Key Points
Question
Is screening for hepatocellular carcinoma (HCC) in patients with cirrhosis associated with a survival benefit after accounting for lead-time and length-time biases?
Findings
In this cohort study of 1313 patients, only 42.3% of cases of HCC were detected by screening. Detection by screening was associated with improved early-stage detection and survival, which persisted after adjusting for lead-time and length-time biases.
Meaning
These findings suggest that HCC screening is associated with reduced mortality, even after accounting for lead-time and length-time biases, and remains an important target for interventions to increase utilization.
This cohort study examines the clinical benefits of screening for hepatocellular carcinoma in at-risk patients, adjusting for lead-time and length-time biases.
Abstract
Importance
Cohort studies demonstrating an association of hepatocellular carcinoma (HCC) screening with reduced mortality are prone to lead-time and length-time biases.
Objective
To characterize the clinical benefits of HCC screening, adjusting for lead-time and length-time biases, in a diverse, contemporary cohort of at-risk patients.
Design, Setting, and Participants
This retrospective cohort study of patients with HCC was conducted between January 2008 and December 2022 at 2 large US health systems. Data analysis was performed from September to November 2023.
Main Outcomes and Measures
The primary outcome was screen-detected HCC, defined by abnormal screening-intent abdominal imaging or α-fetoprotein level within 6 months before diagnosis. Cox regression analysis was used to characterize differences in overall survival between patients with screen-detected and non–screen-detected HCC; lead-time and length-time adjustments were calculated using the Duffy parametric formula.
Results
Among 1313 patients with HCC (mean [SD] age, 61.7 [9.6] years; 993 male [75.6%]; 739 [56.3%] with Barcelona Clinic Liver Cancer stage 0/A disease), HCC was screen-detected in 556 (42.3%) and non–screen detected in 757 (57.7%). Patients with screen-detected HCC had higher proportions of early-stage HCC (393 patients [70.7%] vs 346 patients [45.7%]; risk ratio [RR], 1.54; 95% CI, 1.41-1.70) and curative treatment receipt (283 patients [51.1%] vs 252 patients [33.5%]; RR, 1.52; 95% CI, 1.34-1.74) compared with patients with non–screen-detected HCC. The screen-detected group had significantly lower mortality, which persisted after correcting for lead-time bias (hazard ratio, 0.75; 95% CI, 0.65-0.87) in fully adjusted models. Both groups had similar tumor doubling times (median [IQR], 3.8 [2.2-10.7] vs 5.6 [1.7-11.4] months) and proportions of indolent tumors (28 patients [35.4%] vs 24 patients [38.1%]; RR, 0.93; 95% CI, 0.60-1.43). Adjustment for length-time bias decreased survival estimates, although 3-year and 5-year survival for patients with screen-detected HCC remained longer than that for patients with non–screen-detected HCC.
Conclusions and Relevance
The findings of this cohort study suggest that HCC screening is associated with reduced mortality even after accounting for lead-time and length-time biases. However, these biases should be considered in future studies.
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide.1,2 One of the main determinants of prognosis is tumor stage and eligibility for curative therapy. Patients with early-stage HCC have 5-year survival rates approaching 70% with curative treatments, compared with a median survival of 1 to 2 years after palliative therapies for those with more-advanced tumor burden.3 Guidelines from the American Association for the Study of Liver Diseases and European Association for the Study of the Liver recommend HCC screening using ultrasonography with or without α-fetoprotein (AFP) in high-risk patients, including those with cirrhosis.4,5,6
The efficacy of HCC screening in patients with cirrhosis is controversial because of the lack of randomized data and the inherent biases of cohort studies, including lead-time and length-time biases.7 This controversy was fueled by a case-control study8 from the US Veterans Health Administration that failed to find an association between screening and HCC-related mortality. Lead-time bias occurs when screening leads to earlier cancer detection, so the time from diagnosis to death appears longer without an actual difference in mortality. Length-time bias results from screening being more likely to detect slow-growing indolent tumors that are less likely to be fatal, which confers an artificial perception of improved survival.9 Although HCC is considered an aggressive cancer with 5-year survival rates less than 20%,10 tumor biology is variable. A systematic review11 reported an average HCC doubling time of 4 to 5 months, although indolent growth patterns are more commonly observed with nonviral liver disease.
Understanding the true benefit of HCC screening is important for determining its overall value, considering potential physical, financial, and psychological harms.12,13 Delayed evaluation of the risk-to-benefit ratio has led to controversies in other cancer screening programs, including prostate cancer, colorectal cancer in older individuals, and breast cancer in younger women. Here, we aimed to characterize the benefits of HCC screening after considering lead-time and length-time biases in a contemporary cohort of patients.
Methods
Patient Selection
We performed a retrospective cohort study including patients with cirrhosis from any cause or noncirrhotic chronic hepatitis B virus (HBV) infection with a new diagnosis of HCC between January 2008 and December 2022. Patient recruitment was done at 2 large health systems from the North American Liver Cancer Consortium: UT Southwestern (UTSW) Medical Center (Dallas, Texas) and Parkland Health (Dallas, Texas). UTSW is an academic tertiary-care referral center, and Parkland Health is an integrated safety-net health system.14,15 This study was approved by the institutional review board of UTSW Medical Center, with a waiver of informed consent because the study was retrospective and the research involved minimal risk to participants, in accordance with 45 CFR §46. The study follows Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies.
