Abstract
Exosomes, which are nanosized vesicles secreted by cells, are attracting increasing interest in the field of biomedical research due to their unique properties, including biocompatibility, cargo loading capacity, and deep tissue penetration. They serve as natural signaling agents in intercellular communication, and their inherent ability to carry proteins, lipids, and nucleic acids endows them with remarkable therapeutic potential. Thus, exosomes can be exploited for diverse therapeutic applications, including chemotherapy, gene therapy, and photothermal therapy. Moreover, their capacity for homotypic targeting and self-recognition provides opportunities for personalized medicine. Despite their advantages as novel therapeutic agents, there are several challenges in optimizing cargo loading efficiency and structural stability and in defining exosome origins. Future research should include the development of large-scale, quality-controllable production methods, the refinement of drug loading strategies, and extensive in vivo studies and clinical trials. Despite the unresolved difficulties, the use of exosomes as efficient, stable, and safe therapeutic delivery systems is an interesting area in biomedical research. Therefore, this review describes exosomes and summarizes cutting-edge studies published in high-impact journals that have introduced novel or enhanced therapeutic effects using exosomes as a drug delivery system in the past 2 years. We provide an informative overview of the current state of exosome research, highlighting the unique properties and therapeutic applications of exosomes. We also emphasize challenges and future directions, underscoring the importance of addressing key issues in the field. With this review, we encourage researchers to further develop exosome-based drugs for clinical application, as such drugs may be among the most promising next-generation therapeutics.
Subject terms: Nanoparticles, Drug delivery
Exosomes: overcoming challenges for revolutionary biomedical applications
Nanomedicine, which applies nanotechnology in medicine, has improved drug delivery. Yet, issues like poor drug movement in the body, low absorption, and high toxicity of traditional drugs persist. Scientists are now using nanomedicines, especially nanoparticles, to tackle these problems. Nanoparticles can carry drugs to specific cells, help in imaging and diagnostic methods, or fix body structures at a cellular level. But, they can also cause unwanted immune responses. Hence, researchers are exploring exosomes, substances produced from various cells, as potential drug carriers. These naturally occurring exosomes are less toxic and more compatible with the body than synthetic nanoparticles. This study gives an overview of the current state of exosome research and its potential for drug delivery.
This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
Introduction
The use of many traditional drugs is often limited by their poor pharmacokinetics, low bioavailability, and high toxicity. Significant advances in overcoming these challenges and increasing the therapeutic efficacy of drugs have been made in the burgeoning fields of nanotechnology and nanomedicine. Nanomedicines represent a transformative and pervasive field of science in the 21st century, and nanomedicine is a specialized field that utilizes the knowledge and tools of nanotechnology for disease prevention and treatment. Nanomedicines typically include nanoparticles (NPs) that are 1 to 500 nanometers in size1. These tiny particles can be designed to deliver drugs to specific cells, facilitate imaging and diagnostic techniques, or even repair body structures at the cellular level. In addition, nanomedicines can be functionalized with drugs and targeted to specific sites. They offer advantages including high stability, tunable surface properties, and the possibility of combining therapeutic and diagnostic functionalities. For these reasons, nanomedicine is attracting significant research interest2.
NPs have attracted significant attention in the field of drug delivery due to their unique properties and ability to overcome various challenges associated with conventional drug delivery systems. NP-based DDSs (nanoparticle-based drug delivery systems) can be used to effectively transport therapeutic agents for the targeted treatment of diseases affecting various organ systems3. These DDSs include both organic and inorganic nanoparticles. Treatment agents can be encapsulated within organic nanoparticles, such as polymeric NPs and liposomes, to maximize their therapeutic effects. Surface modification with ligands can enable targeted drug delivery to specific cells or tissues. Like organic NPs, inorganic nanoparticles composed of materials such as gold, silver, iron oxide, and silica have also been utilized to load drugs and facilitate drug delivery for the treatment of diverse diseases. The use of NPs in DDSs can offer benefits such as increased drug solubility, prolonged release, targeted delivery, reduced toxicity, and heightened therapeutic efficacy4.
Although nanoparticles have great potential as drug carriers, adverse immunological responses to nanomedicines, including anaphylaxis, cytokine release syndrome, the neutralization of biological activity, cross-reactivity with endogenous protein counterparts, and delayed immune reactions, have led to termination of the production of various biologicals. Therefore, it is important to perform rigorous preclinical and clinical studies to examine factors including nanoparticle stability, biocompatibility, clearance mechanisms, and potential toxicity in order to ensure their safety and efficacy prior to their use in therapeutic applications5,6. Owing to the occurrence of adverse responses, many scientists have recently focused on exosomes, which are bioproducts generated from various cell lines and thus detected in various body fluids, including blood, urine, bronchoalveolar lavage fluid (BALF), and cerebrospinal fluid (CSF), as new potential drug carriers. Since they are naturally derived, exosomes typically exhibit lower toxicity and greater biocompatibility than synthetic NPs such as liposomes7,8. In addition, exosomes have a unique composition. They consist of a lipid bilayer membrane in which various membrane proteins, such as tetraspanins, glycoproteins, and signaling receptors, are embedded. This structure can naturally encapsulate various genetic substances, including DNAs, RNAs, and proteins. Accordingly, exosomes vary depending on their source and are affected by pathological conditions, which makes them candidate biomarkers for biopsy-based diagnostics9. In addition to their natural properties, exosome surface can be modified to expand the applications of exosomes in nanobiotechnology, particularly in drug delivery systems. The therapeutic application of exosomes depends on the cargoes they transport to target cells as well as on their own characteristics. Exogenous molecules with beneficial effects can be loaded into exosomes using various methods, such as electroporation, sonication, incubation, and/or transfection10.
Despite the current technical challenges related to exosome preparation and the efficiency of cargo loading, exosomes have promising therapeutic potential as a new type of drug delivery system. In this review article, we focused specifically on exosomes, which are the most extensively studied type of small extracellular vesicles11. We summarize basic information on exosomes, also known as small extracellular vesicles (sEVs); introduce cargo-loaded exosomes that are currently undergoing testing; and describe the therapeutic effects and advantages in exosome-based drug delivery. Finally, we discuss the therapeutic potential and future directions of this attractive field.
Characteristics of exosomes
Classification and biogenesis of exosomes
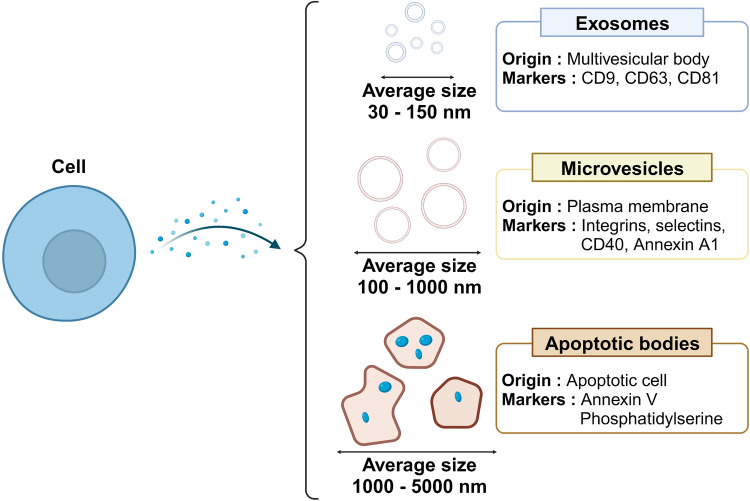
Extracellular vesicles (EVs) are highly heterogeneous membrane-bound carriers of various cargoes, including proteins, lipids, and nucleic acids. They are secreted by various cell types under both normal and pathological conditions, participate in intercellular communication, and influence various physiological and pathological processes. EVs are commonly classified as exosomes, microvesicles, or apoptotic bodies according to the mechanism of production12. The three types of EVs differ in their size, origin, and mechanism of formation, but they all serve as means of communication between cells. In addition, they carry various cargoes that reflect their cells of origin and can be delivered to other cells, influencing their function; thus, EVs are recognized as important biomarkers for diagnosis and therapy in many physiological and pathological conditions13,14.
Exosomes are the smallest type of EV and are typically 30–150 nanometers in diameter. They originate from the endosomal system within cells. Exosomes contain a variety of molecular constituents, including proteins and RNA, from their cells of origin. Exosome can be taken up by neighboring or distant cells and influence their function. Microvesicles, also known as ectosomes, shedding vesicles, or microparticles, are larger than exosomes and have a diameter of ~100–1000 nanometers. They are formed by direct outward budding and fission of the plasma membrane. Like exosomes, microvesicles contain a variety of molecules from their cells of origin and can influence other cells by fusing with their membranes or by being internalized. Apoptotic bodies are the largest type of EV: they are usually 1–5 micrometers in diameter and are generated during apoptosis, a type of programmed cell death. The disassembling cell body forms blebs that then break off to form apoptotic bodies that contain various cellular components, including organelles and DNA. Apoptotic bodies are usually taken up and digested by nearby phagocytic cells and can influence the immune response (Fig. 1)13.
Fig. 1.
Classification of extracellular vesicles.
Although each type of EV exhibits distinct characteristics, separating exosomes and microvesicles can be challenging. Although exosomes are typically smaller, ranging from ~30 to 150 nm in diameter, and microvesicles are usually larger, from ~100 nm to 1 µm, there is a range in which they overlap, making them difficult to separate based solely on size. Although exosomes and microvesicles are generated through different cellular processes, there are no specific biomarkers that distinguish exosomes and microvesicles. Since both types of vesicles can contain similar proteins and RNAs, it is difficult to differentiate them based on their molecular content15. Therefore, the International Society for Extracellular Vesicles recommends classifying EVs into two main categories: small EVs (sEVs, with a diameter less than 200 nm) and medium/large EVs (m/lEVs, with a diameter greater than 200 nm)16. Since exosomes and sEVs are used interchangeably in research (the term “exosome” is preferred by researchers)17, we refer to exosomes and sEVs without distinguishing them in this review paper.
