Abstract
In this study TiO2 and ZnO nanoparticles were employed as a protective coating to impart multifunctional properties, i.e. self-cleaning, water repellency, UV protection and antimicrobial activity onto jute-cotton union fabric. Through the sol-gel method, using titanium (IV) isopropoxide (TTIP) and zinc acetate as precursors, TiO2 and ZnO nanoparticles have been synthesized. Following the dip-pad-dry-cure method along with a 2 wt% of acrylic binder, the synthesized particles were applied on the jute-cotton union fabric. Subsequently, antibacterial activity, self-cleaning properties and physical properties like water absorbency, crease recovery, water vapor permeability, tensile strength, and tear strength of the treated fabric were tested and evaluated. The structural properties of the nano-particles were distinguished by Fourier Transform Infrared Spectroscopy (FTIR) and SEM. Upon comparing the test results of the coated fabric with the uncoated fabric, the results were found to be extremely satisfactory. This study provided valuable insights into the potential of TiO2 and ZnO coating in enhancing properties of jute-cotton union fabric.
Keywords: Antimicrobial activity, Coated fabric, FTIR, Self-cleaning properties, SEM, Union fabric
Highlights
-
•
In this study a protective coating of TiO2 and ZnO was applied on jute-cotton union fabric. Jute-cotton union fabric, a cellulosic fabric containing hydroxyl groups, has a high affinity for water and liquids, making it prone to stains and bacterial growth, ultimately limiting its utility.
-
•
To overcome this shortcoming, in this study, it was coated with TiO2 and ZnO, well known for boosting the physical and chemical properties of fabric.
-
•
Through the sol-gel method using titanium (IV) isopropoxide (TTIP) and Zinc Acetate as precursors TiO2 and ZnO nanoparticles have been synthesized.
-
•
Applying the dip-pad-dry-cure method, along with a 2 wt% of acrylic binder, the synthesized particles were applied on the jute/cotton union fabric.
-
•
The presence of the nanoparticles was affirmed by FTIR and SEM method.
-
•
Various tests like water absorbency, crease recovery, water vapor permeability, tensile strength, elongation at break, tear strength test etc were carried out on the coated sample and the results were compared with the untreated fabric.
-
•
The treated fabric not only provided UV protection and tremendous self-cleaning properties but also exhibited astounding antimicrobial activity and soil release action.
-
•
These advantageous attributes make this union fabric an excellent pick for sportswear, medical textiles, home furnishing etc. Due to its high UV protection, it can an outstanding choice for umbrellas.
-
•
These versatile application of this union fabric can also be advantageous in reducing pressure on jute and cotton cultivation.
-
•
However, a slight decrease in mechanical properties of the treated fabric was also noticed.
-
•
Therefore, the balanced use of TiO2 and ZnO nanoparticles is suggested.
-
•
Further research needs to be carried to fully understand the implications of this treatment for variety in fabric usage.
1. Introduction
Jute-cotton union fabric, proportional blend of jute and cotton fibers, is a cellulosic fabric that contains hydroxyl group (OH), which makes the fabric surface hydrophilic [1]. Therefore, the fabric intends to be wetted quickly by water and other liquids. This makes the fabric prone to stains and bacterial growth, limiting the usage of cellulosic fabric in various applications [2]. Due to this, washing cost also increases. Hence, to improve the quality of the fabric surface and enable it to be used for multiple purposes, modification of these types of cellulosic fabric is of interest [3]. For garments and filter substances, gauzes for curing injury, and sterile food wrapping elements in food processing, nanotextile particles are used [4]. The preparation of smart nanotextiles can alter the production of fibers, the usefulness of cloth, nonwoven fabrics, and numerous categories of fabric merchandise and usage [5]. From smart textiles, and medical textiles to shielding fabrics for soldiers, nanofinishing is very reassuring [6]. As nanoparticles exhibit an enormous area of surface vs. volume ratio and raised surface energy, comprising an enhanced attraction to textiles and advanced toughness, nanotechnology revealed excellent stability for textiles [7]. Functionally finished textiles can be considered a fast-paced and fast-growing industry due to increasing health concerns along with customer satisfaction [8]. Functional finishes illustrate the next generation of finishing in the industry that makes textile materials act by themselves [9]. Functional finishes include multifunctional capabilities in waterproof textiles, antibacterial, UV protection, self-cleaning, flame-retardant, etc. These results can be obtained by adding nanomaterials, such as, TiO2 and ZnO [10]. Various strategies have been adopted to enhance the performance and functionality across different applications by altering the surface of the jute-cotton union fabric [11]. These include treatments such as low-pressure glow discharge air plasma for surface alterations, specific pre-treatments combined with N-methylol-acrylamide resin post-treatment, enzyme treatments with silicone-polyurethane softening, sizing treatments, and cationic adjustments to boost dye ability.
Titanium dioxide (TiO2) is a photocatalytic semiconductor and has proven to be an ideal catalyst for the visual degradation of dyes and other organic contaminants [12]. Due to its different benefits, including non-toxicity, availability, cost efficiency, chemical constancy, and beneficial physical and chemical properties, are widely applied [13]. Studies on the usage of TiO2 for flame-retardant coatings, enhancement of the photostability of wool fabrics, and development of coatings for durable self-cleaning abilities under sunlight and increased stiffness, creation of super hydrophobic surfaces for efficient separation of oil and water contaminants along with purifying water bulk contaminants emphasize their potential in advancing fabric functionalities for diverse applications. Through methods such as ultrasound irradiation, ZnO can be coated onto cotton fabric, and the incorporation of enduring antibacterial properties is vital for preserving a germ-free environment [14]. Another study explored single-step sono-enzymatic processes for coating cotton medical textiles with antibacterial ZnO nanoparticles and gallic acid, resulting in long-lasting antibacterial bio-friendly fabrics [15]. The integration of seaweed-capped ZnO nanoparticles onto cotton fabrics enhanced UV protection and antibacterial features [16]. The discoveries related to flame-retardant coatings on cotton fabrics, highlighting the flexibility of ZnO nanoparticles in enhancing fabric functions, underscore the potential of ZnO-based coatings in improving textile performance and usability in healthcare, UV protection, and antimicrobial protection sectors. Therefore, this study was conducted to evaluate the antibacterial efficacy, durability, and topographical function of TiO2 and ZnO-coated jute-cotton fabric. Various tests, such as, water absorbency, crease recovery, water vapor permeability, tensile strength, elongation at break, and tear strength were performed on the coated sample, and the results were compared with those of the untreated fabric. The treated fabric not only provided UV protection and tremendous self-cleaning properties but also showed astounding antimicrobial activity and soil-release action [17]. These advantageous attributes make this union fabric an excellent choice for sportswear, medical textiles, and home furnishing [18]. Because of its high UV protection, it is an outstanding umbrella choice. These versatile applications of this union fabric can also be advantageous in reducing the pressure on jute and cotton cultivation [19].
