Abstract
Objective:
We aimed to delineate the distinctive characteristics that aid in distinguishing between Kawasaki disease (KD) and multisystem inflammatory syndrome in children (MIS-C) with KD-like manifestations during the pandemic.
Materials and Methods:
We evaluated KD patients and MIS-C patients with KD-like symptoms admitted during the pandemic (between January 2021 and December 2022).
Results:
Thirty-three MIS-C patients and 15 KD patients were included. Kawasaki disease patients were younger than MIS-C patients (3.4 vs. 7.6 years). Rash (P = .044, 100% vs. 75.7%), oral mucosal changes (P = .044, 100% vs. 75.7%), and cervical lymphadenopathy (P = .001, 93.3% vs. 42.4%) were more common in KD. Multisystem inflammatory syndrome in children: patients had more hypotension (P = .002, 45.4% vs. 0), gastrointestinal (P < .001, 72.7% vs. 13.3%), and respiratory symptoms (P = .044, 24.2% vs. 0). Multisystem inflammatory syndrome in children patients also had low lymphocyte and thrombocyte counts and elevated levels of d-dimer, ferritin, and cardiac parameters, unlike KD patients. Multisystem inflammatory syndrome in children patients exhibited a notable reduction in left ventricular systolic function in echocardiography. Another significant difference with regard to management was the anakinra treatment, which was prescribed for MIS-C patients.
Conclusion:
Although MIS-C patients might display a clinical resemblance to KD, several features could help differentiate between MIS-C and classical KD. Specific clinical (hypotension, gastrointestinal, and respiratory symptoms) and laboratory (low lymphocyte and thrombocyte counts with higher C-reactive protein, ferritin, d-dimer, and cardiac parameters) features are characteristic of MIS-C. In addition, divergence in management strategies is evident between the 2 diseases, as biologic drugs were more prevalently employed in MIS-C patients than in classical KD patients.
Keywords: Kawasaki disease, multisystem inflammatory syndrome in children, pandemic
What is already known on this topic?
Due to the resemblances in phenotypic characteristics between multisystem inflammatory syndrome in children (MIS-C) and Kawasaki disease (KD), these conditions are challenging to differentiate, particularly during the pandemic.
What this study adds on this topic?
Some laboratory (low lymphocyte and thrombocyte counts, high ferritin, d-dimer, and cardiac markers) and clinical features (gastrointestinal and respiratory symptoms, hypotension) are characteristic of MIS-C, unlike KD.
Moreover, a noteworthy disparity between the 2 diseases is the common utilization of biologic drugs in the treatment of patients with MIS-C.
Introduction
Multisystem inflammatory syndrome in children (MIS-C) has emerged as a significant component within the realm of pediatric rheumatology practice, and the number of cases reported worldwide has increased as the pandemic progressed.1,2 Different case definitions have been developed by the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) for this entity.3,4 In both definitions, fever, involvement of ≥2 systems/organs, elevated inflammatory markers with indications of prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or exposure, and exclusion of alternative potential causes are required for MIS-C diagnosis.
Pediatricians have faced the challenge of differentiating MIS-C patients from classical Kawasaki disease (KD).5,6 Kawasaki disease is diagnosed primarily through clinical criteria. Classic (typical) KD is diagnosed when a child has a fever persisting for at least 5 days, along with the presence of 4 out of 5 specific findings: bilateral conjunctival injection (redness of the whites of the eyes), oral mucosal changes (cracked and red lips, strawberry tongue, etc.), cervical lymphadenopathy, extremity changes like erythema/edema or peeling of the palms and soles, and rash.7 Incomplete (atypical) KD is diagnosed when a child has a fever lasting 5 or more days and shows 2 or 3 of these findings.7 Kawasaki disease is commonly observed in children under 5 years of age.8 Although it is often self-limiting, it may lead to severe complications such as coronary artery aneurysms, thrombotic events, and myocardial dysfunction.9
During the coronavirus disease 2019 (COVID-19) pandemic, there have been reports of an overlap or similarities between KD and MIS-C.10-24 The most important point is that KD exhibits certain shared clinical characteristics (such as fever, rash, conjunctival injection, erythema and edema of extremities, and cervical lymphadenopathy) with MIS-C. Both MIS-C and KD involve significant systemic inflammation, affecting multiple organ systems. Multisystem inflammatory syndrome in children and KD also have an abnormal immune response, with elevated levels of certain cytokines and immune markers. In addition, both conditions can affect the heart and coronary arteries, potentially leading to serious cardiac complications. Although there are similarities, MIS-C and KD are distinct conditions with differences in clinical presentation, age groups affected, and specific immune markers. Distinguishing MIS-C from KD is essential since there are some therapeutic management and prognosis variations between these 2 conditions.5
Herein, we aimed to identify differences in clinical, laboratory, and echocardiographic findings that may help distinguish KD from MIS-C patients who presented with KD-like symptoms.