The biogenesis of exosomes is a complex multistep process involving the endosomal pathway. It starts with the inward budding of the plasma membrane, a process called endocytosis. This process results in the formation of early endosomes, which subsequently mature into late endosomes. During maturation, late endosomes are formed by inward budding of the limited multivesicular body (MVB) membrane. The invagination of late endosomal membranes induces the formation of intraluminal vesicles (ILVs) within MVBs18. Throughout this process, proteins, nucleic acids, and lipids in the cytosol are incorporated into these small vesicles, which can fuse with the cellular membrane, leading to the release of ILVs as exosomes into the extracellular space. Alternatively, ILVs can merge with the lysosome if their contents are fated for degradation (Fig. 2)19.
Fig. 2.

Biogenesis of exosomes.
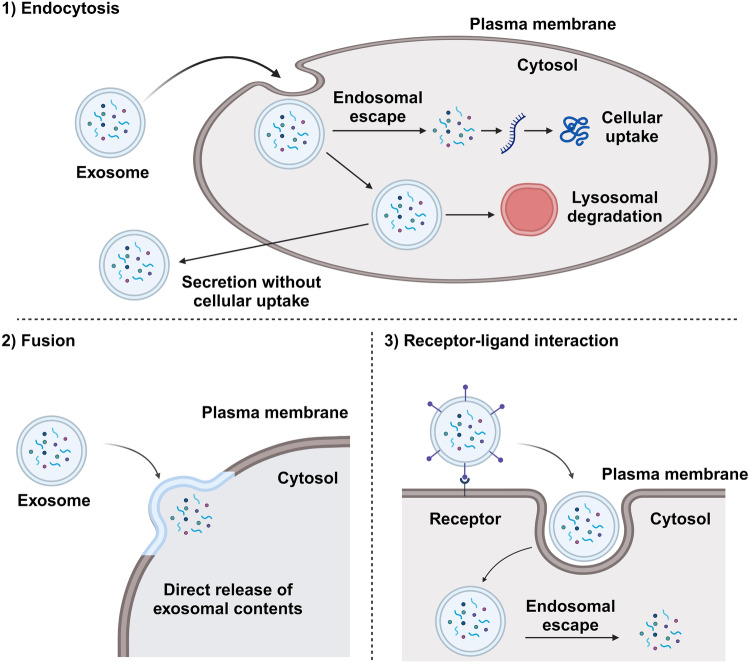
Cellular uptake of exosomes
Once exosomes are released into the extracellular space, they can interact with other cells in multiple ways. The specific mechanism may depend on factors such as the type of cells involved, the molecules present on the surface of the exosome, and the molecules present on the surface of the recipient cell. Currently, the mechanisms by which recipient cells can be targeted by exosomes are not fully understood, and whether the delivery of exosomes is largely random or specific to certain destinations remains unclear20. However, once an exosome reaches its recipient cells, it can be taken up via endocytosis, direct fusion with the plasma membrane, or interaction with cell-surface receptors, initiating intracellular signaling pathways (Fig. 3). Endocytosis is a process by which cells absorb material from outside their cell membrane by engulfing it with their cell membrane. Exosomes can be taken up by recipient cells through several forms of endocytosis, including clathrin-mediated endocytosis, caveolin-mediated endocytosis, macropinocytosis, and phagocytosis21. After endocytosis, exosomes can fuse with the endosome membrane to release their contents into the cytoplasm. Alternatively, exosomes can directly fuse with the plasma membrane of recipient cells, similar to how a viral envelope merges with the cell membrane during viral entry into a host cell22. This process releases the contents of the exosome directly into the cytoplasm of the recipient cell. Although endocytosis is the predominant mechanism for exosome uptake, this direct fusion route is the most effective for delivering cargo into the cell23. For exosomal molecular cargoes to exert effects within the recipient cell, they need to be released from vesicles into the cytoplasm, a process known as endosomal escape. However, if cargoes delivered via the endocytosis pathway remain within endosomes, they will be degraded by a lysosome or secreted into the extracellular space without any therapeutic effect on the target cell24,25. Thus, the direct fusion route of exosomes is more attractive for the use of exosomes as drug delivery vehicles. Once cargoes enter the cell, the contents of the exosome, such as proteins, lipids, RNA, and drugs, can influence the function of the recipient cell. These effects can include changes in gene expression, alterations in signaling pathways, and induction of immune responses. In the third route, specific proteins on the surface of exosomes can interact with specific receptors on the surface of recipient cells. Although the mechanism of interaction is unclear, exosome surface molecules, including fibronectin, tetraspanins, immunoglobulins, proteoglycans, lectin receptors, syncytin-1, and syncytin-2, are known to interact with target cells26–28. PD-L1, TNF, FasL, and TRAIL are currently the most therapeutically intriguing exosomal ligands due to their receptor presence on tumor cell surfaces, rendering them promising candidates for cancer treatment. However, the inconsistency of these procedures across diverse cell types is the main challenge in effectively applying exosome-based drug delivery systems in the clinic29. Nevertheless, many researchers are exploring the use of exosomes as next-generation drug delivery systems, primarily due to their ability to effectively deliver drugs into cells30.
Fig. 3.
Cellular uptake pathways of exosomes.
Drug loading methods
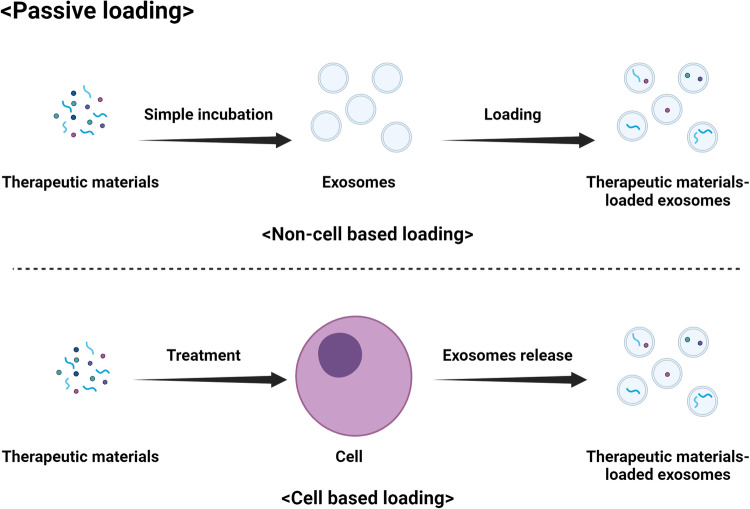
For several reasons, EVs have gained much attention in scientific research as next-generation carriers for drug delivery. Compared to synthetic nanoparticles, exosomes have shown lower toxicity and immunogenicity in preclinical studies. These findings suggested that exosomes may have a reduced risk of causing adverse reactions or immune responses when used as drug delivery vehicles. Exosomes are derived from cells and are formed by lipid bilayers, which are similar to cell membranes. This composition enhances the biocompatibility and stability of exosomes, allowing them to protect cargo molecules from degradation and harsh conditions in the body, such as enzymatic degradation and low-pH environments. Several researchers have reported that exosomes can reach their target organs after they travel in the bloodstream. Exosomes can even deliver drugs by penetrating the blood‒brain barrier (BBB)31. Therefore, many attempts have been made to load various therapeutic materials as well as naturally loaded substances, thereby increasing the therapeutic efficacy of these materials.
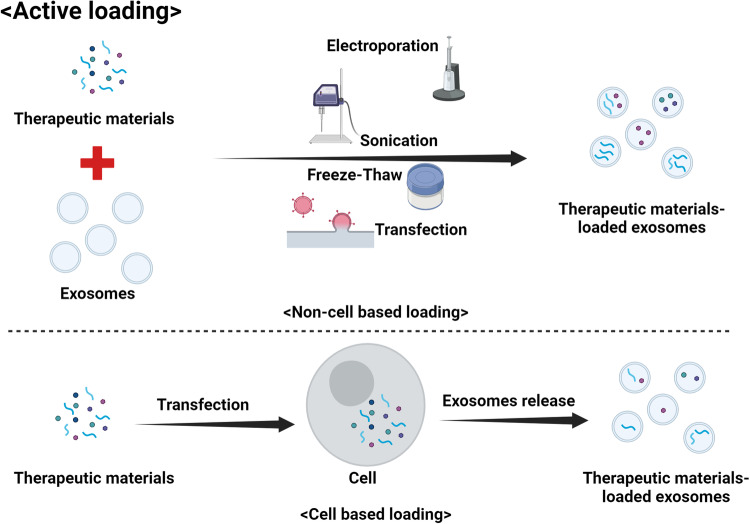
Many ongoing studies have focused on optimizing the cargo loading of exosomes to utilize their full potential as drug delivery carriers. Various methods have been developed to load therapeutic agents, such as small-molecule drugs and genetic substances, into exosomes. There are two main methods used to load drugs into exosomes: passive loading (Fig. 4) and active loading (Fig. 5)32. The choice of method can depend on the specific drug and its properties, as well as the desired release mechanism. Passive loading occurs when cells are cultured with the drug of interest and naturally incorporate the drug into the exosomes as they form. The cells take up the drug, package it into exosomes, and then release the drug-loaded exosomes into the medium. This method is simple and easy to perform, but the loading efficiency is low, and it can be difficult to control the amount of drug loaded into the exosomes. Passive loading generally works best for lipophilic drugs that can easily cross cell and vesicle membranes. However, this method may not be appropriate for all drug types. Another method is active loading, in which the drug is directly loaded into isolated exosomes. This process is typically conducted by various techniques that increase the permeability of the exosome membrane, such as sonication, heat shock, electroporation, incubation with detergents, or the use of saponins. Active loading achieves higher loading efficiency than passive loading and allows more precise control of the drug loading amount. However, this approach can be complex and time-consuming, and there is a risk of damaging the exosomes if the process is not performed properly10,32. In both methods, removing the unloaded drug after loading by various purification techniques, such as ultracentrifugation, size exclusion chromatography, or ultrafiltration, is crucial. Genetic engineering can also be used to load genetic substances into exosomes. Cells can be genetically engineered to express a protein or RNA of interest, which can then be incorporated into exosomes as they form. This method can be used to load specific proteins or RNA molecules into exosomes but requires complex molecular biology techniques and may not be suitable for all types of therapeutic agents10. Each of these methods has advantages and disadvantages, and the best method can depend on factors such as the type of drug, the source of the exosomes, and the intended drug delivery target.