2. Materials and methodology
2.1. Fabric
The fabric used here was Jute-Cotton union woven fabric. This fabric was collected from jute research institute of Bangladesh. The GSM (Gram per Square Meter) of the fabric was 260 with an EPI (Ends Per Inch) of 24 and PPI (Picks Per Inch) of 26.
2.2. Chemicals
-
1.
Zinc Acetate
-
2.
Distilled water
-
3.
Sodium hydroxide (NaOH)
-
4.
Ethanol
-
5.
Titanium (IV) isopropoxide
-
6.
Propanol
2.3. Preparation of TiO2 nano powder
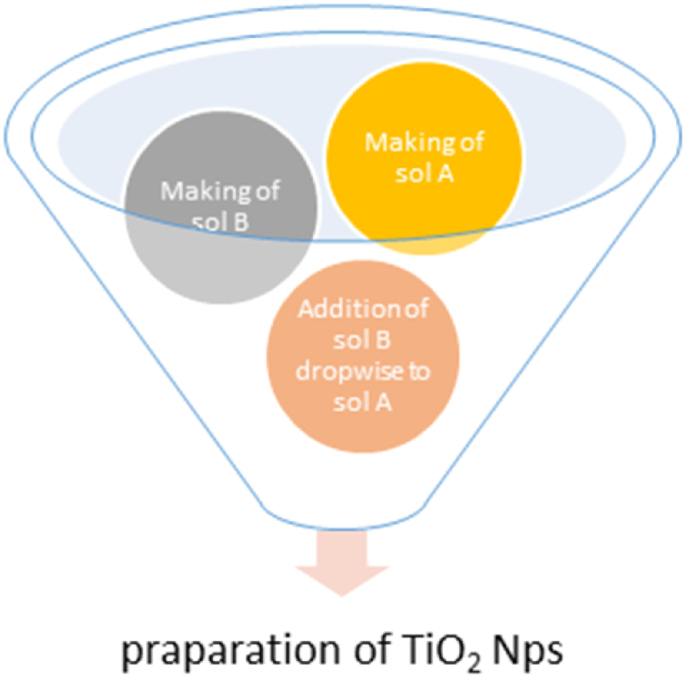
Titanium isopropoxide (C12H28O4Ti) was used as starting material to prepare TiO2 powder [20]. 5 ml of Titanium (IV) isopropoxide was mixed in 60 ml of propanol by using pipet and stirred with magnetic stirrer until a clear solution was obtained and this solution was marked as solution A and kept in beaker. 6 ml of water was taken in conical flask and 60 ml of propanol was added to it and the solution obtained was marked as solution B. The solution B was placed on magnetic stirrer with magnetic bead inside the flask and solution A was added drop by drop using dropper, for 20min at room temperature. After that, the solution was kept on the magnetic stirrer for another 30 min for completion of the reaction. Then solution was taken in a Petri dish and kept in oven at 90 °C for 5 h. Thus TiO2 nano-powder was produced and it was kept in a plastic container.
2.4. Preparation of ZnO nano powder
To synthesize Zinc Oxide following sol-gel method, initially, 2 g of zinc acetate dihydrate was dissolved in 15 ml of distilled water and 8 g of sodium hydroxide was dissolved in 10 ml of distilled water. Each solution was constantly stirred for about 5 min. Afterwards both the solutions were mixed and again stirred for another 5 min using a magnetic stirrer. Then in this new solution 100 ml ethanol was titrated drop-wise, which will eventually create a white gel. This formed gel was dried in an oven for 3 h. After this period, ZnO nanoparticles were collected following [21,22].
2.5. Application of TiO2 and ZnO nano-powder
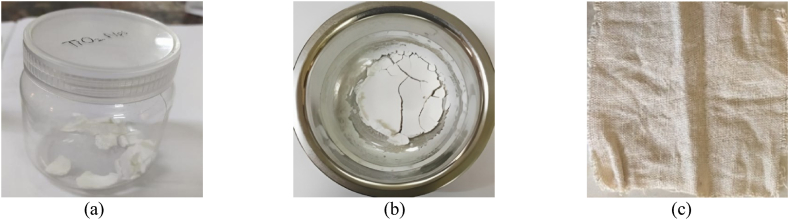
Pad-dry-cure method was applied for coating the jute-cotton union fabric by TiO2 and ZnO nano-particles. 200 ml 2 wt % acrylic acid solution was made and 1 wt% of TiO2 and ZnO nano-powder were taken as per solution. Then the solution was stirred at magnetic stirrer for 30 min to ensure the even application on the fabric. The Jute-Cotton union fabric was soaked for 10 min in aqueous solution and passed over the padding mangle to remove the excess solution. After the padding, curing was done for 5 min at 150 °C by mini-stenter. In Fig. 1(a) and (b), prepared TiO2 and ZnO Nanoparticles are shown respectively, and the treated fabric is shown in Fig. 1(c).
Fig. 1.
(a) Prepared TiO2 Nps (Nanoparticles) (b) Prepared ZnO Nps and (b) Treated fabric.
2.6. Spray rating test
First, the sample was cut into a dimension of 18 cm2. The weight of the sample was measured using an electric balance. Then, the sample was placed in the hopper. The diameter was 15 cm. Then, 250 ml of distilled water was poured into the funnel. It took 30 s to spray in the sample. After that, the weight of the wet cloth was measured. The absorbency of the fabric was measured using the appropriate formula. During this test, the temperature was maintained at 25 °C, and the relative humidity was maintained at 65 % [23].
2.7. Self-cleaning activity, wicking, and water vapor transmission test
The coated and uncoated fabric samples were dusted with 2 % coffee solution and exposed to sunlight for 20 h. Multiple images were taken after this period, and the self-cleaning activity was compared. The ASTM E96 standard was followed for the water vapor transmission test. A cup was filled with distilled water, leaving a small gap (0.75″ to 0.25″) in the air space between the specimen and the water. The cup was then sealed to prevent vapor loss, except through the test sample. An initial weight was taken of the apparatus and then periodically weighed over time until the results became linear. A 4 x 4-inch specimen was used for this. Here the thickness was less than . For the wicking test, the AATCC 197 test method was followed. A 10 × 1 cm strip of test fabric was suspended vertically with its lower end (1 cm) immersed in a reservoir of distilled water with 0.25 g of turquoise color to track the wick-up action of water spreading by capillary action, as per AATCC 197 and the movement of water through the fabric strip was observed for 1 min. The temperature was kept at 25 °C.
