Abstract
Little information exists on the potential of NH3-oxidizing bacteria to cooxidize halogenated hydrocarbons in soil. A study was conducted to examine the cooxidation of methyl bromide (MeBr) by an NH3-oxidizing bacterium, Nitrosomonas europaea, under soil conditions. Soil and its water content modified the availability of NH4+ and MeBr and influenced the relative rates of substrate (NH3) and cosubstrate (MeBr) oxidations. These observations highlight the complexity associated with characterizing soil cooxidative activities when soil and water interact to differentially affect substrate and cosubstrate availabilities.
In recent years considerable research has been conducted to determine the soil physical and chemical factors which control the fate of the agriculturally applied soil fumigant methyl bromide (MeBr) (2, 3, 11, 25, 26, 29). Although the potential of methanotrophic and NH3-oxidizing bacteria to cooxidatively degrade MeBr has been known for some time (12, 17, 20, 24), and facultatively methylotrophic soilborne bacteria have been isolated that grow on MeBr as a C source (6, 15), only recently was it shown that soil bacteria act as a sink for MeBr in situ (7, 15, 18, 22). At this time it is unclear to what extent MeBr consumption in soil occurs by cooxidative rather than energy-gaining metabolism and how soil factors might influence microbiological MeBr transformation. Nitrosomonas europaea, a chemolithotrophic NH3 oxidizer, carries out cooxidation of a variety of halogenated and nonhalogenated hydrocarbons in the presence of NH4+ through the activity of ammonia monooxygenase (AMO) (12, 13, 19, 20, 21). Many factors that influence NH3 oxidation in soil will presumably influence transformation of alternate substrates. For example, soil colloids are known to bind NH4+, which might influence the ratio of substrate (NH3) to cosubstrate during cooxidation and affect competition for the active site of AMO. Hommes et al. (8) examined the ability of N. europaea to oxidize NH4+ and three cosubstrates, ethylene, chloroethane, and 1,1,1-trichloroethane, in vigorously aerated soil slurries. The influence of soil exchangeable acidity on solution pH and NH3-NH4+ equilibrium was the main factor affecting NH3 and cosubstrate oxidation, whereas NH4+ adsorption played a lesser role under slurry conditions. The influence of intact soil on cooxidation by NH3 oxidizers could be more complex. For example, in a study which examined the effect of soil water content on nitrification, it was concluded that the primary negative effect on nitrification of lowering soil water potential from saturation to −0.6 MPa was reduced diffusion of NH4+ and NH3 to the sites of NH3-oxidizing activity (23). Furthermore, it is not difficult to conceptualize that MeBr oxidation might be sensitive to soil water content because of the large differences in the diffusion coefficients of MeBr through water and air (diffusion coefficients, 0.1037 cm2 s−1 for air and 1.35 × 10−5 cm2 s−1 for water) (26). Recent studies showed that relatively small changes in soil water content had a profound effect on microbially mediated MeBr uptake by a forest soil (7). The purpose of our research was to build upon earlier studies (8) and to examine the characteristics of NH3 and MeBr oxidations by N. europaea incubated in intact soil held at different water contents.
Cell growth, experimental manipulations, and analyses.
Surface samples of a Willamette silt loam (0 to 20 cm) were used, and the properties are described elsewhere (14). The soil pH was raised to approximately neutral (7.0 ± 0.2) by incubation for 3 days with 3 g Ca(OH)2 kg of soil−1, air dried, and sterilized with gamma irradiation (4 megarads) at the Oregon State University Radiation Center. Batch cultures of N. europaea (ATCC 19718) were grown as described elsewhere (9). Late-exponential-phase cells (3 to 4 days) were harvested by centrifugation (11,000 × g; 15 min), washed twice in ice-cold buffer (50 mM potassium phosphate buffer, pH 7.2), and resuspended to a cell density of (30 ± 7) × 107 ml−1 (80 ± 20 μg [dry weight] of cells ml−1).