Fig. 4.
Passive loading of therapeutic materials into exosomes.
Fig. 5.
Active loading of therapeutic materials into exosomes.
Exosomes for therapeutic agent delivery
The interest in exosomes as drug carriers stems from several key attributes. Exosomes play a natural role in transporting various biological molecules between cells and have potential for use in the delivery of therapeutic agents33. In addition, exosomes are biocompatible and biodegradable, minimizing the risk of immune responses or toxicity that is often associated with synthetic drug delivery systems. The small size of these vesicles enables them to cross biological barriers that larger carriers cannot cross, and their stability in the bloodstream increases the chance of reaching their targets. Interestingly, exosomes have shown an inherent ability to target specific cells or tissues and can be utilized to deliver drugs directly to diseased cells, thereby increasing effectiveness and minimizing side effects30. Furthermore, exosomes are versatile and can carry cargoes including proteins, nucleic acids, and small drug molecules; thus, multiple therapeutic agents can be delivered simultaneously, and complex therapies involving various types of molecules can be designed10. This section focuses on the utilization of exosomes as a drug delivery system for improving therapeutic effects, categorizing studies based on the type of substances being delivered. The necessity of exosomes for the efficient delivery of each substance is discussed, and recent advances in research on exosomes as a vehicle for drug delivery are highlighted. Furthermore, the section highlights emerging strategies for modifying exosomes, including surface functionalization with targeting ligands or antibodies, to achieve targeted delivery to specific cells or tissues.
Protein
Protein drugs, which include substances such as therapeutic recombinant proteins, monoclonal antibodies, and hormones, can interact with specific targets within the body to treat diseases or restore normal functions. However, they can lose their effectiveness through immune responses, clearance from the bloodstream, degradation by enzymes, or binding to other molecules. To mitigate these challenges, carriers such as nanoparticles and exosomes can be used34. Carriers can increase the stability and bioavailability of these drugs, control their release for sustained effects, enable targeted delivery, facilitate cell penetration, promote elevated therapeutic effects, and prevent immune responses35. Thus, carriers play an indispensable role in improving the effectiveness and efficiency of protein delivery (Table 1).
Table 1.
Loading protein cargo into exosomes.
| Exosome origin | Protein cargo | Loading method | Application | Reference |
|---|---|---|---|---|
| Rat platelet | Yap1 | Direct electroporation into exosomes | Rejuvenation of tendon | Lu et al.37 |
| Breast cancer cells (MCF-7, SK-BR-3, and 4T1) | P53 | Direct electroporation into exosomes | Breast cancer | Jiao et al.40 |
| Mouse serum | DNase I | Copper-free click chemistry | ARDS (neutrophil extracellular trap [NET] inhibition and M2 polarization) | Liu et al.41 |
| Lung epithelial cell (BEAS-2B) | CC16 | Donor cell overexpression | Acute lung injury | Han et al.43 |
| Mesenchymal stem cells | IL-27 | Donor cell overexpression | Inflammatory bowel disease | Nie et al.44 |
| Liver-circulated fluids | TM4SF5 | Generation of overexpression mice | Type 2 diabetes | Jung et al.45 |
| Primary dermal fibroblasts from BALB/c mouse | IL-4, IL-10 | Donor cell overexpression | Acute lung injury | Salazar-Puerta et al.46 |
| HEK293T | Wnt3a | Donor cell overexpression | Alveolar epithelial regeneration | Gao et al.47 |
| HEK293T | Ovalbumin with CpG-DNA | Donor cell overexpression | Allergic rhinitis | Liu et al.48 |
| Mesenchymal stem cell | PD-L1 | Priming donor cells with TNF-α and IFN-γ | Type 1 diabetes | Wang et al.49 |
| HK-2 | TNFAIP8 | Priming donor cells with TGF-β | Kidney fibrosis | Liu et al.50 |
| Mesenchymal stem cell | Brain-derived neurotrophic factor (BDNF) | Donor cell overexpression | Ischemic stroke | Zhou et al.51 |
| Immature dendritic cells | AMPKα1 dominant negative mutant | Direct transfection into exosomes | Genetic obesity | Milbank et al.52 |
In some studies, researchers have attempted to load therapeutic proteins into exosomes indirectly. Misfolded proteins were encapsulated in exosomes by treatment with bafilomycin A1, which can produce many unfolded proteins by impairing lysosomal function36. Therefore, large numbers of misfolded proteins were loaded into exosomes, leading to cell death upon delivery to cancer cells. However, it is difficult to determine which proteins can be loaded, and the proteins being loaded cannot be precisely controlled; thus, there have also been attempts to load desired proteins into exosomes directly. Lu et al. loaded recombinant Yap1 into rat platelet-derived exosomes directly through electroporation37. Yap1-loaded exosomes promote the rejuvenation of tendon stem/progenitor cells for functional tendon regeneration. However, soluble proteins, which have a hydrophilic outer surface with a hydrophobic core, cannot easily penetrate the lipid bilayer structure of exosomes38,39. To increase the efficiency of protein loading into exosomes, Jiao et al. conducted electroporation to empty the exosome of its original contents, thereby maximizing the efficiency of loading exogenous recombinant P53 into exosomes40. There have also been attempts to use proteins to precisely deliver drugs to target cells or tissues. Liu et al. conjugated DNase I to exosome and liposome hybrid particles loaded with methylprednisolone sodium succinate (MPS), an ARDS treatment drug, through copper-free click chemistry. By this method, MPS was effectively delivered to macrophages, where it exerted an enhanced therapeutic effect41.
Although the direct loading of proteins into exosomes is promising, the exposure of the protein surface in solution and the low refolding rate in target cells, which can decrease protein stability and activity, are important obstacles to the delivery of recombinant proteins42. In addition, complex modification processes are often necessary to increase the loading efficiency of proteins into exosomes. As a result, many researchers are trying a different strategy in which donor cells are transfected to overexpress specific therapeutic proteins. This approach allows overexpressed proteins to be naturally incorporated into exosomes, significantly simplifying the process. Han et al. overexpressed CC16 in BEAS-2B lung epithelial cells and then isolated exosomes from the culture medium43. In particular, CC16-loaded exosomes exerted a protective effect against lung injury at concentrations more than 1000 times lower than that required for recombinant CC16 to obtain a similar effect. Nie et al. transfected mesenchymal stem cells (MSCs) with lentivirus to overexpress IL-27 and thereby generated IL-27-loaded exosomes. In this study, IL-27-loaded exosomes inhibited inflammatory responses and rescued intestinal barrier dysfunction44. Jung et al. generated hepatic TM4SF5-overexpressing (Alb-Tm4sf5 TG) mice and isolated TM4SF5-loaded exosomes from liver-circulating fluids. The administration of TM4SF5-loaded exosomes to TM4SF5 knockout mice induced the clearance of excess glucose clearance by targeting brown adipose tissues45. Several researchers have transfected several plasmids together to increase the therapeutic efficacy. Salazar-Puerta et al. transfected IL-4 or IL-10 together with an SPA ligand, which can increase intrapulmonary/alveolar interactions and retention, into primary fibroblasts and isolated exosomes (IL-4 + SPA exo or IL-10 + SPA exo) that had therapeutic effects in acute lung injury46. Gao et al. transfected Wnt3a together with engineered glypican GPC6ΔGPI‐C1C2, which is a stabilizer anchor, and isolated exosomes from the resulting Wnt3a-GPC6ΔGPI‐C1C2 cells that promoted epithelial regeneration more effectively than exosomes isolated from cells transfected with Wnt3a alone47. Liu et al. regulated the Th1 immune response by treating cells with CpG DNA and ovalbumin-loaded exosomes, alleviating allergic symptoms48. Wang et al. attempted to increase the therapeutic efficacy of the PD-L1 protein by loading an additional FDA-approved drug49. Mesenchymal stem cells (MSCs) were primed with TNF-α and IFN-γ for 48 h to obtain PD-L1-loaded exosomes. Then, hexyl 5-aminolevulinate hydrochloride (HAL) was loaded into PD-L1-loaded exosomes by electroporation and used to treat type 1 diabetes by preventing the apoptosis of islet β cells. Unlike other studies, in this study, specific proteins were indirectly loaded by stimulating donor cells. In this indirect method, various unexpected proteins can be loaded in addition to the intended protein. This result was also confirmed in a study by Liu et al.50. Exosomes were isolated from TGF-β-primed tubular epithelial cells (HK-2 cells). Tumor necrosis factor-α-induced protein 8 (TNFAIP8)-loaded exosomes were found to be enriched in TGF-β-primed HK-2 cell culture medium, and these exosomes effectively prevented kidney fibrosis. In this study, TNFAIP8 was not the only protein loaded by the indirect method used. For this reason, the exosome cargo in addition to the intended proteins should be identified. Additionally, any effects arising from exosomes derived from unintended loaded proteins should be confirmed. The indirect loading of specific proteins should be reviewed carefully to ensure the precise contents and effects of the exosomes.