Patients with HCC were identified using databases of patients seen in multidisciplinary liver tumor clinics at each health system. HCC diagnoses were confirmed to meet the diagnostic criteria per American Association for the Study of Liver Diseases guidelines,4 including imaging with Liver Imaging Reporting and Data System category 5 lesions or histological confirmation. We excluded patients with Child Pugh class C cirrhosis or Eastern Cooperative Oncology Group (ECOG) performance status of 2 or higher because screening is not indicated for those populations.
Data Collection
We collected patient demographics, insurance status, and clinical characteristics such as body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), liver disease cause, ECOG performance status, and Child-Pugh class from the electronic health record (EHR). Race and ethnicity in the EHR were self-reported by patients and were classified for this study as Black, Hispanic, non-Hispanic White, and other (ie, Asian, American Indian or Alaska Native, or unknown).16 Data on race and ethnicity are included in this study because of their potential impact on screening outcomes. BMI was classified according to World Health Organization categories.17 We classified liver disease cause as hepatitis C virus (HCV), HBV, alcohol-related liver disease (ALD), metabolic dysfunction–associated steatotic liver disease (MASLD), and other, with the following hierarchical algorithm used for patients with multiple causes: (1) HCV, (2) HBV, (3) ALD, (4) other, and (5) MASLD (eg, patients with HCV and ALD were classified as HCV).18 Tumor-related characteristics included nodule count, maximum diameter, vascular invasion, and presence of metastases. We captured dates of HCC treatments, including liver transplantation, surgical resection, local ablation, transarterial chemoembolization or radioembolization, stereotactic body radiation therapy, or systemic therapy.
Using manual abstraction from the EHR, including scanned external records, we recorded receipt of AFP measurement and liver imaging (ultrasonography, multiphase computed tomography, or contrast-enhanced magnetic resonance imaging) in the year before HCC diagnosis. We excluded the last imaging study leading to HCC diagnosis, which was performed with diagnostic intent. We classified imaging study indication as screening (eg, to rule out HCC or for cirrhosis), diagnostic (eg, abdominal pain, jaundice, or elevated liver enzymes), other, or unknown. Examinations performed to monitor an indeterminate liver lesion were captured under other within a monitoring of liver mass subcategory. Imaging studies were classified as positive when there was a suspicious liver lesion 1 cm or larger, and AFP was positive when it was greater than or equal to 20 ng/mL (to convert to micrograms per liter, multiply by 1.0).19
Screen-detected HCC was defined as (1) prior imaging with screening intent and a positive result, (2) imaging performed for monitoring of a liver lesion (eg, Liver Imaging Reporting and Data System category 3 or 4 lesions)20, or (3) positive AFP within the prior 3 months. We further classified screen-detected HCC as that detected by imaging alone, AFP alone, or imaging plus AFP. Patients with false-negative screening results were classified as having screen failure. Non–screen-detected HCCs included those with incidental or symptomatic detection according to the presence of potential HCC-related symptoms.
Associations With Early Tumor Detection and Treatment
We examined associations of screen detection with clinical outcomes, including tumor stage, curative treatment, and overall survival. Early-stage HCC was assessed using 2 definitions: Barcelona Clinic Liver Cancer (BCLC) stage 0/A and the Milan criteria.21,22 Curative treatments were defined as local ablation, resection, or transplantation. Treatment response was assessed using measurements of the enhancing portion of the tumor23 and was classified as complete response, partial response, stable disease, progressive disease, or unknown. Objective response rate (ORR) was defined as complete or partial response, whereas the disease-control rate was defined as complete response, partial response, or stable disease as the best response.
Associations With Overall Survival
Overall survival was captured from HCC diagnosis to death, and patients who were lost to follow-up were censored at their last clinical encounter. Patients were also censored at time of liver transplantation.
Given that the mediating pathway for screening to improve survival would be early tumor detection and curative treatment receipt, we performed stratified analyses by tumor burden and receipt of curative therapy. In the absence of lead-time and length-time biases and residual confounding, differences in mortality between screen-detected and non–screen-detected tumors should be mitigated in these analyses.
Differential Tumor Growth Patterns
To inform the potential for lead-time and length-time biases, we estimated tumor doubling times (TDTs) among the subset of patients with 2 imaging studies after HCC diagnosis more than 30 days apart without intervening HCC-directed treatment. TDT was calculated using the Schwartz equation, as described elsewhere25,26 (eAppendix 1 in Supplement 1). Tumors were categorized as having indolent (TDT ≥365 days), intermediate (TDT, 90-365 days), and rapid (TDT, ≤90 days) growth.
Statistical Analysis
Data analysis was performed from September to November 2023. We used multivariable Cox proportional hazard analyses to characterize differences in overall survival between screen-detected and non–screen-detected HCC, adjusting for a priori important factors (ie, age, sex, race, BMI, insurance status, liver disease cause, Child Pugh class, and ECOG performance status). Crude and adjusted hazard ratios (HRs) with 95% CIs and 1-year, 3-year, and 5-year survival rates (with 95% CIs) were estimated. Lead-time and length-time bias adjustments were conducted using the parametric method proposed by Duffy and colleagues24 (eAppendix 1 in Supplement 1). For all analyses, statistical significance was defined as 2-sided P < .05. Analyses were performed using R statistical software version 4.2.1 (R Project for Statistical Computing).
