Abstract
The West African Ebola virus (EBOV) epidemic has fast-tracked countermeasures for this rare, emerging zoonotic pathogen. Until 2013–2014, most EBOV vaccine candidates were stalled between the preclinical and clinical milestones on the path to licensure, because of funding problems, lack of interest from pharmaceutical companies, and competing priorities in public health. The unprecedented and devastating epidemic propelled vaccine candidates toward clinical trials that were initiated near the end of the active response to the outbreak. Those trials did not have a major impact on the epidemic but provided invaluable data on vaccine safety, immunogenicity, and, to a limited degree, even efficacy in humans. There are plenty of lessons to learn from these trials, some of which are addressed in this review. Better preparation is essential to executing an effective response to EBOV in the future; yet, the first indications of waning interest are already noticeable.
Keywords: Ebola virus, animal models, vaccines, clinical trials, challenges, lessons learned
INTRODUCTION
Emergence and reemergence of infectious diseases have increased over the last 50 years, challenging local, regional, and global public health. Many of these incidences were caused by zoonotic pathogens such as filoviruses. Marburg virus (MARV) was discovered in 1967 as the cause of an outbreak of hemorrhagic fever among workers of a German vaccine company. The workers were exposed to MARV through preparation of primary cell cultures from organs of African green monkeys imported from Uganda (76). Ebola virus (EBOV) and Sudan virus (SUDV), both members of the Ebolavirus genus, were discovered in 1976 during simultaneous outbreaks of hemorrhagic fever in southern Sudan (now South Sudan) and northern Zaire (now Democratic Republic of the Congo, DRC) (127, 128). Two further species of African ebolaviruses were described with the discovery of Taï Forest virus (TAFV) in Côte d’Ivoire in 1994 (67) and Bundibugyo virus (BDBV) in Uganda in 2007 (119). Outside the African continent one finds Reston virus (RESTV), which was discovered in macaques imported to the United States in 1989 (59); today there is evidence for a wider distribution of RESTV in Asia (89). In 2008, RESTV was found in pigs in the Philippines, raising the concern of food safety, a matter that is still under investigation, as is RESTV pathogenicity in pigs (4). In Spain the RNA from Lloviu virus (LLOV) was isolated from bats; however, no virus has been recovered yet (95). The emergence of EBOV in West Africa in 2013–2014 has caused an unprecedented epidemic, with almost 30,000 cases and approximately 11,000 deaths (129) (for filovirus taxonomy see the sidebar titled Family Filoviridae Taxonomy).
VACCINE LICENSURE.
The US Food and Drug Administration (https://www.fda.gov) has implemented the animal rule, allowing licensing of countermeasures based on efficacy data in animal models combined with safety and immunogenicity studies in humans (108). In general, licensing agencies require different clinical trials evaluating safety, immunogenicity, and efficacy of a product. Clinical trials are classified into four categories: phase 1, product safety and dosage in a small cohort; phase 2, side effects and immunogenicity in larger cohorts; phase 3, efficacy data and monitoring adverse effects in large cohorts; and phase 4, long-term product safety and efficacy in larger cohorts (typically after product licensure).
Filoviruses are zoonotic pathogens that are occasionally transmitted to humans, nonhuman primates (NHPs), and perhaps other wildlife. The field currently follows the hypothesis of bats being potential reservoir species in nature. To this day, however, only MARV has been isolated from fruit bats (Rousettus aegyptiacus) (118). This important link is missing for all of the ebolaviruses. Wildlife or domestic mammalian species, such as duiker antelope or domestic/feral pig, might play a role as amplifying or intermediate hosts, but strong evidence has yet to be provided for this hypothesis (30). In the future it will be important to close these knowledge gaps, as wildlife vaccination might be considered for containing EBOV and other filoviruses (30, 94).
Filoviruses are filamentous, nonsegmented, negative-stranded, enveloped RNA viruses that replicate in the cytoplasm of the cell (5, 30, 35). The genome is approximately 19 kb and displays this gene order: 3′ trailer, nucleoprotein (NP), virion protein 35 (VP35), VP40, glycoprotein (GP), VP30, VP24, polymerase (L), 5′ trailer. Transcription of the genome leads to the expression of seven structural proteins, including NP, VP35, VP30, and L, which make up the nucleocapsid complex (RNP) and drive transcription and replication. VP40 is the matrix protein and important for particle morphology and budding. VP24 bridges between the RNP complex and matrix, with GP being inserted into the virus envelope as the only transmembrane protein mediating virus entry. All ebolaviruses express two additional nonstructural proteins encoded on their GP gene. The primary translation product is soluble GP (sGP) that gets released from infected cells. The transmembrane GP and the small soluble GP (ssGP) are the result of RNA editing (30). No defined functions have been assigned to sGP and ssGP. The transmembrane GP mediates particle binding to cellular surface entities and fusion with the endosomal membrane by interaction with the receptor Niemann-Pick 1 (NCP-1) (14, 18). Besides potential contribution by NP, VP40, and VP24, GP is considered the key immunogenic viral protein and the most important immunogen for vaccine development (for further reading on the molecular biology and evolution of filoviruses, see Reference 30).
Ebolaviruses and marburgviruses cause a clinical syndrome known as viral hemorrhagic fever. To reflect the increased variation in clinical symptoms and disease progression as most evidently observed during the West African EBOV outbreak, the filovirus diseases are now more commonly referred to as Ebola virus disease (EVD) and Marburg virus disease (MVD) (5, 34). So far, all reported African filoviruses are human pathogens, with case fatality rates up to 90% for MARV, Ravn virus (RAVV), and EBOV. Lower case fatality rates in the range of 25–50% are associated with SUDV and BDBV; the public health importance of TAFV is uncertain, with only a single clinical case. RESTV has infected humans but is not known to cause clinical disease. There are no data on human pathogenicity for Lloviu virus (95) (see the sidebar titled Family Filoviridae Taxonomy) or the putative new Chinese filoviruses, which have yet to be classified (50). The West African EBOV epidemic has changed the way we respond with regard to diagnosis, triage, and case patient management; altogether, this and/or unknown factors (e.g., biological features of EBOV Makona) have lowered the case fatality rate below 50%—a first for an EBOV strain. Given the high numbers of survivors during this epidemic, we also have a better understanding on sequelae leading to post–ebolavirus disease syndrome (15). In addition, it was discovered that EBOV temporarily persists in immune-privileged body sites, potentially leading to infrequent transmission through sex, and perhaps other routes (16) (for further reading on EBOV disease and pathogenesis, see Reference 5).
The unforeseen and unprecedented West African epidemic, caused by EBOV strain Makona, has highlighted the urgent need for effective and rapidly deployable countermeasures. The epidemic created a clear necessity to allow fast-tracked antiviral/therapeutic (for further reading see Reference 19) and vaccine testing, providing insight into the feasibility of such approaches for future epidemics. Unfortunately, clinical trials could only begin toward the end of the epidemic and suffered from, among other issues, a lack of preclinical data, questionable designs, low number of patients, and controversial politics. Despite those drawbacks, the vaccine trials have offered valuable data on safety and immunogenicity, with one trial even providing the first data for efficacy in humans (https://www.clinicaltrials.gov; http://www.pactr.org). However, almost two years after the epidemic, only Russia and China (8) have licensed an EBOV vaccine, whereas efforts in other countries are still held up in production and licensing. In this review, we address vaccines focusing on EBOV, as most vaccine developments have been targeted toward this virus. We review current promising vaccine candidates and human clinical trials and then discuss challenges and lessons learned for future development.
VACCINE OVERVIEW
Vaccine development against EBOV (and to a lesser degree other filoviruses) has been hampered in the past by the need for high-level biocontainment, general lack of interest among pharmaceutical companies, and lower priority in the public health sector. EBOV was considered a rare zoonosis with infrequent transmission events into the human species that either were self-limiting or were stopped with basic public health interventions (109). This view started to change with the EBOV-Kikwit outbreak (DRC, 1995), and definitely with the classification of EBOV as a biothreat pathogen and then a U.S. Select Agent and Tier 1 Pathogen (33). EBOV became of increasing interest for biodefense research with the goal to produce countermeasures such as diagnostics, therapeutics, and vaccines. It is interesting to note that despite attention and funding, and except for a few diagnostic tests (99), no product, whether antiviral, therapeutic, or vaccine, is licensed for human use today. Particularly promising vaccine candidates were stalled between the preclinical and clinical milestones on the path to licensure. Of course, the move into clinical trials is an expensive step in product development that often requires partnerships with industry, and there did not seem to be interest then, largely due to an uncertain market. Also, the public health sector seemed hesitant to give high priority to products for rare emerging viruses, such as EBOV, as other infectious and chronic disease issues were burdening the system. Thus, the unprecedented West African EBOV epidemic caught the academic, industrial, and government sectors by surprise, forcing them to respond immediately, even to use unfinished products in humans.
Animal Models
Traditionally, vaccine candidates for EBOV and other filoviruses are screened in rodent models, including mouse, hamster, or guinea pig (132) (Table 1). Unfortunately, all clinical filovirus isolates cause no or very limited disease in these rodent species. Therefore, typically serial adaptation is required to produce uniform lethality in rodents. Adaptation is associated with genome mutations occurring often in, but not limited to, those genes that encode interferon antagonists such as VP24 for ebolaviruses and VP40 for marburgviruses. Rodent models utilizing adapted filoviruses do not always closely mimic human disease manifestations and progression, especially not the mouse models. Nevertheless, rodents are preferred screening models, with the drawback that they may not predict overall efficacy in NHP models and humans (42, 101). NHP models are still considered the gold standard for filoviruses, due to disease presentation similar to what is observed in humans (42). Cynomolgus macaques are preferred for prophylactic vaccine studies, whereas rhesus macaques are often utilized for therapeutic studies because their prolonged time to death allows for an extended window for intervention. Today, most EBOV vaccine candidates have gone through rodent screening followed by NHP confirmation testing prior to clinical trials (see the sidebar titled Vaccine Licensure).