Exosomes can naturally cross the BBB and enter the brain, making them attractive candidates for drug delivery to treat various neurological disorders. Zhou et al. overexpressed brain-derived neurotrophic factor (BDNF) in MSCs to purify BDNF-loaded exosomes. BDNF-loaded exosomes were then administered to mice via intranasal delivery and shown to affect ischemic stroke by penetrating the BBB51. Although exosomes can delivery drugs into the brain by penetrating the BBB, their delivery efficiency is still very low. Therefore, researchers have tried to increase delivery efficiency by engineering exosomes. Milbank et al. used immature dendritic cells overexpressing lysosome-associated membrane protein 2b (Lamp2b) and neurotrophic rabies virus (RVG), which enables more efficient penetration of the BBB by binding to the nicotinic acetylcholine receptor (nAChR). After exosomes were isolated from engineered immature dendritic cells, AMPKα1 dominant negative mutant plasmid-transfected exosomes were delivered to the ventromedial nucleus of the hypothalamus, promoting weight loss in leptin receptor-deficient mice52.
Genetic materials
Genetic materials, such as small interfering RNA (siRNA), microRNA (miRNA), messenger RNA (mRNA), and DNA, have been used extensively in gene therapy. In particular, siRNAs, miRNAs, and antisense oligonucleotides (ASOs) are commonly used in function loss research. However, cells have a protective lipid bilayer membrane that acts as a barrier to the direct entry of large and hydrophilic molecules such as siRNAs, miRNAs, and ASOs. These genetic materials are negatively charged and cannot easily pass through the cell membrane, hindering their access to the intracellular machinery responsible for gene regulation53. They are also susceptible to enzymatic degradation, which hinders their effective transportation to cells; thus, they require a carrier or delivery system for effective transport into cells (Table 2).
Table 2.
Loading genetic materials into exosomes.
| Exosome origin | Loaded material | Loading method | Application | Reference |
|---|---|---|---|---|
| C57BL/6J mice plasma | Anti-H19 (small RNA) | Injection of anti-H19 into mice via tail vein | Colorectal cancer | Sun et al.56 |
| Adipose-derived mesenchymal stem cells | NF-κB siRNA | Donor cell transfection | Skin injury | Lu et al.57 |
| Neural stem cells (iNSCs) | CCL2-siRNA | Direct electroporation into exosomes | Traumatic spinal cord injury | Rong et al.58 |
| Mouse serum | MyD88-siRNA | Direct electroporation or CaCl2 transfection into exosomes | Acute lung injury | Han et al.61 |
| Mesothelial cells | miR-769-5p | Donor cell transfection | Organ fibrosis | Bontempi et al.62 |
| Ckit+ progenitor cells | miR-126 | Direct electroporation into exosomes | Myocardial infarction | Bheri et al.63 |
| Neural stem cells | STAT3 ASO | Donor cell treatment | Glioma | Adamus et al.67 |
| HEK293 | GSDMD-N mRNA | Donor cell transfection with puromycin treatment | Breast tumor | Xing et al.68 |
| HEK293T | LDL-R mRNA | Donor cell transfection | Familial hypercholesterolemia | Mei et al.69, Yang et al.70 |
| Neonatal human dermal fibroblasts | COL1A1 mRNA | Donor cell transfection | Photoaged skin | You et al.72 |
| HEK293T cells | iBax mRNA | Donor cell transfection | Atherosclerosis by targeting senescent cells | Zhang et al.73 |
| HEK293T | ALKBH5 mRNA | Fusion with ALKBH5 mRNA-loaded liposome | Colorectal cancer | Wu et al.74 |
| Mesenchymal stem cell | A151 oligodeoxynucleotide | Direct incubation with exosome | Immunomodulation and skin regeneration | Camoes et al.75 |
| HEK293T | RBP-J decoy oligodeoxynucleotides | Direct electroporation into exosomes | Hepatic fibrosis | He et al.76 |
| Osteosarcoma cells | MEG3 (long noncoding RNA) | Donor cell transfection | Osteosarcoma | Huang et al.77 |
| Mesenchymal stem/stromal cells | HOTAIR (long noncoding RNA) | Donor cell transfection | Angiogenesis and wound healing | Born et al.78 |
| HEK293T | mSCAR (circular RNA) | Donor cell transfection | Sepsis | Fan et al.79 |
| HEK293T | AAV9-SERCA2a | Donor cell transfection | Myocardial infarction | Li et al.80 |
| HEK293FT | Cas9–single-guide-RNA complexes for C-X-C chemokine coreceptor type 4 | Donor cell transfection | HIV-1 | Stranford et al.83 |
Several limitations and challenges have hindered the widespread clinical use of these materials. Although various carriers, including polymers, cyclodextrins, and liposomes, are used in clinical applications due to their high transfection efficiency in vitro, the in vivo performance of most delivery systems is unsatisfactory due to toxicity, nonspecific uptake, and unwanted inflammatory and immune responses54,55. Various delivery systems present problems such as off-target effects, limited tissue penetration, and rapid clearance, which can reduce therapeutic efficacy and cause unintended side effects. Therefore, targeted delivery of siRNAs, miRNAs, or ASOs to specific tissues or cells in the body remains a significant challenge. In efforts to overcome these limitations, exosomes have attracted significant attention as potential carriers for gene therapy. Sun et al. constructed a small RNA, anti-H19, that targets H19 lncRNA. They confirmed that overexpressed anti-H19 could be loaded into exosomes. They loaded anti-H19 into mouse plasma exosomes by injection These exosomes ameliorated colorectal cancer in a nontoxic, nonimmunogenic, and biocompatible manner. Their therapeutic effect was better than that of 5-Fu, one of the most commonly used cancer drugs56. Lu et al. loaded NF-κB siRNA into adipose-derived mesenchymal stem cell exosomes and treated skin injury by inhibiting NF-κB inflammatory signaling57. Since exosomes are natural extracellular vesicles secreted by many cell types, they can offer better biocompatibility and reduced immunogenicity than synthetic nanoparticles. In addition, exosomes can be engineered to carry specific target molecules on their surface, allowing them to interact selectively with specific cell types and to be delivered intracellularly with high efficiency, thereby reducing off-target effects, which commonly induce side effects. CAQK peptide selectively binds chondroitin sulfate proteoglycans (CSPGs), whose expression is upregulated in lesions after spinal cord injury, was anchored to the membranes of neural stem cell (iNSC)-derived exosomes through chemical modification in a study by Rong et al. The exosomes were then loaded with CCL2-siRNA for delivery to the spinal cord to treat injury58. In addition to targeting functions, siRNA-loaded exosomes can be delivered through medical devices such as nebulizers. It has been reported that nebulizers can affect drug stability by impacting molecular integrity, leading to a drug loss of up to 88% during the initial loading into liposomes59,60. However, there was no difference in the amount of siRNA loaded into exosomes before and after nebulization, and the loaded siMyD88 inhibited the NF inflammatory pathway by inhibiting NF-κB inflammatory signaling61. Currently, progress in the development of siRNAs is outpacing that of miRNAs, with a greater number of siRNA candidates already in clinical trials. This trend can be attributed to the complex roles and multiple targets of miRNAs. However, there has been a recent increase in intensive research focusing on miRNAs, and it is anticipated that significant advances will be made in their therapeutic potential61. Bontempi et al. loaded miR-769-5p into mesothelial cell-derived exosomes, inhibiting organ fibrosis by blocking the mesothelial-to-mesenchymal transition62. Bheri et al. loaded miR-126 into cKit+ progenitor cells to ameliorate myocardial infarction63. In addition, attempts to maximize the delivery efficiency of miRNAs have been ongoing. Modifying exosomes by hybridizing them with liposomes, hydrogels, or peptides can increase their stability and targeting ability64–66. Similar to siRNA and miRNA, ASOs, which are small synthetic fragments of nucleic acids block the function of proteins by binding to mRNAs, which can lead to mRNA degradation, thereby altering protein production. To deliver ASOs into cells, carriers are needed. In the research of Adamus et al., glioma-targeted STAT3-ASOs were shown to function only when they were delivered through carriers such as exosomes67.