Materials and Methods
The ethics committee of Hacettepe University approved this study (GO 21/670). Before inclusion in the study, informed consent was obtained from all parents/patients. The study adhered to the ethical guidelines outlined in the 1964 Declaration of Helsinki and its subsequent revisions.
Patients
We evaluated the MIS-C patients who had KD-like symptoms and KD patients admitted during the pandemic between January 2021 and December 2022 at the Pediatric Rheumatology Department. All MIS-C patients met the definitions of the WHO3 or CDC4, while all KD patients fulfilled the criteria established by the American Heart Association (AHA).7 An expert pediatric rheumatologist diagnosed patients with MIS-C and KD retrospectively according to these definitions and criteria. The group of MIS-C patients with KD-like symptoms included patients with at least 2 major clinical features of KD (rash, oral mucosal changes, bilateral conjunctival injection, cervical lymphadenopathy, and erythema and edema of extremities). Multisystem inflammatory syndrome in children, patients who did not have these features, and KD patients diagnosed during the prepandemic period were excluded from the study. Medical records of all patients were reviewed retrospectively. Demographic characteristics, history of COVID-19 or SARS-CoV-2 contact, clinical features, laboratory findings [whole blood count, C-reactive protein (CRP), erythrocyte sedimentation rate, interleukin-6, aspartate transaminase, alanine aminotransferase, ferritin, d-dimer, troponin, and brain natriuretic peptide (BNP)], echocardiographic results, treatments, and hospitalization duration of both KD and MIS-C patients were noted. Then, KD and MIS-C patients were compared regarding all these features.
Statistical Analyses
We conducted statistical analyses utilizing the Statistical Package for Social Science Statistics software, version 24.0 (IBM Corp., Armonk, NY, USA). The distribution of variables was assessed through both visual and analytical techniques (Shapiro–Wilk’s test) to ascertain their normality. Descriptive statistics were applied, presenting medians (interquartile range, IQR) for continuous variables and numbers and percentages for categorical and nominal variables. Differences between the 2 groups were assessed utilizing the Mann–Whitney U-test, chi-square test, or Fisher’s exact test. Statistical significance was established for P-values less than .05.
Results
General Characteristics of the Patients
Fifteen KD patients and 33 MIS-C patients with KD-like symptoms were included in the study. The median ages of MIS-C and KD patients at diagnosis were 7.6 (5.9) and 3.4 (3.8) years, respectively. Kawasaki disease patients were younger than MIS-C patients at diagnosis (P = .004). Fourteen MIS-C patients (42.4%) and 6 KD patients (40%) were female (P = .875). The characteristics of MIS-C and KD patients are summarized in Table 1.
Table 1.