Table 1.
Animal models for Ebola virus
| Animal | Virus | Inoculation route | Human disease display | Lethal | Ease of handling | Tools and reagents | Cost | Use/predictive value |
|---|---|---|---|---|---|---|---|---|
| Laboratory mouse | MA-EBOV | IP, IN, AR | Low | Yes | Easy | Yes | Cheap | Screening model/low |
| Humanized mouse | WT-EBOV | IP | Medium | Yes | Easy | Yes | Expensive | Not well established/unknown |
| CC-RIX mouse | MA-EBOV | IP | Dep. | Dep. | Easy | Yes | NA | Not well established/unknown |
| Hamster | MA-EBOV | IP | Medium | Yes | Easy | Limited | Cheap | Screening model/medium |
| Guinea pig | GPA-EBOV | IP, SC, AR | Low | Yes | Easy to moderate | Limited | Cheap | Screening model/medium |
| Ferret | WT-EBOV | IN | Medium | Yes | Moderate to difficult | Limited | Moderate | Not well established/unknown |
| Nonhuman primate | WT-EBOV | Various | High | Yes | Difficult | Yes | Expensive | Confirmatory model/high |
Abbreviations: AR, aerosol; Dep., depends on CC-RIX strain (asymptomatic, disease, lethality); GPA, guinea pig adapted; IN, intranasal; IP, intraperitoneal; MA, mouse adapted; NA, not applicable (no commercial source); SC, subcutaneous; WT, wild type.
Past and Current Vaccine Efforts
In the 1970s and 1980s, inactivated virus preparations were tested first in animal models, with varying success (41, 74). The strategy was abandoned because its efficacy was limited and there were safety concerns. This was followed in the 1990s with subunit and DNA vaccination. Vaccine development was accelerated around the beginning of the new millennium, with a variety of nonreplicating and replication-competent viral vector platforms mostly targeting EBOV, the prototype filovirus associated with the highest case fatality rates (40). The leading platforms included alphavirus replicons (55, 56, 98), human adenoviruses (37, 115, 116), chimpanzee adenovirus (110), paramyxoviruses (10, 11), rabies virus (6, 125), and different strategies with recombinant vesicular stomatitis virus (rVSV) (61, 86). In addition, virus-like particles (124), a biologically contained EBOV lacking VP30 (82), DNA (43), cytomegalovirus (CMV) vectors (83), modified vaccinia virus Ankara (MVA) (27), and several combinations of DNA, MVA, and adenoviruses have also demonstrated the ability to completely protect NHPs (110, 115). The main viral immunogen for vaccine approaches is the filovirus transmembrane GP, used in various versions (full length, transmembrane anchor deleted, or mucin domain deleted), which is known to be the sole target for classical neutralizing antibodies. Some vaccine approaches include additional immunogens such as VP40 and, less frequently, NP, and VP24 [i.e., DNA and virus-like particle (VLP) vaccines]. The following paragraphs introduce those EBOV vaccine candidates with promising efficacy in NHPs (Figure 1), some of which have proceeded to human clinical trials (Figure 2; Table 2).
Figure 1.
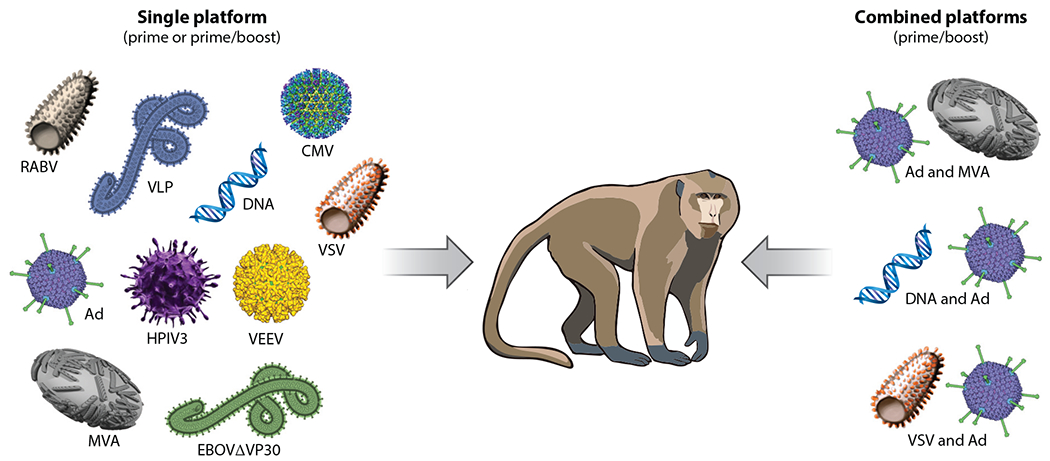
Vaccine approaches with complete and partial efficacy in nonhuman primate models. Successful single platforms in a prime or prime-boost approach are pictured on the left, whereas combined platforms in a prime-boost approach are depicted on the right. Abbreviations: Ad, adenovirus; CMV, cytomegalovirus; EBOVΔVP30, Ebola virus lacking the gene for VP30; HPIV3, human parainfluenza virus type 3; MVA, modified vaccinia virus Ankara; RABV, rabies virus; VEEV, Venezuelan equine encephalitis virus; VLP, Ebola virus–like particles; VSV, vesicular stomatitis virus.
Figure 2.
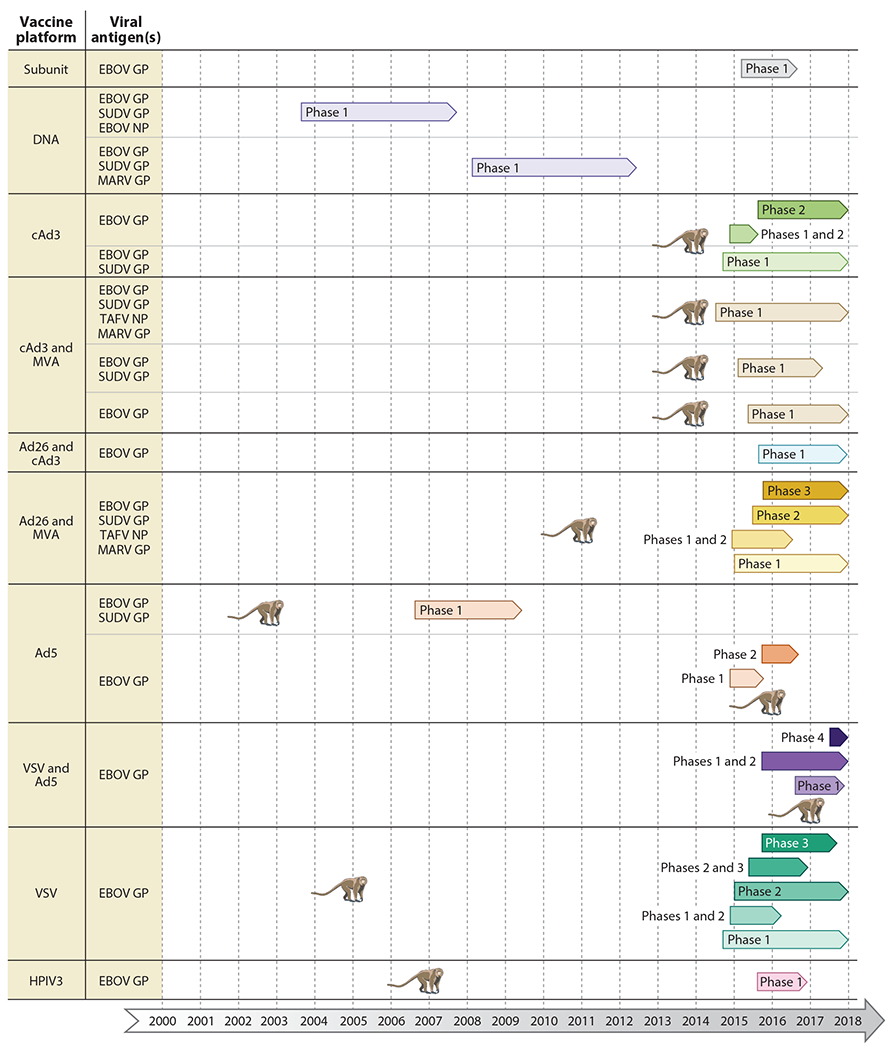
Vaccine platforms in clinical trials. Completed and ongoing phase 1–4 clinical trials are shown for each vaccine platform, with various antigen combinations. The monkey symbolizes the first publication demonstrating complete protective efficacy of the vaccine approach in nonhuman primates. Abbreviations: Ad, human adenovirus; cAd, chimpanzee adenovirus; GP, glycoprotein; HPIV3, human parainfluenza virus type 3; MARV, Marburg virus; MVA, modified vaccinia virus Ankara; NP, nucleoprotein; SUDV, Sudan virus; TAFV, Taï Forest virus; VSV, vesicular stomatitis virus.
Table 2.