Moreover, mRNAs can be loaded into exosomes. Xing et al. loaded GSDMD-N mRNA, one of the key factors involved in pyroptosis. Briefly, they transfected the GSDMD-N plasmid into HEK293 cells with puromycin treatment. Since puromycin inhibits translation, untranslated GSDMD-N mRNA can be easily sorted into exosomes. Through this process, they successfully loaded GSDMD-N mRNA into exosomes68. However, loading mRNA into nanocarriers is still challenging due to the instability and large size of the mRNA, which complicates packaging and controlled release. Even after successful loading and sorting, controlled release of mRNA at the target site is challenging, as rapid release may result in degradation before the desired effect is achieved. Therefore, Yang et al. developed an “All-in-One” exosome engineering and purification strategy for easy packaging. Briefly, they generated HEK-293T cells expressing Flag-TCS-PTGFRN-CTSL-MCP, namely, an exosome sorter and the mRNA Ldlr-MS2. Since MCP can specifically bind to MS2-containing RNA during biogenesis, the therapeutic mRNA Ldlr-MS2 was loaded into exosomes conjugated with Flag-TCS-PTGFRN-CTSL-MCP. Then, the exosomes were purified with anti-Flag magnetic beads. After purification, Flag and MCP were cleaved by thrombin treatment and acidification, respectively69. To release Ldlr mRNA more efficiently, they delivered the Ldlr releaser, which can release Ldlr mRNA from MS2 by competitively interacting with MS270. Through this process, therapeutic Ldlr mRNA can be delivered into cells to exert a therapeutic effect on atherosclerosis. Despite the complex loading process compared to that of other materials, advancements are being made, highlighted by the successful use of lipid nanoparticles for mRNA delivery in COVID-19 vaccines. Currently, many efforts are underway to hybridize exosomes and load them with sufficient amounts of mRNA for clinical use. Several research groups have attempted to hybridize exosomes with other engineering technologies. Yang et al. applied cellular nanoporation by using a biochip that generates electrical pulses that stimulate cells to release additional exosomes with increased mRNA loading71. Through this technology, You et al. loaded COL1A1 mRNA into exosomes and prevented wrinkle formation during photoaging of the skin72. Various studies have involved attempts to increase the efficacy of mRNA function. Zhang et al. generated the iBax mRNA, a modified Bax mRNA that targets the liver-specific miRNA miR-122, to modulate hepatotoxicity caused by excessive translation of iBax. Then, this mRNA was encapsulated into exosomes conjugated with superparamagnetic iron oxide nanoparticles (SMNs). This approach enabled the use of magnetic fields to target lesions precisely to ameliorate atherosclerosis73. In addition, exosomes and liposomes can be used to form hybrid nanoparticles to increase loading efficiency while maintaining low toxicity. Wu et al. constructed exosome–liposome hybrid nanoparticles through the freeze−thaw method. A mixture of exosomes and ALKBH5 mRNA-loaded liposomes (1:1) was repeatedly frozen and thawed three times to induce fusion of the two particles. This process can solve the difficulty of encapsulating mRNA in exosomes and ameliorate the toxicity of liposomes, resulting in a greatly improved effect on colorectal cancer74. In addition to these genetic materials, researchers have successfully loaded various genetic materials into exosomes, including oligodeoxynucleotides75,76, long noncoding RNAs77,78, circular RNAs79, and adenoviruses80. In particular, clustered regularly interspaced short palindromic repeats (CRISPR), which of particular interest in genetic therapy, is also being studied intensively for loading into exosomes for targeted delivery81,82. Stranford et al. improved the exosome-based CRISPR gene delivery system by generating genetically programmed self-assembling multifunctional particles. This system avoided multiple challenges, including purification and the decreased exosome yields resulting from the chemical modification of sgRNA83. At the end of 2023, CRISPR gene therapy for sickle cell disease received initial approval from the FDA, and its use is expected to greatly increase in the future84.
Delivery of small-molecule drugs
Natural vesicle-derived exosomes offer advantages including a decreased immune response, reduced toxicity, enhanced bioavailability, and potential for selective drug delivery. Therefore, exosomes can reduce the drug doses needed to achieve the same therapeutic effects and decrease side effects (Table 3). Reddy et al. loaded bevacizumab into exosomes and observed the resulting therapeutic effects on diabetic retinopathy for 2 months. Bevacizumab alone exhibited observable effects for 1 month. In contrast, bevacizumab-loaded exosomes exhibited effects for 2 months, demonstrating the ability of the exosome drug delivery system to prolong the effectiveness of a drug85. Zhou et al. loaded the caspase-1 inhibitor VX-765 into bone marrow dendritic cell-derived exosomes. In this study, the therapeutic efficacy of VX-765 was increased by exosomal delivery to macrophages86. Exosomes are also promising for overcoming drug resistance and can be modified to achieve targeted delivery and high loading efficiency. Iyaswamy et al. modified the surface of exosomes to express the amyloid-β precursor protein (APP)-binding protein Fe65. Then, corynoxine-B was loaded into exosomes to induce autophagy in APP-expressing neuronal cells, and treatment with these exosomes ameliorated cognitive decline and pathogenesis in a mouse model of Alzheimer’s disease87. Due to these advantages, many researchers are investigating the potential of loading exosomes with anticancer drugs to address the shortcomings of conventional anticancer drugs, including poor bioavailability, the necessity of high doses as a result of nonspecific targeting, low therapeutic indices, and multiple drug resistance88,89. Using exosomes, Rehman et al. maximized the anticancer effect of temozolomide, a brain-specific cancer drug, on temozolomide-resistant glioblastoma90. To target glioblastoma, they decorated exosomes with the short peptide HSSP. HSSP can specifically bind to heme oxygenase-1 (HMOX1), which is highly expressed in temozolomide-resistant glioblastoma. These exosomes were loaded with a combination of temozolomide and siSTAT3, which can inhibit the cancer progression gene STAT3. These HSSP-decorated exosomes carrying temozolomide and siSTAT3 reached the brain in larger quantities than other experimental groups and exhibited the strongest anticancer effects. Zhu et al. modified the surface of exosomes to more effectively target the brain. Angiopep-2 targets LRP1, which is highly expressed in glioblastoma cells, and TAT peptide is a cell-penetrating peptide. Both molecules were overexpressed in HEK293T cells, and angiopep-2/TAT-loaded exosomes were isolated. Doxorubicin, a chemotherapeutic agent for treating various cancers, was directly loaded into these Angiopep-2/TAT-loaded exosomes via electroporation, and survival time was significantly improved in a mouse model of glioma without severe side effects91. To increase the efficacy of cancer immunotherapy, Kim et al. loaded doxorubicin into signal regulatory protein alpha (SIPRα)-loaded exosomes. Since SIPRα can block CD47, a tumor antigen related to cancer immunotherapy, SIPRα-loaded exosomes can target cancer cells for immunotherapy, and the loaded doxorubicin increases the anticancer effect92. Faruque et al. isolated exosomes from human pancreatic cancer cells and loaded them with RGD peptides to bind to αvβ3, which is highly expressed in pancreatic cancer cells. These pancreatic cancer cell-targeting exosomes were then loaded with paclitaxel and were confirmed to have advantageous anticancer effects93.
Table 3.
Loading small molecules into exosomes.
| Exosome origin | Loaded materials | Loading method | Application | Reference |
|---|---|---|---|---|
| Mesenchymal stem cell | Bevacizumab | Direct incubation with exosomes | Diabetic retinopathy | Reddy et al.85 |
| Bone marrow dendritic cells | VX-765 | Direct sonication with exosomes | Myasthenia gravis | Zhou et al.86 |
| OVCAR-8 cells | Benzoyloxy dibenzyl carbonate (B2C) | Direct sonication with exosomes | Multidrug-resistant cancer | Kang et al.89 |
| HT22 hippocampus neurons | Corynoxine-B | Direct sonication with exosomes | Alzheimer’s disease | Iyaswamy et al.87 |
| Bone marrow mesenchymal stem cells | Temozolomide with siSTAT3 | Direct sonication with exosomes | Temozolomide-resistant glioblastoma | Rehman et al.90 |
| HEK293T | Doxorubicin | Direct electroporation into exosomes | Glioma | Zhu et al.91 |
| HEK293T | Doxorubicin | Direct incubation with exosomes | Melanoma | Kim et al.92 |
| Human pancreatic cancer cell (PANC-1) | Paclitaxel | Direct sonication with exosomes | Pancreatic cancer | Al Faruque et al.93 |
| RAW264.7 | Shikonin, photosensitizer IR820, and polymetformin | Fusion with shikonin-, photosensitizer IR820-, and polymetformin-loaded liposomes | Breast cancer and melanoma under laser irradiation | Tang et al.94 |
| Goat milk | Chlorin e6 and 18F-FDG | Direct incubation with exosomes | Breast cancer | Guo et al.95 |
| HEK-293T | FX11 (a glycolysis inhibitor) | Direct incubation with exosomes | Breast cancer | Nguyen Cao et al.96 |
| Brain endothelial cells (bEnd.3 cells | Ce6 | Direct incubation with exosomes | Brain cancer | Nguyen Cao et al.97 |
| HEK293T | Triphenylphosphonium (TPP) conjugated chlorin e6 and piperlongumine | Direct incubation with exosomes | Breast cancer | Nguyen Cao et al.98 |
| HEK293T | Indocyanine green paclitaxel, and sodium bicarbonate | Direct incubation with exosomes | Breast cancer | Nguyen Cao et al.99 |
| 4T1 or CT26 | GM-CSF mRNA | Donor cell transfection | Colon and breast cancers | Ji et al.100 |
| RAW264.7 | Resveratrol | Direct sonication with exosomes | Multiple sclerosis | Zheng et al.102 |
| Milk | Forsythiaside A | Direct ultrasonic incubation with exosomes | Liver fibrosis | Gong et al.103 |
| J774A.1 | Curcumin and albumin | Direct sonication with exosomes | Inflammatory skin diseases | Yerneni et al.104 |
In addition to exosome surface modification, several engineering technologies have been applied to exosomes. Tang et al. inserted the CD47-targeting RS17 peptide into liposomes with the chemotherapeutic agent shikonin, the photosensitizer IR820, and the immunomodulator poly-metformin. These liposomes were then fused with exosomes and used to treat breast cancer and melanoma under laser irradiation94. Several researchers have applied chlorin e6 (Ce6), which is an FDA-approved photosensitizer. Photodynamic therapy (PDT) is a two-stage cancer treatment method that utilizes light energy combined with a photosensitizer that can be activated by light energy. Guo et al. loaded Ce6 with 18F-FDG, which can exert photodynamic effects against cancer cells, into goat milk-derived exosomes and thereby targeted breast cancer for Cerenkov luminescence-induced photodynamic therapy95. Nguyen Cao et al. also loaded Ce6 into exosomes for sonodynamic therapy, which is a novel ultrasound-based noninvasive therapeutic strategy derived from photodynamic therapy96. Briefly, Ce6 was conjugated with a triphenylphosphonium (TPP) moiety to target mitochondria. This conjugate was subsequently loaded into brain endothelial cell-derived exosomes to treat brain cancer97. They also utilized TPP-Ce6-engineered exosomes to load the cancer-specific chemotherapeutic agent pipelongumin (PL). After ultrasonic irradiation, TPP-Ce6-PL-loaded exosomes exerted the strongest anticancer effect on breast cancer98. This research team also coloaded indocyanine green, a sonosensitizer and photoacoustic agent, with paclitaxel and sodium bicarbonate and confirmed the anticancer effect on breast cancer99. Ji et al. also utilized Ce6 with CCL21a and GM-CSF mRNA to treat colon and breast cancers100.