Portions of sterile air-dried soil (11.6 g, equivalent to 11.0 g of oven-dried soil) were dispensed into sterile 74-ml serum vials sealed with gray butyl stoppers and aluminum crimp top seals (Wheaton, Millville, N.J.). Appropriate amounts (2.5, 5.0, or 10.0 μmol) of MeBr were added to the vials. A 4.4-ml aliquot of water is required to bring 11.6 g of air-dried soil to its water-holding capacity (WHC) (454 g of H2O kg−1) and provide a total water volume of 5 ml in 11 g of oven-dried soil. To conduct experiments with saturated soil at WHC, the following mixture (prepared at 4°C and kept in the dark on ice) was injected slowly through the septum of each vial using a plastic syringe fitted with a 23-gauge needle: 25 or 100 mM NH4+ in 50 mM K2HPO4 (pH 7.2), 2 ml; N. europaea cell suspension with an optical density of 0.25, 1 ml; and 50 mM K2HPO4 (pH 7.2), 1.4 ml. Vials without soil were set up in a similar manner with an additional 0.6 ml of sterile deionized water to provide the same total volume of water as in the soil treatments. The final cell density of N. europaea was equivalent to 6 × 107 cells ml of water−1 or 2.7 × 107 cells g of soil−1. To conduct experiments with unsaturated soil at approximately two-thirds of WHC (300 g of H2O kg of soil−1), the same amounts of soil and MeBr were dispensed into vials as described above. A 2.4-ml aliquot of water is required to provide a total volume of 3 ml. The following mixture was injected into each vial: 25 or 100 mM NH4+ in 50 mM K2HPO4 (pH 7.2), 1.2 ml; N. europaea cell suspension with an optical density of 0.25, 0.6 ml; and 50 mM K2HPO4 (pH 7.2), 0.6 ml. Vials without soil were set up in a similar manner with an additional 0.6 ml of water. In this case, we chose to keep the N. europaea cell density constant on the basis of water; the density per gram of soil was lower than in the saturated experiment (1.63 × 107 cells g of soil−1). Triplicate vials of each treatment were incubated horizontally in the dark at 27°C with periodic rotation to facilitate gas distribution. Agitation was avoided to prevent the breakdown of soil structure, which would occur especially under water-saturated conditions. Preliminary experiments were conducted to determine if O2 limitation would occur in unshaken vials and retard NO2− production and MeBr oxidation. No differences were detected in rates of NO2− production assayed in unshaken vials containing either ambient or supplemental levels of O2 (0.4 atm) at 10 or 40 mM NH4+.
For NO2− concentration determinations, 17 or 19 ml of ice-cold 2 M KCl (supplemented with 0.1 mM allyl thiourea, an inhibitor of NH3 oxidation) was added to each vial containing either saturated or unsaturated soil, respectively (2:1 liquid/soil ratio). Vials were shaken vigorously for 5 min and were centrifuged to pellet the soil, and NO2− concentrations were determined in the supernatants (5). To measure the amount of NH4+ in soil solution under saturated conditions, 4.4 ml of buffer containing either 50 or 200 μmol of NH4+ was added to 11.6 g of air-dried soil in a centrifuge tube and was incubated for 1 h. Following the incubation, soil was centrifuged and samples of supernatant were recovered for analysis of NH4+. To measure the amount of NH4+ in solution under unsaturated conditions, larger portions of soil (18.4 g of air-dried soil) were placed in 60-ml syringes, and buffer (6 ml) containing 100 or 400 μmol of NH4+ was dribbled over the soil. Samples were incubated for 1 h and samples of soil solution were forced from the soil by applying pressure with the plunger of a syringe. Samples were centrifuged to pellet soil, and NH4+ concentrations were determined in supernatants after alkaline steam distillation and back titration against standard acid (1).
MeBr oxidation was measured by monitoring its disappearance from the gas phase of the vials using a Shimadzu GC-14 gas chromatograph. To account for abiological MeBr hydrolysis and MeBr sorption to vials, butyl rubber stoppers, and soil, control vials with and without soil were set up containing 1% (vol/vol) acetylene, a specific mechanism-based inactivator of AMO (10). The amount of MeBr in the vials was determined by comparison to standards of known amounts of MeBr prepared in 74-ml vials containing sterile soil and either 5 ml (saturated) or 3 ml (unsaturated) of water. Because the amounts of water in the vials differed between the saturated and unsaturated soil treatments, slightly different concentrations of MeBr developed in the aqueous phases. For example, when 10 μmol of MeBr was added to vials, the aqueous-phase concentrations were calculated to be 0.47 and 0.51 mM for unsaturated and saturated conditions, respectively. Preliminary studies established that the presence of soil had no significant impact on the partitioning of MeBr between the aqueous and gaseous phases and that ≤10% of the MeBr that disappeared during incubations was AMO independent (i.e., acetylene insensitive). We concluded that some abiological hydrolysis or sorption of MeBr occurred in our experimental system, and these values were subtracted from those measured in the experimental treatments without acetylene. No attempt was made to distinguish between MeBr disappearance due to sorption and that due to abiological hydrolysis. In all assays, the amount of MeBr transformed was expressed as total micromoles inclusive of that amount which partitioned into the liquid and soil phases.
