Abstract
Miniaturized growth systems for heterogeneous culture collections are not only attractive in reducing demands for incubation space and medium but also in making the parallel handling of large numbers of strains more practicable. We report here on the optimization of oxygen transfer rates in deep-well microtiter plates and the development of a replication system allowing the simultaneous and reproducible sampling of 96 frozen glycerol stock cultures while the remaining culture volume remains frozen. Oxygen transfer rates were derived from growth curves of Pseudomonas putida and from rates of oxygen disappearance due to the cobalt-catalyzed oxidation of sulfite. Maximum oxygen transfer rates (38 mmol liter−1 h−1, corresponding to a mass transfer coefficient of 188 h−1) were measured during orbital shaking at 300 rpm at a shaking diameter of 5 cm and a culture volume of 0.5 ml. A shaking diameter of 2.5 cm resulted in threefold-lower values. These high oxygen transfer rates allowed P. putida to reach a cell density of approximately 9 g (dry weight) liter−1 during growth on a glucose mineral medium at culture volumes of up to 1 ml. The growth-and-replication system was evaluated for a culture collection consisting of aerobic strains, mainly from the genera Pseudomonas, Rhodococcus, and Alcaligenes, using mineral media and rich media. Cross-contamination and excessive evaporation during vigorous aeration were adequately prevented by the use of a sandwich cover of spongy silicone and cotton wool on top of the microtiter plates.
The general practice during screening of large microbial strain collections for new enzyme activities or bioactive compounds is that each strain is handled individually. The labor and time involved in separately reviving the stock cultures on agar plates and the subsequent inoculation of tubes or Erlenmeyer flasks are major limiting factors in the progress of such screening projects. This bottleneck might be alleviated if large numbers of microbial strains could be handled in parallel rather than in sequence, preferably in a format that is compatible with current standards in the manipulation of large numbers of liquid samples, e.g., in 96- or 384-well microtiter plates.
Until now, the use of microtiter plates for the growth and maintenance of microbial strains has been mainly limited to clonal libraries in Escherichia coli (9, 11, 20) and yeasts (2, 18). For this purpose, the apparent drawbacks of growth in microtiter plates—low aeration rates and small working volumes—are generally less relevant since E. coli and yeasts can grow anaerobically. Furthermore, small amounts of biomass generally suffice as the use of high-copy-number vectors ensures high levels of the desired gene or gene product. The resulting mixed aerobic-anaerobic growth, however, can lead to undesirable variations in biomass levels from well to well, especially since oxygen diffusion rates for an individual well may be affected by its position in the microtiter plate. Such well-to-well variations are especially troublesome in quantitative screenings aimed at identifying high-activity mutants (e.g., generated by directed evolution [3]) that display only slightly altered activities.
For screening heterogeneous culture collections of aerobic microbial strains, the requirements for biomass quantities and aeration rates are usually more stringent than for gene banks because desired enzymes or metabolites are present at natural (low) levels. Aeration rates are crucial not only with regard to the final cell mass that can be obtained but also for avoiding oxygen limitation-associated stress responses and excessive acid formation due to incomplete utilization of the supplied carbon and energy sources.
The present article is focused on two major problems in miniaturization of microbial growth systems: (i) how to reach oxygen transfer rates (OTRs) similar to those achieved in regular growth systems like Erlenmeyer shake flasks and stirred bioreactors, while preventing cross-contamination and excessive evaporation (OTRs were determined under various aeration conditions, using both biological and chemical methods), and (ii) how to set up a reliable and reproducible system for the inoculation of microtiter plates with strains from a frozen, heterogeneous culture collection.
Estimation of OTRs in deep-well microtiter plates from growth curves of Pseudomonas putida CA-3.
OTRs in microtiter plates under various conditions (Fig. 1) were estimated from the increase in cell mass during oxygen-limited growth of P. putida CA-3 (a kind gift of Alan Dobson of Cork University College, Cork, Ireland) with glucose as the sole source of carbon and energy. For this purpose we used polypropylene Megaplate deep-well plates (Polylabo, Geneva, Switzerland), which have 40-mm-deep square wells with a cross-section of 8.2 by 8.2 mm, a rounded bottom, and a total filling volume of 2.4 ml. Before initial use, the plates were soaked sequentially in boiling NaOH (1 M) for 4 h and HCl (1 M, 70°C) for 4 h in order to remove potentially toxic UV absorbers and antioxidants (e.g., phenols and phosphites), whiteners (coumarins and benzoxazoles), and heavy metals which are often used as additives in polypropylene (4).