Results
Patient Characteristics
A total of 1313 patients (mean [SD] age, 61.7 [9.6] years; 993 male [75.6%]; 739 [56.3%] with BCLC stage 0/A disease) were analyzed. Their characteristics are described in Table 1. The most common causes of HCC were HCV (786 patients [59.9%]), ALD (184 patients [14.0%]), and MASLD (163 patients [12.4%]). The cohort was diverse regarding race and ethnicity (390 Black patients [29.7%], 354 Hispanic patients [27.0%], 477 White patients [36.3%], and 92 patients of other races [7.0%]). Nearly two-thirds (820 patients [62.5%]) had Child-Pugh class A cirrhosis, and 492 patients [37.5%] had Child Pugh class B cirrhosis.
Table 1. Characteristics of Patient Population.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Total (N = 1313) | With screen-detected HCC (n = 556) | With non–screen-detected HCC (n = 757) | |
| Age at diagnosis, mean (SD), y | 61.7 (9.6) | 61.9 (9.5) | 61.5 (9.6) |
| Sex | |||
| Male | 993 (75.6) | 406 (73.0) | 587 (77.5) |
| Female | 320 (24.4) | 150 (27.0) | 170 (22.5) |
| Race and ethnicity | |||
| Black | 390 (29.7) | 166 (29.9) | 224 (29.5) |
| Hispanic | 354 (27.0) | 161 (29.0) | 193 (25.5) |
| White | 477 (36.3) | 202 (36.3) | 275 (36.3) |
| Othera | 92 (7.0) | 27 (4.9) | 65 (8.6) |
| Cause | |||
| Hepatitis C virus | 786 (59.9) | 339 (61.0) | 447 (59.0) |
| Hepatitis B virus | 89 (6.8) | 34 (6.1) | 55 (7.3) |
| Alcohol | 184 (14.0) | 82 (14.7) | 102 (13.5) |
| Metabolic dysfunction–associated steatotic liver disease | 163 (12.4) | 73 (13.1) | 90 (11.9) |
| Other | 91 (6.9) | 28 (2.1) | 63 (8.3) |
| Child Pugh cirrhosis class | |||
| A | 820 (62.5) | 358 (64.4) | 462 (61.0) |
| B | 493 (37.5) | 198 (35.6) | 295 (39.0) |
| C | NA | NA | NA |
| Barcelona Clinic Liver Cancer stage | |||
| 0/A | 739 (56.3) | 393 (70.7) | 346 (45.7) |
| B | 261 (19.9) | 87 (15.6) | 174 (23.0) |
| C | 313 (23.8) | 76 (13.7) | 237 (31.3) |
| Insurance status (n = 1305) | |||
| Uninsured | 108 (8.3) | 32 (5.8) | 76 (10.1) |
| Medicaid | 204 (15.6) | 87 (15.8) | 117 (15.5) |
| Medicare | 439 (33.6) | 209 (37.9) | 230 (30.5) |
| Private | 248 (19.0) | 109 (19.7) | 139 (18.5) |
| Other | 306 (23.4) | 115 (20.8) | 191 (25.4) |
| Eastern Cooperative Oncology Group performance status | |||
| 0 | 960 (73.1) | 439 (79.0) | 521 (68.8) |
| 1 | 353 (26.9) | 117 (21.0) | 236 (31.2) |
| Body mass index (n = 1299)b | |||
| Underweight (<18.5) | 33 (2.5) | 10 (1.8) | 23 (3.1) |
| Normal weight (18.5-24.9) | 405 (31.2) | 156 (28.4) | 249 (33.2) |
| Preobesity (25.0-29.9) | 456 (35.1) | 192 (34.9) | 264 (35.2) |
| Obesity class I (30.0-34.9) | 261 (20.1) | 128 (23.3) | 133 (17.8) |
| Obesity class II (35.0-39.9) | 79 (6.1) | 39 (7.1) | 56 (7.5) |
| Obesity class III (≥40.0) | 49 (3.8) | 25 (4.5) | 24 (3.2) |
Abbreviations: HCC, hepatocellular carcinoma; NA, not applicable.
Other race included Asian, American Indian or Alaska Native, or unknown race and ethnicity.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Patterns of HCC Detection
HCC was screen detected in 556 patients (42.3%) and non–screen detected in 757 patients (57.7%). Characteristics of patients with non–screen-detected HCC are shown in eTable 1 in Supplement 1. Among patients with screen-detected HCC, 248 (44.6%) had detection by imaging alone, 59 (10.6%) had detection by AFP alone, and 249 (44.7%) had detection by both imaging and AFP. The time between a positive screening test and HCC diagnosis was within 6 months for most patients (imaging-detected, 464 patients [93.4%]; AFP-detected, 46 patients [77.9%]). Within the non–screen-detected group, 187 (24.7%) were symptomatic and 570 (75.3%) were incidental. Within the incidental group, most patients (530 patients [78.9%]) had no prior imaging. Only 26 (4.6%) were categorized as experiencing screen failure.