2.8. Stiffness and thickness test
The stiffness test was performed according to ASTM D4032. 8″ × 4″ template was used to cut the fabric sample. It was then folded to create a 2 ply sample [24]. A fabric thickness tester was used for this test according to ASTM D1777. Keeping the fabric sample on an anvil, the press foot was gently lowered onto the specimen, and the reading was taken to determine the thickness of the specimen. The flat circular indenter of the micrometer exerted the specified pressure on the fabric sample. The above procedure was repeated to obtain the thickness values at least at 3 different locations. The mean value of the readings of thickness determined was calculated, and the average thickness was taken as the result.
2.9. Crease recovery test
The Shirley Crease Recovery Tester was used for this test according to AATCC TM 66. A specimen was cut from the fabrics using a template 2 inches long by 1 inch wide. It was carefully creased by folding it in half, placing it between two glass plates, and adding a weight of 0.5 kg. After 1 min, the weight was removed, and the specimen was transferred to the fabric clamp on the instrument and allowed to recover from the crease. As it recovered, the dial of the instrument was rotated to keep the free edge of the specimen in line with the knife edge. At the end of the period allowed for recovery, usually 1 min, the recovery angle in degrees was read on the engraved scale. Warp and weft way recovery were reported separately to the nearest degree from the mean values of ten tests in each direction.
2.10. Tensile and tear strength
To measure the breaking force and elongation at break of treated and untreated fabric samples, a UTM TF001 (Testometric) machine was used. The tensile strength of the fabric was tested according to ASTM E−4. During this test, a tensile force was applied to the fabric specimen until it ruptured. The size of the samples was 20 cm × 5 cm. The Elmendorf Tearing Tester was used to measure the tear strength of the fabric samples in this study according to Standard ASTM -D1424. Fabric tore when snagged by a sharp object. The small puncher was converted into a long rip with a small effort. For this testing procedure, the sample size was kept at 4 × 3 inches.
2.11. Antimicrobial test
The samples were tested against two types of bacteria, gram-positive Staphylococcus aureus and gram-negative Salmonella according to AATCC 147–2004 [25]. The AATCC 147–2004 states that anti-bacterial activity is acceptable only if there are no bacterial colonies under the sample in the contact area. To test this, small pieces of treated and untreated fabric samples (1 cm × 1 cm) were placed in 250 ml flasks containing working bacterial dilution. The flasks were shaken at 37 °C and 120 rpm for 1 h. Dilutions were made using buffer solution and 1 ml of each dilution was placed on a nutrient agar plate. The plates were incubated at 37 °C for 24 h and surviving cells were counted. The antimicrobial activity against bacteria was analyzed and presented in Figure after 24 h of inhibition. Bacterial growths around samples were observed to qualitatively determine the antibacterial activity. All this confirms that the sample treated with TiO2 and ZnO nanoparticles exhibited antibacterial activity.
2.12. Fourier transform infrared (FTIR) spectroscopy and UV spectrum test
The Fabric characterization was conducted using an FTIR spectrophotometer to analyze the presence of functional groups or chemical bonds between TiO2 and ZnO nanoparticles according to ASTM E168, and UV spectrum tests were typically performed using a spectrophotometer according to ASTM D4329. Using UV–Vis spectroscopy, the UV–Vis light was passed through the sample, and the transmittance of the light by the sample was measured. From the transmittance (T), the absorbance can be calculated as A = -log (T). An absorbance spectrum was obtained that shows the absorbance of a compound at different wavelengths.
2.13. Scanning electron microscope (SEM)
The ZEISS Gemini Sigma 300 machine was used to perform the SEM test according to ASTM F1372-93.
3. Result and discussion
3.1. Analysis of multifunctional properties
3.1.1. Spray rating test
Here Fig. 2(a) represents the untreated fabric and Fig. 2(b) represents the treated fabric. For the untreated fabric, the result of the spray rating was 50 (ISO 1) and for the treated fabric, the result was 90 (ISO 4).
Fig. 2.
(a) ISO 1 for untreated fabric and (b) ISO 4 for treated fabric.
3.1.2. Visual assessment of self-cleaning activity and water repellency properties
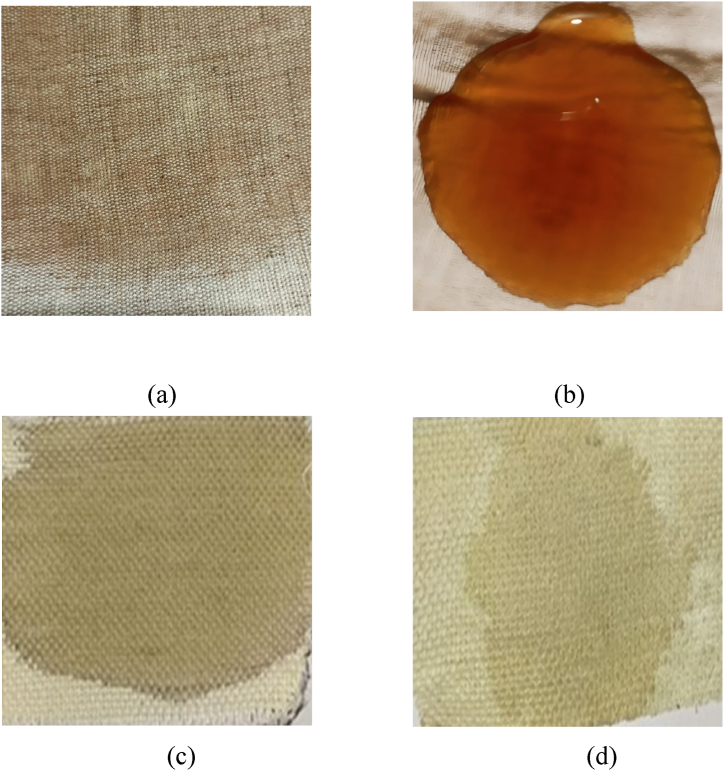
Fig. 3(a–d) depicts the Jute-Cotton union fabric before and after coating, Fig. 3(a–c) displays the fabric before coating, and Fig. 3(b–d) displays the fabric after coating. A 2 % coffee stain was applied to the fabric, Fig. 3(b) represents the treated fabric right after the application of the 2 % coffee solution, and a photograph was taken after 20 h of sunlight exposure, as represented in Fig. 3(d). Based on the samples tested, it can be concluded that the self-cleaning activity is effective for 100 % jute-cotton woven fabric.