Human clinical trials of Ebola virus vaccine candidates
| Start | Status | Phase | Location | Enrollment 01/2018 | Study ID | References |
|---|---|---|---|---|---|---|
| VSV, 100% protection in NHPs (61) | ||||||
| 10/2014 | Completed | 1 | USA | 39 | NCT02269423 | 100 |
| 10/2014 | Completed | 1 | USA | 39 | NCT02280408 | 100 |
| 11/2014 | Completed | 1 | Guinea | 30 | NCT02283099 | 2, 22, 57 |
| 11/2014 | Completed | 1 | Canada | 40 | NCT02374385 | 29 |
| 11/2014 | Completed | 1, 2 | Switzerland | 115 | NCT02287480 | 2, 57, 58 |
| 12/2014 | Active, not recruiting | 1 | Kenya | 40 | NCT02296983 | 2, 57, 58 |
| 12/2014 | Completed | 1 | USA | 512 | NCT02314923 | 54 |
| 01/2015 | Active, not recruiting | 2 | Liberia | 1,500 | NCT02344407 | 63 |
| 03/2015 | Completed | 3 | Guinea | 8,851 | PACTR201503001057193 | 51, 52 |
| 04/2015 | Completed | 2, 3 | Sierra Leone | 8,651 | NCT02378753 PACTR201502001027220 | |
| 08/2015 | Completed | 3 | Canada, Spain, USA | 1,198 | NCT02503202 | 47 |
| 11/2015 | Completed | 1 | Gabon | 155 | PACTR201411000919191 | 1, 2, 57 |
| 12/2015 | Completed | 1 | USA | 38 | NCT02718469 | |
| 01/2016 | Enrolling by invitation | 2 | USA | 18 | NCT02788227 | |
| 11/2016 | Enrolling by invitation | 1 | Switzerland | 100 | NCT02933931 | |
| 03/2017 | Recruiting | 2 | Guinea, Liberia | 5,500 | NCT02876328 PACTR201712002760250 | |
| 08/2017 | Recruiting | 2 | Canada | 200 | NCT03031912 | |
| 12/2017 | Suspended | 3 | Uganda | 500 | NCT03161366 | |
| Ad26 and MVA, 100% protection in NHPs (37) | ||||||
| 12/2014 | Completed | 1, 2 | UK | 87 | NCT02313077 | 88, 126 |
| 01/2015 | Completed | 1 | USA | 164 | NCT02325050 | |
| 03/2015 | Completed | 1 | Kenya | 72 | NCT02376426 | |
| 04/2015 | Completed | 1 | Uganda, Tanzania | 78 | NCT02376400 | |
| 06/2015 | Active, not recruiting | 2 | France, UK | 408 | NCT02416453 | |
| 09/2015 | Recruiting | 3 | Sierra Leone | 1,019 | NCT02509494 PACTR201506001147964 | |
| 09/2015 | Completed | 3 | USA | 525 | NCT02543567 | |
| 09/2015 | Completed | 3 | USA | 329 | NCT02543268 | |
| 11/2015 | Recruiting | 2 | Burkina Faso, Côte d’Ivoire, Kenya, Uganda | 1,056 | NCT02564523 | |
| 12/2015 | Recruiting | 2 | Kenya, Mozambique, Nigeria, Tanzania, Uganda, USA | 575 | NCT02598388 | |
| 05/2016 | Recruiting | 3 | Burkina Faso, Côte d’Ivoire, France, Kenya, Mozambique, Nigeria, Sierra Leone, Tanzania, Uganda, UK, USA | 1,417 | NCT02661464 | |
| 03/2017 | Recruiting | 2 | Guinea, Liberia | 5,500 | NCT02876328 PACTR201712002760250 | |
| 05/2017 | Recruiting | 1 | UK | 56 | NCT03140774 | |
| Active, not recruiting | 1 | USA | 60 | NCT02891980 | ||
| cAd3 and MVA, 100% protection in NHPs (110) | ||||||
| 09/2014 | Active, not recruiting | 1 | UK | 92 | NCT02240875 | 31, 66 |
| 10/2014 | Completed | 1 | Mali | 91 | NCT02267109 | 117 |
| 01/2015 | Completed | 1 | Uganda | 90 | NCT02354404 | |
| 03/2015 | Completed | 1 | USA | 64 | NCT02408913 | |
| 03/2015 | Completed | 1 | Mali | 60 | NCT02368119 | |
| 04/2015 | Active, not recruiting | 1 | UK | 38 | NCT02451891 | |
| 07/2015 | Completed | 1 | Senegal | 40 | NCT02485912 | |
| cAd3, 100% protection in NHPs (110) | ||||||
| 08/2014 | Completed | 1 | USA | 50 | NCT02231866 | 69, 117 |
| 10/2014 | Completed | 1, 2 | Switzerland | 120 | NCT02289027 | 23 |
| 01/2015 | Active, not recruiting | 2 | Liberia | 1,500 | NCT02344407 | 63 |
| 07/2015 | Completed | 2 | Cameroon, Mali, Nigeria, Senegal | 3,024 | NCT02485301 | |
| 11/2015 | Completed | 2 | Mali, Senegal | 600 | NCT02548078 | |
| Active, not recruiting | 1B | Uganda | PACTR201412000957310 | |||
| Ad5, 100% protection in NHPs (112) | ||||||
| 09/2006 | Completed | 1 | USA | 48 | NCT00374309 | 68 |
| 12/2014 | Completed | 1 | China | 120 | NCT02326194 | 71, 133 |
| 03/2015 | Completed | 1 | China | 61 | NCT02401373 | 130 |
| 07/2015 | Completed | 1 | China | 110 | NCT02533791 | 71 |
| 10/2015 | Completed | 2 | Sierra Leone | 500 | NCT02575456 PACTR201509001259869 | 134 |
| VSV and Ad5, 100% protection in NHPs (26) | ||||||
| 09/2015 | Completed | 1, 2 | Russia | 84 | No. 495 (Russia) | 26 |
| 10/2016 | Completed | 1 | Russia | 60 | NCT02911415 | |
| 10/2016 | Completed | 1 | Russia | 60 | NCT02911428 | |
| 08/2017 | Recruiting | 4 | Guinea, Russia | 2,000 | NCT03072030 PACTR201702002053400 | |
| 11/2017 | Recruiting | 1, 2 | Russia | 120 | NCT03333538 | |
| DNA, 83% protection in NHPs (43) | ||||||
| 10/2003 | Completed | 1 | USA | 27 | NCT00072605 | 75 |
| 01/2008 | Completed | 1B | USA | 20 | NCT00605514 | 107 |
| 02/2010 | Completed | 1 | Uganda | 108 | NCT00997607 | 64 |
| Ad26 and cAd3 | ||||||
| 09/2015 | Active, not recruiting | 1 | UK | 32 | NCT02495246 | |
| HPIV3, 100% protection in NHPs (87) | ||||||
| 08/2015 | Completed | 1 | USA | 30 | NCT02564575 | |
| Subunit | ||||||
| 02/2015 | Completed | 1 | Australia | 230 | NCT02370589 | |
Abbreviations: Ad, human adenovirus; cAd, chimpanzee adenovirus; HPIV3, human parainfluenza virus type 3; MVA, modified vaccinia virus Ankara; NHP, nonhuman primate; VSV, vesicular stomatitis virus.
Virus-like particles.
VLPs are a noninfectious, protein subunit–based vaccine platform and can be produced from cells transfected with plasmids encoding EBOV VP40 and GP, which leads to the release of filamentous particles similar in shape to authentic EBOV particles (123) (Figure 1). Coexpression of additional EBOV proteins, such as NP and VP24, can improve VLP production and immunogenicity (72). The VLP platform is highly immunogenic, and vaccination has been shown to induce innate, humoral, and cellular immune responses (122). The humoral immune response, particularly to GP, seems important for protection; T cell–mediated immunity is likely enhanced by including NP in the particle preparation. The VLP platform is considered safe, but production can be difficult and expensive. The baculovirus-based expression technology may partially solve this issue.
DNA plasmids.
DNA vaccines are readily adaptable and noninfectious and can be produced in large quantities, making them a fast, safe, and cheap vaccine approach (Figure 1). DNA vaccines have been shown to induce cellular as well as humoral immune responses, but they regularly require administration of several doses or combination with other vaccine approaches to achieve the desired immunity (44). EBOV DNA vaccine approaches based on GP have shown efficacy in rodent models (103, 120), but efficacy in NHP models has yet to reach 100%. Recently, 83% protection was reported for a codon-optimized GP-DNA vaccine (43). Given new and more powerful delivery technologies for DNA plasmids, this approach should be continued and optimized. In addition, combination approaches with other platforms should be considered, as they have shown promising results.
Venezuelan equine encephalitis virus replicons.
Alphavirus replicons are well-established vaccine vectors (Figure 1). The foreign antigen is cloned to replace the alphavirus structural genes. These replicons transcribe mRNA and replicate the genome, but they do not make virus particles, due to the absence of alphaviral structural proteins. Venezuelan equine encephalitis virus (VEEV) replicons expressing EBOV-GP, EBOV NP, or both have been assessed in NHPs with inconsistent results (41, 55). However, increasing the vaccination regimen by two or more injections might improve protection.
Recombinant EBOVΔVP30.