Characteristics of Patients Diagnosed with Multisystem Inflammatory Syndrome in Children and Kawasaki Disease
| MIS-C Patients (n = 33) | KD Patients (n = 15) | P | |
|---|---|---|---|
| Age, years, median (IQR) | 7.6 (5.9) | 3.4 (3.8) | .004 |
| Sex, n (%) Female Male |
14 (42.4) 19 (57.6) |
6 (40) 9 (60) |
.875 .875 |
| Clinical symptoms | |||
| Days of fever, median (IQR) | 5 (2) | 5.5 (2.5) | .327 |
| Rash, n (%) | 25 (75.7) | 15 (100) | .044 |
| Oral mucosal changes, n (%) | 25 (75.7) | 15 (100) | .044 |
| Bilateral conjunctival injection, n (%) | 32 (96.9) | 14 (93.3) | .532 |
| Erythema and edema of extremities, n (%) | 8 (24.2) | 5 (33.3) | .509 |
| Cervical lymphadenopathy, n (%) | 14 (42.4) | 14 (93.3) | .001 |
| Acute gastrointestinal symptoms, n (%) | 24 (72.7) | 2 (13.3) | <.001 |
| Acute respiratory symptoms, n (%) | 8 (24.2) | 0 | .044 |
| Hypotension, n (%) | 15 (45.4) | 0 | .002 |
| Laboratory findings, median (IQR) | |||
| Hemoglobin, g/dL | 11.2 (2.3) | 11.7 (0.9) | .274 |
| White blood cell count (×103/µL) | 9.5 (6.6) | 14.4 (4.9) | .004 |
| Lymphocyte count (×103/µL) | 0.9 (0.5) | 2.1 (1.3) | <.001 |
| Platelet count (×103/uL) | 159 (84) | 370 (166) | <.001 |
| C-reactive protein, mg/dL (<0.8) | 19.1 (12.6) | 8.2 (7.5) | .013 |
| Erythrocyte sedimentation rate, mm/h (<20) | 38 (39) | 56 (34) | .415 |
| Interleukin-6, pg/mL (<6.4) | 66.4 (149.5) | 31.2 (34.3) | .059 |
| Aspartate transaminase, IU/L (<51) | 38 (25) | 39 (38) | .180 |
| Alanine aminotransferase, IU/L (<39) | 30 (19) | 44 (101) | .344 |
| Ferritin, mg/L (<307) | 505 (742) | 125 (57) | <.001 |
| d-Dimer, mg/L (<0.55) | 3.8 (5) | 0.8 (0.4) | <.001 |
| Troponin, ng/L (<42.9) | 28.3 (100.4) | 4.1 (2.8) | <.001 |
| BNP, pg/mL (<100) | 329 (965) | 75 (27) | .002 |
| Echocardiographic findings, n (%) | |||
| Coronary dilation/aneurysm | 1 (3) | 1 (6.7) | .532 |
| Decreased left ventricle functions | 11 (33.3) | 0 | .010 |
| Myocarditis | 3 (9.1) | 0 | .542 |
| Pericarditis | 2 (6.1) | 0 | 1.000 |
| Treatments, n (%) | |||
| Intravenous immunoglobulin (IVIG)* | 33 (100) | 15 (100) | NA |
| Corticosteroids (MPZ), IV** | 32 (97) | 5 (33.3) | <.001 |
| Anakinra, SC*** | 16 (48.5) | 0 | <.001 |
| Plasma Exchange | 11 (33.3) | 0 | .010 |
| Oxygen support, n (%) | 9 (27.3) | 0 | .041 |
| Vasoactive drugs, n (%) | 15 (45.4) | 0 | .002 |
| Hospitalization duration, days, median (IQR) | 8 (4) | 6 (3) | .037 |
BNP, brain natriuretic peptide; IQR, interquartile range; IV, intravenous; KD, Kawasaki disease; MIS-C, multisystem inflammatory syndrome in children; MPZ, methylprednisolone; NA, not assessed; PCR, polymerase chain reaction; SC, subcutaneous.
Statistically significant values (P < .05) are indicated in bold.
*2 g/kg.
**Usually 1-4 mg/kg, sometimes 30 mg/kg.
***2-4 mg/kg.
Among the MIS-C patients, 23 (69.7%) reported well-defined exposure to SARS-CoV-2. Thirty-two patients (96.9%) showed positive SARS-CoV-2 serology, and 1 (3.1%) exhibited positive PCR results. The patient with PCR positivity also had a history of exposure to SARS-CoV-2. In addition, he had a history of COVID-19 1 month ago, and his PCR was positive at that time. In contrast, none of the KD patients had a COVID-19 or SARS-CoV-2 exposure history. The SARS-CoV-2 PCR and serology results of all KD patients were negative. All KD patients met the complete KD criteria, while among the MIS-C patients, 30 (90.9%) met the criteria for complete KD, and the remaining 3 (9.1%) met the criteria for incomplete KD.