Fig. 3.
a) Untreated and (b) treated fabric right after the application of 2 % coffee solution, (c) untreated and (d) treated fabric after 20 h of applying 2 % coffee.
From Fig. 4(a) it has been visualized that the untreated fabric has no water-repellent properties. But after treatment, the fabric has shown the water repellency properties, which can be seen from Fig. 4(b).
Fig. 4.
Dropping liquid in the untreated fabric and (b) Dropping liquid in the treated fabric.
3.1.3. Analysis of antimicrobial properties
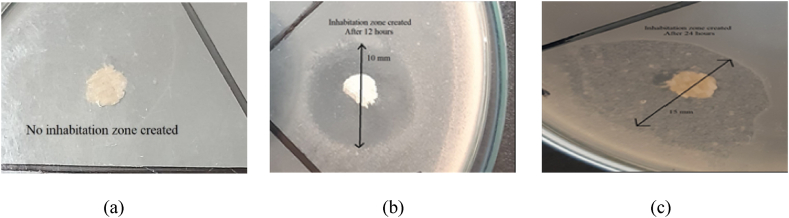
As part of this study, an antimicrobial test was conducted to investigate the effectiveness of fabric samples treated with TiO2 and ZnO nanoparticles against gram-positive and gram-negative bacteria. Fig. 5(a) depicts that the untreated fabric did not show any inhibition zone even after 24 h. Fig. 5(b) and (c) show the created inhibition zone after 12 h and 24 h respectively. Results showed that the treated samples had a greater effect on gram-positive St. Aureus than on gram-negative Salmonella, with a higher value of clear zone observed for samples treated with TiO2 and ZnO against St. Aureus. UV-spectrum analysis confirms the self-cleaning properties of the treated fabric.
Fig. 5.
(a) Untreated Fabric(After 24 h) (b) Treated fabric created Inhabitation zone (After 12 h) (c) Treated fabric created Inhabitation zone after 24 h..
3.1.4. Analysis of UV spectrum
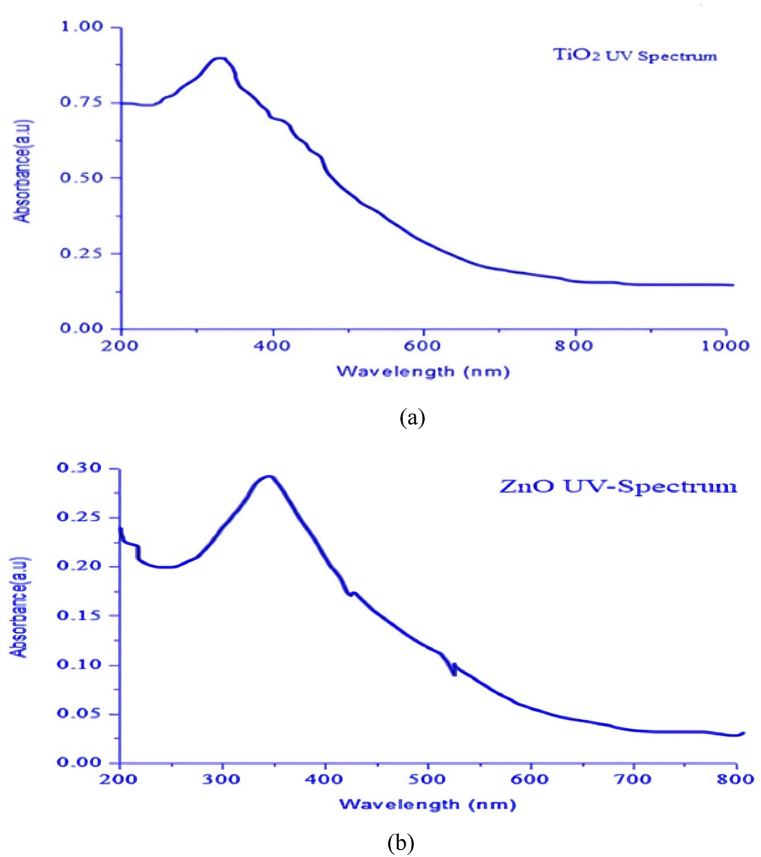
UV–Vis spectroscopy was employed to investigate the optical properties of nanoparticles. For ZnO the peak absorption was at 354 nm and a band gap of 3.26 eV, as depicted in Fig. 6(b). There was a successful formation of ZnO as there are no other peaks observed throughout the entire spectrum. For TiO2 the peak wavelength was observed to be at 376 nm with an absorbance of 0.86, as shown in Fig. 6(a), and this being less than one confirms good absorbance.
Fig. 6.
(a) UV spectrum of TiO2 Nps and (b) UV spectrum of ZnO Nps.
3.2. Experimental analysis of physical properties
3.2.1. Effect of TiO2 and ZnO nanoparticles coating on tensile strength and elongation breaking
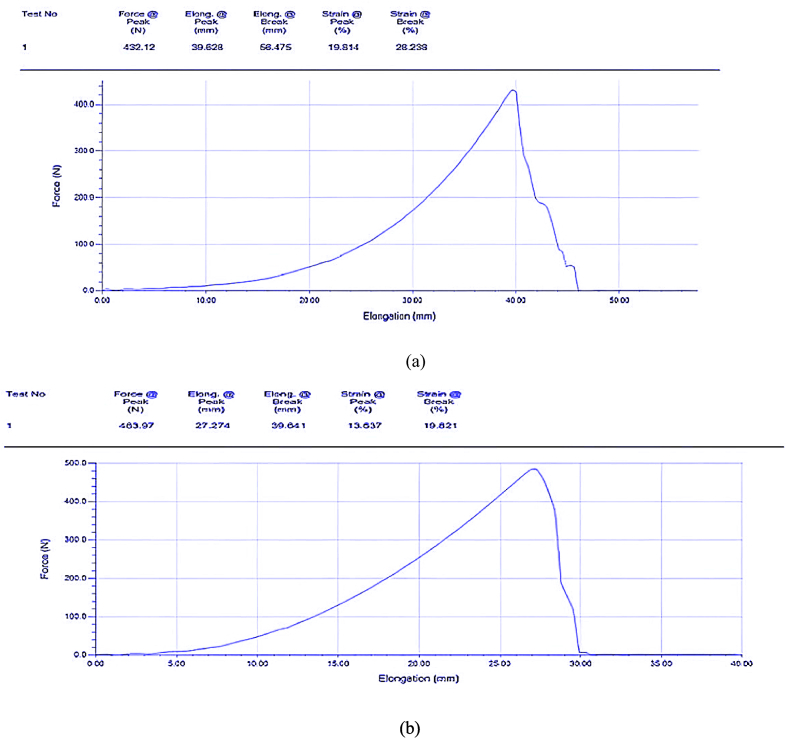
The result obtained for the untreated fabric was 483.87 N, as shown in Fig. 7(b), and for the treated fabric was 432.12 N, as shown in Fig. 7(a).