Early-Stage Detection and Curative Treatment Receipt
Overall, the distribution of HCC was 8.5% (111 patients) BCLC stage 0, 47.8% (628 patients) BCLC stage A, 19.9% (261 patients) BCLC stage B, and 23.8% (313 patients) BCLC stage C; approximately one-half (669 patients [51.1%]) were detected within the Milan criteria. The proportions of early-stage HCC were significantly higher in the screen-detected than non–screen-detected group (BCLC stage 0/A, 393 patients [70.7%] vs 346 patients [45.7%]; risk ratio [RR], 1.54; 95% CI, 1.41-1.70; Milan criteria, 365 patients [66.0%] vs 304 patients [40.2%]; RR, 1.64; 95% CI, 1.48-1.82). The proportions of early-stage HCC across subgroups are reported in eAppendix 2 in Supplement 1.
Overall, 535 patients (40.9%) underwent curative treatment, including liver transplant (123 patients [9.4%]), resection (234 patients [17.9%]), and local ablation (178 patients [13.6%]). Curative treatment was higher in those with screen-detected vs non–screen-detected tumors (283 patients [51.1%] vs 252 patients [33.5%]; RR, 1.52; 95% CI, 1.34-1.74). Among non–screen-detected tumors, curative treatment receipt was higher among patients with incidental tumors than those with symptomatic tumors (204 patients [36.0%] vs 48 patients [25.7%]; RR, 1.40; 95% CI, 1.07-1.83). As anticipated, this association appeared to be mediated by early detection, with similar proportions of patients undergoing curative treatment among those with BCLC stage 0/A vs symptomatic disease (37 patients [50.0%]) and screen-detected HCC (257 patients [65.7%]; RR, 1.31; 95% CI, 1.03-1.67) vs incidental HCC (165 patients [61.1%]; RR, 1.22; 95% CI, 0.95-1.56).
In exploratory analyses, response to first treatment appeared similar between screen-detected, incidental, and symptomatic tumors, with all exhibiting ORR exceeding 80% with curative-intent treatments; ORRs after embolic treatments were similar at 54.8% (RR, 1.22; 95% CI, 0.95-1.56) among screen-detected HCCs and 59.9% (RR, 1.54; 95% 1.08-2.20) among incidental HCC, but lower at 38.9% among HCCs with symptomatic presentations.
Tumor Growth Patterns and Response to Treatment
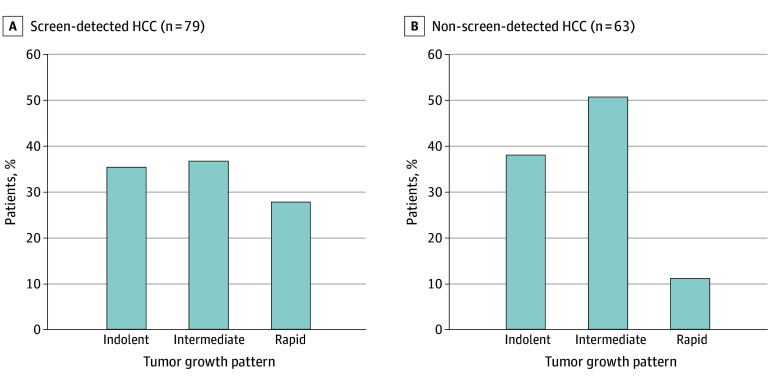
The distribution of TDT for patients with available data (79 screen-detected and 63 non–screen-detected HCC) is demonstrated in Figure 1. Screen-detected HCCs had a median (IQR) TDT of 3.8 (2.2-10.7) months compared with 5.6 (1.7-11.4) months for non–screen-detected HCCs. The proportions of indolent tumors did not significantly differ between the groups (28 patients [35.4%] vs 24 patients [38.1%]; RR, 0.93; 95% CI, 0.60-1.43).
Figure 1. Tumor Growth Patterns Among Early-Stage Group.
Screen-detected hepatocellular carcinoma (HCC) had a median (IQR) tumor doubling time of 3.8 (2.2-10.7) months vs 5.6 (1.7-11.4) months for non–screen-detected HCC (P = .40).
Overall Survival
Median survival was higher in the screen-detected group than in the non–screen-detected group (37.0 months [95% CI, 30.9-47.9 months] vs 19.0 months [95% CI, 16.9-21.9 months]) (Figure 2). Similarly, restricted mean survival time was higher in the screen-detected group (eTable 2 and eFigure in Supplement 1). Among patients with non–screen-detected tumors, survival was higher for those with incidental detection than those with symptomatic presentation (19.9 vs 16.8 months). In multivariable analysis, screen-detected HCC was significantly associated with reduced mortality (HR, 0.65; 95% CI, 0.56-0.75), including after adjustment for curative treatment receipt (HR, 0.75; 95% CI, 0.65-0.87). In exploratory subgroup analyses, imaging detection (HR, 0.67; 95% CI, 0.55-0.80) and AFP detection (HR, 0.69; 95% CI, 0.48-0.98) were both found to be associated with reduced mortality.
Figure 2. Kaplan-Meier Curves Comparing Patients With Non–Screen-Detected vs Screen-Detected Hepatocellular Carcinoma, With Lead-Time Bias Adjustments for Various Sojourn Times.