Natural product-derived chemicals have also been loaded into exosomes to increase their therapeutic efficacy. Natural product-derived chemicals often have lower potency than synthetic drugs101. However, natural product-derived chemicals loaded into exosomes exhibited much higher therapeutic efficacy than the natural product-derived chemicals alone. For example, resveratrol-loaded exosomes showed increased anti-inflammatory effects compared to resveratrol alone102. Gong et al. loaded Forsythiae Fructus-derived forsythiaside A into hyaluronic acid-modified exosomes; because hyaluronic acid can bind to CD44, which is overexpressed in fibrotic tissue, the forsythiaside A-loaded exosomes were targeted to fibrotic sites and showed the ability to regulate NLRP3-mediated pyroptosis103. In addition, curcumin-loaded exosomes have been administered via dissolvable microneedle arrays, further expanding the use of natural products by improving their therapeutic function104. Numerous similar studies on delivering natural products combined with genetic materials are in progress, and high-impact research similar to that mentioned in this review is steadily being published. Thus, exosome-based approaches are expected to introduce a new chapter in natural product research.
Conclusion and future directions
Because of their biocompatibility, deep tissue penetration, and versatile cargo-loading capacity, exosomes are emerging as promising carriers for targeted drug delivery. Owing to their homotypic targeting effects and self-recognition capabilities, exosomes and their hybrid systems are being increasingly explored as alternatives to conventional drug carriers31. These materials have potential for various applications, including chemotherapy, gene therapy, and photothermal therapy30. In addition, multifunctional exosomes can be generated by the simultaneously loading of various therapeutic cargoes94,105,106. Moreover, while mammalian-derived exosomes have been extensively researched in recent years, increasing evidence also indicates that plant-derived exosome-like nanovesicles exhibit structural and functional similarities to mammalian-derived exosomes107, suggesting that the potential of utilizing exosomes is still far from fully understood. However, exosome research and development still present significant challenges related to loading efficiency, structural stability, and defining exosome origins. The existing strategies for cargo loading, such as incubation, transfection, electroporation, sonication, and in situ synthesis, present limitations in terms of efficiency, cost, potential exosome damage, and versatility of the loaded cargo108. Furthermore, substantial losses can occur during the preparation of modified exosomes or the encapsulation of drugs, leading to low yield and poor reproducibility. To optimize the use of exosomes for targeted delivery, various strategies for assembling homing molecules on exosomal surfaces have been developed. Biological strategies leveraging ligand–receptor interactions offer high specificity but demand broader identification of cell-, tissue-, and organ-specific receptors58,92–94. Additionally, clinical application requires continued research, larger animal studies, clinical trials, and intensive efforts to achieve large-scale, quality-controllable production. Despite these challenges, the unique attributes of exosomes, including high targeting ability, nontoxicity, and biocompatibility, underscore their substantial potential for drug delivery with broad public health benefits5,6. Therefore, continued exploration and innovation in developing efficient, stable, and safe exosome-based delivery systems are encouraged.
First, a more comprehensive understanding of exosome biology is needed. For instance, detailed studies to uncover the precise mechanisms governing the biogenesis, cargo sorting, release, and uptake of exosomes could be paramount in deciphering the roles of exosomes in intercellular communication and pathological conditions, such as cancer metastasis and neurodegenerative diseases109. Overexpressing specific proteins and genes in exosomes may unexpectedly result in the expression of subdomain proteins. Therefore, understanding how defined stimuli affect the sorting of proteins into modified exosomes is highly important for elucidating therapeutic mechanisms in more detail110. Second, increasing the loading efficiency and stability of exosomes is crucial. Current techniques such as electroporation, sonication, or incubation with permeabilization agents have shown potential but also limitations, such as effects on exosome integrity10. Further investigations of these techniques or the development of novel methods will play important roles in optimizing exosome-based therapeutic delivery. Third, the scalability of exosome production and purification poses a formidable challenge30. Currently, exosomes are typically isolated from cell culture media or bodily fluids through ultracentrifugation, ultrafiltration, or size exclusion chromatography, methods that often suffer from low yield or purity. Developing techniques that can efficiently yield high-quality exosomes on a larger scale is important for future clinical application111. Zhang et al.112 developed cationic lipid‒polymer hybrid nanoparticles that encapsulate a cascade system involving catalytic hairpin assembly and CRISPR–Cas12a (CLHN–CCC), allowing exosome enrichment in a three-dimensional space. The enrichment of exosomes via this method is advantageous for miRNA detection and early-stage cancer diagnosis113. Furthermore, the translation of exosome research into clinical practice necessitates successful clinical trials. Several exosome-based products, such as ExoFlo for acute respiratory distress syndrome and dexosomes for melanoma treatment, are in various stages of clinical trials. The continuation of these trials and the initiation of new ones will be crucial for assessing the safety, efficacy, and optimal dosage of exosome-based therapeutics. In a study by Meng et al.114, the intradermal maintenance of exosomes was extended for more than twice as long (up to 7 days) by delivering the exosomes through micro-subcutaneous injection via a dissolvable microneedle. This approach enabled exosomes to effectively ameliorate skin damage. However, the preparation and storage lifetime of microneedles have not yet been fully elucidated. Therefore, techniques for exosome storage, like those available for liposomes, should be developed to address the inconvenience of exosome storage115. Finally, the potential off-target effects and long-term safety of exosome-based therapies must be thoroughly investigated116. For instance, exosomes derived from tumor cells have been shown to potentially promote tumor growth or metastasis, which necessitates a cautious approach to their therapeutic application117. In addition, the development of standardized protocols for exosome research is vital for future progress. Currently, the lack of uniformity in exosome isolation, characterization, and storage techniques often hampers the comparison of results across studies. Efforts to establish consensus and standardize these procedures will benefit the research community. In conclusion, while exosome research holds immense potential, the path forward must address these important challenges and opportunities to fully realize the transformative possibilities of this field.
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF23C0120 to Y.H.), and by a grant from the National Research Foundation of Korea (NRF), funded by the Korea government (MSIT) (RS-2023-00213024 and 2021R1A5A8029876 to Y.H.). The National Heart, Lung, and Blood Institute (NHLBI) provided grant R00HL141685 to D.Z. Images in this paper were created with Biorender (https://biorender.com/).
Author contributions
Conceptualization and supervision: D.Z. and Y.H.; writing: Y.H. and H.I.K., with contributions from D.Z.; critical revision of the manuscript: H.I.K., J.P., Y.Z., and X.W. All the authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hyo In Kim, Jinbong Park.
Contributor Information
Yohan Han, Email: dygks1867@wku.ac.kr.
Duo Zhang, Email: duozhang@uga.edu.