Clinical and Laboratory Findings
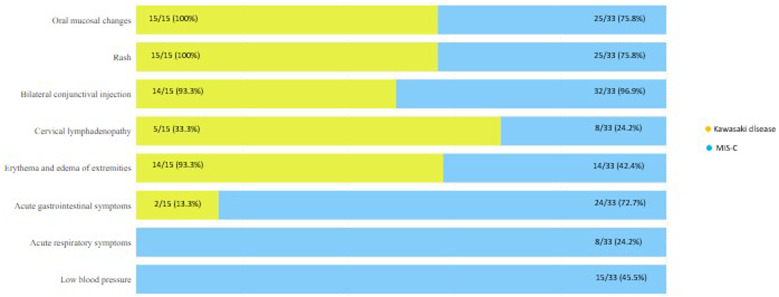
Patients with KD displayed a higher frequency of rash (P = .044), oral mucosal changes (P = .044), and cervical lymphadenopathy (P = .001). Conversely, MIS-C patients demonstrated a higher incidence of acute gastrointestinal symptoms (P < .001), acute respiratory symptoms (P = .044), and hypotension (P = .002) compared to KD patients (Figure 1). Additionally, lymphocyte and platelet counts were notably lower in MIS-C patients than in KD patients (P < .001 for both). C-reactive protein values were notably elevated in MIS-C patients (P = .013). They also exhibited high levels of ferritin, d-dimer, troponin, and BNP (P < .001). A decrease in left ventricular systolic function was more frequent in echocardiograms of MIS-C patients in comparison to KD patients (P = .010).
Figure 1.
Differences in clinical symptoms between multisystem inflammatory syndrome in children and Kawasaki disease.
Treatment
All patients were administered intravenous immunoglobulin (IVIG) as the initial treatment. Notably, most MIS-C patients (97%) underwent corticosteroid therapy, whereas only 5 KD patients (33.3%) with refractory fever were prescribed corticosteroids. Anakinra was used in 16 (48.5%) MIS-C patients. On the other hand, no patients with KD received biologic drugs.
Discussion
Although there are several studies analyzing the distinctions between MIS-C and classical KD10-24, they often compare current MIS-C patients with pre-pandemic KD patients. In our study comparing MIS-C patients with pandemic KD patients, the phenotypic resemblance between MIS-C and KD was evident. However, some clinical (hypotension, gastrointestinal, and respiratory symptoms) and laboratory (low lymphocyte and thrombocyte counts with higher CRP, ferritin, d-dimer, and cardiac parameters) features were primarily associated with MIS-C.
Multisystem inflammatory syndrome in children and KD primarily affect children, although KD is more common in younger children, while MIS-C has been reported in older children and adolescents. Around 80% of KD patients are under the age of 5, with a median diagnosis age of 2 years.25 However, the reported median age for most MIS-C patients falls between 7.3 and 10 years,26,27 and the median age of our MIS-C patients was 7.6 years. Consistent with the literature, KD patients were younger than MIS-C patients in our study. Acute gastrointestinal and respiratory symptoms and hypotension were frequently present in our MIS-C patients, unlike KD patients. Indeed, previous reports have highlighted that gastrointestinal symptoms (abdominal pain, vomiting, and/or diarrhea) are more pronounced in children diagnosed with MIS-C compared to those with KD.2,28-30
Our study showed that MIS-C patients exhibited lower lymphocyte and thrombocyte counts while displaying elevated levels of CRP, ferritin, d-dimer, troponin, and BNP compared to KD patients. Verdoni et al30 reported that leukopenia and thrombocytopenia were more prevalent in MIS-C patients who met the diagnostic criteria for KD in contrast to patients with classical KD. Also, the MIS-C patients had higher CRP and ferritin levels than the pre-pandemic KD patients. Whittaker et al2 reported that MIS-C patients displayed heightened levels of neutrophils, CRP, ferritin, d-dimer, and troponin along with reduced levels of lymphocytes and platelets compared with KD patients from a historic cohort. Two previous studies focused on the differentiating features of MIS-C and contemporaneous KD.31,32 Fernández-Cooke et al31 compared the KD cases diagnosed during the COVID-19 period (from March 1 to May 30, 2020) that were either SARS-CoV-2 positive (n = 26) or negative (n = 20) to those from the same period during 2018 and 2019 (PreCoV) and with each other. Twenty-three (88.5%) of the SARS-CoV-2-positive patients fulfilled the MIS-C criteria. Severe acute respiratory syndrome coronavirus 2-positive patients were significantly older and mainly non-Caucasian and had lower leukocyte and platelet counts, higher inflammatory markers and BNP, less aneurysm development, and more myocardial dysfunction than others. Fridman et al32 compared 38 MIS-C patients with 70 KD patients, 38 of whom were diagnosed during the pandemic. They showed that KD clinical features were less prevalent, while ventricular dysfunction, shock, inotrope requirement, and ICU admission were more common in MIS-C patients than in KD patients.