Effects of intact soil on NO2− production.
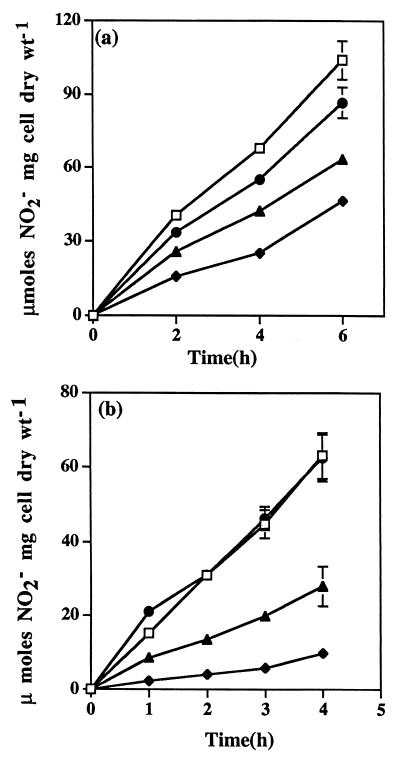
The presence of Willamette silt loam soil affected the rate of NH3 oxidation by N. europaea under both unsaturated and saturated conditions. When incubations were conducted at WHC with 10 mM NH4+, NO2− production occurred at ∼30% of the rate of the minus-soil control and was restored to ≥85% of the minus-soil rate by increasing the concentration of NH4+ to ≥40 mM (Fig. 1a). When N. europaea was incubated with the same weight of soil at approximately two-thirds of WHC, NO2− production occurred at 15 to 35% of the minus-soil rate, and a fourfold increase in NH4+ increased the rate to that of the minus-soil control (Fig. 1b). Under unsaturated conditions, we noted occasionally that 40 mM NH4+ would not completely compensate for the effect of soil on NO2− production (≥55 to 60% of the minus-soil rate). When this phenomenon was observed, larger additions of NH4+ (50 or 60 mM) did not correct the problem. To avoid the possibility of either NH4+ or salt stresses on N. europaea, 40 mM NH4+ was used routinely as our maximum NH4+ level for unsaturated conditions. The presence of Willamette silt loam substantially reduced the concentrations of NH4+ in soil solution from 40 and 10 mM to approximately 10 and 1 mM, respectively, regardless of soil water content. Using a Ks value of 4.8 mM for NH4+ oxidation at pH 7.0 (the pH of a 1:1 [vol/wt] soil suspension) and the concentrations of NH4+ experimentally determined in soil solutions, theoretical estimates of the rates of NO2− production matched reasonably well those experimentally determined, i.e., 24 to 26% and 73 to 80% of the rates expected in the absence of soil at pH 7.2 and 10 or 40 mM NH4+, respectively.
FIG. 1.
NO2− production by N. europaea in the presence of soil under water-saturated (5 ml) (a) and unsaturated (3 ml) (b) conditions. Cells (3 × 108) of N. europaea were incubated with the equivalent of 11 g of oven-dried soil and either 10 mM NH4+ (⧫), 20 mM NH4+ (▴), or 40 mM NH4+ (●). The minus-soil control contained 10 mM NH4+ and the same amount of cells in either 5 or 3 ml of buffer plus water (□). Error bars represent the standard deviations of the means of three analytical replicates. Most standard deviations were <5% of the means and are not shown.
Effects of soil water content on MeBr oxidation and NO2− production.