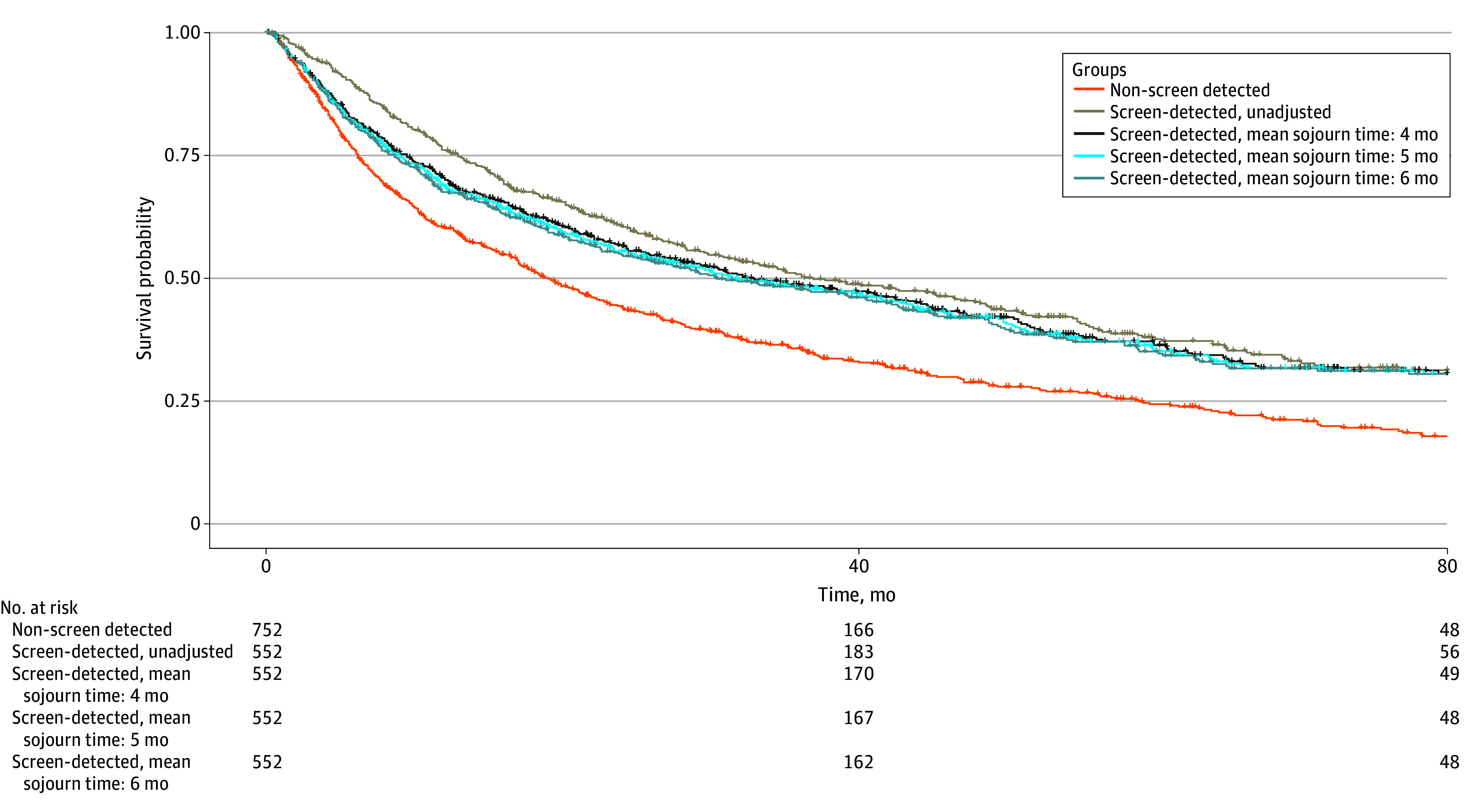
After adjusting for lead-time bias (sojourn time 5 months), the median survival of patients with screen-detected HCC decreased to 31.4 months (95% CI, 26.0-42.9 months) (Figure 2). In sensitivity analyses using sojourn times of 4 to 6 months, screen detection remained associated with reduced mortality (Table 2). With a 6-month sojourn time, screen detection was associated with 33% decreased risk of mortality (HR, 0.77; 95% CI, 0.67-0.89). Further adjustment for length-time bias decreased survival estimates, although 3-year and 5-year survival remained longer than for patients with non–screen-detected HCC (Table 3). Patients with screen-detected tumors had 3-year and 5-year survival estimates of 37% and 26%, respectively, compared with 25% and 8%, respectively, for those with non–screen-detected tumors. The association with improved survival remained significant after lead-time bias adjustment among those with HCC detected by imaging, across sojourn times of 4 to 6 months (4 months, HR, 0.75; 95% CI, 0.62-0.90; 5 months, HR, 0.76; 95% CI, 0.63-0.92; 6 months, HR, 0.78; 95% CI, 0.64-0.94); however, there was no association among those with AFP-detected HCC.
Table 2. Multivariable Cox Proportional Hazards Model for Association of Screen Detection With Overall Survival, Adjusting for Lead-Time Bias.
| Variable | HR (95% CI)a |
|---|---|
| Crude (unadjusted) | |
| Non–screen-detected hepatocellular carcinoma | 1 [Reference] |
| Screen-detected hepatocellular carcinoma | 0.65 (0.56-0.75) |
| Adjusted for lead-time bias | |
| Mean sojourn time of 4 mo | 0.74 (0.64-0.85) |
| Mean sojourn time of 5 mo | 0.75 (0.65-0.87) |
| Mean sojourn time of 6 mo | 0.77 (0.67-0.89) |
Abbreviation: HR, hazard ratio.