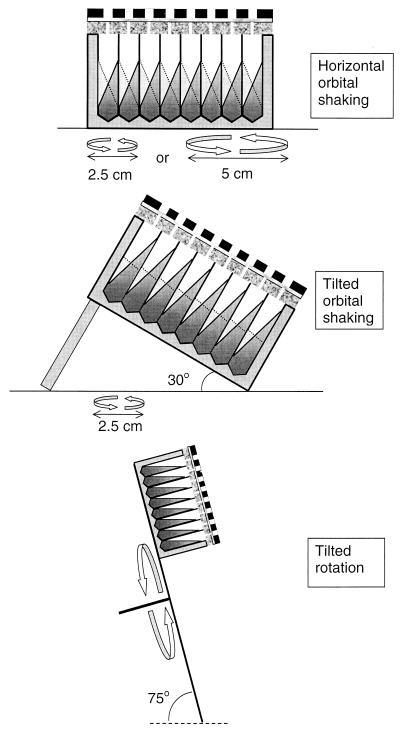
FIG. 1.
Methods of aeration that were assessed for maximum OTRs as shown in Table 1 and Fig. 2. The solid lines inside the wells in the upper two panels represent the culture surface when the orbital shaker is at the outer right side of its rotating movement (as determined by photographic analysis). The dashed lines represent the culture surface when the orbital shaker is at the outer left side of its rotating movement.
P. putida CA-3 was grown overnight in a 300-ml Erlenmeyer flask (without baffles) filled with 35 ml of mineral medium (8) supplied with nitrilotriacetic acid (4 mM) as the complexing agent, glucose (150 mM) as the sole carbon and energy source, and a K2HPO4-KH2PO4 buffer (200 mM) at pH 7.2. The flask was incubated at 25°C on an orbital shaker (300 rpm; shaking diameter, 5 cm). This preculture was used to inoculate a second-batch culture with the same properties. When this second culture had reached an optical density at 540 nm (OD540) of approximately 1.0 (corresponding to 0.40 g [dry weight] liter−1, as determined by drying the washed pellet of a 20-ml cell suspension [OD540 = 24] at 110°C for 18 h and subsequent weighing on an analytical balance), aliquots of 500, 750, and 1,000 μl were transferred to the wells of several microtiter plates. To prevent cross-contamination between suspended cultures in adjacent wells (e.g., via aerosols) during shaking, the microtiter plate was covered with a spongy silicone plate (thickness, 8 mm; nonpermeable film on both sides; quality, 2,660 shore; Maag Technik AG, Dübendorf, Switzerland) perforated with 96 holes 1.5-mm in diameter (holes positioned exactly above the centers of the wells). This spongy silicone plate in turn was covered with a rigid polypropylene plate perforated with 96 holes 6 mm in diameter. A thin layer (2 mm) of cotton wool was placed between the plates. The microtiter plate and the sandwich cover were clamped together with a force of approximately 500 N using an appropriate clamp, causing the silicone plate to become uniformly compressed by a factor of 2 (from a thickness of 8 to 4 mm). Subsequently, the microtiter plate was incubated at 25°C on an orbital shaker with a shaking diameter of either 2.5 or 5 cm (Kühner AG, Basel, Switzerland) or on a rotating platform at 60 rpm (angle of platform with horizontal plane, 75°; center of the microtiter plate, 18 cm from the center of the platform [Fig. 1]). At regular intervals, small samples (10 to 30 μl) were taken from the cultures using a 50-μl syringe (Hamilton, Reno, Nev.). The samples were diluted in a physiological salt solution (0.9% [wt/vol] NaCl, 20 mM K2HPO4-KH2PO4 buffer [pH 7.2]), and the OD540 was measured in glass microcuvettes in a Spectramax plus spectrophotometer (Molecular Devices, Menlo Park, Calif.), which shows a linear response for cell suspensions of P. putida CA-3 at 540 nm between 0 and 0.4 (five data points, R2 = 0.9995). In order to translate biomass formation rates in the linear, oxygen-limited part of the growth curve (slope determined by linear regression) into OTRs, a conversion factor is required. This conversion factor was derived from the decrease in oxygen concentration in the headspace of a 290-ml culture of P. putida CA-3 growing on the glucose mineral medium described above in a 3,000-ml Erlenmeyer flask incubated at 280 rpm on an orbital shaker (5-cm shaking diameter; Kühner AG) at 25°C. The headspace air from the closed Erlenmeyer flask was continuously refreshed at a constant rate of 310 ml/min using a pneumatic unit (Bioengineering, Wald, Switzerland) as a mass-flow controller. The oxygen concentration in the outflow from the headspace was measured continuously using an amperometric oxygen probe (Mettler Toledo, Greifensee, Switzerland) which was calibrated with air and a gas mixture containing 18.95% oxygen (Pangas, Winterthur, Switzerland). Each hour a 1-ml sample was taken from the culture, and the OD540 was measured after appropriate dilution as described above. A linear growth phase occurred between OD540 values of 4.1 and 16.2 (six data points, R2 = 0.9928), during which the average biomass formation rate was 1.99 OD540 units/h and the oxygen concentration in the outflow from the headspace was 19.84 ± 0.05%, corresponding to an oxygen consumption of 0.43 mmol liter of air−1 and an oxygen consumption rate in the culture of 29.1 mM h−1. Division of this value by the biomass formation rate yields the conversion factor of 14.6 mM O2 OD540 unit−1 h−1 or 36.4 mmol of O2 g (dry weight) formed−1.