Reverse genetics for EBOV have enabled the establishment of a recombinant biologically contained ebolavirus (46). The viral genome lacks the VP30 gene (EBOVΔVP30), which is essential for transcription, and thus the virus is no longer replication competent. Providing VP30 in trans allows for easy and safe propagation of EBOVΔVP30 in cell lines, whereas no progeny virus is produced in the absence of VP30 (45). This vaccine vector was shown to confer complete protection of NHPs against EBOV challenge in a single dose, i.e., a prime-boost approach; a prime-boost regimen using hydrogen peroxide–inactivated vaccine also completely protected NHPs (82). The inactivated prime-boost vaccine strategy is likely to go forward, as it addresses remaining safety concerns with EBOVΔVP30 (Figure 1).
Recombinant cytomegalovirus-based vectors.
A new promising vaccine approach is the use of CMV vectors. A recently published study using rhesus CMV (RhCMV) in rhesus macaques established the efficacy of CMV as an EBOV vaccine platform (83) (Figure 1). In contrast to previous studies (48, 49), this is the first one showing an unexpected induction of high antibody levels against a CMV-expressed foreign antigen, suggesting a way to bias immunity toward either antibody or T cells through selective transgene promoter usage. This has far-reaching potential for many vaccine and immunotherapeutic platforms beyond CMV vectors. High preexisting immunity is not an issue, as CMV reinfects individuals despite existing immunity. Therefore, development of CMV-EBOV vaccines for use in humans, and perhaps wildlife, should continue.
Modified vaccinia virus Ankara.
While MVA-based EBOV vaccines have been developed over the past decade, protective immunity against lethal EBOV challenge was only achieved in combination with an adenovirus-based vaccine (92). This strategy has been assessed with good immunogenicity in human clinical trials (Table 2). However, recently an MVA-based vector expressing EBOV-GP and VP40 leading to VLP release from the infected cell was shown to protect NHPs against lethal disease after a single-dose vaccination (27). This MVA-EBOV vaccine is moving toward human clinical trials.
Recombinant human parainfluenza virus.
Live-attenuated vectors based on human parainfluenza virus type 3 (HPIV3) are actively being investigated as vaccines for HPIV3 and other pediatric pathogens (28, 62). The HPIV3 system is replication competent; therefore, safety concerns require testing such as determining tolerance in immunocompromised individuals and neurovirulence testing. As with Ad5, preexisting immunity to this common childhood pathogen is a concern. However, it was demonstrated that vaccination with HPIV3 expressing the EBOV-GP under preexisting immunity conditions resulted in an immune response to the foreign antigen (13). A novel approach based on HPIV type 1 demonstrated good immunogenicity, but no challenge data were provided (73). Recently, an HPIV3 vector expressing the EBOV-GP has shown promising protective efficacy in NHPs following combined intranasal-intratracheal as well as aerosol vaccination (Figure 1) (87).
Recombinant rabies virus–based vectors.
The rabies-based vectors for EBOV have been developed with the idea of a dual vaccine approach against rabies virus (RABV) and EBOV infection, which overlap in many EBOV-endemic areas (Figure 1). The vector is based on the reverse genetics system for the RABV vaccine strain SAD B19, which is currently used for wildlife immunizations. A point mutation in the RABV glycoprotein attenuates this vector (7). A single dose of the live-attenuated RABV vaccine vector expressing the EBOV-GP (rRABV/EBOV-GP) has shown complete protection in the EBOV NHP model (6). In contrast, one dose of rRABVΔG/EBOV-GP and a prime-boost regimen with inactivated rRABV/EBOV-GP resulted in only 50% protection. Protection by RABV vectors was largely dependent on the quality of antibody responses to EBOV-GP (6). Recently, the inactivated RABV vaccine vector was improved using a codon-optimized antigen (125) and showed promising results in an adjuvanted prime-boost regimen in the EBOV NHP challenge model (60). Production of this vaccine for human clinical trials has been initiated in adherence to good manufacturing practices (60).
Recombinant adenovirus-based vectors.
Replication-defective adenoviruses, mainly human adenovirus serotype 5 (Ad5), have been used as vaccine vectors for viral diseases, including EVD (80) (Figure 1). However, a significant concern with this vaccine platform is the preexisting immunity in certain human populations (85). In vivo studies utilizing Ad5-based vaccines have shown that protective vaccine efficacy is significantly lowered with preexisting immunity to the vector (20, 102). Mucosal delivery of Ad5-based vaccines, however, can circumvent preexisting immunity without affecting protective efficacy; interestingly, this also led to a beneficial improvement of T cell responses (20, 102). A milestone vaccine approach that first protected NHPs from EBOV infection was a result of a DNA-prime and Ad5-boost vaccination (115) (Figure 1). Protection seemed associated with humoral immunity and T memory helper responses, while cell-mediated immunity was not a requirement (115). Subsequently, it was shown that a single injection of high-titered Ad5 expressing the EBOV-GP alone resulted in complete protection against high-dose intramuscular EBOV challenge (112). As a nonreplicating vector, the Ad5 platform is considered safer than replication-competent vectors. The need for high-titered (>1010 infectious units) vaccine, however, might cause problems with vaccine production and safety in immunocompromised people. The preexisting immunity issue pushed the search for replacement vectors based on different human serotypes, such as Ad26 and Ad35 (37), or to primate-specific adenoviruses such as chimpanzee adenovirus [(c)Ad] and simian Ad21 (106, 110). Varying success was achieved with the less-prevalent human adenovirus vectors as vaccines for ebolaviruses, but optimization of vaccine approaches is still ongoing. More promising was the cAd3 vaccine vector approach expressing EBOV-GP, which provided 100% protection with a single dose (110).
Recombinant vesicular stomatitis virus–based vectors.
Since the mid-1990s, the Rose laboratory has spearheaded the use of recombinant vesicular stomatitis virus (VSV) as live-attenuated and replication-incompetent vaccine vectors (104, 105). The main vector used for the filovirus vaccines is an attenuated version of the VSV Indiana serotype expressing the filovirus GP instead of the VSV glycoprotein (G), which normally mediates VSV entry and contributes to neurotropism and pathogenicity of the virus (36, 105) (Figure 1). While this vaccine virus is attenuated, it can be propagated to high titers and is highly immunogenic (2, 58, 61, 100). To date, VSV-EBOV has proven to be one of the more successful candidates (detailed in Reference 111). Briefly, a single intramuscular or mucosal vaccination of NHPs elicited complete protection against a high-dose challenge of homologous EBOV given 28 days later (61). Depletion studies have identified antibodies as a key mechanism of protection (78). This platform has been used to generate vaccines for all known ebolaviruses and several members of the marburgvirus species (81). Recently, it was shown that in NHPs the VSV-EBOV vector conferred 100% protection when administered within a week of a lethal EBOV challenge (84). These data are intriguing as they suggest the vaccine can be used as an emergency vaccine. To address safety, pigs representing a susceptible livestock species and SIV-infected NHPs were infected with VSV-EBOV; the animals showed no signs of disease, and VSV-EBOV shedding was minimal (25, 38). Interestingly, the simian HIV-infected macaques were protected against lethal EBOV challenge and protection correlated with the level of the humoral immune response, again supporting the importance of antibodies elicited by this vaccine for protection (38). An alternative VSV vaccine vector is VSV-N4CT1-ZEBOV-GP, which carries a mutated VSV-G and expresses VSV-N and the EBOV-GP at positions four and one in the VSV genome, respectively. This vaccine has recently shown protective efficacy in NHPs (93).
Human Clinical Trials
Human clinical studies (see the sidebar titled Vaccine Licensure) are costly, and before the 2013–2016 West African EBOV epidemic only four EBOV phase 1 vaccine trials had been conducted evaluating either DNA- or Ad5-based vaccines (64, 68, 75, 107) (Figure 2; Table 2). However, since the year 2005 several other vaccine platforms have shown promising efficacy in NHPs (Figure 1), and during and after the 2013–2016 EBOV epidemic several of these vaccine candidates were assessed in phase 1–3 clinical trials (Figure 2; Table 2). These trials included, but were not limited to, VSV-EBOV (1, 2, 22, 29, 47, 51, 52, 54, 57, 58, 63, 100), Ad5-EBOV (71, 130, 133, 134), rVSV-EBOV combined with Ad5-EBOV (26), cAd3-EBOV with or without MVA-EBOV (23, 31, 66, 69, 117), and Ad26-EBOV combined with MVA-EBOV (88, 126). Most of the conducted trials analyzed immunogenicity of the EBOV-GP antigen; however, some evaluated immune responses to GP and/or NP from different ebolavirus species (Figure 2). In general, the EBOV vaccines in these phase 1 and 2 trials showed no serious adverse effects and elicited good immunogenicity against the immunogen. Several VSV-EBOV phase 1 trials, however, reported adverse effects including temporary arthritis with high-dose vaccination (2, 58), something that was less documented in the later trials with VSV-EBOV (1, 29, 51, 52, 54, 63). A more recent review summarizes most aspects of VSV-EBOV clinical trials (111).
Although there are over 50 phase 1 and 2 trials registered (completed or ongoing) with the US National Library of Medicine (https://www.clinicaltrials.gov) (Table 2) or the Pan African Clinical Trials Registry (http://www.pactr.org) (Table 2), there were only a few opportunities to conduct phase 3 trials as the West African EBOV epidemic ceased. Only one of those phase 3 trials reported results on vaccine efficacy—the ring vaccination, open-label, cluster-randomized trial in Guinea utilizing VSV-EBOV (51, 52). This study showed statistically significant protection against EBOV infection, with no new cases among immunized individuals from ten days after vaccination in randomized and nonrandomized clusters (51). However, despite the promising results, VSV-EBOV is still not licensed today, two years after the first trial (8). Instead, Russia licensed a combination vaccine approach based on a VSV-EBOV prime and Ad5-EBOV boost, with limited preclinical data and a single phase 1/2 trial (26). Similarly, China licensed an Ad5-based EBOV vaccine based on limited preclinical data (131) and more robust phase 1 and 2 clinical trials (71, 130, 133, 134). Intriguingly, both products are very similar to the two leading earlier and well-studied EBOV vaccine approaches, VSV-EBOV and Ad5-EBOV (Figure 1), developed and preclinically evaluated in North America/Europe and tested in phase 1–3 trials at multiple sites before and during the West African epidemic (Figure 2; Table 2). It will be interesting to see if the World Health Organization (WHO) and national regulatory authorities will promote the use of the two licensed products, and when those other promising vaccine approaches will finally be approved for human use.