Adjusted for age at diagnosis, sex, race, body mass index, insurance status, liver disease etiology, Child Pugh class as a continuous variable, and Eastern Cooperative Oncology Group class.
Table 3. Survival Estimates for Patients With HCC, Adjusting for Lead-Time and Length-Time Biases.
| Variable | Survival rate, % (95% CI)a | ||
|---|---|---|---|
| 1 y | 3 y | 5 y | |
| Crude (not adjusted for lead-time or length-time biases) | |||
| Non–screen-detected HCC | 63 (51-77) | 25 (14-46) | 8 (3-26) |
| Screen-detected HCC | 75 (62-90) | 46 (29-75) | 32 (16-65) |
| Adjusted for lead time | |||
| Mean sojourn time of 4 mo | 68 (54-87) | 44 (26-73) | 31 (15-65) |
| Mean sojourn time of 5 mo | 66 (51-86) | 43 (26-73) | 30 (14-64) |
| Mean sojourn time of 6 mo | 65 (50-85) | 43 (25-72) | 29 (14-63) |
| Adjusted for lead and length timeb | |||
| Mean sojourn time of 4 mo | 59 (47-76) | 38 (23-64) | 27 (13-57) |
| Mean sojourn time of 5 mo | 57 (44-75) | 37 (23-64) | 26 (12-56) |
| Mean sojourn time of 6 mo | 57 (44-74) | 37 (22-63) | 25 (12-55) |
Abbreviation: HCC, hepatocellular carcinoma.
Adjusted for age at diagnosis, sex, race, body mass index, insurance status, liver disease etiology, Child Pugh class as a continuous variable, and Eastern Cooperative Oncology Group class.
Length-time adjustment is based on calculated Φ = 0.87.
Overall Survival, Stratified by Tumor Burden
When stratified by BCLC stage, screen detection was associated with a survival benefit among those with early-stage HCC (BCLC 0/A, HR, 0.79; 95% CI, 0.64-0.97) but not those with larger tumor burden (BCLC B, HR, 1.04; 95% CI, 0.75-1.44; BCLC C, HR, 0.81; 95% CI, 0.59-1.11). Among patients with early-stage HCC, median survival was 56.6 months (95% CI, 51.1-68.9 months) for those with screen-detected HCC vs 48.7 months (95% CI, 40.1-59.4 months) for those with non–screen-detected HCC. Curative treatments appeared to be associated with survival differences among patients with early-stage HCC, because survival was similar between those with screen-detected and non–screen-detected early-stage HCC who received curative treatment (HR, 0.91; 95% CI 0.68-1.23). When stratified by Milan criteria in adjusted analyses, there was no association of HCC screening with survival for either those whose disease fell within the Milan criteria (HR, 0.85; 95% CI, 0.68-1.07) and those whose disease did not (HR, 0.88; 95% CI, 0.72-1.08).
Among those with BCLC stage A HCC, 389 patients (67.0%) had a unifocal HCC between 2 and 5 cm, 51 (8.8%) had a unifocal lesion larger than 5 cm, and 141 (24.3%) had multifocal HCC with each 3 cm or smaller in maximum diameter. Survival did not significantly differ between those with screen-detected and non–screen-detected tumors in all subgroups (unifocal 2-5 cm HCC, HR, 0.97; 95% CI, 0.73-1.29, unifocal >5 cm HCC, HR, 0.70; 95% CI, 0.18-2.69; multifocal ≤3 cm HCC, HR, 0.75; 95% CI, 0.41-1.35) in adjusted models. Among patients with early-stage, non–screen-detected HCC, those with incidental detection had a reduced HR for mortality compared with those with symptomatic presentation, but this difference did not reach statistical significance (HR, 0.84; 95% CI, 0.57-1.22).
Discussion
In this cohort study, we found that fewer than one-half of at-risk patients with HCC at 2 large US health centers had screen-detected disease. Screen detection was associated with improved early tumor detection, curative treatment receipt, and reduced mortality. The association of screen detection with reduced mortality persisted after adjusting for lead-time and length-time biases across a range of sojourn times.
Prior studies27,28 reported a variable association of HCC screening with improved survival after adjustment for lead-time bias. Notably, a case-control study27 from the US Veterans Affairs health system failed to find an association of screening with HCC-related mortality. The lack of an association may be related to downstream failures in the cancer care continuum, including underuse of curative treatments in patients with early-stage HCC. Our data reinforce a significant association between screening and improved outcomes, including HCC-related mortality, even after adjusting for lead-time bias across several sojourn times.