Using this procedure, the highest OTR was found during orbital shaking at 300 rpm with a shaking diameter of 5 cm and culture volume of 500 μl (38 mmol liter−1 h−1 [Table 1]). In the following three sections, the effect of the culture volume, the shaking diameter, and the shaking angle are discussed.
TABLE 1.
Effect of shaking parameters on OTRs in square deep-well microtiter plates
| Type of movement | Angle with horizontal plane | Rotating velocity (rpm) | Diam (cm) | Froude no.a | OTRs (mmol of O2 liter−1 h−1)b at 3 different aqueous vols (μl)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 500
|
750
|
1,000
|
||||||||
| Biol.c | Chem.d | Biol. | Chem. | Biol. | Chem. | |||||
| None | 0° | 0 | 0 | 2.1 | NDe | 1.6 | ND | 1.6 | ND | |
| Orbital shaking | 0° | 300 | 2.5 | 1.3 | 12.5 | 11.0 ± 0.8 | 7.4 ± 0.9 | 7.5 ± 0.2 | 3.5 | 5.8 ± 0.4 |
| 30° | 300 | 2.5 | 1.3 | 22.0 ± 0.6 | ND | 12.0 ± 0.6 | ND | 10.2 | ND | |
| 0° | 375 | 2.5 | 2.0 | 19.4 | ND | 15.1 | ND | 10.9 | ND | |
| 0° | 300 | 5 | 2.5 | 37.8 ± 2.6 | 27.3 ± 0.4 | 23.8 ± 2.2 | 19.8 ± 0.4 | 17.7 ± 0.7 | 16.3 ± 0.1 | |
| Rotation | 75° | 60 | 18 | 0.7 | 20.7 | ND | 15.7 | ND | 12.5 | ND |
The Froude number represents the ratio between centrifugal and gravitational acceleration.
Values are averages of three or more measurements ± standard deviations. Single values are results of single measurements.
Biol. indicates OTRs determined from growth curve of P. putida CA-3 on a glucose mineral medium.
Chem. indicates OTRs determined by the sulfite oxidation method.
ND, Not determined.
Effect of the culture volume on oxygen transfer.
The culture volume appeared to have a clear-cut effect on the OTR: OTRs at a culture volume of 500 μl were about twice those in 1,000-μl cultures for the various conditions tested (Table 1), implying that the absolute amount of oxygen transferred per well per unit of time was not affected by the working volume. These results are in agreement with previous studies using shake flasks and test tubes (unpublished results) and indicate that the main determinant of the amount of oxygen transferred into a culture is the oxygen transfer area, which is not significantly affected by the aqueous volume.
Effect of the shaking diameter on oxygen transfer.
The ratio of the 300-rpm OTRs measured at a shaking diameter of 5 cm to those measured at a shaking diameter of 2.5 cm (Table 1; Fig. 2) averaged 3.2. This large difference may be partially explained by the larger mass transfer area at a shaking diameter of 5 cm. Table 1 shows the Froude number for different culture conditions. This pure number represents the ratio between centrifugal and gravitational acceleration. From the arc tangent of this number, the theoretical angle of the liquid inside the wells can be calculated. The relative increase of the mass transfer area inside the wells due to shaking is given by the formula (1 + Fr2)1/2, where Fr is the Froude number. Thus, the expected ratio between the mass transfer areas at 300 rpm for shaking diameters of 5 and 2.5 cm can be calculated to be 1.7. The discrepancy between 3.2 and 1.7 (the OTRs at a shaking diameter of 5 cm are larger than expected on the basis of differences of calculated mass transfer areas) may have two sources. First, the actual mass transfer area at a shaking diameter of 5 cm might actually be larger than calculated on the basis of the angle of the surface with the horizontal plane; photographs showed that the liquid surface was irregular (a wave pattern was visible). Second, the actual angle of the aqueous surface with the horizontal plane (measured from photographs of a shaking microtiter well filled with 750 μl of colored medium) at a shaking diameter of 2.5 cm was found to be lower than the theoretical angle based on the Froude number (40 to 45° rather than 51°), whereas there was no such discrepancy at a shaking diameter of 5 cm (68°). The lower-than-expected angle at a shaking diameter of 2.5 cm might be due to “out-of-phase conditions,” which are characterized by the failure of the bulk of the liquid volume to follow the movement of the shaker table (6). As a result the surface area and the mass transfer rates are reduced. This phenomenon appears in regular Erlenmeyer flasks only at an elevated viscosity, but in the case of baffled shaken bioreactors, it may also appear at a water-like viscosity depending on the operating conditions (5). The square shape of the wells of the microtiter plates used may be of particular interest in this respect: it can be assumed that the corners of the square wells disturb the flow in a way similar to that of baffles in shake flasks or fermentors.