Limited studies within the existing literature have delved explicitly into the variations in immunopathogenesis between KD and MIS-C. Gruber et al33 and Consiglio et al17 have shown that the inflammatory response in MIS-C differs from that in KD, particularly concerning T cell subsets. Carter et al34 reported activation of CD4 + CCR7 + T cells and γδ T cell subsets in MIS-C, not previously observed in KD. In another study, Ghosh et al35 demonstrated that the host immune response nature in MIS-C mirrors that of the pre-pandemic KD; both are characterized by an IL-15/IL-15 receptor subunit alpha-centric cytokine storm. However, they differ in other laboratory tests and cardiac findings.
In our study, significantly reduced left ventricular systolic function was observed in MIS-C patients when compared to KD patients. While left ventricular systolic dysfunction is rarely seen in KD, decreased systolic functions were reported in 18%-87% of MIS-C patients.29,36,37 In their study comparing KD and MIS-C patients, Matsubara et al38 also reported that patients with MIS-C exhibited poorer left ventricular systolic and diastolic functions in comparison to those with KD. Fridman et al32 also showed more frequent ventricular dysfunction in MIS-C patients than in KD patients. Another study showed that cardiac involvement of MIS-C, although more severe and widespread compared to KD, has a relatively favorable prognosis, potentially leading to a decreased necessity for long-term follow-up after recovery.24 In addition, approximately 33%-87% of patients with MIS-C encounter hypotension that necessitates inotropic support. In contrast, shock develops in only 2%-7% of children with KD.2,29,30,39-42 Furthermore, while respiratory distress is infrequent among KD patients, 12%-46% of MIS-C patients require oxygen, and some even require mechanical ventilation support.2,29,30,39-43 Similarly, in our study, some MIS-C patients needed oxygen support, and some needed vasoactive drugs.
In our cohort, all MIS-C and KD patients received IVIG, while corticosteroids were administered concomitantly to some patients. Intravenous immunoglobulin is the standard treatment of KD.44 It is also frequently used in MIS-C patients.45 Corticosteroids are almost routinely used in MIS-C, and many reports support their use in KD, especially in high-risk patients.41,42 In our study, as expected, corticosteroids were more frequently administered to KD-like MIS-C patients than to KD patients. Another major difference in the management of the 2 groups was the anakinra treatment. Sixteen MIS-C patients (48.5%) received anakinra, while none of the KD patients received this treatment. In addition, although anakinra is frequently used in the literature mainly in MIS-C treatment, its use has also been documented in KD, especially in patients with IVIG-resistant KD.46
Lee et al47 reported 45 MIS-C patients in their systematic review, and 60% of them met the criteria for incomplete or complete KD. They highlighted the challenge of distinguishing MIS-C from KD or severe COVID-19. However, they emphasized the importance of promptly initiating appropriate treatments with early diagnosis.47
Distinguishing the 2 syndromes is also essential for implementing personalized medicine in management. In MIS-C, there could be a need to escalate the treatment to biologic drug use in the early disease course.
Several of the characteristics we have discussed thus far could potentially aid in distinguishing KD-like MIS-C from KD unrelated to SARS-CoV-2. However, the definitive differentiation between MIS-C and KD relies on SARS-CoV-2 testing and the history of exposure. Throughout this pandemic, individuals with positive SARS-CoV-2 testing (or those exposed to someone with COVID-19) who also meet the criteria for incomplete or complete KD are classified as having MIS-C. Additionally, none of the KD patients in our study had previous COVID-19 history or exposure to SARS-CoV-2, and both SARS-CoV-2 PCR and serology were negative. Therefore, we moved away from MIS-C in these patients as well.
The main limitation of this study was that the number of KD patients was very low. Other studies have also reported that the number of classic KD patients decreased significantly during the pandemic.48 Therefore, we faced some inadequacies in comparing KD and MIS-C appropriately. Another limitation is that it is not always possible to rule out the possibility of MIS-C in KD patients during the pandemic,23 so the diagnosis process was challenging. However, we meticulously analyzed the features of the patients to conclude on the diagnosis, and we did not include any patients with a history of COVID-19, positive serology, or PCR in the KD group. Moreover, negative PCR and serology results were available in all included KD patients, which decreases the probability of a misdiagnosis of KD to a minimum. Lastly, comparisons regarding cardiac function were somehow restricted due to the low frequency of cardiac involvement in KD patients included in the study.