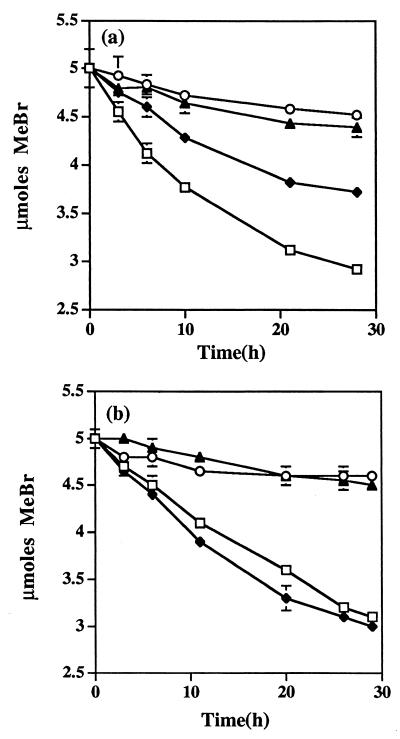
N. europaea oxidized MeBr under both unsaturated and saturated soil conditions. The rates of MeBr disappearance were constant for approximately 12 h and declined to zero between 24 and 36 h (Fig. 2). Because MeBr oxidation was not sustainable at >0.5 mM aqueous concentration (MeBraq), subsequent experiments were restricted to three aqueous concentrations of 0.13, 0.25, and 0.51 mM MeBr (saturated) or 0.12, 0.24, and 0.47 mM MeBr (unsaturated). As mentioned earlier, there were slight differences between the aqueous-phase concentrations in the saturated and unsaturated experiments because different amounts of water were used. Experiments were conducted to compare the effect of soil on NO2− production and MeBr oxidation under water-saturated (Table 1) and water-unsaturated (Table 2) conditions. Saturated soil reduced NO2− production by 10 mM NH4+–MeBr combinations to 29, 23, and 17%, respectively, of their corresponding minus-soil values while also reducing the amounts of 0.24 and 0.47 mM MeBr oxidized to 64 and 43%, respectively, of their corresponding minus-soil values (Table 1). Saturated soil reduced NO2− production by 40 mM NH4+–MeBr combinations to a much lesser degree than it did the 10 mM NH4+–MeBr combinations, and it increased the amounts of MeBr oxidized by 25, 41, and 33% over the corresponding minus-soil values. The interactions between saturated soil, NH4+, and MeBr concentrations were quite striking for both NO2− production and MeBr oxidation. For example, 40 mM NH4+ completely compensated for the negative effect of saturated soil on the amount of MeBr oxidized by the 0.24 mM MeBr–10 mM NH4+ combination (9.6 versus 8.0 μmol mg [dry weight] of cells−1). In contrast, 40 mM NH4+ only partially compensated for the effect of saturated soil on the 0.47 mM MeBr–10 mM NH4+ combination (9.6 versus 12.8 μmol mg [dry weight] of cells−1) despite increasing the rate of NO2− production by almost the same degree (fourfold) as it did in the presence of 0.24 mM MeBr.
FIG. 2.
MeBr oxidation by N. europaea in the presence of soil under water-saturated (5 ml) (a) and unsaturated (3 ml) (b) conditions. Cells (3 × 108) of N. europaea were incubated with the equivalent of 11 g of oven-dried soil, 0.25 mM MeBr, and 10 mM NH4+. □, minus soil; ⧫, plus soil; ○, minus soil and plus 1% acetylene; ▴, plus soil and 1% acetylene. Error bars represent the standard deviations of the means of three analytical replicates.
TABLE 1.
Influence of soil and NH4+ on NO2− production and MeBr oxidation by N. europaea under water-saturated conditions
| MeBr (mM) | Amt (μmol/mg [dry wt] of cells) witha:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| NH4+ (10 mM)b
|
NH4+ (40 mM)c
|
|||||||
| −Soild
|
+Soile
|
−Soil
|
+Soil
|
|||||
| NO2− | MeBr | NO2− | MeBr | NO2− | MeBr | NO2− | MeBr | |
| 0 | 180 (10) | 100 (10) | 220 (20) | 230 (30) | ||||
| 0.12 | 170 (10) | 7.2 (0.4) | 50 (5) | 6.4 (0.4) | 190 (20) | 5.1 (0.5) | 150 (10) | 6.4 (0.6) |
| 0.24 | 130 (10) | 8.0 (0.6) | 30 (3) | 5.1 (0.4) | 170 (10) | 6.8 (0.9) | 110 (10) | 9.6 (0.7) |
| 0.47 | 120 (30) | 12.8 (0.9) | 20 (2) | 5.5 (0.5) | 160 (10) | 7.2 (0.6) | 80 (10) | 9.6 (0.1) |
Values in parentheses are the standard errors of the means of three replicates per treatment.
