohydrazide. J. Mol. Struct. 2021;1241:130558. doi: 10.1016/j.molstruc.2021.130558. [DOI] [Google Scholar]
- 43.Release S. Schrödinger Suite 2017–4 Induced Fit Docking protocol. Glide, Schrödinger. 2017;2017:4. [Google Scholar]
- 44.B. Liskov, M. Day, M. Herlihy, P. Johnson, G. Leavens, G. Argus Reference Manual (No. MIT/LCS/TR-400). Massachusetts Inst of Tech Cambridge Lab for Computer Science, 1987.
- 45.Spessard GO. ACD Labs/LogP dB 3.5 and ChemSketch 3.5. J. Chem. Infor. comp. Sci. 1998;38(6):1250–1253. doi: 10.1021/ci980264t. [DOI] [Google Scholar]
- 46.Studio D. Discovery Studio. Accelrys. 2008;2008:420. [Google Scholar]
- 47.Broughton HB. A method for including protein flexibility in protein-ligand docking: Improving tools for database mining and virtual screening. J. Mol. Graph Modell. 2000;18(3):247–257. doi: 10.1016/S1093-3263(00)00036-X. [DOI] [PubMed] [Google Scholar]
- 48.Yuan S, Chan HS, Hu Z. Using PyMOL as a platform for computational drug design. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2017;7(2):e1298. doi: 10.1002/wcms.1298. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data other than those reported in this manuscript are available.