Notwithstanding these limitations, a significant strength of our study lies in the direct comparison between KD-like MIS-C patients and classical KD patients diagnosed within the context of the pandemic. Thus, the differences we determined will probably help clinicians differentiate between these 2 similar conditions in clinical practice.
Distinguishing between KD-like MIS-C and classical KD may be challenging since there is a significant overlap in the phenotype. However, certain clinical features, such as lower lymphocyte and thrombocyte counts and higher CRP, ferritin, d-dimer, troponin, and BNP levels, are characteristics of MIS-C. These clues may help for both the correct diagnosis and the differential diagnosis of MIS-C from KD. By paying close attention to these distinct clinical and laboratory features, clinicians can differentiate between these 2 diseases and, therefore, offer patients the most appropriate management and care in clinical practice.
Funding Statement
This study received no funding.
Footnotes
Ethics Committee Approval: This study was approved by the Ethics Committee of Hacettepe University (Approval No: GO 21/670, Date: 06/04/2021).
Informed Consent: Written informed consent was obtained from the parents/patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.Ş., E.D.B., S.Ö.; Design – S.Ş., E.D. B., S.Ö.; Supervision – S.Ş., E.D.B., Ö.B., T.K., Y.Ö., Y.B., S.Ö.; Resources – S.Ş., E.D.B., Ü.K.A., E.A., M.K.C., Z.B.; Materials – S. Ş., E.D.B.; Data Collection and/or Processing – S.Ş., Ü.K.A.; Analysis and/or Interpretation – S.Ş., E.D.B.; Literature Search – S.Ş.; Writing – S.Ş., E.D.B.; Critical Review – S.Ş., E.D.B., Ö.B., T.K., Y.Ö., Y.B., S.Ö.
Declaration of Interests: Yelda Bilginer is an Associate Editor and Seza Özen is a member in the Editorial Board at the Turkish Archives of Pediatrics, however, their involvement in the peer review process was solely as an author. Other authors have no conflict of interest to declare.
References
- 1. Haslak F, Yıldız M, Adrovic A, Şahin S, Barut K, Kasapçopur Ö. A recently explored aspect of the iceberg named COVID-19: multisystem inflammatory syndrome in children (MIS-C). Turk Arch Pediatr. 2021;56(1):3 9. ( 10.5152/TurkArchPediatr.2020.20245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259 269. ( 10.1001/jama.2020.10369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific Brief 2020. Available at: https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed May 17, 2020. [Google Scholar]
- 4. Centers for Disease Control and Prevention health alert network (HAN). Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Available at: https://emergency.cdc.gov/han/2020/han00432.asp Accessed May 15, 2020. [Google Scholar]
- 5. Sharma C, Ganigara M, Galeotti C, et al. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol. 2021;17(12):731 748. ( 10.1038/s41584-021-00709-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haslak F, Gunalp A, Kasapcopur O. A cursed goodbye kiss from severe acute respiratory syndrome-coronavirus-2 to its pediatric hosts: multisystem inflammatory syndrome in children. Curr Opin Rheumatol. 2023;35(1):6 16. ( 10.1097/BOR.0000000000000910) [DOI] [PubMed] [Google Scholar]
- 7. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927 e999. ( 10.1161/CIR.0000000000000484) [DOI] [PubMed] [Google Scholar]
- 8. Zhu FH, Ang JY. The clinical diagnosis and management of Kawasaki disease: a review and update. Curr Infect Dis Rep. 2016;18(10):32. ( 10.1007/s11908-016-0538-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dietz SM, van Stijn D, Burgner D, et al. Dissecting Kawasaki disease: a state-of-the-art review. Eur J Pediatr. 2017;176(8):995 1009. ( 10.1007/s00431-017-2937-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cherqaoui B, Koné-Paut I, Yager H, Bourgeois FL, Piram M. Delineating phenotypes of Kawasaki disease and SARS-CoV-2-related inflammatory multisystem syndrome: a French study and literature review. Rheumatol (Oxf Engl). 2021;60(10):4530 4537. ( 10.1093/rheumatology/keab026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzuki J, Abe K, Matsui T, et al. Kawasaki disease Shock Syndrome in Japan and Comparison with Multisystem inflammatory Syndrome in Children in European countries. Front Pediatr. 