Few studies have evaluated the impact of length-time bias, although most suggested that impact was minimal.29 We found lower survival estimates for patients with screen-detected HCC after adjusting for length-time bias, although screening continued to have an association with improved survival. We also examined biological differences in tumor growth patterns to inform discussions of length-time bias and overdiagnosis. We noted variation in TDTs and growth patterns, consistent with a prior meta-analysis11 that reported an average TDT of 4 to 5 months and approximately one-third of HCCs having indolent growth patterns. Our study adds to this literature by demonstrating similar proportions of indolent tumors between screen-detected and non–screen-detected groups. The similar proportions may relate to the high proportion of incidental detection in the non–screen-detected group, likely because of frequent use of nonscreening imaging among patients with cirrhosis. These data reinforce the impact of length-time bias on screening benefits, and the risk of HCC overdiagnosis is likely small. Overdiagnosis in HCC is further mitigated through guideline recommendations that promote screening in at-risk populations, avoidance of screening in those with high competing risks of mortality (eg, Child-Pugh class C cirrhosis), and strict diagnostic criteria for HCC cases.30
Overall, these data highlight the importance of promoting HCC screening implementation in practice. Several studies31,32 have demonstrated persistent underuse of screening, related to a combination of patient and practitioner barriers. Although most practitioners believe screening improves early detection and survival, many report a continued need for data evaluating the benefits and harms of HCC screening.
Screening improves overall survival by facilitating early tumor detection. However, the association between screening and survival in our study was only partially mitigated after adjusting for tumor stage. After adjusting for curative treatment, survival differences between patients with screen-detected and non–screen-detected early-stage HCC disappeared. These data reinforce that screening is only the first step in the cancer care continuum,33 and patients with early-stage detection must undergo timely curative treatment to have improved survival.34,35,36 Efforts are needed to promote patients receiving high-quality care at multidisciplinary, high-volume settings to reduce issues regarding underuse of curative treatments.34,37,38,39
Limitations
Our study has a few limitations. First, it is prone to ascertainment bias for imaging studies not performed in our health systems. This risk was mitigated by a review of outside clinical records. Second, our study is subject to misclassification bias for screen-detected vs non–screen-detected HCC classification, as well as symptomatic vs incidental presentation, although this was minimized by using detailed study indications. Third, our study includes patients from 2 large diverse health systems, but our results may not generalize to broader populations, especially those outside the US. Fourth, there is a risk of residual confounding because other factors, such as recognition of underlying cirrhosis, are needed for HCC screening receipt. Fifth, tumor growth patterns were derived from patients without interval treatment, which may have a higher proportion of indolent tumors, and tumor growth patterns within each patient may vary over time.40 We feel these limitations are outweighed by the strengths of the study including its large sample size, diversity regarding race and liver disease cause, and rigorous statistical and biological consideration of lead-time and length-time biases.
Conclusions
In this cohort study of at-risk patients, lead-time and length-time biases had an impact on survival estimates and should be considered in future studies. However, HCC screening was associated with improved survival after accounting for these biases and remains an important target for multilevel interventions.
eAppendix 1. Supplemental Methods
eAppendix 2. Supplemental Results
eTable 1. Characteristics of Non–Screen-Detected HCC Patients
eTable 2. Restricted Mean Survival Times for HCC, Adjusting for Lead Time Bias
eFigure. Kaplan Meier Curves Comparing Patients With Incidental Versus Symptomatic Non–Screen-Detected HCC
eReferences
Data Sharing Statement
References
- 1.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650-2666. doi: 10.1016/j.cgh.2019.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20(12):864-884. doi: 10.1038/s41571-023-00825-3 [DOI] [PubMed] [Google Scholar]
- 3.Dhir M, Lyden ER, Smith LM, Are C. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: a meta-analysis. HPB (Oxford). 2012;14(9):635-645. doi: 10.1111/j.1477-2574.2012.00500.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Llovet JM, Yarchoan M, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922-1965. doi: 10.1097/HEP.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317-370. doi: 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 7.Abrahamsson L, Isheden G, Czene K, Humphreys K. Continuous tumour growth models, lead time estimation and length bias in breast cancer screening studies. Stat Methods Med Res. 2020;29(2):374-395. doi: 10.1177/0962280219832901 [DOI] [PubMed] [Google Scholar]
- 8.Moon AM, Weiss NS, Beste LA, et al. No association between screening for hepatocellular carcinoma and reduced cancer-related mortality in patients with cirrhosis. Gastroenterology. 2018;155(4):1128-1139.e6. doi: 10.1053/j.gastro.2018.06.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen MF, Tabár L, Vitak B, Smith RA, Chen HH, Duffy SW. Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. Eur J Cancer. 2003;39(12):1746-1754. doi: 10.1016/S0959-8049(03)00260-0 [DOI] [PubMed] [Google Scholar]