CHALLENGES FOR VACCINE DEVELOPMENT
Despite the progress in EBOV vaccine development, plenty of challenges remain to be addressed. The following discussion raises some of those issues and is meant to motivate the reader rather than provide a complete list.
Animal Models
Animal modeling is being critically discussed these days. EVD and MVD are not uniformly fatal in humans, and several investigators are speaking out about the importance of having animal models that reflect this. The challenge strain is a matter of discussion. EBOV-Mayinga was the reference strain in the early days but was replaced by EBOV-Kikwit after the 1995 outbreak; nowadays, the EBOV-Makona strain seems favored. Of note, growth of ebolaviruses on Vero cells favors the conversion of a wild-type virus with seven uridine residues at the GP editing site to an eight-uridine-residue mutant. This mutant strain is still lethal but slightly attenuated in NHP models and may lower the bar in efficacy studies (42). The challenge dose in NHP models has been high (1,000 plaque-forming units, ~1,000 LD50). Lower challenge doses are being discussed and in fact have already been used. Furthermore, the EBOV challenge route is arguable. Most previous NHP studies used intramuscular challenge, but mucosal challenge is thought to mimic natural infection more closely. The aerosol route is favored for biodefense countermeasure development.
It will be difficult to reach consensus among researchers on the challenge strain, and it may not matter much which EBOV strains are used, as they all are uniformly lethal in NHPs upon intramuscular inoculation (77, 79). This is not the case with rodent models, where adapted strains are needed. For many years, only mouse-adapted EBOV-Mayinga was available (9), but a few additional viruses/strains have been adapted recently. Lower challenge doses might be more applicable for efficacy testing of antivirals and therapeutics than for vaccine efficacy testing. As we already have multiple vaccine platforms that protect against the high challenge dose, lowering the bar might result in suboptimal products. The mucosal route of challenge does not result in uniform lethality, something that might be favored by some investigators because it would be more similar to infection outcome in humans. However, lack of uniform lethality will increase the number of animals needed to obtain meaningful results, unless one changes the parameters for efficacy (i.e., viral load), which has so far largely been survival. The Filovirus Animal Non-Clinical Group (FANG) is trying to define parameters on animal work that should ideally be applied to filovirus countermeasure development (https://report.nih.gov/crs/View.aspx?Id=2323). Such parameters could be helpful, and many argue they are needed, but they should not impede science because important things might be missed.
Immunogen (Antigen) Choice
Most approaches today utilize the EBOV-GP as the immunogen (antigen). Other antigens that have been utilized largely in combination with GP are NP, VP40, and VP24. So far, only vaccine approaches based on GP have shown complete protection in the NHP models (101). Based on this, currently only GP-based vaccine approaches have entered clinical trials and advanced for licensure (121) (Figure 2; Table 2).
It might be helpful to study the contribution of other EBOV antigens to protection, in particular T cell–mediated protective responses. This might be more important regarding cross-protection and durability, as these antigens are usually more conserved and potent T cell responses may elicit stronger, longer-lasting, and broader immune responses.
Short Time to Immunity (Fast-Acting Vaccination)
An important feature of vaccines against rare, high-consequence pathogens such as EBOV is rapid protection with a single dose, because outbreaks, epidemics, and biothreat events usually do not allow for multiple-injection strategies. Several filovirus vaccines prevent infection in NHPs when administered as a single injection (6, 55, 61, 82, 87, 110, 112) (Figure 1). In particular, VSV-EBOV is very potent in this regard and can completely protect NHPs when administered only a week prior to high-dose homologous challenge (84). The ring vaccination trial in Guinea is a prime example of the success of such an approach (51, 52).
Given the epidemiology and ecology of rare zoonotic diseases such as EBOV, the development and optimization of single-dose or prime-boost approaches with shortened immunization intervals should continue. The current interest is shifting toward long-lasting immunity, which is likely easier to achieve and more reliable with prime-boost approaches. However, it is not even clear what target populations will be addressed with these time-consuming, labor-intensive, and expensive vaccination approaches over the use of emergency (ring) vaccination.
Cross-Protection
There is little to no cross-protective immunity between natural infections with marburgviruses and ebolaviruses, nor with vaccines targeting these distinct viruses. Limited cross-protection may occur among different species within a genus such as Ebolavirus. For example, a single injection of VSV-EBOV provided partial protection against BDBV in NHPs (32). One way to achieve cross-protection is to blend individual vaccine vectors. This was successful for VSV vaccines expressing several filovirus GPs, showing the utility of this platform as a single-dose, multivalent vaccine (39). DNA-prime and Ad5-boost with plasmids/vectors expressing the EBOV and SUDV GPs protected macaques against BDBV challenge (53). Additionally, combining VSV-SUDV and VSV-EBOV vectors using either a single-dose blended vaccination approach or a prime-boost regimen against heterologous BDBV challenge in NHPs resulted in 100% protection (91). Solid cross-protection, however, does occur within a single species. For example, within the species Marburg marburgvirus, several vaccines protected NHPs against both MARV and RAVV challenges (21, 116). The same held true for heterologous vaccine/challenge studies with distinct EBOV isolates. For example, VSV-EBOV is based on the EBOV-Kikwit glycoprotein and shows good protection against EBOV-Makona (51, 52, 84).
The overlapping endemicity zones of filoviruses, arenaviruses, and other pathogens in parts of sub-Saharan Africa raise the need for cross-protective vaccine approaches. Current research is actively addressing multivalent vaccines for filoviruses and/or other related pathogens with overlapping endemicities (for example Lassa virus). However, to our knowledge there are no reports of dual infections with different filoviruses or filoviruses and arenaviruses; thus, the medical and public health importance seems questionable. In addition, immune responses to multivalent vaccines might be complicated, favoring certain immunogens over others and thus affecting efficacy. As appealing as a multivalent, single vaccine might be, an easier, less expensive, and more effective alternative might already be possible by blending successful monovalent vaccines or combining vaccines in a prime-boost strategy. However, coinfections with bacteria and parasites (such as Plasmodium spp.) certainly occur (24). Multivalent vaccines that also target pathogens that are more prevalent and more important to public health might be worthwhile, considering that people are more likely to comply with vaccination against rare pathogens if the vaccines provide simultaneous protection against more common pathogens.
Durability
The durability of filovirus vaccines is of concern, as nearly all past preclinical studies have focused on short-term protection. A promising result was achieved when NHPs were vaccinated with a single dose of VSV-MARV 14 months prior to challenge (90). Less potent was a vaccination using a cAd3 vector expressing the EBOV and SUDV GPs that completely protected macaques against EBOV challenge 5 weeks after vaccination but failed to protect when challenge occurred 10 months after vaccination (110). However, complete protection against EBOV was observed in the same study when animals received a cAd3 EBOV/SUDV vaccine prime followed by an MVA EBOV/SUDV boost. The data suggest that long-term protection may require strong memory CD8 T cells to reestablish effector and memory CD8 T cell responses after challenge. A small study using an Ad5 codon-optimized EBOV vaccine delivered by the respiratory route showed protection against homologous EBOV challenge 5 months after the final vaccination, suggesting that durability may also be improved by certain routes of vaccination (17).
Durability is certainly an important feature of any vaccination approach. However, given that filoviruses rarely strike twice in the same region so as to cause reinfection of humans with the same or a related virus, the importance of the durability of a vaccine is questionable. Current promising vaccine candidates providing solid short-term protection should not be dismissed for limited durability. The final goal, of course, is to achieve both fast and long-lasting immunity.
Mechanism of Protection
The mechanism of protection against natural EBOV infection is not understood and might differ from that of vaccination (97). It was shown for the Ad5-EBOV vaccine that CD8+ T cells appear to be critical for protection against lethal infection (113). In contrast, the VSV-EBOV vaccine elicits antigen-specific IgG responses that are essential for protection from lethal disease (78). Thus, the mechanism of protection may depend on the vaccine platform, vaccination route, and immunogen and may differ from natural infection.
Understanding the mechanism(s) of protection of any vaccine candidate would be highly desirable and should be pursued in preclinical studies and clinical trials. As mentioned above, the mechanism(s) may be platform and/or antigen dependent. In the absence of known mechanisms of protection, correlates of protection might help to define the likelihood of a protective immune response in an individual. This has been studied for several vaccine candidates, in particular Ad5-EBOV (114). If phase 3 efficacy trials cannot be performed in the future due to lack of cases, we may need more animal studies to define at least potential correlates of protection. These data can then be utilized to correlate findings on immune responses from the many phase 2 clinical trials to define those correlates in humans.
Target Population
Unlike AIDS or tuberculosis, EVD is not a disease with global distribution. Therefore, population-based vaccination may be unjustified and unwanted. However, the West African epidemic gave a taste of how quickly EBOV can spread among a naive human population when introduced into large urban settings. Now with identified and evaluated vaccine candidates available, it is time to develop vaccination strategies and to define the target population for vaccination.