References
- 1.Din FU, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017;12:7291–7309. doi: 10.2147/IJN.S146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domingues C, et al. Where is nano today and where is it headed? A review of nanomedicine and the dilemma of nanotoxicology. ACS Nano. 2022;16:9994–10041. doi: 10.1021/acsnano.2c00128. [DOI] [PubMed] [Google Scholar]
- 3.Yang F, Xue J, Wang G, Diao Q. Nanoparticle-based drug delivery systems for the treatment of cardiovascular diseases. Front. Pharmacol. 2022;13:999404. doi: 10.3389/fphar.2022.999404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alshammari BH, et al. Organic and inorganic nanomaterials: fabrication, properties and applications. RSC Adv. 2023;13:13735–13785. doi: 10.1039/D3RA01421E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egbuna C, et al. Toxicity of nanoparticles in biomedical application: nanotoxicology. J. Toxicol. 2021;2021:9954443. doi: 10.1155/2021/9954443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022;17:53–69. doi: 10.1016/j.ajps.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, et al. Exosomes, a new star for targeted delivery. Front. Cell Dev. Biol. 2021;9:751079. doi: 10.3389/fcell.2021.751079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega, A., Martinez-Arroyo, O., Forner, M. J. & Cortes, R. Exosomes as drug delivery systems: endogenous nanovehicles for treatment of systemic lupus erythematosus. Pharmaceutics1310.3390/pharmaceutics13010003 (2020). [DOI] [PMC free article] [PubMed]
- 10.Han, Y. et al. Overview and update on methods for cargo loading into extracellular vesicles. Processes910.3390/pr9020356 (2021). [DOI] [PMC free article] [PubMed]
- 11.Jeppesen DK, Zhang Q, Franklin JL, Coffey RJ. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 2023;33:667–681. doi: 10.1016/j.tcb.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willms E, Cabanas C, Mager I, Wood MJA, Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018;9:738. doi: 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler JT, Abdelhamed S, Kurre P. Extracellular vesicles in the hematopoietic microenvironment. Haematologica. 2018;103:382–394. doi: 10.3324/haematol.2017.183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EL Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 15.Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A. 2021;1636:461773. doi: 10.1016/j.chroma.2020.461773. [DOI] [PubMed] [Google Scholar]
- 16.Thery C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witwer KW, Thery C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles. 2019;8:1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie, S., Zhang, Q. & Jiang, L. Current knowledge on exosome biogenesis, cargo-sorting mechanism and therapeutic implications. Membranes1210.3390/membranes12050498 (2022). [DOI] [PMC free article] [PubMed]
- 21.Tian T, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolte-‘t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: are they close relatives? Proc. Natl Acad. Sci. USA. 2016;113:9155–9161. doi: 10.1073/pnas.1605146113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulcahy, L. A., Pink, R. C. & Carter, D. R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles10.3402/jev.v3.24641 (2014). [DOI] [PMC free article] [PubMed]
- 24.Joshi BS, de Beer MA, Giepmans BNG, Zuhorn IS. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano. 2020;14:4444–4455. doi: 10.1021/acsnano.9b10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polanco JC, Hand GR, Briner A, Li C, Gotz J. Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol. 2021;141:235–256. doi: 10.1007/s00401-020-02254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purushothaman A, et al. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J. Biol. Chem. 2016;291:1652–1663. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazawa M, et al. Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem. Biophys. Res. Commun. 2014;446:1165–1171. doi: 10.1016/j.bbrc.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 28.Prada, I. & Meldolesi, J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int. J. Mol. Sci.1710.3390/ijms17081296 (2016). [DOI] [PMC free article] [PubMed]
- 29.Krylova, S. V. & Feng, D. The machinery of exosomes: biogenesis, release, and uptake. Int. J. Mol. Sci.2410.3390/ijms24021337 (2023). [DOI] [PMC free article] [PubMed]
- 30.Cecchin R, Troyer Z, Witwer K, Morris KV. Extracellular vesicles: the next generation in gene therapy delivery. Mol. Ther. 2023;31:1225–1230. doi: 10.1016/j.ymthe.2023.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Y, et al. Cell-derived nanovesicle-mediated drug delivery to the brain: principles and strategies for vesicle engineering. Mol. Ther. 2023;31:1207–1224. doi: 10.1016/j.ymthe.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luan X, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorshkov, A., Purvinsh, L., Brodskaia, A. & Vasin, A. Exosomes as natural nanocarriers for RNA-based therapy and prophylaxis. Nanomaterials1210.3390/nano12030524 (2022). [DOI] [PMC free article] [PubMed]
- 34.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell MJ, et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao, Z. et al. Encapsulation of nano-bortezomib in apoptotic stem cell-derived vesicles for the treatment of multiple myeloma. Small e2301748 10.1002/smll.202301748 (2023). [DOI] [PubMed]
- 37.Lu J, et al. Rejuvenation of tendon stem/progenitor cells for functional tendon regeneration through platelet-derived exosomes loaded with recombinant Yap1. Acta Biomater. 2023;161:80–99. doi: 10.1016/j.actbio.2023.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Banach, M., Fabian, P., Stapor, K., Konieczny, L. & Roterman, A. I. Structure of the hydrophobic core determines the 3D protein structure-verification by single mutation proteins. Biomolecules1010.3390/biom10050767 (2020). [DOI] [PMC free article] [PubMed]
- 39.Kalinowska B, Banach M, Wisniowski Z, Konieczny L, Roterman I. Is the hydrophobic core a universal structural element in proteins? J. Mol. Model. 2017;23:205. doi: 10.1007/s00894-017-3367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao Y, et al. Tumor cell-derived extracellular vesicles for breast cancer specific delivery of therapeutic P53. J. Control Release. 2022;349:606–616. doi: 10.1016/j.jconrel.2022.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, et al. An inhalable hybrid biomimetic nanoplatform for sequential drug release and remodeling lung immune homeostasis in acute lung injury treatment. ACS Nano. 2023;17:11626–11644. doi: 10.1021/acsnano.3c02075. [DOI] [PubMed] [Google Scholar]
- 42.Yim N, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han Y, et al. Extracellular vesicle-encapsulated CC16 as novel nanotherapeutics for treatment of acute lung injury. Mol. Ther. 2023;31:1346–1364. doi: 10.1016/j.ymthe.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nie M, Huang D, Chen G, Zhao Y, Sun L. Bioadhesive microcarriers encapsulated with IL-27 high expressive MSC extracellular vesicles for inflammatory bowel disease treatment. Adv. Sci. 2023;10:e2303349. doi: 10.1002/advs.202303349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung JW, et al. Liver-originated small extracellular vesicles with TM4SF5 target brown adipose tissue for homeostatic glucose clearance. J. Extracell Vesicles. 2022;11:e12262. doi: 10.1002/jev2.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salazar-Puerta AI, et al. Engineered extracellular vesicles derived from dermal fibroblasts attenuate inflammation in a murine model of acute lung injury. Adv. Mater. 2023;35:e2210579. doi: 10.1002/adma.202210579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao L, et al. Wnt3a-loaded extracellular vesicles promote alveolar epithelial regeneration after lung injury. Adv. Sci. 2023;10:e2206606. doi: 10.1002/advs.202206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, Ota M, Tabushi M, Takahashi Y, Takakura Y. Development of allergic rhinitis immunotherapy using antigen-loaded small extracellular vesicles. J. Control Release. 2022;345:433–442. doi: 10.1016/j.jconrel.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Wang, L. et al. Engineered cytokine-primed extracellular vesicles with high PD-L1 expression ameliorate type 1 diabetes. Small e2301019 10.1002/smll.202301019 (2023). [DOI] [PubMed]
- 50.Liu X, et al. Kidney tubular epithelial cells control interstitial fibroblast fate by releasing TNFAIP8-encapsulated exosomes. Cell Death Dis. 2023;14:672. doi: 10.1038/s41419-023-06209-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou X, et al. Intranasal delivery of BDNF-loaded small extracellular vesicles for cerebral ischemia therapy. J. Control Release. 2023;357:1–19. doi: 10.1016/j.jconrel.2023.03.033. [DOI] [PubMed] [Google Scholar]
- 52.Milbank E, et al. Small extracellular vesicle targeting of hypothalamic AMPKalpha1 promotes weight loss in leptin receptor deficient mice. Metabolism. 2023;139:155350. doi: 10.1016/j.metabol.2022.155350. [DOI] [PubMed] [Google Scholar]
- 53.Ramasamy T, Munusamy S, Ruttala HB, Kim JO. Smart nanocarriers for the delivery of nucleic acid-based therapeutics: a comprehensive review. Biotechnol. J. 2021;16:e1900408. doi: 10.1002/biot.201900408. [DOI] [PubMed] [Google Scholar]
- 54.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Lam JK, Chow MY, Zhang Y, Leung SW. siRNA versus miRNA as therapeutics for gene silencing. Mol. Ther. Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Y, et al. In vivo self-assembled small RNA targets H19 lncRNA for the treatment of colorectal cancer. J. Control Release. 2023;358:142–160. doi: 10.1016/j.jconrel.2023.04.026. [DOI] [PubMed] [Google Scholar]
- 57.Lu W, et al. Engineered NF-kappaB siRNA-encapsulating exosomes as a modality for therapy of skin lesions. Front. Immunol. 2023;14:1109381. doi: 10.3389/fimmu.2023.1109381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rong Y, et al. Engineered extracellular vesicles for delivery of siRNA promoting targeted repair of traumatic spinal cord injury. Bioact. Mater. 2023;23:328–342. doi: 10.1016/j.bioactmat.2022.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Respaud R, et al. Effect of formulation on the stability and aerosol performance of a nebulized antibody. MAbs. 2014;6:1347–1355. doi: 10.4161/mabs.29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elhissi AM, Faizi M, Naji WF, Gill HS, Taylor KM. Physical stability and aerosol properties of liposomes delivered using an air-jet nebulizer and a novel micropump device with large mesh apertures. Int. J. Pharm. 2007;334:62–70. doi: 10.1016/j.ijpharm.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Han Y, et al. Nebulization of extracellular vesicles: a promising small RNA delivery approach for lung diseases. J. Control Release. 2022;352:556–569. doi: 10.1016/j.jconrel.2022.10.052. [DOI] [PubMed] [Google Scholar]
- 62.Bontempi G, et al. Restoration of WT1/miR-769-5p axis by HDAC1 inhibition promotes MMT reversal in mesenchymal-like mesothelial cells. Cell Death Dis. 2022;13:965. doi: 10.1038/s41419-022-05398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bheri S, et al. Customized loading of microRNA-126 to small extracellular vesicle-derived vehicles improves cardiac function after myocardial infarction. ACS Nano. 2023;17:19613–19624. doi: 10.1021/acsnano.3c01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elkhoury, K. et al. Hybrid extracellular vesicles-liposome incorporated advanced bioink to deliver microRNA. Biofabrication1410.1088/1758-5090/ac8621 (2022). [DOI] [PMC free article] [PubMed]
- 65.Hade MD, Suire CN, Suo Z. An effective peptide-based platform for efficient exosomal loading and cellular delivery of a microRNA. ACS Appl. Mater. Interfaces. 2023;15:3851–3866. doi: 10.1021/acsami.2c20728. [DOI] [PubMed] [Google Scholar]
- 66.Yin Z, et al. Injectable hyperbranched PEG crosslinked hyaluronan hydrogel microparticles containing mir-99a-3p modified subcutaneous ADSCs-derived exosomes was beneficial for long-term treatment of osteoarthritis. Mater. Today Bio. 2023;23:100813. doi: 10.1016/j.mtbio.2023.100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adamus T, et al. Glioma-targeted delivery of exosome-encapsulated antisense oligonucleotides using neural stem cells. Mol. Ther. Nucleic Acids. 2022;27:611–620. doi: 10.1016/j.omtn.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xing Y, et al. Efficient delivery of GSDMD-N mRNA by engineered extracellular vesicles induces pyroptosis for enhanced immunotherapy. Small. 2023;19:e2204031. doi: 10.1002/smll.202204031. [DOI] [PubMed] [Google Scholar]
- 69.Mei R, et al. “All-in-One” exosome engineering strategy for effective therapy of familial hypercholesterolemia. ACS Appl. Mater. Interfaces. 2022;14:50626–50636. doi: 10.1021/acsami.2c15785. [DOI] [PubMed] [Google Scholar]
- 70.Yang Z, et al. Improved extracellular vesicle-based mRNA delivery for familial hypercholesterolemia treatment. Theranostics. 2023;13:3467–3479. doi: 10.7150/thno.82873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Z, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020;4:69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.You Y, et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat. Biomed. Eng. 2023;7:887–900. doi: 10.1038/s41551-022-00989-w. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L, et al. Targeted elimination of senescent cells by engineered extracellular vesicles attenuates atherosclerosis in ApoE(-/-) mice with minimal side effects. Theranostics. 2023;13:5114–5129. doi: 10.7150/thno.87484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu S, et al. Therapeutic m(6)A eraser ALKBH5 mRNA-loaded exosome-liposome hybrid nanoparticles inhibit progression of colorectal cancer in preclinical tumor models. ACS Nano. 2023;17:11838–11854. doi: 10.1021/acsnano.3c03050. [DOI] [PubMed] [Google Scholar]
- 75.Camoes SP, et al. 3D-MSCs A151 ODN-loaded exosomes are immunomodulatory and reveal a proteomic cargo that sustains wound resolution. J. Adv. Res. 2022;41:113–128. doi: 10.1016/j.jare.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He F, et al. Exosome-mediated delivery of RBP-J decoy oligodeoxynucleotides ameliorates hepatic fibrosis in mice. Theranostics. 2022;12:1816–1828. doi: 10.7150/thno.69885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang X, et al. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J. Control Release. 2022;343:107–117. doi: 10.1016/j.jconrel.2022.01.026. [DOI] [PubMed] [Google Scholar]
- 78.Born LJ, et al. HOTAIR-loaded mesenchymal stem/stromal cell extracellular vesicles enhance angiogenesis and wound healing. Adv. Healthc. Mater. 2022;11:e2002070. doi: 10.1002/adhm.202002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fan L, et al. Exosome-based mitochondrial delivery of circRNA mSCAR alleviates sepsis by orchestrating macrophage activation. Adv. Sci. 2023;10:e2205692. doi: 10.1002/advs.202205692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X, et al. Extracellular vesicle-encapsulated adeno-associated viruses for therapeutic gene delivery to the heart. Circulation. 2023;148:405–425. doi: 10.1161/CIRCULATIONAHA.122.063759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitley JA, Cai H. Engineering extracellular vesicles to deliver CRISPR ribonucleoprotein for gene editing. J. Extracell Vesicles. 2023;12:e12343. doi: 10.1002/jev2.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, et al. Engineered extracellular vesicle-delivered CRISPR/Cas9 for radiotherapy sensitization of glioblastoma. ACS Nano. 2023;17:16432–16447. doi: 10.1021/acsnano.2c12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stranford, D. M. et al. Genetically encoding multiple functionalities into extracellular vesicles for the targeted delivery of biologics to T cells. Nat. Biomed. Eng.10.1038/s41551-023-01142-x (2023). [DOI] [PMC free article] [PubMed]
- 84.U.S. Food and Drug Administration. FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease (2023).