50 μmol of NH4+ added per vial, equivalent to 10 μmol of NH4+/ml of buffer plus water or 4.55 μmol of NH4+/g of soil.
200 μmol of NH4+ added per vial, equivalent to 40 μmol of NH4+/ml of buffer plus water or 18.2 μmol of NH4+/g of soil.
Minus-soil treatments contained 5 ml of buffer plus water per vial.
Plus-soil treatments contained the equivalent of 11 g of oven-dried soil and 5 ml of buffer plus water per vial.
TABLE 2.
Influence of soil and NH4+ on NO2− production and MeBr oxidation by N. europaea under unsaturated water conditions
| MeBr (mM) | Amt (μmol/mg [dry wt] of cells) witha:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| NH4+ (10 mM)b
|
NH4+ (40 mM)c
|
|||||||
| −Soild
|
+Soile
|
−Soil
|
+Soil
|
|||||
| NO2− | MeBr | NO2− | MeBr | NO2− | MeBr | NO2− | MeBr | |
| 0 | 280 (30) | 54 (5) | 280 (10) | 155 (5) | ||||
| 0.13 | 240 (20) | 8.0 (1) | 29 (3) | 8.1 (1) | 285 (15) | 4.8 (0.2) | 80 (0) | 7.7 (0.6) |
| 0.25 | 160 (18) | 11.2 (2) | 20 (2) | 11.2 (1) | 210 (10) | 8.0 (2) | 60 (10) | 13.7 (0.2) |
| 0.51 | 146 (13) | 16.8 (2) | 16 (2) | 9.6 (1) | 185 (15) | 11.0 (2) | 55 (5) | 18.8 (2.7) |
Values in parentheses are the standard errors of the means of three replicates per treatment.
30 μmol of NH4+ added per vial, equivalent to 10 μmol/ml of water plus buffer or 2.73 μmol of NH4+/g of soil.
120 μmol of NH4+ added per vial, equivalent to 40 μmol/ml of water plus buffer or 10.91 μmol of NH4+/g of soil.
Minus-soil treatments contained 3 ml of buffer plus water per vial.
Plus-soil treatments contained the equivalent of 11 g of oven-dried soil and 3 ml of buffer plus water per vial.
Unsaturated soil reduced NO2− production by 10 mM NH4+–MeBr combinations more severely than did saturated soil (11 to 12.5% of the corresponding MeBr-minus-soil combinations) (Table 2). Despite the severe reductions in NO2− production, oxidations of 0.13 and 0.25 mM MeBr were unaffected, while the amount of 0.51 mM MeBr oxidized was reduced to 57% of the corresponding minus-soil treatment (9.6 versus 16.8 μmol mg [dry weight] of cells−1). In the presence of 40 mM NH4+, unsaturated soil lowered NO2− production by the 40 mM NH4+–MeBr combinations more than occurred in the saturated-soil experiment (Table 1). The amounts of MeBr oxidized, however, were increased substantially (60 to 70%) above the minus-soil values at each of the three MeBr concentrations.
The results of this study clearly reveal the potential of the NH3 oxidizer N. europaea to oxidize MeBr under intact soil conditions. Although the rates of oxidation of halogenated hydrocarbons by soilborne populations of NH3 oxidizers are not currently available in the literature, we intentionally used relatively low-density cell suspensions of N. europaea in an attempt to generate rates of MeBr oxidation and NO2− production that could be placed into context with rates documented elsewhere in the microbial ecology literature. For example, we measured rates of soilborne NO2− production of 25 to 60 nmol g of soil−1 h−1, which are on the high end of the range of values generally accepted for nitrification potentials of actively nitrifying soils (1 to 100 nmol of N g of soil−1 h−1) (16). The rates of MeBr oxidation fell into the range of ∼5 to 10 nmol of MeBr transformed g of soil−1 h−1. These values are similar to rates of MeBr degradation reported for a methanotrophic peat exposed to MeBr concentrations similar to those used in this study (17). They are much greater than the rates of 1 to 3 nmol of MeBr transformed g of soil−1 day−1 reported for a fumigated agricultural soil (15) and of ∼2 pmol g of soil−1 h−1 for degradation of atmospheric levels of MeBr by a forest soil (7).