Emergency immunization in the form of ring vaccination might be one workable approach. Prophylactic vaccination of certain high-risk exposure groups should certainly be considered. Among those groups are first responders, health care workers, and perhaps military personnel. Family members of patients, another high-risk exposure group, can likely be protected more easily with ring vaccination. Distinct approaches may apply to these different scenarios. Therefore, it is necessary to not focus on a single vaccine candidate in order to have options. For emergency vaccination, a single-shot, fast-acting vaccine is needed; for prophylactic vaccination a more broad and durable immune response may be beneficial and can likely be better achieved with a prime-boost approach.
Licensing
China and Russia have gone ahead and licensed EBOV vaccines, based on concepts principally developed in other countries that have yet to license their own products (8). These vaccines have yet to be evaluated by WHO for use in humans. It remains to be seen if or when licensing of other products will occur.
The licensing pathway of a vaccine is lengthy and cumbersome. Unfortunately, the intent can change over time for marketing or other reasons. Let’s hope that this won’t be the case with some of the prime EBOV vaccines.
LESSONS LEARNED
The West African EBOV epidemic has demonstrated the limitations of our public health response to rare emerging, highly pathogenic infectious diseases. It also has shown that public health measures compete and interfere with human behavior, religion, traditions, and politics (70). This is not unusual, but in this case, it has had a disastrous outcome. Public health intervention came late, but not unusually late when compared to previous outbreaks. The interventions simply failed when applied to an extremely mobile population with intensive cross-border movement and fast and easy access to metropolitan areas. Overall, one must admit that local/regional public health and global response systems were ill prepared for such an overwhelming and catastrophic event. Nevertheless, the response was certainly justified and will likely be better prepared in the future should such a devastating epidemic ever happen again. On a positive note, the epidemic has allowed the utilization of new and sophisticated state-of-the-art diagnostic and surveillance tools in the field that had never been applied to outbreak management before; this relates to assays diagnosing patients in minutes or hours rather than days or months and on-site sequencing that allowed tracking of transmission events (3, 96). In addition, clinical trials tested safety, immunogenicity, and in rare occasions even efficacy of countermeasures (121).
Since the West African EBOV epidemic, new approaches have been added and promising existing approaches have advanced to phase 1, 2, and even 3 clinical trials (121). The Coalition for Epidemic Preparedness Innovations (http://cepi.net/) has been founded to speed up the development of vaccines for rare and emerging pathogens with epidemic/pandemic potential, such as EBOV, MARV, Lassa virus, Nipah virus, and Middle East respiratory syndrome coronavirus. This initiative is highly welcome and globally supported by governmental, academic, industrial, and other nongovernmental entities, a coalition that is unique in its constellation and support. On the other hand, it is disappointing to hear about the delay in licensing of the VSV-EBOV vaccine (8). Let’s hope that this is just a temporary delay for a plausible reason and not an indication of further lowering prioritization and waning interest in countermeasure development, similar to what we have experienced following previous outbreaks.
Stalling countermeasures at the level of preclinical work was a mistake that hopefully will not be repeated. At the very minimum, we need to move into phase 1—or even better, phase 2—clinical trials. Limited stockpiles of vaccines should be available for immediate use in humans and stored at locations that are not vulnerable to financial and political constraints when it comes to immediate release. Licensure would be preferred, but use on compassionate grounds would be sufficient.
A plan for administering vaccines should be developed, and appropriate protocols should be prepared or in place for immediate ethics approval. Discussion on how to best run clinical trials should occur ahead of time and not when vaccination is required, as happened during the West African outbreak. Regardless of disagreements over clinical study designs in emergency situations, one would think that public health and ethics would always prevail.
Currently, one can observe a shift from emergency to prophylactic vaccination, which may require different types of vaccines and immunization concepts. This raises the question of the target population, which is better defined for emergency vaccination. The ring vaccination trial in Guinea can serve as a prime example for that approach (51, 52). It will be tough to argue for population-based vaccination against EBOV. The region of EBOV endemicity is not well defined, and outbreaks have usually not recurred at the same location. The unknown durability and mechanisms of protection as well as potential adverse effects of most/some vaccine candidates do not yet favor a wide distribution. Thus, we are likely limited to vaccination of certain high-risk groups such as medical personnel and aid workers from entities that support outbreak management, laboratory workers, and medical personnel in countries or regions that have experienced outbreaks. However, this will only work if regular follow-ups and perhaps booster immunizations are offered and people comply with recommendations.
From a public health point of view, we would see vaccines as the priority EBOV countermeasure that will hopefully prevent infection and consequences such as post–ebolavirus disease syndrome and temporary EBOV persistence. Nevertheless, the development of antivirals and therapeutics needs to continue for certain applications and intervention strategies (19), but therapy might have limitations when it comes to larger public health responses.
FAMILY FILOVIRIDAE TAXONOMY (12, 65, 95).
The family Filoviridae comprises three genera.
Marburgvirus
Marburg virus (MARV) and Ravn virus (RAVV) belong to the species Marburg marburgvirus.
Ebolavirus
Five species are recognized in the genus Ebolavirus: Bundibugyo ebolavirus [Bundibugyo virus (BDBV)], Reston ebolavirus [Reston virus (RESTV)], Sudan ebolavirus [Sudan virus (SUDV)], Tai Forest ebolavirus [Taï Forest virus (TAFV)], and Zaire ebolavirus [Ebola virus (EBOV)].
Cuevavirus
Lloviu virus (LLOV) is assigned to the species Lloviu cuevavirus.
ACKNOWLEDGMENTS
We thank Anita Mora (Research Technologies Branch, NIAID) for assistance with the generation of the display items. Work on filoviruses in the Laboratory of Virology at Rocky Mountain Laboratories is funded by the Intramural Research Program of the NIAID, NIH.
Footnotes
DISCLOSURE STATEMENT
H.F. claims intellectual property regarding the vesicular stomatitis virus–based filovirus vaccine. The opinions, conclusions, and recommendations in this report are those of the authors and do not necessarily represent the official position of the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH).
LITERATURE CITED
- 1.Agnandji ST, Fernandes JF, Bache EB, Obiang Mba RM, Brosnahan JS, et al. 2017. Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: a phase I randomised trial. PLOS Med. 14:e1002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, et al. 2015. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. New Engl. J. Med 374:1647–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias A, Watson SJ, Asogun D, Tobin EA, Lu J, et al. 2016. Rapid outbreak sequencing of Ebola virus in Sierra Leone identifies transmission chains linked to sporadic cases. Virus Evol. 2:vew016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, et al. 2009. Discovery of swine as a host for the Reston ebolavirus. Science 325:204–6 [DOI] [PubMed] [Google Scholar]
- 5.Baseler L, Chertow DS, Johnson KM, Feldmann H, Morens DM. 2017. The pathogenesis of Ebola virus disease. Annu. Rev. Pathol 12:387–418 [DOI] [PubMed] [Google Scholar]
- 6.Blaney JE, Marzi A, Willet M, Papaneri AB, Wirblich C, et al. 2013. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLOS Pathogens 9:e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, et al. 2011. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J. Virol 85:10605–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branswell H. 2017. As foreign powers approve Ebola vaccines, U.S. drug makers lag in development pipeline. STAT, Dec. 8. https://www.statnews.com/2017/12/08/ebola-vaccine-development/ [Google Scholar]
- 9.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. 1999. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis 179(Suppl. 1):S248–58 [DOI] [PubMed] [Google Scholar]
- 10.Bukreyev A, Marzi A, Feldmann F, Zhang L, Yang L, et al. 2009. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology 383:348–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukreyev A, Yang L, Zaki SR, Shieh WJ, Rollin PE, et al. 2006. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J. Virol 80:2267–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukreyev AA, Chandran K, Dolnik O, Dye JM, Ebihara H, et al. 2014. Discussions and decisions of the 2012–2014 International Committee on Taxonomy of Viruses (ICTV) Filoviridae Study Group, January 2012–June 2013. Arch. Virol 159:821–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukreyev AA, Dinapoli JM, Yang L, Murphy BR, Collins PL. 2010. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology 399:290–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, et al. 2011. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477:340–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carod-Artal FJ. 2015. Post-Ebolavirus disease syndrome: What do we know? Expert Rev. Anti Infect. Ther 13:1185–87 [DOI] [PubMed] [Google Scholar]
- 16.Caviness K, Kuhn JH, Palacios G. 2017. Ebola virus persistence as a new focus in clinical research. Curr. Opin. Virol 23:43–48 [DOI] [PubMed] [Google Scholar]
- 17.Choi JH, Jonsson-Schmunk K, Qiu X, Shedlock DJ, Strong J, et al. 2015. A single dose respiratory recombinant adenovirus-based vaccine provides long-term protection for non-human primates from lethal Ebola infection. Mol. Pharm 12:2712–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cote M, Misasi J, Ren T, Bruchez A, Lee K, et al. 2011. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 477:344–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cross RW, Mire CE, Feldmann H, Geisbert TW. 2018. Post-exposure treatments for Ebola and Marburg virus infections. Nat. Rev. Drug Discov 17:413–34. Corrigendum. 2018. Nat. Rev. Drug. Discov. 10.1038/nrd.2018.73 [DOI] [PubMed] [Google Scholar]