- 10.Khalaf N, Ying J, Mittal S, et al. Natural history of untreated hepatocellular carcinoma in a US cohort and the role of cancer surveillance. Clin Gastroenterol Hepatol. 2017;15(2):273-281.e1. doi: 10.1016/j.cgh.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 11.Nathani P, Gopal P, Rich N, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut. 2021;70(2):401-407. doi: 10.1136/gutjnl-2020-321040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narasimman M, Hernaez R, Cerda V, et al. Hepatocellular carcinoma surveillance may be associated with potential psychological harms of in patients with cirrhosis. Hepatology. 2024;79(1):107-117. doi: 10.1097/HEP.0000000000000528 [DOI] [PubMed] [Google Scholar]
- 13.Narasimman M, Hernaez R, Cerda V, et al. Financial burden of hepatocellular carcinoma screening in patients with cirrhosis. Clin Gastroenterol Hepatol. Published online August 5, 2023. doi: 10.1016/j.cgh.2023.07.018 [DOI] [PubMed] [Google Scholar]
- 14.Singal AG, Rich NE, Mehta N, et al. Direct-acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology. 2019;157(5):1253-1263.e2. doi: 10.1053/j.gastro.2019.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singal AG, Ghaziani TT, Mehta N, et al. Recall patterns and risk of primary liver cancer for subcentimeter ultrasound liver observations: a multicenter study. Hepatol Commun. 2023;7(3):e0073. doi: 10.1097/HC9.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi: 10.1016/j.cgh.2018.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shields M, Tremblay MS, Connor Gorber S, Janssen I. Measures of abdominal obesity within body mass index categories, 1981 and 2007-2009. Health Rep. 2012;23(2):33-38. [PubMed] [Google Scholar]
- 18.Hester CA, Rich NE, Singal AG, Yopp AC. Comparative analysis of nonalcoholic steatohepatitis- versus viral hepatitis- and alcohol-related liver disease-related hepatocellular carcinoma. J Natl Compr Canc Netw. 2019;17(4):322-329. doi: 10.6004/jnccn.2018.7105 [DOI] [PubMed] [Google Scholar]
- 19.Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154(6):1706-1718.e1. doi: 10.1053/j.gastro.2018.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanneganti M, Marrero JA, Parikh ND, et al. Clinical outcomes of patients with Liver Imaging Reporting and Data System 3 or Liver Imaging Reporting and Data System 4 observations in patients with cirrhosis: a systematic review. Liver Transpl. 2022;28(12):1865-1875. doi: 10.1002/lt.26562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699. doi: 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 22.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681-693. doi: 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galle PR, Forner A, Llovet JM, et al. ; European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 24.Duffy SW, Nagtegaal ID, Wallis M, et al. Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol. 2008;168(1):98-104. doi: 10.1093/aje/kwn120 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz M. A biomathematical approach to clinical tumor growth. Cancer. 1961;14:1272-1294. doi: [DOI] [PubMed] [Google Scholar]
- 26.Wapnir IL, Wartenberg DE, Greco RS. Three dimensional staging of breast cancer. Breast Cancer Res Treat. 1996;41(1):15-19. doi: 10.1007/BF01807032 [DOI] [PubMed] [Google Scholar]
- 27.Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;77(1):128-139. doi: 10.1016/j.jhep.2022.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269. doi: 10.7326/M14-0558 [DOI] [PubMed] [Google Scholar]
- 29.Choi DT, Kum HC, Park S, et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17(5):976-987.e4. doi: 10.1016/j.cgh.2018.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich NE, Singal AG. Overdiagnosis of hepatocellular carcinoma: prevented by guidelines? Hepatology. 2022;75(3):740-753. doi: 10.1002/hep.32284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquardt P, Liu PH, Immergluck J, et al. Hepatocellular carcinoma screening process failures in patients with cirrhosis. Hepatol Commun. 2021;5(9):1481-1489. doi: 10.1002/hep4.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singal AG, Tiro JA, Murphy CC, et al. Patient-reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin Gastroenterol Hepatol. 2021;19(5):987-995.e1. doi: 10.1016/j.cgh.2020.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual model for the hepatocellular carcinoma screening continuum: current status and research agenda. Clin Gastroenterol Hepatol. 2022;20(1):9-18. doi: 10.1016/j.cgh.2020.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi: 10.1016/j.amjmed.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 35.Wagle NS, Park S, Washburn D, et al. Racial, ethnic, and socioeconomic disparities in curative treatment receipt and survival in hepatocellular carcinoma. Hepatol Commun. 2022;6(5):1186-1197. doi: 10.1002/hep4.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao A, Rich NE, Marrero JA, Yopp AC, Singal AG. Diagnostic and therapeutic delays in patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2021;19(9):1063-1071. doi: 10.6004/jnccn.2020.7689 [DOI] [PubMed] [Google Scholar]
- 37.Govalan R, Luu M, Lauzon M, et al. Therapeutic underuse and delay in hepatocellular carcinoma: prevalence, associated factors, and clinical impact. Hepatol Commun. 2022;6(1):223-236. doi: 10.1002/hep4.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seif El Dahan K, Reczek A, Daher D, et al. Multidisciplinary care for patients with HCC: a systematic review and meta-analysis. Hepatol Commun. 2023;7(5):e0143. doi: 10.1097/HC9.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mokdad AA, Zhu H, Marrero JA, Mansour JC, Singal AG, Yopp AC. Hospital volume and survival after hepatocellular carcinoma diagnosis. Am J Gastroenterol. 2016;111(7):967-975. doi: 10.1038/ajg.2016.181 [DOI] [PubMed] [Google Scholar]
- 40.Rich NE, John BV, Parikh ND, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multicenter cohort of patients with cirrhosis. Hepatology. 2020;72(5):1654-1665. doi: 10.1002/hep.31159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Supplemental Methods
eAppendix 2. Supplemental Results
eTable 1. Characteristics of Non–Screen-Detected HCC Patients
eTable 2. Restricted Mean Survival Times for HCC, Adjusting for Lead Time Bias
eFigure. Kaplan Meier Curves Comparing Patients With Incidental Versus Symptomatic Non–Screen-Detected HCC
eReferences
Data Sharing Statement