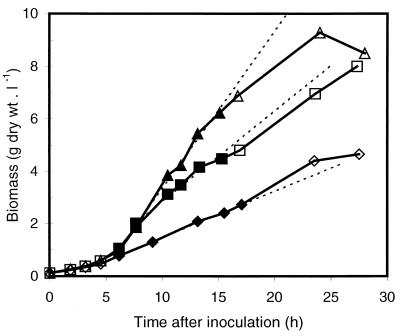
FIG. 2.
Growth curves of P. putida CA-3 on a glucose mineral medium (750 μl) in deep-well microtiter plates incubated under various conditions: orbital shaking, 2.5-cm diameter, 300 rpm (⧫ and ◊); orbital shaking, 2.5-cm diameter, 375 rpm (■ and □); and orbital shaking, 5-cm diameter, 300 rpm (▴ and ▵). The dashed lines denote the slopes resulting from linear regression of the data points represented with solid symbols in the linear, oxygen-limited part of the growth curve. These slopes were used to calculate the OTRs given in Table 1.
Effect of the shaking angle on oxygen transfer.
The OTRs at a shaking diameter of 2.5 cm could be augmented either by tilting the microtiter plate under an angle of 30° or by increasing the shaking frequency to 375 rpm but were still lower than those measured during horizontal shaking at a shaking diameter of 5 cm and 300 rpm (Table 1). Rotation of the microtiter plate on a platform placed at an angle of 75° with the horizontal plane, although impractical for large numbers of plates, gave surprisingly high OTRs (Table 1) despite the low rotation speed (60 rpm). This result further supports the earlier conclusion that the surface area is a more important determinant for OTRs than the frequency of movement.
Validation of biological oxygen transfer data using a chemical method.
The catalyzed oxidation of sodium sulfite to sulfate was employed to measure maximum OTRs chemically in the same type of microtiter plates. A solution of 0.5 M sodium sulfite (98% purity; Roth, Karlsruhe, Germany) buffered by a Na2HPO4-NaH2PO4 buffer (12 mM, pH 8.0) was used. The reaction was catalyzed by 0.1 μM cobalt sulfate (Fluka, Buchs, Switzerland). The rate of oxygen disappearance in the headspace of a well was determined using an oxygen electrode (Fast Silver Bullet, part number 6850930; Dräger, Lübeck, Germany) which was fitted to the plate in a gastight manner using a flexible silicon plate (Riplate; Ritter, Schwabmünchen, Germany) perforated with a hole 3.5 mm in diameter. From the slope of the signal of the electrode, the OTR was calculated. The OTRs obtained with the sulfite method showed the same trends as those obtained using the biological method (Table 1). The OTRs measured by the sulfite method, however, were generally slightly lower than those derived from the growth curves of P. putida CA-3. Similar differences have been observed previously in shake flasks (unpublished results) and can be attributed to the higher salt concentration used in the sulfite method. A higher salt concentration or ionic strength leads to lower oxygen solubility (17) and lower oxygen transfer coefficients (14, 21) due to smaller diffusion coefficients (1, 12).
Maximal biomass attainable and evaporation rates.
At a shaking diameter of 5 cm and 300 rpm, P. putida CA-3 reached final biomass concentrations of approximately 9 g (dry weight) liter−1 for all 3 culture volumes used. No residual glucose could be detected in the medium after growth had ceased, suggesting that even higher biomass concentrations could be attained if more carbon and energy were supplied. The switch from exponential to linear, oxygen-limited growth occurred at biomass concentrations of 5, 4, and 2 g (dry weight) liter−1 for culture volumes of 500, 750, and 1,000 μl respectively. No significant loss of culture volume was observed (less than 10 μl per well per day) under any of the conditions tested. The holes in the silicone plate were apparently sufficiently small to prevent excessive evaporation or the escape of aerosols.
Comparison to oxygen transfer in other growth systems.