- 85.Reddy SK, et al. Small extracellular vesicle-loaded bevacizumab reduces the frequency of intravitreal injection required for diabetic retinopathy. Theranostics. 2023;13:2241–2255. doi: 10.7150/thno.78426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y, et al. Extracellular vesicles encapsulated with caspase-1 inhibitor ameliorate experimental autoimmune myasthenia gravis through targeting macrophages. J. Control Release. 2023;364:458–472. doi: 10.1016/j.jconrel.2023.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Iyaswamy A, et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct. Target Ther. 2023;8:404. doi: 10.1038/s41392-023-01657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L, et al. Exosomes as drug carriers in anti-cancer therapy. Front. Cell Dev. Biol. 2022;10:728616. doi: 10.3389/fcell.2022.728616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang C, et al. Harnessing small extracellular vesicles for pro-oxidant delivery: novel approach for drug-sensitive and resistant cancer therapy. J. Control Release. 2023;365:286–300. doi: 10.1016/j.jconrel.2023.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rehman FU, et al. Heme oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. J. Control Release. 2022;345:696–708. doi: 10.1016/j.jconrel.2022.03.036. [DOI] [PubMed] [Google Scholar]
- 91.Zhu Z, et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J. Extracell Vesicles. 2022;11:e12255. doi: 10.1002/jev2.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim YK, et al. Advantage of extracellular vesicles in hindering the CD47 signal for cancer immunotherapy. J. Control Release. 2022;351:727–738. doi: 10.1016/j.jconrel.2022.09.042. [DOI] [PubMed] [Google Scholar]
- 93.Al Faruque H, Choi ES, Kim JH, Kim E. Enhanced effect of autologous EVs delivering paclitaxel in pancreatic cancer. J. Control Release. 2022;347:330–346. doi: 10.1016/j.jconrel.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 94.Tang, L. et al. Extracellular vesicles-derived hybrid nanoplatforms for amplified CD47 blockade-based cancer immunotherapy. Adv. Mater. e2303835 10.1002/adma.202303835 (2023). [DOI] [PubMed]
- 95.Guo R, et al. Chlorin e6-loaded goat milk-derived extracellular vesicles for Cerenkov luminescence-induced photodynamic therapy. Eur. J. Nucl. Med. Mol. Imaging. 2023;50:508–524. doi: 10.1007/s00259-022-05978-4. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen Cao TG, et al. Bioreducible exosomes encapsulating glycolysis inhibitors potentiate mitochondria-targeted sonodynamic cancer therapy via cancer-targeted drug release and cellular energy depletion. Biomaterials. 2023;301:122242. doi: 10.1016/j.biomaterials.2023.122242. [DOI] [PubMed] [Google Scholar]
- 97.Nguyen Cao TG, et al. Brain endothelial cell-derived extracellular vesicles with a mitochondria-targeting photosensitizer effectively treat glioblastoma by hijacking the blood‒brain barrier. Acta Pharm. Sin. B. 2023;13:3834–3848. doi: 10.1016/j.apsb.2023.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen Cao TG, et al. Mitochondria-targeting sonosensitizer-loaded extracellular vesicles for chemo-sonodynamic therapy. J. Control Release. 2023;354:651–663. doi: 10.1016/j.jconrel.2023.01.044. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen Cao TG, et al. Engineered extracellular vesicle-based sonotheranostics for dual stimuli-sensitive drug release and photoacoustic imaging-guided chemo-sonodynamic cancer therapy. Theranostics. 2022;12:1247–1266. doi: 10.7150/thno.65516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ji P, et al. Modular hydrogel vaccine for programmable and coordinate elicitation of cancer immunotherapy. Adv. Sci. 2023;10:e2301789. doi: 10.1002/advs.202301789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karimi A, Majlesi M, Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015;4:27–30. [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng X, et al. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J. Control Release. 2023;353:675–684. doi: 10.1016/j.jconrel.2022.12.026. [DOI] [PubMed] [Google Scholar]
- 103.Gong L, et al. CD44-targeting drug delivery system of exosomes loading forsythiaside A combats liver fibrosis via regulating NLRP3-mediated pyroptosis. Adv. Healthc. Mater. 2023;12:e2202228. doi: 10.1002/adhm.202202228. [DOI] [PubMed] [Google Scholar]
- 104.Yerneni SS, et al. Skin-targeted delivery of extracellular vesicle-encapsulated curcumin using dissolvable microneedle arrays. Acta Biomater. 2022;149:198–212. doi: 10.1016/j.actbio.2022.06.046. [DOI] [PubMed] [Google Scholar]
- 105.Gong L, et al. An off-the-shelf small extracellular vesicle nanomedicine for tumor targeting therapy. J. Control Release. 2023;364:672–686. doi: 10.1016/j.jconrel.2023.11.013. [DOI] [PubMed] [Google Scholar]
- 106.Yang J, et al. Ultra-efficient radio-immunotherapy for reprogramming the hypoxic and immunosuppressive tumor microenvironment with durable innate immune memory. Biomaterials. 2023;302:122303. doi: 10.1016/j.biomaterials.2023.122303. [DOI] [PubMed] [Google Scholar]
- 107.Huang R, et al. Plant exosomes fused with engineered mesenchymal stem cell-derived nanovesicles for synergistic therapy of autoimmune skin disorders. J. Extracell Vesicles. 2023;12:e12361. doi: 10.1002/jev2.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang X, et al. Current and prospective strategies for advancing the targeted delivery of CRISPR/Cas system via extracellular vesicles. J. Nanobiotechnol. 2023;21:184. doi: 10.1186/s12951-023-01952-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ngan SC, McCarthy NE, Sze SK. pSILAC-based determination of cellular protein sorting into extracellular vesicles. Methods Mol. Biol. 2023;2603:43–58. doi: 10.1007/978-1-0716-2863-8_4. [DOI] [PubMed] [Google Scholar]
- 111.Han L, et al. Removing the stumbling block of exosome applications in clinical and translational medicine: expand production and improve accuracy. Stem Cell Res. Ther. 2023;14:57. doi: 10.1186/s13287-023-03288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang T, et al. CRISPR-Cas12a powered hybrid nanoparticle for extracellular vesicle aggregation and in-situ microRNA detection. Biosens. Bioelectron. 2024;245:115856. doi: 10.1016/j.bios.2023.115856. [DOI] [PubMed] [Google Scholar]
- 113.Reynolds DE, Galanis G, Wang Y, Ko J. Single extracellular vesicle analysis using droplet microfluidics. Methods Mol. Biol. 2023;2689:211–220. doi: 10.1007/978-1-0716-3323-6_16. [DOI] [PubMed] [Google Scholar]
- 114.Meng, S. et al. MiR-141-3p-functionalized exosomes loaded in dissolvable microneedle arrays for hypertrophic scar treatment. Small e2305374 10.1002/smll.202305374 (2023). [DOI] [PubMed]
- 115.Popowski KD, et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter. 2022;5:2960–2974. doi: 10.1016/j.matt.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He J, et al. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2022;12:2385–2402. doi: 10.1007/s13346-021-01087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]