Fig. 7.
(a) Elongation breaking force for treated fabric and (b) Elongation breaking force for untreated fabric.
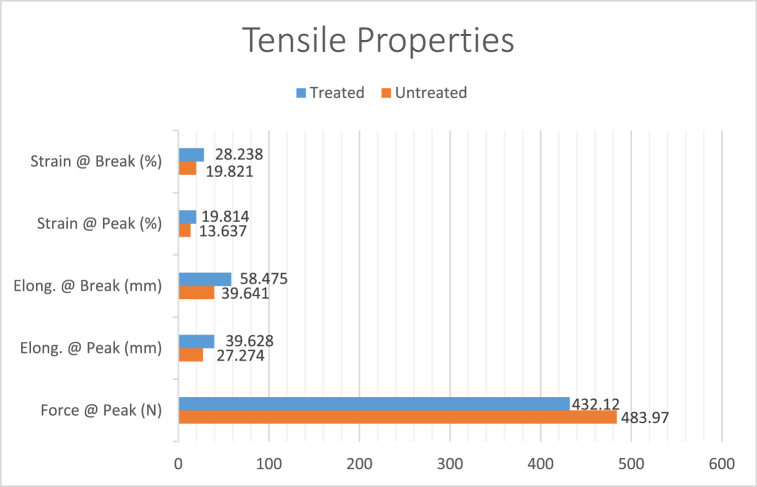
From Fig. 8 & Table 1, it can be seen that elongation at peak for the treated fabric was 39.828 mm which is much greater compared to untreated fabric. Not only elongation at peak we got greater value of elongation and strain at break for treated value while there was a slight increase in the strain value at peak as compared to the other properties. These values denote slight decrease in the mechanical properties of the treated fabric.
Fig. 8.
Tensile properties of treated and untreated fabric.
Table 1.
Tensile strength test results.
| Types of sample | Force @ Peak (N) | Elongation @ Peak (mm) | Elongation @ Break (mm) | Strain @ Peak (%) | Strain @ Break (%) |
|---|---|---|---|---|---|
| Untreated Fabric | 483.97 | 27.274 | 39.641 | 13.637 | 19.821 |
| Treated Fabric | 432.12 | 39.828 | 58.475 | 19.814 | 28.238 |
3.2.2. Effect of TiO2 and ZnO nanoparticles coating on tearing strength
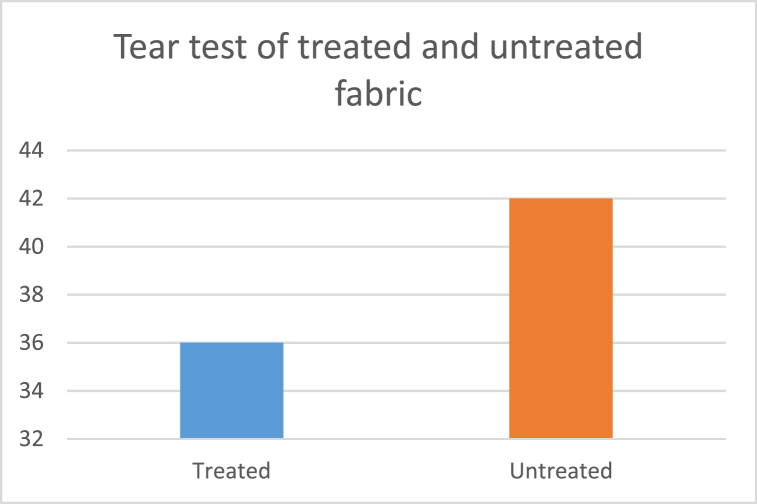
After the tear test, for the untreated fabric, the tear strength was 42 kN and for the treated fabric the result was 36 kN, which is shown in Fig. 9.
Fig. 9.
Tear test of treated and untreated fabric.
Formation of bonds after treatment of TiO2 and ZnO was identified by FTIR KBr spectroscopy. For physical tests it was observed that the tensile strength for untreated fabric was 483.87 N which decreased to 432.87 N for treated samples. Similarly, there was a decrease in tear strength from 42 N for untreated fabric to 36 N for treated fabric. The reduction in physical strength was attributed to reactions caused by the treatment.
From the calculation of Table 2, we got that the water vapor transmission rate decreased in the treated fabric, while the thickness of the treated fabric was found to be greater than that of the untreated fabric.
Table 2.
Effect of TiO2 and ZnO nanoparticles coating on water vapor permeability and fabric thickness.
| Fabric Types | Effect of TiO2 and ZnO nanoparticles coating on water vapor permeability: |
Effect of TiO2 and ZnO nanoparticles coating on fabric thickness: | |||||
|---|---|---|---|---|---|---|---|
| Sample weight | Machine sample weight | Area | Time | Weight change | Water vapor transmission | ||
| Untreated fabric | 182.606 g | 182.378 g | 0.4 square ft. | 12 min/0.2 h | (182.606–182.378) = 0.228 g | g/hr-ft2 | Average measured thickness = 0.96 mm |
| Treated fabric | 183.245 g | 183.112 g | 0.4 square ft. | 12 min/0.2 h | (183.245–183.112) = 0.133 g | g/hr-ft2 | Average measured thickness = 1.23 mm |
3.2.3. Effect of TiO2 and ZnO nanoparticles coating on fabric stiffness
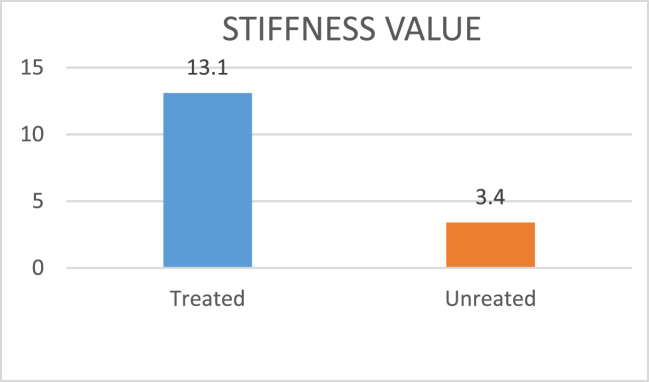
ASTM spec D4032 standard was used to test stiffness of both the treated and untreated Jute-Cotton union fabric. This test was conducted at room temperature and humidity. From Fig. 10, it can be seen that the result was 3.4 N for the untreated Jute-Cotton union fabric, while, the result was 13.1 N for TiO2 and ZnO nanoparticles treated fabric. The value of stiffness has been depicted in Fig. 10, showing that the treated fabric was much stiffer than the untreated fabric.