The maximum OTR reached (38 mmol liter−1 h−1) in the biological system at a culture volume of 0.5 ml is similar to that in 250-ml Erlenmeyer flasks filled with 25 ml of medium under identical shaking conditions (40 mmol liter−1 h−1 [15]). By applying methods developed for the evaluation of the mass transfer resistance of the sterile plug in shaking flasks (10, 16), the minimum concentration in the headspace of the wells (representing the mass transfer resistance of our lid) was estimated. At a maximum OTR of 38 mmol liter−1 h−1 and a filling volume of 500 μl, the minimum headspace oxygen concentration was calculated to be 18% (vol/vol). Taking into account the oxygen solubility for our growth media (2.01 × 10−4 mol liter−1 at 25°C [17]), a mass transfer coefficient of 188 h−1 can be calculated, which is in the range of values (100 to 300 h−1) reported for standard stirred-tank bioreactors with volumes between 0.03 and 50 m3 (19).
Maintenance of a heterogeneous culture collection in a microtiter plate.
The premise in our approach was that miniaturization of growth systems for heterogeneous microbial culture collections will save significant time in high-throughput screening programs only if all essential stages and handling steps (storage, inoculation, growth in liquid medium, incubation with substrate, and analysis) are miniaturized in the same format. To develop and test a system meeting these requirements, we used a heterogeneous set of 48 bacterial strains isolated from various polluted and pristine soil samples for their ability to grow aerobically on aromatic hydrocarbons; the taxonomy given in Table 2 was based on partial 16S RNA sequencing by the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). A master plate containing cell suspensions (approximately 2 g [dry weight] liter−1) of these 48 strains in the presence of glycerol was prepared as follows. Each well of a sterile Bel-Art deep-well microtiter plate (Bel-Art, Pequannock, N.J.; this type of plate has 1.6-mm-thick walls and is mechanically more stable at −80°C than the plates used in the previous sections) was filled with 300 μl of nutrient broth (8 g/liter; Difco, Detroit, Mich.) and agar (20 g/liter; Difco). After solidification, the 48 strains were inoculated using sterile syringe needles onto the agar surfaces, directly from the frozen glycerol stocks in which the strains had been maintained since their isolation. The strains were inoculated in a checkered pattern; all wells directly flanking an inoculated well were used as control blanks. After the strains had been allowed to grow for 3 days on the agar surfaces at an ambient temperature, 500 μl of sterile nutrient broth medium (8 g/liter) was added to all wells. Subsequently, the microtiter plate was closed using a spongy silicone cover as described above and incubated in an orbital shaker (300 rpm; shaking diameter, 5 cm) at 25°C. After growth for 3 days, 200 μl of a 60% (vol/vol) glycerol solution (final concentration, 15% [vol/vol]) was added to each well using a 12-channel pipette (mixed by filling and emptying the tips five times). The microtiter plate was then covered with a polypropylene lid (Bel-Art) and stored at −80°C.
TABLE 2.
Replication from frozen master cultures and subsequent growth of various strains
| Organism | No. of strains (code[s]a) | Avg no. of CFU replicated (103)b | Avg OD540
|
|
|---|---|---|---|---|
| After inoculationc | After 3 days of shakingd | |||
| Achromobacter xylosoxidans | 1 (h6) | 10 | 0.07 | 20 |
| Alcaligenes sp. | 3 (d12, e1, g3) | 9 | 0.08 ± 0.02 | 0.20 ± 0.13 |
| Pseudomonas azotoformans | 2 (d4, g5) | 35 | 0.14 | 29 |
| Pseudomonas cepacia | 1 (h2) | 50 | 0.19 | 46 |
| Pseudomonas fluorescens | 2 (g1, h12) | 35 | 0.13 | 17 |
| Pseudomonas fulva | 2 (d8, f6) | 20 | 0.06 | 32 |
| Pseudomonas marginalis | 6 (c9, c11, e3, e5, f8, f10) | 50 | 0.07 ± 0.06 | 21 ± 10 |
| Pseudomonas mendocina | 1 (g11) | 20 | 0.05 | 0.26 |
| Pseudomonas putida | 5 (a9, g9, h4, h8, h10) | 4 | 0.12 ± 0.07 | 26 ± 8 |
| Pseudomonas stutzeri | 3 (d6, f4, g7) | 26 | 0.13 ± 0.03 | 32 ± 9 |
| Pseudomonas syringae | 6 (b10, c1, c7, d10, f2, f12) | 39 | 0.14 ± 0.06 | 30 ± 7 |
| Pseudomonas viridiflava | 1 (b12) | 5 | 0.06 | 34 |
| Rhodococcus erythropolis | 2 (a3, a5) | 58 | 0.20 ± 0.04 | 12 |
| Rhodococcus globerulus | 5 (a1, a11, b4, e7, e11) | 8 | 0.13 ± 0.05 | 9.6 ± 1.3 |
| Rhodococcus opacus | 6 (a7, b2, b6, b8, c5, d2) | 3 | 0.01 ± 0.01 | 16 ± 11 |
| Rhodococcus sp. | 1 (e9) | 1 | 0.09 | 38 |
| Variovorax sp. | 1 (c3) | 50 | 0.12 | 0.16 |
Strain codes from the authors' laboratory (corresponding to their position on the master plate).