- 20.Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, et al. 2008. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLOS ONE 3:e3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daddario-DiCaprio KM, Geisbert TW, Geisbert JB, Stroher U, Hensley LE, et al. 2006. Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J. Virol 80:9659–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahlke C, Kasonta R, Lunemann S, Krahling V, Zinser ME, et al. 2017. Dose-dependent T-cell dynamics and cytokine cascade following rVSV-ZEBOV immunization. EBioMedicine 19:107–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, et al. 2016. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, doubleblind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect. Dis 16:311–20 [DOI] [PubMed] [Google Scholar]
- 24.de Wit E, Falzarano D, Onyango C, Rosenke K, Marzi A, et al. 2016. The merits of malaria diagnostics during an Ebola virus disease outbreak. Emerg. Infect. Dis 22:323–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Wit E, Marzi A, Bushmaker T, Brining D, Scott D, et al. 2015. Safety of recombinant VSV-Ebola virus vaccine vector in pigs. Emerg. Infect. Dis 21:702–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, et al. 2017. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum. Vaccines Immunotherapeutics 13:613–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domi A, Feldmann F, Basu R, McCurley N, Shifflett K, et al. 2018. A single dose of modified vaccinia Ankara expressing Ebola virus like particles protects nonhuman primates from lethal Ebola virus challenge. Sci. Rep 8:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durbin AP, Skiadopoulos MH, McAuliffe JM, Riggs JM, Surman SR, et al. 2000. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. J. Virol 74:6821–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ElSherif MS, Brown C, MacKinnon-Cameron D, Li L, Racine T, et al. 2017. Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. CMAJ 189:E819–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emanuel J, Marzi A, Feldmann H. 2018. Filoviruses: ecology, molecular biology, and evolution. Adv. Virus Res 100:189–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, et al. 2016. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. New Engl. J. Med 374:1635–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falzarano D, Feldmann F, Grolla A, Leung A, Ebihara H, et al. 2011. Single immunization with a monovalent vesicular stomatitis virus-based vaccine protects nonhuman primates against heterologous challenge with Bundibugyo ebolavirus. J. Infect. Dis 204(Suppl. 3):S1082–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fed. Sel. Agent Program. 2018. Select agents and toxins list. CFR 7 Part 331, 9 Part 121, 42 Part 73, U. S. Dep. Health Hum. Serv., U. S. Dep. Agric., Washington, DC. https://www.selectagents.gov/SelectAgentsandToxinsList.html [Google Scholar]
- 34.Feldmann H, Geisbert TW. 2011. Ebola haemorrhagic fever. Lancet 377:849–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldmann H, Sanchez A, Geisbert TW. 2013. Filoviridae: Marburg and Ebola viruses. In Fields Virology, ed. Knipe DM, Howley PM, pp. 923–56. Philadelphia: Lippincott Williams Wilkins [Google Scholar]
- 36.Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, et al. 2004. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol 78:5458–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, et al. 2011. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against Ebolavirus challenge. J. Virol 85:4222–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, et al. 2008. Vesicular stomatitis virus-based Ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLOS Pathog. 4:e1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, et al. 2009. Single-injection vaccine protects nonhuman primates against infection with Marburg virus and three species of Ebola virus. J. Virol 83:7296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisbert TW, Jahrling PB. 2003. Towards a vaccine against Ebola virus. Expert Rev. Vaccines 2:777–89 [DOI] [PubMed] [Google Scholar]
- 41.Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB. 2002. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg. Infect. Dis 8:503–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisbert TW, Strong JE, Feldmann H. 2015. Considerations in the use of nonhuman primate models of Ebola virus and Marburg virus infection. J. Infect. Dis 212(Suppl. 2):S91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant-Klein RJ, Altamura LA, Badger CV, Bounds CE, Van Deusen NM, et al. 2015. Codon-optimized filovirus DNA vaccines delivered by intramuscular electroporation protect cynomolgus macaques from lethal Ebola and Marburg virus challenges. Hum. Vaccines Immunotherapeutics 11:1991–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant-Klein RJ, Altamura LA, Schmaljohn CS. 2011. Progress in recombinant DNA-derived vaccines for Lassa virus and filoviruses. Virus Res. 162:148–61 [DOI] [PubMed] [Google Scholar]
- 45.Halfmann P, Ebihara H, Marzi A, Hatta Y, Watanabe S, et al. 2009. Replication-deficient Ebolavirus as a vaccine candidate. J. Virol 83:3810–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halfmann P, Kim JH, Ebihara H, Noda T, Neumann G, et al. 2008. Generation of biologically contained Ebola viruses. PNAS 105:1129–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halperin SA, Arribas JR, Rupp R, Andrews CP, Chu L, et al. 2017. Six-month safety data of recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein vaccine in a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J. Infect. Dis 215:1789–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, et al. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med 15:293–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He B, Feng Y, Zhang H, Xu L, Yang W, et al. 2015. Filovirus RNA in fruit bats, China. Emerg. Infect. Dis 21:1675–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, et al. 2017. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça suffit!). Lancet 389:505–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, et al. 2015. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 386:857–66 [DOI] [PubMed] [Google Scholar]
- 53.Hensley LE, Mulangu S, Asiedu C, Johnson J, Honko AN, et al.2010. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus species. PLOS Pathog. 6:e1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heppner DG Jr., Kemp TL, Martin BK, Ramsey WJ Nichols R, et al.2017. Safety and immunogenicity of the rVSVG-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect. Dis 17:854–66 [DOI] [PubMed] [Google Scholar]
- 55.Herbert AS, Kuehne AI, Barth JF, Ortiz RA, Nichols DK, et al. 2013. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J. Virol 87:4952–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28–37 [DOI] [PubMed] [Google Scholar]
- 57.Huttner A, Combescure C, Grillet S, Haks MC, Quinten E, et al. 2017. A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa. Sci. Transl. Med 9:eaaj1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, et al.2015. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect. Dis 15:1156–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jahrling PB, Geisbert TW, Dalgard DW, Johnson ED, Ksiazek TG, et al. 1990. Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet 335:502–5 [DOI] [PubMed] [Google Scholar]
- 60.Johnson RF, Kurup D, Hagen KR, Fisher C, Keshwara R, et al. 2016. An inactivated rabies virus-based Ebola vaccine, FILORAB1, adjuvanted with glucopyranosyl lipid A in stable emulsion confers complete protection in nonhuman primate challenge models. J. Infect. Dis 214:S342–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, et al. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med 11:786–90 [DOI] [PubMed] [Google Scholar]
- 62.Karron RA, Belshe RB, Wright PF, Thumar B, Burns B, et al. 2003. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr. Infect. Dis. J 22:394–405 [DOI] [PubMed] [Google Scholar]
- 63.Kennedy SB, Bolay F, Kieh M, Grandits G, Badio M, et al. 2017. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. New Engl. J. Med 377:1438–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kibuuka H, Berkowitz NM, Millard M, Enama ME, Tindikahwa A, et al.2015. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet 385:1545–54 [DOI] [PubMed] [Google Scholar]
- 65.Kuhn JH, Becker S, Ebihara H, Geisbert TW, Jahrling PB, et al. 2012. Filoviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses; Ninth Report of the International Committee on Taxonomy of Viruses, eds. King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, pp. 665–71. Leiden, Neth.: Elsevier. [Google Scholar]
- 66.Lambe T, Rampling T, Samuel D, Bowyer G, Ewer KJ, et al. 2016. Detection of vaccine-induced antibodies to Ebola virus in oral fluid. Open Forum Infect. Dis 3:ofw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Guenno B, Formenty P, Wyers M, Gounon P, Walker F, Boesch C. 1995. Isolation and partial characterisation of a new strain of Ebola virus. Lancet 345:1271–74 [DOI] [PubMed] [Google Scholar]
- 68.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, et al. 2010. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 29:304–13 [DOI] [PubMed] [Google Scholar]
- 69.Ledgerwood JE, DeZure AD, Stanley DA, Coates EE, Novik L, et al. 2017. Chimpanzee adenovirus vector Ebola vaccine. New Engl. J. Med 9:928–38 [DOI] [PubMed] [Google Scholar]
- 70.Lewis H, Chaudry A, Ndow G, Crossey MM, Garside D, et al. 2015. Ebola: Is the response justified? Pan Afr. Med. J 22(Suppl. 1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li JX, Hou LH, Meng FY, Wu SP, Hu YM, et al. 2017. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob. Health 5:e324–34 [DOI] [PubMed] [Google Scholar]
- 72.Licata JM, Johnson RF, Han Z, Harty RN. Contribution of Ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J. Virol 78:7344–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lingemann M, Liu X, Surman S, Liang B, Herbert R, et al. 2017. Attenuated human parainfluenza virus type 1 expressing Ebola virus glycoprotein GP administered intranasally is immunogenic in African green monkeys. J. Virol 91:e02469–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lupton HW, Lambert RD, Bumgardner DL, Moe JB, Eddy GA. 1980. Inactivated vaccine for Ebola virus efficacious in guineapig model. Lancet 2:1294–95 [DOI] [PubMed] [Google Scholar]
- 75.Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, et al. 2006. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin. Vaccine Immunol 13:1267–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martini GA, Knauff HG, Schmidt HA, Mayer G, Baltzer G. 1968.A hitherto unknown infectious disease contracted from monkeys: “Marburg-virus” disease. Ger. Med. Mon 13:457–70 [PubMed] [Google Scholar]