Our data highlight how soil physical and chemical properties can modify the characteristics of MeBr oxidation by NH3 oxidizers through their influence on the bioavailability of the two substrates. Previous studies illustrated that exchangeable soil acidity was the primary factor influencing NH3 availability to N. europaea under soil slurry conditions (8). In the present study, which was conducted with acid-neutralized soil, it became quite clear that much larger additions of NH4+ were needed to compensate for the effect of structurally intact soil on NO2− production than was apparent under slurry conditions. Our data clearly indicate the interactive effect of soil water content on cooxidation of a halogenated hydrocarbon through its differential influence on the availability of NH4+ and MeBr (i.e., two substrates with quite different chemical properties). In general, there was a greater negative impact of unsaturated soil on NO2− production than on MeBr oxidation, while saturated soil had a greater inhibitory effect on MeBr oxidation than on NO2− production. These findings are consistent with the observation that nitrification declines as soil water content is lowered because of increased restrictions on the diffusion of NH4+ to the sites of the NH3-oxidizing bacteria (23). Furthermore, it is not difficult to conceptualize that MeBr oxidation might be restricted by diffusion at higher soil water content, provided the cooxidative process is not already NH4+ limited. Indeed, several closely related studies have shown that CH4 consumption by soil occurs optimally at 20 to 40% of WHC and usually declines as water content is raised beyond this range (4, 27, 28). In one of these soil studies, the optimum water content for CO2 production was found to be significantly higher than for CH4 consumption (4). The authors concluded that because CH4 consumption was reliant on gaseous diffusion, it would be negatively affected by an increase in soil water content, while soil respiration would respond positively because of its reliance on the diffusion of water-soluble substrates. More studies are required to establish to what extent differences among soils in their properties of NH4+ generation, adsorption, and availability might interact with water-holding characteristics to influence the relationships between NH3 and gaseous hydrocarbon oxidation.
Acknowledgments
This work was supported by the Oregon Agricultural Experiment Station and by EPA grant R821405 to P.J.B. and D.J.A. Additional support was provided to K.N.D. through the Department of Microbiology of Oregon State University and the N. L. Tarter Fellowship.
We express appreciation to David Myrold and Chris Yeager for constructive comments on early drafts of the manuscript.
Footnotes
Technical paper no. 11,573 of the Oregon Agricultural Experiment Station.
REFERENCES
- 1.Bremner J M, Mulvaney R L. Nitrogen total. In: Page A L, et al., editors. Methods of soil analysis, part 2. Chemical and microbiological properties. 2nd ed. Madison, Wis: American Society of Agronomy; 1982. pp. 595–624. [Google Scholar]
- 2.Gan J, Yates S R, Anderson M A, Spencer W F, Ernst F F, Yates M V. Effect of soil properties on degradation and sorption of methyl bromide in soil. Chemosphere. 1994;29:2685–2700. [Google Scholar]
- 3.Gan J, Yates S R, Wang D, Spencer W M. Effect of soil factors on methyl bromide volatilization after soil application. Environ Sci Technol. 1996;30:1629–1636. [Google Scholar]
- 4.Gulledge J, Schimel J P. Moisture control over atmospheric CH4 consumption and CO2 production in diverse Alaskan soils. Soil Biol Biochem. 1998;30:1127–1132. [Google Scholar]
- 5.Hageman R H, Hucklesby D P. Nitrate reductase in higher plants. Methods Enzymol. 1971;23:491–503. [Google Scholar]
- 6.Hancock T L C, Costello A M, Lidstrom M E, Oremland R S. Strain IMB-1, a novel bacterium for the removal of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1998;64:2899–2905. doi: 10.1128/aem.64.8.2899-2905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hines M E, Crill P M, Varner R K, Talbot R W, Shorter J H, Kolb C E, Harriss R C. Rapid consumption of low concentrations of methyl bromide by soil bacteria. Appl Environ Microbiol. 1998;64:1864–1870. doi: 10.1128/aem.64.5.1864-1870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hommes N G, Russell S A, Bottomley P J, Arp D J. Effects of soil on ammonia, ethylene, chloroethane, and 1,1,1-trichloroethane oxidation by Nitrosomonas europaea. Appl Environ Microbiol. 1998;64:1372–1378. doi: 10.1128/aem.64.4.1372-1378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman M R, Arp D J. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992;267:1534–1545. [PubMed] [Google Scholar]