Number of CFU transferred from the frozen master cultures using the spring-loaded replicator, as measured by the number of colonies on nutrient broth agar.
OD540 of 750-μl cultures just after inoculation with colonies from a nutrient broth agar plate.
OD540 of 750-μl cultures (mineral medium, 150 mM glucose, 10 mM aspartate) after 3 days of incubation at 25°C (300 rpm; shaking diameter, 5 cm).
Simultaneous sampling of strains stored in microtiter plates.
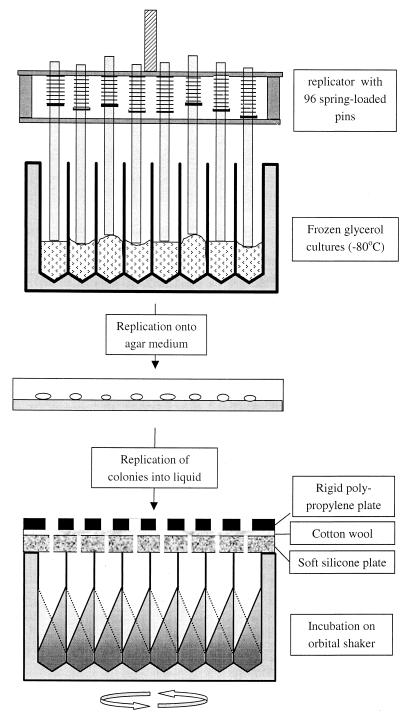
Since repeated freezing and thawing of glycerol cultures may result in rapid loss of viability of the stored bacterial cells, a replicator that allows the removal of small aliquots from all the frozen stocks without thawing the remaining culture was developed (Fig. 3). This replicator (7) consists of 96 steel pins (diameter, 3 mm) able to move up individually under the back pressure (approximately 0.2 N) of light springs, enabling all pins to touch the surfaces of the frozen cultures even though these surfaces may be at different levels due to small differences in volume and unpredictable patterns during freezing (Fig. 3). This replicator (flame sterilized and allowed to cool to room temperature) was pressed onto the frozen surface of the cultures in the master plate for approximately 3 s at a force sufficient to move up each pin for at least 5 mm relative to the frame of the replicator in which the pins are allowed to travel. The transfer of heat from a pin (initially at room temperature) to the frozen surface during the 3-s contact period was found sufficient to cause the melting of approximately 0.3 μl of the culture, which subsequently formed a thin film (thickness of approximately 50 μm) on the end of the pin. This sampling procedure was quantitatively validated by diluting this volume in a physiological salt solution followed by plating onto nutrient broth agar medium. The resulting number of CFU varied between 180 and 100,000 for the 48 strains tested (Table 2). Most stock cultures gave rise to more than 5,000 CFU. Only stock cultures of a number of P. putida, Rhodococcus opacus, Rhodococcus globerulus, and Rhodococcus sp. strains resulted in lower CFU (Table 2).
FIG. 3.
System for the retrieval and growth of a bacterial strain collection in microtiter plate format. The spring-loaded replicator is used for the parallel sampling of up to 96 stock cultures frozen in glycerol; the surfaces of all stock cultures are contacted by the pins even though the surfaces are not exactly at the same level. The sampled cells are allowed to grow on an agar plate for several days. The colonies formed are then transferred to a square-well microtiter plate filled with liquid medium, covered as shown, and incubated on an orbital shaker.
In order to “revive” the sampled cell material, the replicator was transferred immediately from the glycerol stock culture onto an agar plate (2% agar and nutrient broth [3 g/liter], 15 cm in diameter) resulting in a regular array of colonies within 3 days. At such low concentrations of nutrient broth, the colonies remained sufficiently small (approximately 4 mm in diameter) to prevent cross-contamination. This procedure is suitable for strains that form distinct colonies and are capable of growth on the same medium. For swarming strains or collections in which individual members have special antibiotic or growth requirements, one can consider the use of agar medium dispensed in microtiter plates. Direct inoculation of frozen stock cultures in liquid nutrient broth medium resulted in significant growth within 3 days for only 50% of the tested strains (results not shown). The intermediate step of reviving the strains on an agar-based medium that we routinely use is apparently required for many strains.
Parallel growth of the heterogeneous culture collection in suspended culture.