2021;9:625456. ( 10.3389/fped.2021.625456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toubiana J, Cohen JF, Brice J, et al. Distinctive features of Kawasaki disease following SARS-CoV-2 infection: a controlled study in Paris, France. J Clin Immunol. 2021;41(3):526 535. ( 10.1007/s10875-020-00941-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cattalini M, Della Paolera S, Zunica F, et al. Defining Kawasaki disease and pediatric inflammatory multisystem syndrome-temporally associated to SARS-CoV-2 infection during SARS-CoV-2 epidemic in Italy: results from a national, multicenter survey. Pediatr Rheumatol Online J. 2021;19(1):29. ( 10.1186/s12969-021-00511-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carbajal R, Lorrot M, Levy Y, et al. Multisystem inflammatory syndrome in children rose and fell with the first wave of the COVID-19 pandemic in France. Acta Paediatr. 2021;110(3):922 932. ( 10.1111/apa.15667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999 1006. ( 10.1136/annrheumdis-2020-217960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corwin DJ, Sartori LF, Chiotos K, et al. Distinguishing multisystem inflammatory syndrome in children from Kawasaki disease and benign inflammatory illnesses in the SARS-CoV-2 pandemic. Pediatr Emerg Care. 2020;36(11):554 558. ( 10.1097/PEC.0000000000002248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4):968 981.e7. ( 10.1016/j.cell.2020.09.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esteve-Sole A, Anton J, Pino-Ramirez RM, et al. Similarities and differences between the immunopathogenesis of COVID-19–related pediatric multisystem inflammatory syndrome and Kawasaki disease. J Clin Invest. 2021;131(6):1 10. ( 10.1172/JCI144554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohsin SS, Abbas Q, Chowdhary D, et al. Multisystem inflammatory syndrome (MIS-C) in Pakistani children: A description of the phenotypes and comparison with historical cohorts of children with Kawasaki disease and myocarditis. PLOS ONE. 2021;16(6):e0253625. ( 10.1371/journal.pone.0253625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bar-Meir M, Guri A, Godfrey ME, et al. Characterizing the differences between multisystem inflammatory syndrome in children and Kawasaki disease. Sci Rep. 2021;11(1):13840. ( 10.1038/s41598-021-93389-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee J, Kim BJ, Cho KS, Rhim JW, Lee SY, Jeong DC. Similarities and differences between multisystem inflammatory syndrome in children (MIS-C) and Kawasaki disease shock syndrome. Children (Basel). 2023;10(9):1527. ( 10.3390/children10091527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yavuz L, AlHamdani S, Alasrawi S, et al. Kawasaki disease (KD) and multisystem inflammatory syndrome in children (MIS-C) in a Middle Eastern patient cohort. Pediatr Rheumatol Online J. 2023;21(1):64. ( 10.1186/s12969-023-00834-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Batu ED. Multisystem inflammatory syndrome in children vs Kawasaki disease: A never-ending spectrum of phenotypes. Can J Cardiol. 2023. (S0828-282X(23)01517-9). (https://doi.org/10.1016/j.cjca.2023.07.012) [DOI] [PubMed] [Google Scholar]
- 24. Felsenstein S, Duong P, Lane S, Jones C, Pain CE, Hedrich CM. Cardiac pathology and outcomes vary between Kawasaki disease and PIMS-TS. Clin Immunol. 2021;229:108780. ( 10.1016/j.clim.2021.108780) [DOI] [PubMed] [Google Scholar]
- 25. Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016;67(14):1738 1749. ( 10.1016/j.jacc.2015.12.073) [DOI] [PubMed] [Google Scholar]
- 26. Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: A systematic review. J Pediatr. 2020;226:45 54.e1. ( 10.1016/j.jpeds.2020.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haslak F, Barut K, Durak C, et al. Clinical features and outcomes of 76 patients with COVID-19-related multisystem inflammatory syndrome in children. Clin Rheumatol. 