- 10.Hyman M R, Wood P M. Suicidal inactivation and labeling of ammonia monooxygenase by acetylene. Biochem J. 1985;227:719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Y, Jury W A. Methyl bromide diffusion and emission through soil columns under various management techniques. J Environ Qual. 1995;24:1002–1009. [Google Scholar]
- 12.Keener W K, Arp D J. Kinetic studies of ammonia monooxygenase inhibition in Nitrosomonas europaea by hydrocarbons and halogenated hydrocarbons in an optimized whole-cell assay. Appl Environ Microbiol. 1993;59:2501–2510. doi: 10.1128/aem.59.8.2501-2510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keener W K, Arp D J. Transformations of aromatic compounds by Nitrosomonas europaea. Appl Environ Microbiol. 1994;60:1914–1920. doi: 10.1128/aem.60.6.1914-1920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendes I C, Bandick A K, Dick R P, Bottomley P J. Microbial biomass and activities in soil aggregates affected by winter cover crops. Soil Sci Soc Am J. 1999;63:873–881. [Google Scholar]
- 15.Miller L G, Connell T L, Guidetti J R, Oremland R S. Bacterial oxidation of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1997;63:4346–4354. doi: 10.1128/aem.63.11.4346-4354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myrold D D. Transformations of nitrogen. In: Sylvia D M, Fuhrmann J J, Hartel P G, Zuberer D A, editors. Principles and applications of soil microbiology. Upper Saddle River, N.J: Prentice Hall; 1998. pp. 259–294. [Google Scholar]
- 17.Oremland R S, Miller L G, Culbertson C W, Connell T L, Jahnke L. Degradation of methyl bromide by methanotrophic bacteria in cell suspensions and soils. Appl Environ Microbiol. 1994;60:3640–3646. doi: 10.1128/aem.60.10.3640-3646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou L-T, Joy P J, Thomas J E, Hornsby A G. Stimulation of microbial degradation of methyl bromide in soil during oxidation of an ammonia fertilizer by nitrifiers. Environ Sci Technol. 1997;31:717–722. [Google Scholar]
- 19.Rasche M E, Hicks R E, Hyman M R, Arp D J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990;172:5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasche M E, Hyman M R, Arp D J. Biodegradation of halogenated hydrocarbon fumigants by nitrifying bacteria. Appl Environ Microbiol. 1990;56:2568–2571. doi: 10.1128/aem.56.8.2568-2571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasche M E, Hyman M R, Arp D J. Factors limiting aliphatic chlorocarbon degradation by Nitrosomonas europaea: cometabolic inactivation of ammonia monooxygenase and substrate specificity. Appl Environ Microbiol. 1991;57:2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shorter J H, Kolb C E, Crill P M, Kerwin R A, Talbot R W, Hines M E, Harriss R C. Rapid degradation of atmospheric methyl bromide in soils. Nature. 1995;377:717–719. [Google Scholar]
- 23.Stark J M, Firestone M K. Mechanisms for soil moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol. 1995;61:218–221. doi: 10.1128/aem.61.1.218-221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stirling D L, Dalton H. The fortuitous oxidation and cometabolism of various carbon compounds by whole-cell suspensions of Methylococcus capsulatus (Bath) FEMS Microbiol Lett. 1979;5:315–318. [Google Scholar]
- 25.Wang D, Yates S R, Ernst F F, Gan J, Gao F, Becker J O. Methyl bromide emission reduction with field management practices. Environ Sci Technol. 1997;31:3017–3022. [Google Scholar]
- 26.Wang D, Yates S R, Gan J. Temperature effect on methyl bromide volatilization in soil fumigation. J Environ Qual. 1997;26:1072–1079. [Google Scholar]
- 27.Whalen S C, Reeburgh W S. Moisture and temperature sensitivity of CH4 oxidation in boreal soils. Soil Biol Biochem. 1996;28:1271–1281. [Google Scholar]
- 28.Whalen S C, Reeburgh W S, Sandbeck K A. Rapid methane oxidation in a landfill cover soil. Appl Environ Microbiol. 1990;56:3405–3411. doi: 10.1128/aem.56.11.3405-3411.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yates S R, Gan J, Ernst F F, Matziger A, Yates M V. Methyl bromide emissions from a covered field. 1. Experimental conditions and degradation in soil. J Environ Qual. 1996;25:184–192. [Google Scholar]