Fig. 10.
Graph of stiffness value for treated and untreated fabric.
3.2.4. Effect of TiO2 and ZnO nanoparticles coating on fabric crease recovery properties
Shirley crease recovery machine was used for this testing. We found that the crease recovery angle for the untreated fabric was 105°, while, for the treated fabric we got an angle of 85°.
3.2.5. Effect of TiO2 and ZnO nanoparticles coating on fabric absorbency
From Fig. 11 it can be seen that the absorbency of untreated fabric was 87 %, while, the treated fabric showed a reduced absorbency of 27 %, indicating that the treated fabric had water-repellent properties.
Fig. 11.
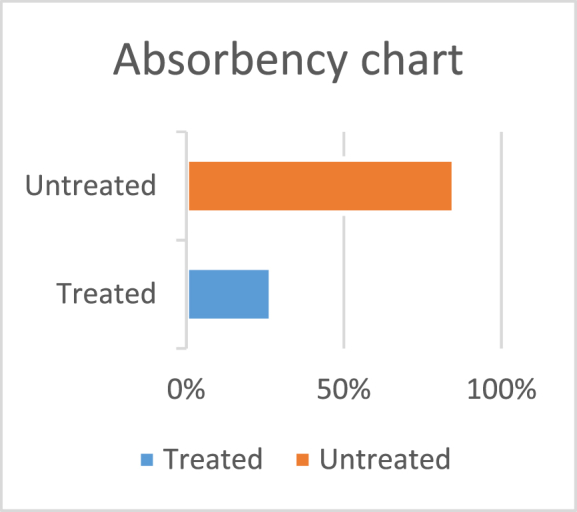
Absorbency chart for treated and untreated fabric.
3.3. Characterization of treated fabric
3.3.1. Characterization of fabric by FTIR
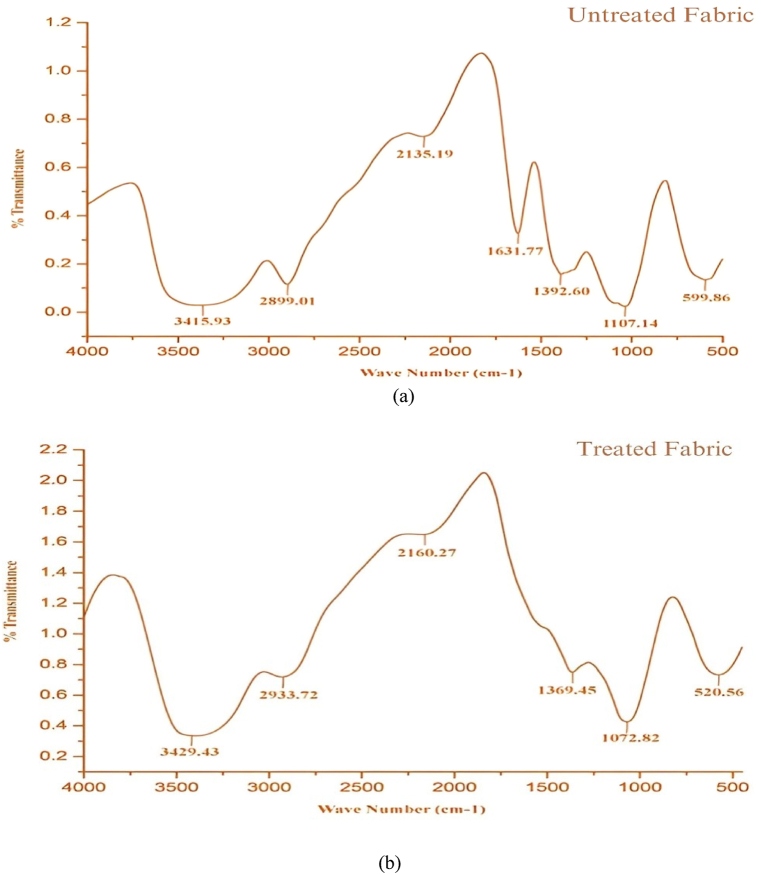
KBr FTIR spectroscopy analyzes bonds between atoms to identify organic materials and the molecular structure of the sample. Specific chemical bonds are identified by the peaks of the spectrum at a certain position. To identify atomic arrangements and concentrations of chemical bonds Fourier transform was applied to the intensity-time output of the interferometer. The sample was scanned using infrared light and the presence of specific chemical bonds was identified from the presence of a peak at a particular wave number. FTIR spectra of untreated and treated fabric samples have been presented in Fig. 12(a) and (b) respectively. From Fig. 12(a) peak shifts can be seen at 599.86, 1107.14, 1392.60, 1631.77, 2135.19, 2899.01, and 3415.93 cm−1 with intense absorption bands. Hydroxyl groups were identified by the presence of a peak at 3415.93 cm−1 and –C N was identified by the presence of a band at 2899.01. Likewise, the peak at 1631.77 cm−1 specifies C O, and the peak at 2135.19 cm−1 specifies the N–H bond of peptide linkage. In Fig. 12(b), the FTIR spectrum for treated fabric shows peak shifts at 520.56, 1072.82, 1369.45, 2160.27, 2933.72, and 3429.43 cm−1 with intense absorption. The peak at 520.56 cm−1 denotes the presence of ZnO and at 1072.82 cm−1 denotes the presence of TiO2 linkage. Thus this FTIR test confirmed the presence of TiO2 and ZnO in the fabric.
Fig. 12.
FTIR results for (a) untreated fabric and (b) treated fabric.
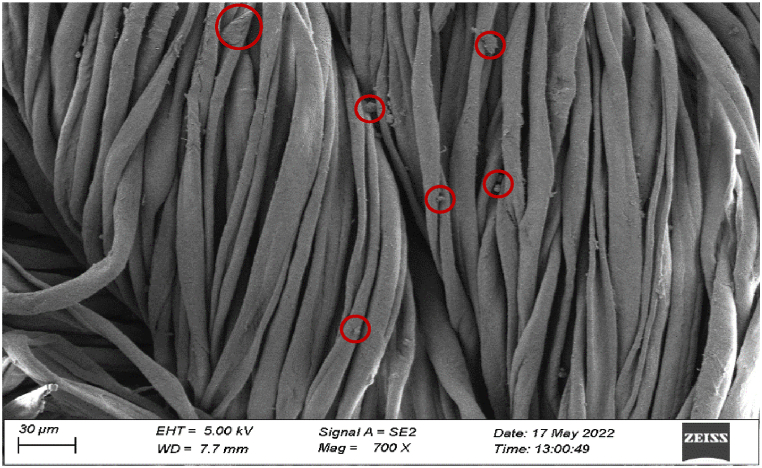
3.3.2. Characterization of fabric by SEM
The morphology of the Jute-Cotton union fabric after the application of TiO2 and ZnO nanoparticles has been shown in the SEM image as observed in Fig. 13. The fabric surface was observed to confirm the presence of TiO2 and ZnO after the application of nanoparticles under SEM, as the red circles highlight the areas where the nanoparticles were observed.