After 3 days of growth at an ambient temperature, the formed colonies were simultaneously transferred to a microtiter plate supplied with a mineral medium (as described above) containing 150 mM glucose and 10 mM aspartate (the latter was found to decrease the lag time) as carbon sources (750 μl per well). For this purpose the same spring-loaded replicator was used. The pins were simultaneously loaded with biomass from the agar plate and subsequently pressed down on the bottom of the wells. Lateral movement of the replicator in the wells resulted in the transfer of most of the cell mass from the pins into the liquid medium. The amount of cell mass transferred resulted in initial OD540 values (measured after appropriate dilution in a physiological salt solution as described above) ranging from 0.3 to 2 for Pseudomonas strains and most Rhodococcus strains and being below 0.1 for the dry-crusted colonies of R. opacus strains, for which only small but sufficient numbers of cells were transferred by the pins (Table 2). Subsequently, the wells were covered with a pierced silicone cover plate as described above and aerated by shaking on a 5-cm orbital shaker for 3 days. A major advantage of such a dense inoculation of the liquid culture medium is its high viability: more than 90% of the strains grew to an OD540 of more than 5 within 3 days (Table 2). No growth occurred in the 48 uninoculated wells.
Reproducibility of growth of the heterogeneous culture collection in nutrient broth medium.
In order to further validate the system, especially its reproducibility and susceptibility to cross-contamination and contamination from outer air during growth in suspended culture, we performed tests in duplicate with the same set of 48 strains using a rich medium that supported growth of a wide range of possible contaminants (nutrient broth). The collection was replicated from the frozen master plate onto two nutrient broth agar plates in the same way as described above. On both agar plates, all 48 strains appeared to have given rise to colonies after 3 days of incubation at room temperature. These were used to inoculate two microtiter plates containing nutrient broth (8 g/liter in 50 mM KH2PO4-K2HPO4 buffer [pH 7.2]) medium (750 μl per well). Subsequently, the wells were covered with a sandwich cover plate as described above and aerated by shaking on a 5-cm orbital shaker for 3 days (300 rpm, 25°C). The noninoculated wells were checked for contamination by replicating the contents of the 96 wells of both plates onto nutrient broth-agar plates. After 3 days of incubation at room temperature, colonies appeared only at positions corresponding to the inoculated wells. No colonies appeared at the 48 positions corresponding to the uninoculated wells, even though these blank wells were all flanked by at least two wells containing actively growing cultures. This proves that the risk of cross-contamination during 3 days of vigorous shaking is sufficiently contained by the sandwich cover.
In addition to these checks for cross-contamination, the OD540 values of the 48 suspended cultures of the duplicate microtiter plates were measured and compared. For all uninoculated wells, the OD540 was below 0.01. OD540 values for the 48 different strains ranged from 3.3 (a P. putida strain) to 1.16 (an Alcaligenes sp. strain) with an average of 1.98 ± 0.63. Reproducibility was assessed by statistically comparing the duplicate values of individuals trains in the two microtiter plates. For 18 strains the OD540 values of the duplicates differed by less than 5% (the absolute difference divided by the highest value), while for 13 strains they differed by 5 to 10% and for 13 other strains they differed by 10 to 20%. Only 4 of the 48 strains showed a difference larger than 20% (all differences were lower than 34%). The cell suspensions of three of these four strains were observed not to be homogeneous (aggregate formation), which might explain the relatively large differences between the duplicates. This additional set of experiments was performed 14 months after the frozen master plate had been prepared and had been sampled at least 20 times in the intervening period. The reproducible viability of the sampled cell material is an indication of the quality and longevity of master cultures prepared and handled as described here.
Applications.
The replication and growth system described here has been routinely used to screen our culture collection for the presence of specific enzyme activities. A number of monooxygenases catalyzing the regio- and enantiospecific hydroxylation of N-benzylpyrrolidine were discovered within a collection of bacterial strains maintained and grown according to the currently described methods (13). For screenings using our complete collection (1,500 bacterial strains including actinomycetes) in microtiter plate format, the time needed to arrive at the stage of washed cell suspensions was generally 7 to 8 days and involved approximately 20 working hours, including medium preparation and dispension. One orbital shaker (75 by 60 cm) was sufficient to accommodate 20 microtiter plates. Further miniaturization (e.g., to 384-well plates) seems to be of limited value for our purposes, especially since analysis for product formation is now the major time-limiting factor. Possible improvements of the system described here may come from the use of orbital shakers with larger shaking amplitudes (e.g., 7.5 or 10 cm) and the use of replicators capable of sampling multiple microtiter plates simultaneously. Besides the presently described use for heterogeneous culture collections, the system could also prove valuable for screening gene and mutant banks in which the amounts of cell mass and/or reproducibility are important factors, e.g., in projects involving directed evolution (3), gene shuffling (3), or functional genomics. Since the OTRs are in the same range as those reported for stirred-tank bioreactors, the described growth system may also be highly applicable for studies aimed at optimizing the composition of medium used for large-scale bioprocesses.