2021;40(10):4167 4178. ( 10.1007/s10067-021-05780-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Felsenstein S, Willis E, Lythgoe H, et al. Presentation, treatment response and short-term outcomes in paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS). J Clin Med. 2020;9(10):1 20. ( 10.3390/jcm9103293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Otar Yener G, Paç Kısaarslan A, Ulu K, et al. Differences and similarities of multisystem inflammatory syndrome in children, Kawasaki disease and macrophage activating syndrome due to systemic juvenile idiopathic arthritis: a comparative study. Rheumatol Int. 2022;42(5):879 889. ( 10.1007/s00296-021-04980-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771 1778. ( 10.1016/S0140-6736(20)31103-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernández-Cooke E, Grasa CD, Domínguez-Rodríguez S, et al. Prevalence and clinical characteristics of SARS-CoV-2 confirmed and negative Kawasaki disease patients during the pandemic in Spain. Front Pediatr. 2020;8:617039. ( 10.3389/fped.2020.617039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fridman MD, Tsoukas P, Jeewa A, Yeung RSM, Gamulka BD, McCrindle BW. Differentiation of COVID-19-associated multisystem inflammatory syndrome from Kawasaki disease with the use of cardiac biomarkers. Can J Cardiol. 2023;39(6):815 823. ( 10.1016/j.cjca.2022.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gruber CN, Patel RS, Trachtman R, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell. 2020;183(4):982 995.e14. ( 10.1016/j.cell.2020.09.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26(11):1701 1707. ( 10.1038/s41591-020-1054-6) [DOI] [PubMed] [Google Scholar]
- 35. Ghosh P, Katkar GD, Shimizu C, et al. An Artificial Intelligence-guided signature reveals the shared host immune response in MIS-C and Kawasaki disease. Nat Commun. 2022;13(1):2687. ( 10.1038/s41467-022-30357-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alsaied T, Tremoulet AH, Burns JC, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143(1):78 88. ( 10.1161/CIRCULATIONAHA.120.049836) [DOI] [PubMed] [Google Scholar]
- 37. Bulut M, Ekici F, Kara TT, et al. Echocardiographic findings in children with multisystem inflammatory syndrome from initial presentation to the first years after discharge. Turk Arch Pediatr. 2023;58(5):546 552. ( 10.5152/TurkArchPediatr.2023.23070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsubara D, Kauffman HL, Wang Y, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. 2020;76(17):1947 1961. ( 10.1016/j.jacc.2020.08.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dhanalakshmi K, Venkataraman A, Balasubramanian S, et al. Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome - temporally associated with SARS-CoV-2 (PIMS-TS) in Indian children. Indian Pediatr. 2020;57(11):1010 1014. ( 10.1007/s13312-020-2025-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belot A, Antona D, Renolleau S, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25(22):1 6. ( 10.2807/1560-7917.ES.2020.25.22.2001010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the Covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. ( 10.1136/bmj.m2094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607 1608. ( 10.1016/S0140-6736(20)31094-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lima-Setta F, Magalhães-Barbosa MC, Rodrigues-Santos G, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J Pediatr (Rio J). 2021;97(3):354 361. ( 10.1016/j.jped.2020.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25 e28. ( 10.3928/19382359-20161212-01) [DOI] [PubMed] [Google Scholar]
- 45. Ouldali N, Toubiana J, Antona D, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325(9):855 864. ( 10.1001/jama.2021.0694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kessel C, Koné-Paut I, Tellier S, et al. An Immunological Axis Involving interleukin 1β and leucine-Rich-α2-glycoprotein Reflects Therapeutic Response of Children with Kawasaki disease: implications from the KAWAKINRA Trial. J Clin Immunol. 2022;42(6):1330 1341. ( 10.1007/s10875-022-01301-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee KH, Li H, Lee MH, et al. Clinical characteristics and treatments of multisystem inflammatory syndrome in children: a systematic review. Eur Rev Med Pharmacol Sci. 2022;26(9):3342 3350. ( 10.26355/eurrev_202205_28754) [DOI] [PubMed] [Google Scholar]
- 48. Kaya Akca U, Atalay E, Cuceoglu MK, et al. Impact of the COVID-19 pandemic on the frequency of the pediatric rheumatic diseases. Rheumatol Int. 2022;42(1):51 57. ( 10.1007/s00296-021-05027-7) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a