Fig. 13.
SEM result of treated fabric.
3.4. Comparative analysis of test results
Comparison of test results (Spray rating test, self-cleaning activity test, water repellent property test, antimicrobial property test, water vapor transmission test, fabric absorbency test, average thickness, tear strength test, stiffness test, tensile properties) for treated and untreated fabric has been shown in Table 3.
Table 3.
Comparison of test results for treated and untreated fabric.
| Name of tests | Untreated fabric | Treated fabric | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spray rating | 50 (ISO 1) | 90 (ISO 4) | ||||||||
| Self-cleaning activity | less | more | ||||||||
| Water repellent property | null | good | ||||||||
| Antimicrobial property | null | Good (better for gram-positive St. Aureus than for gram-negative Salmonella) | ||||||||
| Water vapor transmission | g/hr-ft2 | g/hr-ft2 | ||||||||
| Fabric absorbency | 87 % | 27 % | ||||||||
| Average thickness | 0.96 mm | 1.23 mm | ||||||||
| Tear strength | 42 N | 36 N | ||||||||
| Stiffness | 3.4 N | 13.1 N | ||||||||
| Tensile properties | Force @ Peak (N) | Elongation @ Peak (mm) | Elongation @ Break (mm) | Strain @ Peak (%) | Strain @ Break (%) | Force @ Peak (N) | Elongation @ Peak (mm) | Elongation @ Break (mm) | Strain @ Peak (%) | Strain @ Break (%) |
| 483.97 | 27.274 | 39.641 | 13.637 | 19.821 | 432.12 | 39.828 | 58.475 | 19.814 | 28.238 | |
3.5. Discussion
In this study, antimicrobial tests were performed to investigate the antimicrobial properties of fabric samples treated with TiO2 and ZnO nanoparticles. Samples were tested against gram-positive (St. Aureus) and gram-negative (Salmonella) bacteria. The treated samples had a greater effect on gram-positive bacteria than gram-negative ones, as shown by the value of the clear zone in St. Aureus for samples treated with TiO2 and ZnO was higher than the samples in Salmonella. Spray rating test results and visual assessment of fabric cleanliness before and after exposure to staining agents confirm the self-cleaning properties of the fabric, attributed to the photocatalytic properties of TiO2 and ZnO nanoparticles when exposed to light, particularly UV radiation, decomposing organic stains and contaminants, resulting in the effective removal of such impurities from the fabric surface. The UV spectrum is also shown for this experiment, where the peak absorption wavelengths for these nanoparticles (354 nm for ZnO and 376 nm for TiO2) indicate their ability to absorb UV radiation. A significant decrease in UV transmission or an increase in UV absorption at specific wavelengths indicates the enhanced UV protection capabilities of the fabric due to the nanoparticle coating. FTIR KBr spectroscopy was performed to characterize the treated fabric samples. The FTIR results confirm the formation of bonds after treatment with TiO2 and ZnO. Physical testing was also performed to investigate the physical characteristics of the treated samples compared with those of the untreated samples. Tensile strength, elongation at break, and tear strength were tested. It was found that after treating the samples, there was a slight decrease in the physical strength of the sample. For example, the tensile strength of the untreated sample was 483.87 N. The strength becomes 432.87 N for samples treated with TiO2 and ZnO. The tear strength for samples without any treatment was 42 N. For fabric treated with nanoparticles, it became 36 N. The deduction in the physical strengths was due to the reactions. The absorbency of the untreated fabric was 87 %, whereas that of the treated fabric it was 27 %. This indicates that the water-repellent property grows in the treated fabric. The untreated fabric exhibited a crease recovery angle of 105 °, whereas for the treated fabric, we obtained an angle of 85°. This reduced angle indicates the superior crease recovery property of the treated fabric compared with that of the untreated fabric. The stiffness of the treated fabric was higher than that of the untreated fabric. For untreated fabric, the value is 3.4 N, but for treated fabric, the value is 13.1 N. The thickness of the treated fabric also slightly increased compared with that of the untreated fabric.
4. Conclusion
The surface of Jute-Cotton union fabric was modified by TiO2 and ZnO and this modification was specified by SEM, FTIR and other tests like tensile and tear strength, UV and antimicrobial tests. Presence of IR bands at specific wavelengths indicated the existence of these nanoparticles in the finished product. The treated fabric not only provided UV protection and tremendous self-cleaning properties but also showed astounding antimicrobial activity and soil release action. However, a slight decrease in the mechanical properties of the treated fabric was also noticed. The tests were performed at room temperature and some results may vary with procedures and experimental conditions. Still, it can be said that the coating of ZnO and TiO2 nanoparticles depicts the possibility of multifunction for textiles with a single treatment procedure. To fully understand the implications and variability of the TiO2 and ZnO coating on different types of fabric parameters such as precursor concentrations, coating methods (e.g. sol-gel, spray coating, dip coating), curing conditions, and post-treatment processes can be varied. The evaluation of long-term durability and stability can be performed under different environmental conditions, including exposure to UV radiation, washing cycles, mechanical stress, and chemical exposure.
Data availability section
-
•
Has data associated with your study been deposited into a publicly available repository?
-
-
No. The data that has been used is confidential
Ethics declaration statement
-
•
Review and/or approval by an ethics committee was not needed for this study because the research did not involve human or animal subjects and solely involved material science experimentation on fabrics, hence, did not fall under the purview of ethical review.
-
•
Informed consent was not required for this study because the research did not involve human participants or any personal or sensitive data collection.