- 77.Marzi A, Chadinah S, Haddock E, Feldmann F, Arndt N, et al. 2018. Recently identified mutations in the Ebola virus-Makona genome do not alter pathogenicity in animal models. Cell Rep. 23:1806–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, et al. 2013. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. PNAS 110:1893–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marzi A, Feldmann F, Hanley PW, Scott DP, Gunther S, Feldmann H. 2015. Delayed disease progression in cynomolgus macaques infected with Ebola virus Makona strain. Emerg. Infect. Dis 21:1777–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marzi A, Feldmann H. 2014. Ebola virus vaccines: an overview of current approaches. Expert Rev. Vaccines 13:521–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marzi A, Feldmann H, Geisbert TW, Falzarano D. 2011. Vesicular stomatitis virus-based vaccines for prophylaxis and treatment of filovirus infections. J. Bioterrorism Biodefense 2011(Suppl. 1):004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marzi A, Halfmann P, Hill-Batorski L, Feldmann F, Shupert WL, et al. 2015. Vaccines: An Ebola whole-virus vaccine is protective in nonhuman primates. Science 348:439–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marzi A, Murphy AA, Feldmann F, Parkins CJ, Haddock E, et al. 2016. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Sci. Rep 6:21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, et al. 2015. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 349:739–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, et al.2010. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28:950–57 [DOI] [PubMed] [Google Scholar]
- 86.Matassov D, Marzi A, Latham T, Xu R, Ota-Setlik A,et al. 2015. Vaccination with a highly attenuated recombinant vesicular stomatitis virus vector protects against challenge with a lethal dose of Ebola virus. J. Infect. Dis 212(Suppl. 2):S443–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meyer M, Garron T, Lubaki NM, Mire CE, Fenton KA, et al. 2015. Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J. Clin. Investig 125:3241–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, et al. 2016. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA 315:1610–23 [DOI] [PubMed] [Google Scholar]
- 89.Miranda ME, Miranda NL. 2011. Reston ebolavirus in humans and animals in the Philippines: a review. J. Infect. Dis 204(Suppl. 3):S757–60 [DOI] [PubMed] [Google Scholar]
- 90.Mire CE, Geisbert JB, Agans KN, Satterfield BA, Versteeg KM, et al. 2014. Durability of a vesicular stomatitis virus-based Marburg virus vaccine in nonhuman primates. PLOS ONE 9:e94355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mire CE, Geisbert JB, Marzi A, Agans KN, Feldmann H, Geisbert TW. 2013. Vesicular stomatitis virus-based vaccines protect nonhuman primates against Bundibugyo ebolavirus. PLOS Negl. Trop. Dis 7:e2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mire CE, Geisbert TW, Feldmann H, Marzi A. 2016. Ebola virus vaccines—reality or fiction? Expert Rev. Vaccines 15:1421–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mire CE, Matassov D, Geisbert JB, Latham TE, Agans KN, et al.2015. Single-dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature 520:688–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy AA, Redwood AJ,Jarvis MA. 2016. Self-disseminating vaccines for emerging infectious diseases. Expert Rev. Vaccines 15:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Negredo A, Palacios G, Vazquez-Moron S, Gonzalez F, Dopazo H, et al. 2011. Discovery of an ebolavirus-like filovirus in Europe. PLOS Pathogens 7:e1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perkins MD, Dye C, Balasegaram M, Brechot C, Mombouli JV, et al. 2017. Diagnostic preparedness for infectious disease outbreaks. Lancet 390:2211–14 [DOI] [PubMed] [Google Scholar]
- 97.Prescott JB, Marzi A, Safronetz D, Robertson SJ, Feldmann H, Best SM. 2017. Immunobiology of Ebola and Lassa virus infections. Nat. Rev. Immunol 17:195–207 [DOI] [PubMed] [Google Scholar]
- 98.Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, et al. 2000. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142–53 [DOI] [PubMed] [Google Scholar]
- 99.RealStar Ebolavirus RT-PCR Kit 1.0 [instructions for use]. San Francisco, CA: Altona Diagnostics; 2014. https://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM425024.pdf [Google Scholar]
- 100.Regules JA, Beigel JH, Paolino KM,Voell J, Castellano AR, et al.2017.A recombinant vesicular stomatitis virus Ebola vaccine. New Engl. J. Med 376:330–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reynolds P, Marzi A. 2017. Ebola and Marburg virus vaccines. Virus Genes 53:501–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richardson JS, Pillet S, Bello AJ, Kobinger GP. 2013. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J. Virol 87:3668–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riemenschneider J, Garrison A, Geisbert J, Jahrling P, Hevey M, et al. 2003. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine 21:4071–80 [DOI] [PubMed] [Google Scholar]
- 104.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, et al.2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539–49 [DOI] [PubMed] [Google Scholar]
- 105.Rose NF, Roberts A, Buonocore L, Rose JK. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol 74:10903–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roy S, Zhi Y, Kobinger GP, Figueredo J, Calcedo R, et al. 2006. Generation of an adenoviral vaccine vector based on simian adenovirus 21. J. Gen. Virol 87:2477–85 [DOI] [PubMed] [Google Scholar]
- 107.Sarwar UN, Costner P, Enama ME, Berkowitz N, Hu Z, et al. 2015. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. J. Infect. Dis 211:549–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Snoy PJ. 2010. Establishing efficacy of human products using animals: the US Food and Drug Administration’s “animalrule”. Vet. Pathol 47:774–78 [DOI] [PubMed] [Google Scholar]
- 109.Sprecher A, Feldmann H, Hensley LE, Kobinger G, Nichol ST, et al. 2016. Ebola virus is unlikely to become endemic in West Africa. Nat. Microbiol 1:16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau-Kilby AW, et al. 2014. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med 20:1126–29 [DOI] [PubMed] [Google Scholar]
- 111.Suder E, Furuyama W, Feldmann H, Marzi A, de Wit E. 2018. The vesicular stomatitis virus-based Ebola virus vaccine: from concept to clinical trials. Hum. Vaccines Immunother 10.1080/21645515.2018.1473698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, et al. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sullivan NJ,Hensley L,Asiedu C, Geisbert TW,Stanley D,et al. 2011. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med 17:1128–31 [DOI] [PubMed] [Google Scholar]
- 114.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. 2009. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat. Rev. Microbiol 7:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605–9 [DOI] [PubMed] [Google Scholar]
- 116.Swenson DL, Wang D, Luo M, Warfield KL, Woraratanadharm J, et al. 2008. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin. Vaccine Immunol 15:460–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, et al. 2016. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, singleblind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis 16:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Towner JS, Amman BR, Sealy TK, Carroll SA, Comer JA, et al. 2009. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLOS Pathog. 5:e1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S,et al.2008. Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda. PLOS Pathog. 4:e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vanderzanden L, Bray M, Fuller D, Roberts T, Custer D, et al. 1998. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology 246:134–44 [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Li J, Hu Y, Liang Q, Wei M, Zhu F. 2017. Ebola vaccines in clinical trial: the promising candidates. Hum. Vaccines Immunotherapeutics 13:153–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Warfield KL, Olinger G, Deal EM, Swenson DL, Bailey M, et al. 2005. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J. Immunol 175:1184–91 [DOI] [PubMed] [Google Scholar]
- 123.Warfield KL, Posten NA, Swenson DL, Olinger GG, Esposito D, et al. 2007. Filovirus-like particles produced in insect cells: immunogenicity and protection in rodents. J. Infect. Dis 196(Suppl. 2):S421–29 [DOI] [PubMed] [Google Scholar]
- 124.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. 2007. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J. Infect. Dis 196(Suppl. 2):S430–37 [DOI] [PubMed] [Google Scholar]
- 125.Willet M, Kurup D, Papaneri A, Wirblich C, Hooper JW, et al. 2015. Preclinical development of inactivated rabies virus-based polyvalent vaccine against rabies and filoviruses. J. Infect. Dis 212(Suppl. 2):S414–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Winslow RL, Milligan ID,Voysey M, Luhn K, Shukarev G,et al.2017. Immune responses to novel adenovirus type 26 and modified vaccinia virus Ankara-vectored Ebola vaccines at 1 year. JAMA 317:1075–77 [DOI] [PubMed] [Google Scholar]
- 127.World Health Organ. 1978. Ebola heaemorrhagic fever in Sudan, 1976. Bull. World Health Organ. 56:247–70 [PMC free article] [PubMed] [Google Scholar]
- 128.World Health Organ. 1978.Ebola haemorrhagic fever in Zaire, 1976. Bull. World Health Organ 56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 129.World Health Organ. 2016. Ebola situation report, 30 March 2016. Rep., World Health Organ., Geneva [Google Scholar]
- 130.Wu L, Zhang Z, Gao H, Li Y, Hou L, et al. 2017. Open-label phase I clinical trial of Ad5-EBOV in Africans in China. Hum. Vaccines Immunotherapeutics 13:2078–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu S, Kroeker A, Wong G, He S, Hou L,et al.2016. An adenovirus vaccine expressing Ebola virus variant Makona glycoprotein is efficacious in guinea pigs and nonhuman primates. J. Infect. Dis 214:S326–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamaoka S, Banadyga L, Bray M, Ebihara H. 2017. Small animal models for studying filovirus pathogenesis. Curr. Top. Microbiol. Immunol 411:195–227 [DOI] [PubMed] [Google Scholar]
- 133.Zhu FC, Hou LH, Li JX, Wu SP, Liu P, et al. 2015. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 385:2272–79 [DOI] [PubMed] [Google Scholar]
- 134.Zhu FC, Wurie AH, Hou LH, Liang Q, Li YH, et al. 2017. Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: a single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 389:621–28 [DOI] [PubMed] [Google Scholar]