REFERENCES
- 1.Akita K. Diffusivities of gases in aqueous electrolyte solutions. Ind Eng Chem Fundam. 1981;20:89–94. [Google Scholar]
- 2.Anand R, Riley J H, Butler R, Smith J C, Markham A F. A 3.5 genome equivalent multi access YAC library: construction, characterisation, screening and storage. Nucleic Acids Res. 1990;18:1951–1956. doi: 10.1093/nar/18.8.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold F H, Volkov A A. Directed evolution of biocatalysts. Curr Opin Chem Biol. 1999;3:54–59. doi: 10.1016/s1367-5931(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 4.Becker R F, Burton L P J, Amos S E. Additives. In: Moore E P Jr, editor. Polypropylene handbook. Cincinnati, Ohio: Hanser-Gardner; 1996. pp. 177–210. [Google Scholar]
- 5.Büchs J, Maier U, Milbradt C, Zoels B. Power consumption in shaking flasks on rotary shaking machines, part II: non-dimensional description of the specific power consumption and flow-regimes in unbaffled flasks at elevated liquid viscosity. Biotechnol Bioeng. 2000;68:594–601. [PubMed] [Google Scholar]
- 6.Büchs, J., S. Lotter, and C. Milbradt. Out-of-phase operating conditions, a hitherto unknown phenomenon in shaken bioreactors. Biochem. Eng. J., in press. [DOI] [PubMed]
- 7.Duetz W A, Witholt B. Werkwijze en inrichting voor het gelijktijdig en onafhankelijk van elkaar bemonsteren van ingevroren cultures van micro-organismen en/of eukaryote cellen. Dutch Patent Office; June 1999. [Google Scholar]
- 8.Evans C T G, Herbert D, Tempest D W. The continuous cultivation of microorganisms. 2. Construction of a chemostat. Methods Microbiol. 1970;2:277–327. [Google Scholar]
- 9.Giege P, Konthur Z, Walter G, Brennicke A. An ordered Arabidopsis thaliana mitochondrial cDNA library on high-density filters allows rapid systematic analysis of plant gene expression: a pilot study. Plant J. 1998;15:721–726. doi: 10.1046/j.1365-313x.1998.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Henzler H J, Schedel M. Suitability of the shaking flask for oxygen supply to microbiological cultures. Bioprocess Eng. 1991;7:123–131. [Google Scholar]
- 11.Hersh B M, Farooq F T, Barstad D N, Blankenhorn D L, Slonczewski J L. A glutamate-dependent acid resistance gene in Escherichia coli. J Bacteriol. 1996;178:3978–3981. doi: 10.1128/jb.178.13.3978-3981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung G W, Dinius R H. Diffusivity of oxygen in electrolyte solutions. J Chem Eng Data. 1972;4:449–458. [Google Scholar]
- 13.Li Z, Feiten H-J, van Beilen J B, Duetz W, Witholt B. Preparation of optically active N-benzyl-3-hydroxy-pyrrolidine. Tetrahedron Asymmetry. 1999;10:1323–1333. [Google Scholar]
- 14.Linek V, Mayrhoferova J, Mosnerova J. The influence of diffusivity on liquid phase mass transfer in solutions of electrolytes. Chem Eng Sci. 1970;25:1033–1045. [Google Scholar]
- 15.Maier, U., and J. Büchs. Characterization of the gas-liquid mass transfer in shaken bioreactors. Biochem. Eng. J., in press. [DOI] [PubMed]
- 16.Mrotzek, C., T. Anderlei, J. Büchs, and H. J. Henzler. Diffusion resistance of sterile plugs of shaken bioreactors. Biochem. Eng. J., in press. [DOI] [PubMed]
- 17.Schumpe A, Quicker G, Deckwer W D. Gas solubilities in microbial culture media. In: Binder H, et al., editors. Reaction engineering. Berlin, Germany: Springer-Verlag; 1982. pp. 1–38. [Google Scholar]
- 18.Takeda H, Yamakuchi H, Ihara N, Hara K, Watanabe T, Sugimoto Y, Oshiro T, Kishine H, Kano Y, Kohno K. Construction of a bovine yeast artificial chromosome (YAC) library. Anim Genet. 1998;29:216–219. doi: 10.1046/j.1365-2052.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 19.Trilli A. Scale-up of fermentations. In: Demain A L, Solomon N A, editors. Manual of industrial microbiology and biotechnology. Washington, D.C.: American Society for Microbiology; 1986. pp. 277–307. [Google Scholar]
- 20.Wetzel R, Perry L J, Veilleux C, Chang G. Mutational analysis of the C-terminus of human interferon-γ. Protein Eng. 1990;3:611–623. doi: 10.1093/protein/3.7.611. [DOI] [PubMed] [Google Scholar]
- 21.Zieminski S A, Whittemore R C. Behavior of gas bubbles in aqueous electrolyte solutions. Chem Eng Sci. 1971;26:509–520. [Google Scholar]