CRediT authorship contribution statement
Mohammad Naim Hassan: Writing – original draft, Methodology, Conceptualization. Mehrin Beg Mou: Writing – review & editing, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interest nor personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ullah M.H., Akther H., Rahman M.M., Foisal A.B.M., Hasan M.M., Amir-Al Zumahi S.M., Amri A. Surface modification and improvements of wicking properties and dyeability of grey jute-cotton blended fabrics using low-pressure glow discharge air plasma. Heliyon. 2021;7(8) doi: 10.1016/j.heliyon.2021.e07893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyanhongo G., Herrero Acero E., Matuchaki M.D.D.J., Rau M., Guebitz G., Andreaus J. Cambridge University Library; 2022. Microbial Applications for Fabric and Textile Industries. [Google Scholar]
- 3.Wei D.W., Wei H., Gauthier A.C., Song J., Jin Y., Xiao H. Superhydrophobic modification of cellulose and cotton textiles: methodologies and applications. Journal of Bioresources and Bioproducts. 2020;5(1):1–15. [Google Scholar]
- 4.Barman J., Tirkey A., Batra S., Paul A.A., Panda K., Deka R., Babu P.J. The role of nanotechnology based wearable electronic textiles in biomedical and healthcare applications. Mater. Today Commun. 2022;32 [Google Scholar]
- 5.Júnior H.L.O., Neves R.M., Monticeli F.M., Dall Agnol L. Smart fabric textiles: recent advances and challenges. Textiles. 2022;2(4):582–605. [Google Scholar]
- 6.Jadoun S., Verma A., Arif R. Modification of textiles via nanomaterials and their applications. Frontiers of textile materials: polymers, nanomaterials, enzymes, and advanced modification techniques, 2020:135–152. [Google Scholar]
- 7.Shah M.A., Pirzada B.M., Price G., Shibiru A.L., Qurashi A. Applications of nanotechnology in smart textile industry: a critical review. J. Adv. Res. 2022;38:55–75. doi: 10.1016/j.jare.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehghani M., Abubakar A.M., Pashna M. Market-driven management of start-ups: the case of wearable technology. Appl. Comput. Inform. 2022;18(1/2):45–60. [Google Scholar]
- 9.Toro A.U., Gupta V., Shukla S.K., Bansal P. Antiviral and Antimicrobial Coatings Based on Functionalized Nanomaterials. Elsevier; 2023. Functional finishing of textile materials using silver-based functionalized nanoparticles: health perspectives; pp. 333–363. [Google Scholar]
- 10.Ali T., Najam-ul-Haq M., Mohyuddin A., Musharraf S.G., Hussain D. Next-generation functional nanotextiles—prospects and challenges. Sustainable Materials and Technologies. 2023 [Google Scholar]
- 11.Sengupta S. Novel Sustainable Raw Material Alternatives for the Textiles and Fashion Industry. Springer Nature Switzerland; Cham: 2023. Sustainable products from natural fibers/biomass as a substitute for single-use plastics: Indian context; pp. 81–119. [Google Scholar]
- 12.Krakowiak R., Musial J., Bakun P., Spychała M., Czarczynska-Goslinska B., Mlynarczyk D.T., Koczorowski T., Sobotta L., Stanisz B., Goslinski T. Titanium dioxide-based photocatalysts for degradation of emerging contaminants including pharmaceutical pollutants. Appl. Sci. 2021;11(18):8674. [Google Scholar]
- 13.Diaa M., Hassabo A.G. Self-cleaning properties of cellulosic fabrics (a review) Biointerf. Res. Appl. Chem. 2022;12(2):1847–1855. [Google Scholar]
- 14.Sanjarnia P., Picchio M.L., Solis A.N.P., Schuhladen K., Fliss P.M., Politakos N., Metterhausen L., Calderón M., Osorio-Blanco E.R. Bringing innovative wound care polymer materials to the market: challenges, developments, and new trends. Adv. Drug Deliv. Rev. 2024 doi: 10.1016/j.addr.2024.115217. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadipour-Nodoushan R., Shekarriz S., Shariatinia Z., Heydari A., Montazer M. Improved cotton fabrics properties using zinc oxide-based nanomaterials: a review. Int. J. Biol. Macromol. 2023 doi: 10.1016/j.ijbiomac.2023.124916. [DOI] [PubMed] [Google Scholar]
- 16.Rabiei H., Montazer M., Farhang Dehghan S., Khaloo S.S., Yousefinejad S., Hassanipour S., Sharifi A. Comparison of the effectiveness of textiles containing metal nanoparticle and metal-organic frameworks for protection against ultraviolet radiation: a systematic review and meta-analysis. J. Text. Inst. 2023;114(12):1951–1966. [Google Scholar]
- 17.Mather R.R., Rana S., Wardman R.H. The chemistry of textile fibres. Royal Society of Chemistry. 2023 [Google Scholar]
- 18.Čuden A.P., Urbas R. Functional and Technical Textiles. Woodhead Publishing; 2023. Advances in ultraviolet (UV) ray blocking textiles; pp. 213–273. [Google Scholar]
- 19.Azam F., Ahmad F., Ahmad S., Haji A.D. Circularity in Textiles. Springer Nature Switzerland; Cham: 2023. Sustainable raw materials; pp. 59–128. [Google Scholar]
- 20.Awad M.E., Farrag A.M., El‐Bindary A.A., El‐Bindary M.A., Kiwaan H.A. Photocatalytic degradation of rhodamine B dye using low‐cost pyrofabricated titanium dioxide quantum dots‐kaolinite nanocomposite. Appl. Organomet. Chem. 2023;37(7):e7113. [Google Scholar]
- 21.Aalami Z., Hoseinzadeh M., Manesh P.H., Aalami A.H., Es' haghi Z., Darroudi M., Sahebkar A., Hosseini H.A. Synthesis, characterization, and photocatalytic activities of green sol-gel ZnO nanoparticles using Abelmoschus esculentus and Salvia officinalis: a comparative study versus co-precipitation-synthesized nanoparticles. Heliyon. 2024;10(2) doi: 10.1016/j.heliyon.2024.e24212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasnidawani J.N., Azlina H.N., Norita H., Bonnia N.N., Ratim S., Ali E.S. Synthesis of ZnO nanostructures using sol-gel method. Procedia Chem. 2016;19:211–216. [Google Scholar]
- 23.Ma J., Zhang C., Xi F., Chen W., Jiao K., Du Q., Bai F., Liu Z. Experimental study on the influence of environment conditions on the performance of paper-based microfluidic fuel cell. Appl. Therm. Eng. 2023;219 [Google Scholar]
- 24.Gdoutos E., Konsta-Gdoutos M. Mechanical Testing of Materials. Springer Nature Switzerland; Cham: 2024. Compression, bending, torsion and multiaxial testing; pp. 35–61. [Google Scholar]
- 25.Hossain I., Parvez M.S., Mahmud T., Rahman T., Moniruzzaman M. Investigation of antimicrobial and physical properties of polyester/cotton blended knitted fabric treated with AgNO3 and aloe vera. Clean. Eng. Technol. 2023;16 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
Has data associated with your study been deposited into a publicly available repository?
-
-
No. The data that has been used is confidential